Abstract
Neutrophil chemotaxis plays an essential role in innate immunity, but the underlying cellular mechanism is still not fully characterized. Here, using a small-molecule functional screening, we identified NADPH oxidase–dependent reactive oxygen species as key regulators of neutrophil chemotactic migration. Neutrophils with pharmacologically inhibited oxidase, or isolated from chronic granulomatous disease (CGD) patients and mice, formed more frequent multiple pseudopodia and lost their directionality as they migrated up a chemoattractant concentration gradient. Knocking down NADPH oxidase in differentiated neutrophil-like HL60 cells also led to defective chemotaxis. Consistent with the in vitro results, adoptively transferred CGD murine neutrophils showed impaired in vivo recruitment to sites of inflammation. Together, these results present a physiological role for reactive oxygen species in regulating neutrophil functions and shed light on the pathogenesis of CGD.
Keywords: chronic granulomatous disease, innate immunity, NADPH oxidase
Neutrophils are major players in innate immunity and constitute the first line of host defense against invading bacteria and other pathogens. In response to inflammatory stimuli, neutrophils migrate from the blood to infected tissues, where they protect their host by engulfing, killing, and digesting invading bacterial and fungal pathogens. Conversely, excessive neutrophil accumulation can be detrimental to the system. Hence, neutrophil recruitment in response to inflammatory stimuli needs to be well controlled.
Neutrophils are recruited to the site of infection by responding to a variety of chemokines, leukotrienes, complement peptides, and some chemicals released by bacteria directly, such as peptides bearing the N-formyl group (i.e., formyl-peptides). Neutrophil chemotaxis is mediated by heterotrimeric guanine nucleotide-binding regulatory proteins (G protein)–coupled receptors (GPCRs). One essential downstream target of GPCR is PtdIns(3,4,5)P3. Chemoattractants bind receptors on cell membrane and induce the dissociation of a specific G protein into α- and βγ-subunits. Released βγ-subunits initiate accumulation of PtdIns(3,4,5)P3 and subsequent actin polymerization at the leading edge of chemotaxing cells. Earlier studies have suggested that PtdIns(3,4,5)P3 plays the essential role of a cellular compass, localizing to the leading edge of pseudopodia, mediating direction sensing during chemotactic migration and cell polarity (1 –4). However, several recent studies have shown that loss of PI3K and reduced PtdIns(3,4,5)P3 level lead to decreased polarity, but does not affect the ability of the cell to sense chemoattractant gradients. In both human neutrophils (5, 6) and Dictyostelium (7 –9), chemotaxis could occur independently of the PI3K-dependent actin polymerization, although it was somewhat delayed, suggesting extra pathways are required for neutrophil chemotaxis.
To identify these putative signal-induced chemotactic pathways, we conducted a functional screening for chemical compounds that disrupt neutrophil directionality. We have identified NADPH oxidase dependent reactive oxygen species (ROS) as key regulators of neutrophil chemotaxis. Neutrophils with pharmacologically inhibited NADPH oxidase, or isolated from chronic granulomatous disease (CGD) patients and mice, displayed more frequent multiple pseudopodia formation and impaired directionality during chemotaxis. This finding provides a cellular mechanism for CGD pathogenesis and might lead to development of new therapeutic strategies for this disease.
Results
Screening for Inhibitors of Neutrophil Chemotaxis.
The screening was performed using an EZ-TAXIScan chemotaxis device in which a stable chemoattractant gradient was formed in a 260-μm–wide channel (Fig. S1A). Freshly purified human primary neutrophils migrated robustly up the gradient and most cells crossed the channel in 20 min (Fig. S1B). A Tocris screening library containing 386 biologically active compounds was used for screening (Table S1). To achieve the maximal inhibition of each targeted pathway in the primary screening, we treated neutrophils with each drug at a concentration equivalent to 10 times the IC50 of the drug. Although most compounds did not affect neutrophil chemotaxis (Dataset S1), 83 compounds displayed inhibitory effects. (The video files for each compound will be released to a public database after the publication of this article.) The inhibitory effects were elicited via a variety of mechanisms (Table S1), such as induction of cell death (Dataset S2), complete inhibition of polarization and migration, slow migration, and impairment of directionality. Selected compounds from the primary screen were then used at 0.2 to 10 times the IC50 in a secondary screening (Dataset S3). Most positive compounds identified from the primary screening showed the same inhibitory effect at lower concentrations, suggesting that the drug-induced phenotype changes were most likely caused by specific inhibition of each targeted pathway.
We focused on the 12 compounds that led to impaired directionality, but did not inhibit neutrophil migration completely (Figs. S2 and S3). Five of these drugs are compounds that inhibit microtubule polymerization, which is consistent with recent reports indicating that microtubules negatively regulate uropod signaling and enhance directional sensing in neutrophils (10, 11). Interestingly, the most dramatic inhibitory effect was induced by diphenyleneiodonium chloride (DPI), a well characterized and commonly used flavoprotein inhibitor that was known to suppress activity of NADPH oxidase and NOS (12 –14). As neutrophil chemotaxis was not affected by other NOS inhibitors (e.g., 7-nitroindazole, L-NIO dihydrochloride, 1-[2-(trifluoromethyl)phenyl]imidazole, 2-amino-5,6-dihydro-6-methyl-4H-1,3 thiazine, ethylisothiourea, S-isopropylisothiourea hydrobromide, NG-methyl-L-arginine, nω-nitro-l-arginine methyl ester, nω-nitro-L-arginine, and L-canavanine) or NO donors (3-morpholinosydnonimine, s-nitrosoglutathione, and spermine NONOate; Dataset S1), it is most likely that the effect of DPI on neutrophil chemotaxis was mediated by the inhibition of NADPH oxidase, suggesting that chemoattractant elicited ROS production might play a role in regulating neutrophil chemotactic migration.
ROS Are Physiological Regulators of Neutrophil Chemotactic Migration.
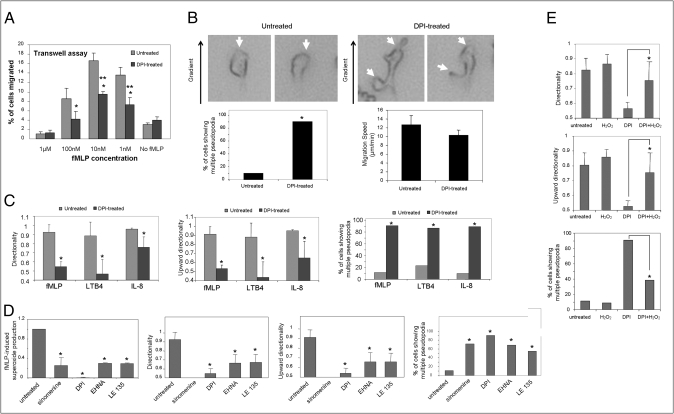
We further investigated the effect of DPI on neutrophil directional migration using a transwell migration system. Cells were plated on transwell filters and induced to migrate in response to chemoattractant added to wells beneath the filters. The migration of neutrophils to these lower wells requires 2D chemotaxis on top of the filter (toward the holes), followed by migration through the holes into the bottom well of chemoattractant. The number of cells in the bottom well was then used to calculate percentage of cells migrated. Consistent with the EZ-TAXIScan results, treatment with DPI significantly inhibited neutrophil migration into the lower wells (Fig. 1A). To take a closer look at the morphological changes elicited by DPI treatment, multiple pseudopod formation was measured in chemotaxing neutrophils. We observed that DPI-treated neutrophils showed multiple pseudopodia much more frequently compared with untreated neutrophils, although the migration speed of these two populations was essentially the same (Fig. 1B). The DPI-induced inhibitory effect appeared not to be specific to chemotaxis-elicited by chemoattractant N-formyl-methionyl-leucyl-phenylalanine (fMLP), which was used for the initial screening. Treatment with DPI also significantly inhibited chemotaxis elicited by leukotriene B4 (LTB4) and IL-8, suggesting that DPI might block a general pathway in directional migration (Fig. 1C). Interestingly, IL-8–mediated chemotaxis was more resistant to DPI treatment compared with fMLP and LTB4. It seems that this is not a sensitivity issue, as we checked several chemoattractant concentrations and the effect of DPI treatment on IL-8–induced chemotaxis is always weaker (Fig. S4). This effect might be caused by relatively lower ROS production in IL-8–treated cells. Chemical inhibitors often have multiple targets. DPI may also inhibit other flavin-containing enzymes. To ensure that the DPI-induced neutrophil chemotaxis defect was indeed caused by inhibition of NADPH oxidase, we examined another NADPH oxidase inhibitor, sinomenine (15). Similar to DPI, sinomenine induced multiple pseudopodia in chemotaxing neutrophils and significantly reduced chemotaxis efficiency. Interestingly, two of the compounds identified from the initial screening, EHNA hydrochloride and LE135, previously known as adenosine deaminase inhibitor and retinoic acid antagonist, respectively, also drastically suppressed chemoattractant-elicited NADPH oxidase activation, again indicating the involvement of ROS in chemotactic migration (Fig. 1D and Fig. S5). Further supporting this hypothesis is the observation that DPI-induced chemotaxis defects were partially rescued by including H2O2 in chemotaxis buffer (Fig. 1E).
Fig. 1.
Pharmacological inhibition of NADPH oxidase–mediated ROS production in human neutrophils leads to more frequent formation of multiple pseudopodia and reduced chemotaxis efficiency. (A) Inhibition of NADPH oxidase decreases transwell migration of human neutrophils. Human neutrophils were left untreated or pretreated with 50 μM DPI for 30 min at 37 °C, and then allowed to migrate in response to the indicated concentration of fMLP. Percentage of cells that migrated into the bottom well was recorded. Data shown are means ± SD from n = 3 wells, from one experiment representative of three. (*P < 0.05 vs. untreated neutrophils.) (B) Chemotaxing NADPH oxidase–inhibited human neutrophils display multiple pseudopodia more frequently, but do not show difference in cell speed. Representative images of human neutrophils treated (Right) or not treated (Left) with 50 μM DPI and allowed to chemotax in response to a chemoattractant gradient generated by addition of 1 μL of 100 nM fMLP in the EZ-TAXIScan device. White arrowheads specify pseudopodia in the cells. Percentage of cells that display multiple pseudopodia (bottom left, n = 20 cells; Fisher 2 × 2 test, *P < 0.05 vs. untreated) and migration speed (bottom right, mean ± SD from n = 20 cells; Student t test, *P > 0.05 vs. untreated) during the course of the EZ-TAXIScan chemotaxis assay in the DPI-treated or untreated case were quantified as described in Experimental Procedures. (C) Inhibition of NADPH oxidase leads to multiple pseudopod formation and defective directionality in fMLP-, LTB4-, and IL-8–mediated chemotaxis. Neutrophil chemotaxis in response to addition of 100 nM fMLP, 100 nM LTB4, or 10 nM IL-8 (1 μL each), with 50 μM DPI treatment (or without treatment), was analyzed (n = 20 cells) for directionality, upward directionality, and percentage of neutrophils displaying multiple pseudopodia as described earlier (n = 20 cells, *P < 0.05). (D) Pharmacological agents that inhibit ROS production cause chemotaxis defect in human neutrophils. fMLP-induced ROS production (Fig. S5 Top) in human neutrophils (5 × 105) pretreated with sinomenine (10 μM), DPI (50 μM), EHNA hydrochloride (40 μM), or LE135 (14 μM), or without pretreatment, was evaluated by stimulating neutrophils with 100 nM fMLP and monitoring chemiluminescence (for 1 s) every 7 s for 280 s, in the presence of 50 μM luminol and 0.8 U HRP in a luminometer at 37 °C. Data represent maximal chemiluminescence in drug-treated neutrophils normalized to maximal chemiluminescence in untreated neutrophils (mean ± SD, n = 3 wells from one experiment representative of three). Drug-treated (and untreated) neutrophils were also exposed to an fMLP gradient generated by addition of 100 nM fMLP (1 μL) in the EZ-TAXIScan device. Images and cell tracks of migrating neutrophils were evaluated for directionality, upward directionality and percentage of neutrophils displaying multiple pseudopodia as described earlier (n = 20 cells; *P < 0.05). (E) H202 treatment of NADPH oxidase–inhibited human neutrophils rescues defect in pseudopod formation and chemotaxis. Human neutrophils were pretreated with 50 μM DPI (or without) and then treated with (or without) 100 μM H202 for 5 min and then allowed to chemotax in response to a fMLP gradient in the EZ-TAXIScan device as described earlier. Migrating neutrophils were evaluated (n = 20 cells; *P < 0.05 vs. DPI-treated neutrophils) for directionality, upward directionality, and percentage of neutrophils displaying multiple pseudopodia as described earlier.
NADPH Oxidase Is Required for Efficient Neutrophil Chemotaxis.
Although DPI is a well known and commonly used NADPH oxidase, its effect on cell migration may be mediated by other undefined mechanisms. To definitely prove that the DPI-induced neutrophil chemotaxis defect is at least partially caused by inhibition of NADPH oxidase, we next explored neutrophil chemotaxis using a CGD mouse in which gp91 subunit of NADPH oxidase holoenzyme was deleted and thus chemoattractant-elicited superoxide production was completely abolished (Fig. 2A). Similar to chemical inhibition, neutrophils isolated from these mice displayed multiple pseudopods (Fig. 2C) and reduced chemotaxis efficiency (Fig. 2 B and D). Consistently, neutrophils isolated from the CGD mice also displayed a migration defect in the transwell assay compared with WT neutrophils (Fig. 2E). Finally, we investigated the chemotaxis of neutrophil-like differential HL60 cells in which the p22phox subunit of NADPH oxidase was knocked down by a specific siRNA, and essentially the same results were observed (Fig. 2 F–H). Collectively, all these results suggest that signal-induced NADPH oxidase-mediated ROS production plays an essential role in regulating neutrophil chemotaxis.
Fig. 2.
Disruption of NADPH oxidase leads to chemotaxis defects. (A–E) Neutrophils from CGD mice do not produce ROS in response to chemoattractant stimulation, display multiple pseudopodia, and show loss of directionality during chemotaxis. (A) ROS production in neutrophils (5 × 105) from WT or CGD mice after stimulation with 1 μM fMLP was evaluated by monitoring chemiluminescence (for 1 s) every 7 s for 280 s, in the presence of 50 μM luminol and 0.8 U HRP in a luminometer at 37 °C. Data represents mean ± SD from three wells from one experiment representative of three. (B) Neutrophils from WT and CGD mice (3,000 cells) were plated into the EZ-TAXIScan device and exposed to a shallow chemoattractant gradient generated by addition of 1 μL LTB4 (100 nM). Cell tracks of migrating WT (Left) and CGD (Right) neutrophils (cells that move ≥65 μm from the bottom of the channel; n = 20) were traced from captured images, realigned such that all cells started from the same starting point (0,0) and plotted. Chemoattractant concentration increases in the positive y direction. (C) Representative images of WT (Left) and CGD migrating neutrophils (Right) are also shown; white arrowheads specify pseudopodia. (D) Migrating neutrophils were evaluated (n = 20 cells; *P < 0.05 vs. WT neutrophils) for directionality, upward directionality, percentage of neutrophils displaying multiple pseudopodia, and migration speed as described earlier. (E) Transwell migration of CGD mice neutrophils. WT or CGD murine neutrophils were allowed to migrate in response to the indicated concentration of LTB4. Percentage of cells that migrated into the bottom well was recorded. Data shown are means ± SD from three wells from one experiment representative of three. (*P < 0.05 vs. WT neutrophils.) (F–H) Knocking down p22phox via siRNA results in impaired cell migration and chemotaxis. HL60 cells were differentiated with 1.75% DMSO for 1 d, transfected with 1 μM control siRNA or p22phox siRNA, and further differentiated until d 5 or 6. (F) Knockdown of p22phox in dHL60 cells. At d 5 (Left) or d 6 (Right), dHL60 cells were lysed and probed with p22phox antibody to evaluate knockdown and GAPDH antibody to evaluate loading. (G) Decreased ROS production in p22phox-knockdown dHL60 cells. ROS production in control siRNA or p22phox siRNA transfected dHL60 cells (2 × 105, 5 d of differentiation) after stimulation with 10 nM C5a was evaluated by monitoring chemiluminescence (for 1 s) every 30 s for 300 s, in the presence of 50 μM luminol and 0.8 U HRP in a luminometer at 37 °C. (H) Control siRNA or p22phox siRNA transfected dHL60 cells (d 5) were exposed to a chemoattractant gradient generated by addition of 25 nM or 100 nM C5a (1 μL) in the EZ-TAXIScan device and imaged every 0.5 min for 20 min. Cell tracks of migrating dHL60 cells (n = 15) were traced from the captured images, realigned to start from the same point (0,0), and plotted (Left). Migration paths of the dHL60 cells were evaluated for a 0- to 20-min time frame (n > 15 cells, *P < 0.005 vs. control siRNA dHL60 cells) for directionality, upward directionality, and migration speed as described in Experimental Procedures (Right).
Neutrophils Isolated from CGD Patients Also Show Severe Chemotaxis Defect.
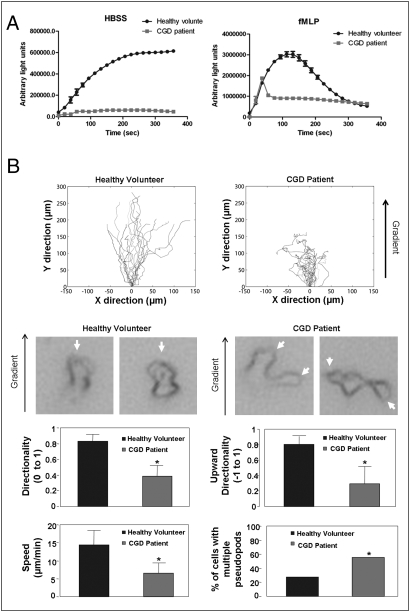
To further explore the physiological and clinical significance of the regulation of neutrophil migration by ROS, we examined chemotaxis behaviors of neutrophils isolated from a CGD patient that contain mutated alleles of the gene encoding gp91phox. As expected, neutrophils from the CGD patient displayed impaired chemoattractant-elicited ROS production in comparison with neutrophils from a healthy volunteer (Fig. 3A). The ROS peak in these neutrophils is significant, but is much smaller than that in the WT neutrophils. Adhesion-induced ROS production in the absence of chemoattractant was also abolished in the CGD neutrophils. The CGD neutrophils displayed a striking chemotaxis defect, showing lack of directionality, more frequent formation of multiple pseudopodia, and slow migration toward the direction of higher chemoattractant (Fig. 3B). It is noteworthy that the CGD patient in this study was receiving IFN-γ treatment. Nevertheless, it is unlikely that the observed neutrophil chemotaxis defect was a result of this treatment, as IFN-γ–treated WT neutrophils showed normal directionality during chemotaxis. Collectively, these results suggest that the defective neutrophil chemotaxis might be contributive to the compromised bactericidal activity in CGD patients, providing a cellular mechanism for CGD pathogenesis.
Fig. 3.
Neutrophils isolated from the CGD patient are defective in ROS production and chemotactic migration. (A) Decreased ROS production in neutrophils from a CGD patient. ROS production in neutrophils (5 × 105) from a CGD patient or a healthy volunteer after addition of HBSS (Top) or 100 nM fMLP (Bottom) was evaluated by monitoring chemiluminescence (for 1 s) every 20 s for 360 s, in the presence of 50 μM isoluminol and 0.8 U HRP in a luminometer at 37 °C. Data represent mean ± SD from three wells. (B) Neutrophils from the CGD patient or healthy volunteer were exposed to a chemoattractant gradient generated by addition of 100 nM fMLP (1 μL) in the EZ-TAXIScan device and imaged every 0.5 min for 20 min. Cell tracks of migrating neutrophils (n = 20) were traced from the captured images, realigned to start from the same point (0,0), and plotted (Top). Images of chemotaxing neutrophils from the CGD patient or healthy volunteer are shown (Middle). White arrowheads specify pseudopodia in the cells. Neutrophils were evaluated (n = 20 cells, *P < 0.01 vs. WT neutrophils) for directionality, upward directionality, percentage of neutrophils displaying multiple pseudopodia, and migration speed as described earlier (Bottom).
CGD Mice Display Impaired in Vivo Neutrophil Recruitment.
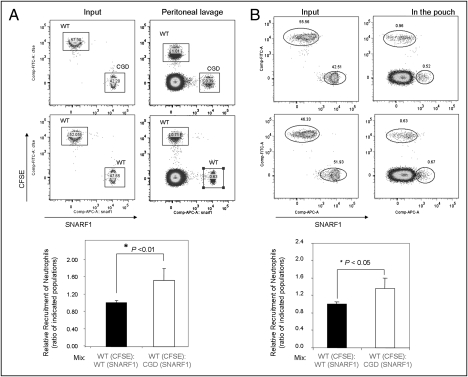
Our in vitro experiments showed that neutrophils depleted of ROS display reduced chemotaxis efficiency. We next investigated whether this defect in chemotaxis will lead to impaired neutrophil recruitment to sites of inflammation in live mice using a murine acute peritoneal inflammation (i.e., peritonitis) model. To compare neutrophil recruitment under exactly the same environment, an adoptive transfer experiment was conducted (Fig. S6). We labeled in vitro purified CGD neutrophils with intracellular fluorescent dye 5- (and 6-) carboxyfluorescein diacetate succinimidyl esters (CFSE; green) and WT neutrophils with another dye, 5- (and 6-) chloromethyl SNARF-1 acetate (red), or vice versa. The mixed (1:1) population was i.v. injected into a WT recipient mouse 2.5 h after the i.p. thioglycolate (TG) injection. By doing this, variability caused by difference in inflammatory environment in each individual recipient mouse will be eliminated. CGD (green) and WT (red) neutrophils were identified by their unique fluorescent labels. As we measured neutrophil numbers at 4 h after the TG injection, when neutrophil death is minimal, the ratio of CGD neutrophils to WT neutrophils most likely reflected their relative capability to migrate to the inflamed peritoneal cavity. Consistent with the in vitro results, we detected a much reduced peritoneal recruitment of CGD neutrophils compared with WT neutrophils (Fig. 4A). A similar effect was also detected in a murine air pouch model in which recruitment of adoptively transferred neutrophils to a preformed air pouch was induced by TNF-α (Fig. 4B). These results further support the conclusion that ROS generated by NADPH oxidase are key physiological regulators of actin dynamics in neutrophils.
Fig. 4.
CGD neutrophils exhibit an intrinsic defect in recruitment to sites of inflammation. (A) Recruitment of adoptively transferred neutrophils in the TG-induced peritonitis model. Neutrophils isolated from WT and CGD mice were labeled with intracellular fluorescent dye CFSE (final concentration, 1 μM; Molecular Probes) or 5- (and 6-) chloromethyl SNARF-1 acetate (final concentration, 1 μM; Molecular Probes) at 37 °C for 10 min. Labeled cells were mixed (1:1) as indicated and then injected i.v. (via tail vein) into WT mice that have been challenged with 1 mL 3% TG for 2.5 h. Peritoneal fluids were harvested 1.5 h after the injection of cell mixture. The amount of adoptively transferred neutrophils recruited to the peritoneal cavity was analyzed using a BD FACSCanto II flow cytometer (Becton Dickinson) and BD FACSDiva software. Relative recruitment of neutrophil was calculated as the ratio of indicated populations in the peritoneal cavity. (B) Recruitment of adoptively transferred neutrophils to a preformed air pouch. The dorsal air pouch was generated on WT recipient mice as described in Experimental Procedures. Neutrophil recruitment to the pouch was induced by TNF-α, which was directly injected into the pouch 2.5 h before the neutrophil injection. The pouch was flushed 1.5 h after the injection of cell mixture. The relative recruitment of WT and CGD neutrophil was calculated as described earlier.
Discussion
In this study, using an unbiased screening approach, we have identified ROSs as essential players for modulating neutrophil chemotaxis. Chemotaxis is a process in which cells sense and move up a gradient of molecules (chemoattractants). It plays a central role in the regulation of host defense and inflammatory reactions by recruiting circulating effector leukocytes, including neutrophils, monocytes, and effector T cells, to the sites of injury or infection. During chemotaxis, chemoattractants elicit a number of changes in neutrophils. These include a localized polymerization of F-actin at the site of cell cortex closest to the chemoattractant source, a morphological change characterized by cell elongation, the formation of new lamellipodia or pseudopods at the leading edge, and the forward protrusion of the leading edge followed by retraction of posterior of the cell. We have found that neutrophils with inhibited ROS production that were isolated from CGD patients or mice or pharmacologically/siRNA–treated to inhibit the NADPH oxidase complex displayed defective migration. These neutrophils formed more frequent multiple pseudopodia and lost their directionality as they migrated up a chemoattractant concentration gradient.
CGD is an inherited disorder characterized by recurrent bouts of infection as well as chronic inflammation with granuloma formation. Consistently, neutrophil recruitment to sites of inflammation is dramatically elevated in the CGD mice (16). This hyperinflammatory phenotype is likely caused by dysfunctional kynurenine pathway of tryptophan catabolism (17) and suppression of ROS-induced deactivation of proinflammatory chemokines such as C5a, fMLP (18), LTB4 (19), and IL8 (20). At the sites of infection, the inability of CGD neutrophils to produce ROS in response to chemoattractant stimulation may contribute to the impaired bactericidal activity of these cells. Our discovery that depletion of signal-elicited ROS production in fact inhibits neutrophil chemotactic migration provides another cellular mechanism for CGD pathogenesis and might lead to development of new therapeutic strategies for this disease.
How is chemotactic migration regulated by ROSs? ROSs have been identified as important second messengers that can regulate intracellular signal transduction under a variety of physiological and pathophysiological conditions. This has been shown to occur predominantly via oxidation of thiols (-SH) on protein cysteine residues, resulting in reversible protein posttranslational modifications such as glutathionylation, disulfide bond formation, and sulfenic acid formation. Many of these modifications control signal transduction by altering functionality/activity of the protein involved. Redox regulation of numerous proteins such as Ras, protein tyrosine kinases (Src kinases), and protein tyrosine phosphatases have been reported and are known to alter protein functions. PTEN has also been identified as a target of ROSs (21 –23). ROSs can also directly regulate actin polymerization via modifying G-actin monomers (21 –23). In addition, the NADPH oxidase is also essential for chemoattractant-elicited depolarization of membrane potential and can regulate Ca2+, K+ homeostasis in neutrophils (24). This may contribute to directional sensing in an indirect way. The exact mechanism by which ROS regulate neutrophil chemotaxis needs to be further investigated. During chemotaxis, many signaling events take place locally within the cell. For example, Rac-related signaling and actin polymerization are detected at the leading edge of chemotaxing cells, whereas RhoA activation and contractile actin–myosin complexes appear only at the back of the cells. Interestingly, we found NADPH oxidase was highly enriched near the leading edge of migrating neutrophils (Fig. S7). Whether this specific localization is essential for chemotaxis needs to be further investigated.
Experimental Procedures
Neutrophil Purification and Functional Assays.
Human blood neutrophil purification, murine bone marrow neutrophil purification, quantification of F-actin levels by phalloidin labeling, measurement of superoxide production by luminol chemiluminescence, micropipette chemotaxis, and transwell migration assays were performed as described previously (25 –27). Peripheral blood was obtained from a human CGD patient and healthy volunteer, with informed consent. EZ-TAXIScan chemotaxis assay, analysis of cell tracks and morphology, siRNA knockdown, and other related assays are described in SI Materials and Methods.
Recruitment of Adoptively Transferred Neutrophils in TG-Induced Peritonitis.
Peritonitis was induced as previously described (26). Neutrophils isolated from WT and CGD mice were labeled with intracellular fluorescent dye CFSE (final concentration, 1 μM; Molecular Probes) or 5- (and 6-) chloromethyl SNARF-1 acetate (final concentration, 1 μM; Molecular Probes) at 37 °C for 10 min. Labeled cells were mixed as indicated in Fig. S6 then injected i.v. (via tail vein) into WT mice that have been challenged with 1 mL 3% TG for 2.5 h. Mice were euthanized by CO2 inhalation 1.5 h after the injection of cell mixture (4 h after TG injection) and peritoneal exudate cells were recovered by peritoneal lavage with 10 mL of ice-cold PBS solution containing 5 mM EDTA. The amount of adoptively transferred neutrophils recruited to the peritoneal cavity was analyzed using a BD FACSCanto II flow cytometer (Becton Dickinson) and BD FACSDiva software. Relative recruitment of neutrophil was calculated as the ratio of indicated populations in the peritoneal cavity.
Recruitment of Adoptively Transferred Neutrophils in a Murine Dorsal Air Pouch Model.
A dorsal air pouch was created by injecting mice with 5 mL of air s.c. on the back at d 0. On d 3 and 5, the pouches were reinflated with 2 mL of air. At 6 d after the initial air injection, TNF-α (in 0.5 mL sterile 0.9% saline solution) was directly injected into the pouch. Four hours after TNF-α injection, mice were anesthetized, and the pouch was flushed with 2 mL saline solution. The relative recruitment of WT and CGD neutrophils was calculated as described earlier.
Statistics.
Analysis of statistical significance for indicated data sets was performed using the Student t test capability on Microsoft Excel.
Supplementary Material
Acknowledgments
The authors thank Leslie Silberstein, John Manis, Li Cai, and Narayanaswamy Ramesh for helpful discussions; and Dan Stevens from Hirata Corp for assistance with the EZ-TAXIScan device. B.S. was supported by National Institutes of Health (NIH) training Grant HL066987. H.L. was supported by NIH Grants HL085100, AI076471, HL092020, and GM076084, and a Research Scholar Grant from the American Cancer Society.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914351107/DCSupplemental.
References
- 1.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 2.Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, et al. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 5.Chodniewicz D, Zhelev DV. Chemoattractant receptor-stimulated F-actin poly-merization in the human neutrophil is signaled by 2 distinct pathways. Blood. 2003;101:1181–1184. doi: 10.1182/blood-2002-05-1435. [DOI] [PubMed] [Google Scholar]
- 6.Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated indepen-dently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. doi: 10.1038/ncb1536. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, et al. Two phases of actin polymerization display different dependencies on PI(3,4,5)P3 accumulation and have unique roles during chemotaxis. Mol Biol Cell. 2003;14:5028–5037. doi: 10.1091/mbc.E03-05-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loovers HM, et al. Distinct roles of PI(3,4,5)P3 during chemoattractant signaling in Dictyostelium: a quantitative in vivo analysis by inhibition of PI3-kinase. Mol Biol Cell. 2006;17:1503–1513. doi: 10.1091/mbc.E05-09-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, et al. PLA2 and PI3K/PTEN pathways act in parallel to mediate che-motaxis. Dev Cell. 2007;12:603–614. doi: 10.1016/j.devcel.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Wang F, Van Keymeulen A, Rentel M, Bourne HR. Neutrophil microtubules suppress polarity and enhance directional migration. Proc Natl Acad Sci USA. 2005;102:6884–6889. doi: 10.1073/pnas.0502106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niggli V. Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signalling path-ways. J Cell Sci. 2003;116:813–822. doi: 10.1242/jcs.00306. [DOI] [PubMed] [Google Scholar]
- 12.Stuehr DJ, et al. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991;5:98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- 13.Cross AR, Jones OT. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986;237:111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis JA, Cross AR, Jones OT. Studies on the electron-transfer mechanism of the human neutrophil NADPH oxidase. Biochem J. 1989;262:575–579. doi: 10.1042/bj2620575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian L, et al. Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007;4:23. doi: 10.1186/1742-2094-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollock JD, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 17.Romani L, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 18.Clark RA, Klebanoff SJ. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1979;64:913–920. doi: 10.1172/JCI109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segal BH, Kuhns DB, Ding L, Gallin JI, Holland SM. Thioglycollate peritonitis in mice lacking C5, 5-lipoxygenase, or p47(phox): complement, leukotrienes, and reactive oxidants in acute inflammation. J Leukoc Biol. 2002;71:410–416. [PubMed] [Google Scholar]
- 20.Lekstrom-Himes JA, Kuhns DB, Alvord WG, Gallin JI. Inhibition of human neutrophil IL-8 production by hydrogen peroxide and dysregulation in chronic granulomatous disease. J Immunol. 2005;174:411–417. doi: 10.4049/jimmunol.174.1.411. [DOI] [PubMed] [Google Scholar]
- 21.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 22.Clavreul N, et al. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2006;26:2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- 23.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 24.Rada BK, Geiszt M, Hably C, Ligeti E. Consequences of the electrogenic function of the phagocytic NADPH oxidase. Philos Trans R Soc Lond B Biol Sci. 2005;360:2293–2300. doi: 10.1098/rstb.2005.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu D, et al. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci USA. 2006;103:14836–14841. doi: 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian KK, et al. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia Y, et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phos-phatidylinositol-3,4,5- trisphosphate signaling in neutrophils. Immunity. 2007;27:453–467. doi: 10.1016/j.immuni.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.