Abstract
It has been proposed that the cellular corepressor protein CoREST is involved in repressing herpes simplex virus type 1 (HSV-1) infection in the absence of the viral regulatory protein ICP0. This proposal predicts that depletion of CoREST should improve the plaque-forming efficiency and replication of ICP0 null mutant virus. To test this hypothesis, human HepaRG cells that were highly depleted of CoREST were isolated using RNA interference technology. Depletion of CoREST had no effect on the replication of ICP0 null mutant HSV-1, demonstrating that CoREST does not play an influential role in regulating HSV-1 infection in this cell type.
Natural infections of individuals by herpes simplex virus type 1 (HSV-1) are characterized by the establishment of latency in sensory neurons, interspersed by episodes of active infection at the periphery (for reviews, see reference 19). The balance between latent and lytic infection is regulated by both cellular and viral factors, for which the viral immediate-early (IE) protein ICP0 is of significant importance (2, 4, 6, 18). ICP0 is a complex protein for which a large number of functions have been reported (reviewed in references 4, 5, 18, and 22). It is a member of the RING finger class of E3 ubiquitin ligases, and this activity is known to be essential for its core biological functions (1, 3, 6, 10, 26). However, a number of other functions have been proposed for ICP0, of which the most topical is disruption of a cellular corepressor complex, which includes the proteins CoREST, REST, and histone deacetylases 1 and 2 (HDAC1/2) (14-17).
ICP0 contains a serine- and alanine-rich sequence between residues 550 and 600 that has similarity to a region in CoREST, and based on this information, it was hypothesized that ICP0 might disrupt the CoREST/REST complex (14). This was indeed observed at mid- to late times of HSV-1 infection (14) and further worked mapped sequences of ICP0 (which do not coincide with the original sequence of interest) that are required for an interaction with CoREST (16). Evidence that this interaction might contribute to the biological functions of ICP0 came from improved (but not fully complemented) yields in single-step growth curves of a recombinant virus expressing a fragment of CoREST (predicted to act in a dominant-negative manner) in place of ICP0 (16) and from a slight decrease in replication at intermediate times of a mutant virus with a lesion in the CoREST interaction region of ICP0 (17). On the basis of these observations, it was proposed that disruption of the CoREST/REST complex is a major function of ICP0 that underlies its core biological properties. However, a number of observations do not fit easily with this hypothesis. For example, ICP0 null mutant HSV-1 exhibits severely compromised gene expression from the earliest stages of infection (6), whereas the disruption of the CoREST/REST complex was observed only at mid- to late times and also occurred, albeit less efficiently, during ICP0 null mutant infection (14). Furthermore, the proposed CoREST interaction region of ICP0 is within a region where several other functions have been described, and whereas complete deletion of this region compromises ICP0 function, this mutant still functions about 2 orders of magnitude more efficiently than a RING finger mutant in assays of both lytic infection and reactivation from quiescence (10).
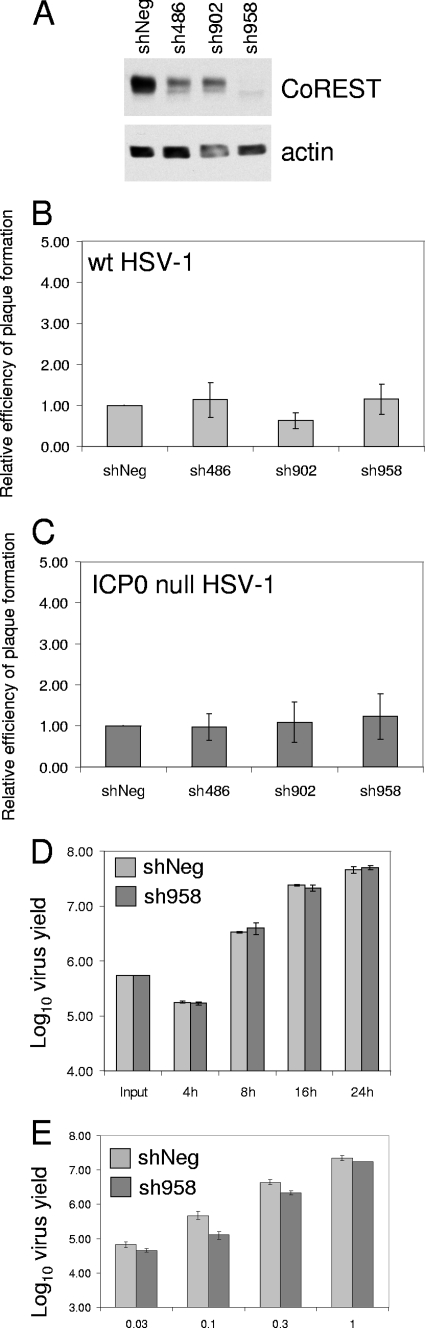
An RNA interference approach was adopted to test the proposal that CoREST is involved in regulating ICP0 null mutant HSV-1 infection. A clear prediction of this hypothesis is that depletion of CoREST should weaken cell-mediated repression of the mutant virus gene expression and replication. In consequence, the plaque-forming efficiency and growth yields of the mutant virus should be increased in CoREST-depleted cells. Lentiviruses expressing control (shNeg) and candidate anti-CoREST shRNAs (purchased from Sigma-Aldrich) were used to transduce HepaRG cells, in which ICP0 is required for efficient HSV-1 replication, using cell culture and transduction methods described previously (9, 10). Two short hairpin RNAs (shRNAs; sh486 and sh902) gave partial depletion, while another (sh958) resulted in a very substantial decrease in CoREST expression (Fig. 1A). The coding strand sequences of sh486, sh902, and sh558 are TTGGATGAATACATTGCCAT, GTCTTATTTGAGCAAGCCTTT, and GTACAAGCCATCAGGAAATAT, respectively, starting at nucleotides 435, 592, and 1174 of the CoREST cDNA (taking the A of the initiating ATG as nucleotide 1). The negative-control shRNA sequence (shNeg; coding strand sequence TTATCGCGCATATCACGCG) was designed so that it does not target any human or herpesvirus gene. The plaque-forming efficiencies of neither the wild type (wt) nor ICP0 null mutant HSV-1 strains were affected by depletion of CoREST (Fig. 1B and C). Single-step growth curves of ICP0 null mutant virus in shNeg and sh958 cells showed no significant effect of CoREST depletion (Fig. 1D). Since the ICP0 null mutant phenotype is most pronounced in low-multiplicity infections, the yields of ICP0 null mutant virus were investigated in shNeg and sh958 cells at 24 h after infection with decreasing virus inputs. Consistent with the other experiments, the yields of the mutant virus were not improved by depletion of CoREST (Fig. 1E). These data are incompatible with the hypothesis that CoREST plays an influential role in regulating HSV-1 infection, at least in this cell type.
FIG. 1.
(A) Whole-cell extracts of HepaRG cells expressing control (shNeg) and anti-CoREST shRNAs were analyzed for CoREST expression by Western blotting, using the anti-CoREST antibody (product no. 07-455; Millipore) used in previous studies (15-17). Actin (Sigma-Aldrich monoclonal antibody AC-40) was used for the loading control. (B and C) The four cell lines analyzed in panel A were used for plaque assays with wt HSV-1 strain in1863 (B) and ICP0 null mutant strain dl1403/CMVlacZ (C), as described previously (9, 11). The plaque-forming efficiencies of the viruses in the CoREST-depleted cells are presented as a proportion of those in control shNeg cells. The data present the mean results and standard deviations from several independent experiments. (D) Single-step growth curve of ICP0 null mutant virus dl1403 (24) in shNeg and sh958 cells. Infections of 1 × 105 cells were performed at an input multiplicity of 2.5 PFU per cell, based on titers on U2OS cells. At the indicated time points, samples of cells were scraped into the medium and sonicated, and virus yields were measured by titration in U2OS cells. The data show the mean results from two independent experiments, with the error bars showing the range of the actual experimental values. (E) Yields of ICP0 null mutant HSV-1 at 24 h after infection of 1 × 105 shNeg cells and sh958 cells at the indicated multiplicities. Cells and medium were harvested, and virus yields were determined in U2OS cells. The mean results from two independent experiments are shown. Error bars represent the range of the actual values.
The phenotype of ICP0 null mutant HSV-1 is heavily influenced by cell type, with the plaque-forming defect differing widely at about 1,000-fold in human fibroblasts (HFs), 5- to 100-fold in HeLa, Vero, and BHK cells, and absent in U2OS cells (3, 6, 23, 24, 27). HFs are the cell line of choice for many experiments because they are nontransformed, but they are more difficult to use for the production of transduced cell lines. HepaRG cells are much more amenable to this type of experimentation, and although they are a permanent cell line, they are minimally transformed (13). It is possible that CoREST has a regulatory role in HSV-1 infections in cell types other than HepaRG cells. However, it is unlikely that HepaRG cells differ substantially from HFs in this respect for the following reasons. The defect of ICP0 null mutant HSV-1 in HepaRG cells is of the order of 500-fold (9, 10) (only 2-fold down from that in HFs), and the burst sizes of the ICP0 null mutant in single-step growth curve experiments are similar in the two cell types (compare data shown in Fig. 1 with those shown in Fig. 6 and 7 of reference 12). Furthermore, mutations that affect ICP0 function do so to similar extents in HFs and HepaRG cells (10). Based on this evidence, it is a reasonable hypothesis that the same cellular factors are involved in restricting ICP0 null mutant HSV-1 in the two cell types. In addition, we have not detected any major differences between HepaRG cells and HFs in terms of plating efficiency or replication of wt HSV-1 or establishment of quiescent HSV-1 infections with defective HSV-1 mutants (10, 11) or in the use of many other assays of viral and cellular proteins in HSV-1-infected cells (8-12).
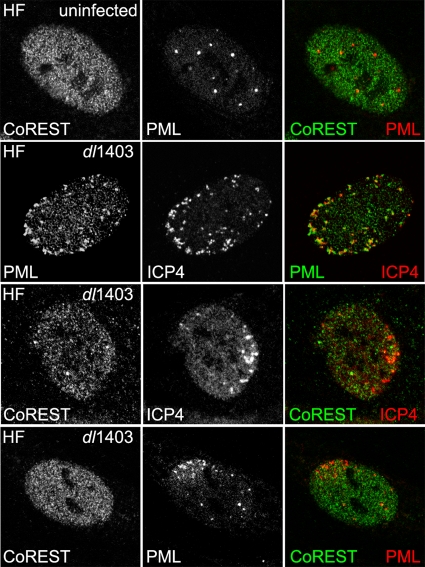
An event that occurs during the earliest stages of HSV-1 infection is the recruitment of the promyelocytic leukemia protein PML to sites that are closely associated with incoming HSV-1 genomes (7). Several other components of cellular nuclear substructures known as ND10 or PML nuclear bodies behave similarly (7). In a wt HSV-1 infection, this process is rapidly counteracted by ICP0 (7), and the evidence suggests that this function of ICP0 correlates well with its ability to stimulate viral gene expression and reactivation from quiescence (10). A plausible hypothesis is that the cellular response that leads to the recruitment of selected cellular proteins to sites associated with HSV-1 genomes is involved in a mechanism that represses transcription from foreign DNA and that by inhibiting this recruitment, ICP0 enables more efficient expression of viral genes. It has been proposed that CoREST, which is diffusely distributed in the nuclei of uninfected cells, is partially relocated to sites adjacent to PML in ICP0 null mutant HSV-1 infections of human embryo lung fibroblasts and is recruited into viral replication compartments as infection progresses (15). These data raised the possibility that CoREST might, like PML and other ND10 proteins, be recruited to sites associated with incoming HSV-1 genomes during the early stages of ICP0 null mutant infection. As observed previously (15), CoREST was diffusely distributed in the nuclei of HFs (Fig. 2, top row). However, compared to the control of PML recruitment (Fig. 2, second row), no evidence of recruitment of CoREST to viral genome foci in ICP0 null mutant infections was observed (Fig. 2, third row), even when recruitment of PML was evident (Fig. 2, bottom row). Essentially identical results were obtained in HepaRG cells (data not shown). Therefore, CoREST is not a representative of those proteins that accumulate markedly in viral genome-associated foci.
FIG. 2.
Distribution of CoREST in uninfected and ICP0 null mutant HSV-1-infected human foreskin fibroblasts (obtained from Thomas Stamminger, University of Erlangen). Cell growth and immunofluorescence methods were performed as described previously (11). Top row, uninfected HFs stained for CoREST (product no. 07-455; Millipore) and PML (monoclonal antibody 5E10 (25). Second row, HFs were infected with HSV-1 ICP0 null mutant dl1403 at low multiplicity, and then cells at the edges of developing plaques were stained for PML and ICP4 (monoclonal antibody 58S) the following day. ICP4 acts as a marker for viral genomes and early replication compartments (7). Third row, infection and staining done as in the second row, but cells were stained for CoREST and ICP4. Bottom row, infection and staining done as in the second and third rows, except cells were stained for CoREST and PML. The asymmetric pattern of PML staining is due to recruitment to viral genome-associated foci (7), as seen in the second row.
Previous work demonstrated that PML and a number of other ND10 component proteins are involved in the repression of HSV-1 gene expression that occurs in the absence of ICP0 (9, 11, 20, 21). These studies used lentivirus-mediated shRNA expression to make stable cells lines depleted of PML, Sp100, hDaxx, or ATRX. In all cases, a modest but reproducible increase in replication efficiency (up to 10-fold) of ICP0 null mutant HSV-1 was observed. Further increases in ICP0 null mutant plaque-forming efficiency were observed in cells depleted of both PML and Sp100, suggesting that the repressive effects of this group of proteins may be additive (9). Although these increases are insufficient to explain the full defect of ICP0 null mutant HSV-1, these data demonstrate that this technology is capable of revealing relevant cellular regulators of HSV-1 infection. The data presented here show that depletion of CoREST has no effect on the efficiency of plaque formation or replication of ICP0 null mutant HSV-1 infection in HepaRG cells, thereby putting into doubt the hypothesis that CoREST has a significant universal role in regulating HSV-1 infection.
Acknowledgments
The work done in my laboratory is funded by the Medical Research Council. Part of the work of this study was funded by the EC FP6 SME-STREP project TargetHerpes (contract LSHG-CT-2006-037517).
The negative-control shRNA used in shNeg cells was designed by Ivan Rossi (BioDec, Bologna, Italy), and anti-PML monoclonal antibody 5E10 was provided by Roel van Driel. I am grateful for technical assistance from Anne Orr and Jill Murray and the constructive comments on the manuscript from Chris Preston and Chris Boutell.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efstathiou, S., and C. M. Preston. 2005. Towards an understanding of the molecular basis of herpes simplex virus latency. Virus Res. 111:108-119. [DOI] [PubMed] [Google Scholar]
- 3.Everett, R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70:1185-1202. [DOI] [PubMed] [Google Scholar]
- 4.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 5.Everett, R. D. 2006. The roles of ICP0 during HSV-1 infection, p. 39-64. In R. M. Sandri-Goldin (ed.), Alpha herpesviruses. Molecular and cellular biology. Caister Academic Press, Wymondham, United Kingdom.
- 6.Everett, R. D., C. Boutell, and A. Orr. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 78:1763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett, R. D., and J. Murray. 2005. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 79:5078-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everett, R. D., and A. Orr. 2009. Herpes simplex virus type 1 regulatory protein ICP0 aids infection in cells with a preinduced interferon response but does not impede interferon-induced gene induction. J. Virol. 83:4978-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett, R. D., C. Parada, P. Gripon, H. Sirma, and A. Orr. 2008. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 82:2661-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett, R. D., M. L. Parsy, and A. Orr. 2009. Analysis of the functions of herpes simplex virus type 1 regulatory protein ICP0 that are critical for lytic infection and derepression of quiescent viral genomes. J. Virol. 83:4963-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, R. D., S. Rechter, P. Papior, N. Tavalai, T. Stamminger, and A. Orr. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 80:7995-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everett, R. D., D. F. Young, R. E. Randall, and A. Orr. 2008. STAT-1- and IRF-3-dependent pathways are not essential for repression of ICP0-null mutant herpes simplex virus type 1 in human fibroblasts. J. Virol. 82:8871-8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gripon, P., S. Rumin, S. Urban, J. Le Seyec, D. Glaise, I. Cannie, C. Guyomard, J. Lucas, C. Trepo, and C. Guguen-Guillouzo. 2002. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. U. S. A. 99:15655-15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, H., Y. Liang, G. Mandel, and B. Roizman. 2005. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. U. S. A. 102:7571-7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu, H., and B. Roizman. 2009. Engagement of the lysine-specific demethylase/HDAC1/CoREST/REST complex by herpes simplex virus 1. J. Virol. 83:4376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu, H., and B. Roizman. 2007. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc. Natl. Acad. Sci. U. S. A. 104:17134-17139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu, H., and B. Roizman. 2009. The two functions of herpes simplex virus 1 ICP0, inhibition of silencing by the CoREST/REST/HDAC complex and degradation of PML, are executed in tandem. J. Virol. 83:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagglund, R., and B. Roizman. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 78:2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knipe, D. M., P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strauss (ed.). 2006. Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 20.Kyratsous, C. A., and S. J. Silverstein. 2009. Components of nuclear domain 10 bodies regulate varicella-zoster virus replication. J. Virol. 83:4262-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukashchuk, V., A. Orr, and R. D. Everett. Regulation of ICP0 null mutant HSV-1 infection by ND10 components ATRX and hDaxx. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 22.Roizman, B., and B. Taddeo. 2006. HSV-1 and the host cell: a story of global conspiracies, plots and counterplots, p. 261-282. In R. M. Sandri-Goldin (ed.), Alpha herpesviruses. Molecular and cellular biology. Caister Academic Press, Wymondham, United Kingdom.
- 23.Sacks, W. R., and P. A. Schaffer. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 61:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 25.Stuurman, N., A. de Graaf, A. Floore, A. Josso, B. Humbel, L. de Jong, and R. van Driel. 1992. A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J. Cell Sci. 101:773-784. [DOI] [PubMed] [Google Scholar]
- 26.Thompson, R. L., and N. M. Sawtell. 2006. Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J. Virol. 80:10919-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 69:6249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]