Abstract
The gastrointestinal tract represents a major site for human and simian immunodeficiency virus (HIV and SIV) replication and CD4+ T-cell depletion. Despite severe depletion of mucosal CD4+ T cells, FOXP3+ regulatory CD4+ T cells (Treg) are highly increased in the gut mucosa of chronically HIV-infected individuals and may contribute to HIV pathogenesis, either by their immunosuppressive function or as a significant target cell population for virus production. Little is known about the susceptibility of mucosal Treg to viral infection and the longitudinal effect of HIV/SIV infection on Treg dynamics. In this study, we determined the level of SIV infection in Treg and nonregulatory CD4+ T cells (non-Treg) isolated from the colon of SIV-infected rhesus macaques. The dynamics of mucosal Treg and alterations in the mucosal CD4+ T-cell pool were examined longitudinally. Our findings indicate that mucosal Treg were less susceptible to productive SIV infection than non-Treg and thus were selectively spared from SIV-mediated cell death. In addition to improved survival, local expansion of Treg by SIV-induced proliferation of the mucosal CD4+ T-cell pool facilitated the accumulation of mucosal Treg during the course of infection. High frequency of mucosal Treg in chronic SIV infection was strongly related to a reduction of perforin-expressing cells. In conclusion, this study suggests that mucosal Treg are less affected by productive SIV infection than non-Treg and therefore spared from depletion. Although SIV production is limited in mucosal Treg, Treg accumulation may indirectly contribute to viral persistence by suppressing antiviral immune responses.
The gut-associated lymphoid tissue (GALT) represents a key site for viral replication and CD4+ T-cell depletion in human and simian immunodeficiency virus (HIV and SIV) infection (7, 11, 18, 23, 28, 34-36, 46, 54). Immunologic dysfunction and impairment of the intestinal barrier function cause enhanced translocation of luminal antigens and thus drive the systemic immune hyperactivation associated with HIV disease progression (10, 16, 24, 44). Although the gastrointestinal mucosa is of major importance in HIV/SIV infection, the mechanisms contributing to GALT-associated HIV/SIV pathogenesis are not fully understood.
FOXP3+ regulatory CD4+ T cells (Treg), a specialized subset of CD4+ T cells whose function depends on the expression of the master transcription factor FOXP3 (forkhead box P3) (20, 26, 29), control host immune responses by suppressing autoreactive and pathogen-specific effector T-cell functions (8, 45, 50-52). We and others have previously demonstrated increased frequencies of Treg among CD4+ T cells in gastrointestinal mucosal tissues in HIV-1 and SIV infection (9, 15, 19). Moreover, we have shown that mucosal Treg are markedly increased in chronic HIV infection but not in norovirus infection, suggesting that high accumulation of Treg in GALT is pronounced in HIV infection and not just a common consequence of any intestinal viral infection (15). The accumulation of Treg in gut mucosal tissues may reflect a beneficial mechanism of the immune system that aims to reduce the level of chronic immune action. However, several studies of humans and nonhuman primates strongly imply that Treg may limit the ability of adaptive immune responses to control HIV and SIV replication (1, 27, 30-32). Increased frequencies of mucosal Treg may therefore play a pivotal role in the failure of the immune system to control HIV/SIV replication in gut mucosal tissues and facilitate persistent infection (4, 5, 17, 43, 48).
Little is known about the mechanisms contributing to accumulation of Treg at mucosal sites in HIV infection and about the effect of viral replication on mucosal Treg dynamics. The fact that the percentage of Treg within the mucosal CD4+ T-cell pool is highly increased in chronic HIV infection in spite of massive mucosal CD4+ T-cell depletion raised the question of whether mucosal Treg are selectively spared from the effects of viral infection (15). Several studies determined the susceptibility of Treg to HIV infection in vitro, but the findings were controversial (6, 22, 25, 37, 40, 47). The level of HIV/SIV infection in mucosal Treg compared to nonregulatory CD4+ T cells (non-Treg) in vivo has not been addressed so far.
In the present study we investigated the effect of SIV infection on Treg compared to non-Treg in the colon of rhesus macaques. Our findings strongly suggest that one factor contributing to the increase of mucosal Treg in SIV-infected macaques is their low susceptibility to productive SIV infection and subsequent cell death compared to those of mucosal non-Treg. Moreover, in a longitudinal analysis we found that in chronic SIV infection the local accumulation of Treg is further supported by increased proliferation of the total mucosal CD4+ T-cell pool.
MATERIALS AND METHODS
Animals and experimental SIV infection.
Rhesus macaques (Macaca mulatta) of Chinese origin were housed at the German Primate Center, Goettingen, Germany. Animal care and handling were performed under the German Animal Protection Law and in accordance with the guidelines of the German Primate Center and were reviewed and approved by the appropriate authorities. Test animals were seronegative for SIV, simian T-lymphotropic virus type 1, and simian retrovirus. Animals were infected with 300 50% tissue culture infective doses (TCID50) of SIVmac251 intravenously. Three animals (group 1) were infected for at least 9 months before euthanasia, which was performed in accordance with federal guidelines and institutional policies. Colon tissue was obtained at necropsy. For longitudinal analysis, colonic biopsy specimens from five additional animals (group 2) were collected under combined ketamine, xylazine, and atropine anesthesia 1 week before and 1, 2, 3, 4, 7, 10, and 12 weeks after infection as described previously (2). Plasma viral load peaked at 2 weeks postinfection and remained detectable throughout the course of SIV infection in all animals. None of the animals developed AIDS-like disease during the observation period. The levels of viral load and CD4+ T cells in peripheral blood and colonic mucosa are given in Table 1.
TABLE 1.
Kinetics of SIV plasma viral RNA load and CD4+ T-cell subsets in the rhesus macaques infected with SIVmac251a
| Group | Wk of SIV infection | Viral load (SIV RNA copies/ml plasma) |
CD4+ T cells (% of CD3+ T cells ± SD) |
||
|---|---|---|---|---|---|
| Median | Range | Peripheral blood | Colonic mucosa | ||
| 1 (n = 3) | Preinfection | NA | NA | 54 ± 6 | 55 ± 3 |
| 2 | 3.1 × 106 | 1.8 × 106-6.0 × 106 | 40 ± 12 | 6 ± 4 | |
| 13 | 3.4 × 103 | 1.4 × 103-3.8 × 104 | 42 ± 7 | 18 ± 5 | |
| 23 | 8.4 × 103 | 8.3 × 102-8.1 × 104 | 43 ± 10 | 22 ± 8 | |
| 31 | 2.3 × 103 | 8.3 × 102-1.8 × 105 | ND | 14 ± 5 | |
| 38-41* | 2.5 × 103 | 7.1 × 102-2.1 × 104 | 37 ± 18 | 17 ± 3 | |
| 2 (n = 5) | Preinfection | NA | NA | 55 ± 5 | 59 ± 13 |
| 2 | 3.4 × 106 | 1.8 × 106-1.0 × 107 | 36 ± 7 | 7 ± 2 | |
| 7 | 2.3 × 105 | 1.7 × 104-8.8 × 105 | 36 ± 4 | 12 ± 8 | |
| 12 | 3.1 × 105 | 1.7 × 104-1.4 × 106 | 34 ± 4 | 18 ± 8 | |
*, necropsy; NA, not applicable; ND, not determined.
Isolation of mucosal cell subsets.
Mucosal lamina propria lymphocytes were isolated from colon sections by EDTA treatment and enzymatic digestion (49). CD4+ CD25+ and CD4+ CD25− lymphocytes were isolated by using the nonhuman primate CD4+ CD25+ regulatory T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol, using two passages on magnetic columns. Immediately thereafter, the purity of isolated cell subsets was verified by flow cytometric analysis, and remaining cells were quickly snap-frozen and stored at −80°C for subsequent DNA/RNA extraction.
Flow cytometric analysis.
Flow cytometric analysis of Treg was performed using antibodies against CD4 (clone L200; BD Biosciences, Heidelberg, Germany), CD8 (DK25; Dako, Hamburg, Germany), CD25 (ACT-1; Dako), and FOXP3 (PCH101; eBioscience, San Diego, CA) according to the manufacturer's FOXP3 staining protocol. Antibodies were conjugated to fluorescein, phycoerythrin, peridinin chlorophyll protein, or allophycocyanin. Data were acquired on the FACSCalibur flow cytometer (BD) and analyzed with CellQuest software (BD). Treg were phenotypically identified by their coexpression of FOXP3 and CD25 within the CD4+ CD8− lymphocyte gate.
Quantification of viral RNA and DNA in mucosal tissue and mucosal cells.
Total DNA and RNA were simultaneously purified from biopsy specimens or mucosal cell subsets with the use of the Allprep DNA/RNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's protocol, except that both DNA and RNA were eluted in 70 μl. On-column digestion of DNA was performed during RNA purification using the RNase-free DNase set (Qiagen) in order to remove DNA carryover. Viral RNA was reverse transcribed by using the SuperScriptTM III reverse transcriptase kit (Invitrogen, Karlsruhe, Germany) with 0.5 μM of the oligonucleotide SIVgag1521rc (5′-TCAATTTTACCCAGGCATTTAATGT-3′). Viral DNA or viral RNA was quantified by a TaqMan-based real-time PCR (ABI-Prism 7500 real-time PCR system; Applied Biosystems, Darmsatdt, Germany) using 50 to 500 ng and 0.5 to 1 μg template DNA extracted from biopsy specimens and cell subsets, respectively, or 10 μl cDNA and the following oligonucleotides specific for the gag region of SIVmac251: SIVgag1444fw (5′-ACCCAGTACAACAAATAGGTGG-3′), SIVgag1521rc, and the 6-carboxyfluorescein (FAM)-labeled probe SIVgag1472 (5′-FAM-TGTCCACCTGCCATTAAGCCCGAG-BBQ-3′). In parallel with each DNA sample, simian CD4 gene copies were quantified in order to normalize the SIV DNA input using the following oligonucleotides: CD4mm163776fw (5′-TGTAGTGTGCCAGTTTAGTGC-3′), CD4mm163918rc (5′-GCACTATGGCAGTTAACATCATC-3′), and the YAK-labeled probe CD4mm163798 (5′-YAK-TGGACCCTGGTTGAAATCCTGGTTCTGC-BBQ-3′). Samples were analyzed in a total volume of 50 μl of a mixture containing PlatinumQ PCR SuperMix-UDG (Invitrogen), 400 nM each oligonucleotide, and 100 nM probe. Thermal cycling conditions consisted of 2 min at 50°C and 2 min at 95°C, followed by 50 cycles of 15 s at 95°C and 45 s at 60°C. Amplification, data acquisition, and analysis were performed using the ABI Prism 7500 sequence detection system (Applied Biosystems). The limit of detection of the SIVgag assay and the CD4 assay was 20 copies/reaction. Based on two CD4 gene copies per cell, the input number of cells was calculated in each assay, and SIV DNA copy numbers were related to cell number. Results are given as SIV DNA copies/106 cells. SIV RNA copies/106 cells were calculated in each assay from SIV RNA copy numbers/10 μl based on the volume of input DNA used for the corresponding quantification of SIV DNA copies/106 cells.
Gene expression analysis.
RNA was extracted from isolated mucosal CD4+ CD25+ and CD4+ CD25− cell subsets using the NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions. Quality control for the integrity and purity of the RNA preparation was performed on a capillary electrophoresis system (Agilent 2100 Bioanalyzer; Agilent Technologies, Böblingen, Germany). RNA was amplified and labeled with the fluorescent dye carbocyanine 3 (Cy3) using the Agilent low-RNA input linear amplification kit (Agilent Technologies) following the manufacturer's protocol. The amount of cRNA and the Cy3 incorporation rate were measured using the ND-100 spectrophotometer (NanoDrop Technologies, Wilmington, DE). cRNA microarray analysis was carried out using the 44K Agilent whole rhesus-genome oligo microarray (Agilent Technologies). Hybridization was performed according to the Agilent 60-mer oligo microarray processing protocol using the Agilent gene expression hybridization kit (Agilent Technologies), and fluorescence signals were detected using Agilent's scanner system (Agilent Technologies). Raw microarray image files were read out and processed using Agilent feature extraction (Agilent Technologies) and analyzed with the Rosetta Resolver gene expression data analysis system (Rosetta Biosoftware, Seattle, WA). All procedures were performed by customer service of Miltenyi Biotec, Germany. Normalized signal intensities for two sets of selected genes were analyzed for transcriptional characterization of mucosal Treg and non-Treg.
Immunohistochemistry and immunofluorescence staining.
For immunostaining, 2- or 3-μm sections were cut, deparaffinized, and subjected to a heat-induced epitope retrieval step before incubation with antibodies. Sections were immersed in sodium citrate buffer solution at pH 6.0 and heated in a high-pressure cooker. The slides were rinsed in cool running water, washed in Tris-buffered saline (pH 7.4), and incubated with primary antibodies. The primary antibodies included monoclonal antibodies against CD3 (clone UCHT1, dilution 1:25; Dako, Glostrup, Denmark), CD4 (1F6, 1:5; Novocastra Laboratories, Newcastle upon Tyne, United Kingdom), FOXP3 (PCH101, 1:100; eBioscience), Ki67 (MIB-1, 1:2,000; Dako), and Perforin (Pf-344). For detection, the streptavidin-AP kit (K5005; Dako) and the Envision PO kit (K5007; Dako) were used. Only the lamina propria was analyzed, and lymphoid aggregates and intraepithelial lymphocytes were excluded. For quantification of perforin+ cells, lamina propria and intraepithelial cells were analyzed. The frequency of Treg per mucosal CD4+ T cells was determined by dividing the number of CD3+ FOXP3+ cells by the number of mucosal CD4+ cells. The frequency of proliferating cells within mucosal CD4+ cells was determined by dividing the number of CD4+ Ki67+ cells by the number of mucosal CD4+ cells. For immunofluorescence labeling, sections were incubated with rat anti-FOXP3 antibody (PCH101, 1:20; eBioscience) followed by Alexa Fluor 555-conjugated anti-rat antibody (1:100; Invitrogen, Carlsbad, CA), washed three times in PBS, and incubated with mouse anti-Ki67 (MIB-1, 1:1,000; Dako,) followed by Alexa Fluor 488-conjugated anti-mouse antibody (1:100; Invitrogen). Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (1:1,500; Roche, Mannheim, Germany), and slides mounted in Fluoromount-G (Southern Biotech, Birmingham, AL). Images were acquired using a fluorescence microscope (AxioImager Z1) equipped with a charge-coupled-device (CCD) camera (AxioCam MRm) and processed with Axiovision software (Carl Zeiss MicroImaging, Inc.). Negative controls were performed by omitting the primary antibodies. Positive cells were quantified in intestinal tissues per high-power field (hpf) (1 hpf = 0.237 mm2), and 10 hpf were averaged in each case.
Statistical analysis.
Data are represented as medians ± the standard deviation (SD) and were analyzed by using the two-tailed Student t test or the Wilcoxon test. A P value of less than 0.05 was considered to be statistically significant. Correlations were determined using linear regression (Pearson correlation; GraphPad Prism; GraphPad Software, San Diego, CA).
RESULTS
Isolation of mucosal Treg and non-Treg subsets.
Mucosal CD4+ CD25+ and CD4+ CD25− cells were isolated from the colon sections of three chronically SIV-infected animals (Table 1, group 1) and a healthy SIV-uninfected animal. SIV-infected animals were examined immunohistochemically for absolute numbers of mucosal Treg and their frequency within the absolute number of mucosal CD4+ T cells (relative frequency of mucosal Treg) 1 week before the infection and at necropsy. As expected and consistent with our previous findings for chronic HIV infection, absolute and relative frequencies of Treg in colonic mucosa were markedly increased in the chronically SIV-infected animals compared to preinfection levels: absolute and relative frequency increased from 9.1 ± 6.6/10 hpf and 5.6 ± 2.1% to 25.0 ± 13.4/10 hpf and 53.8 ± 12.8%, respectively.
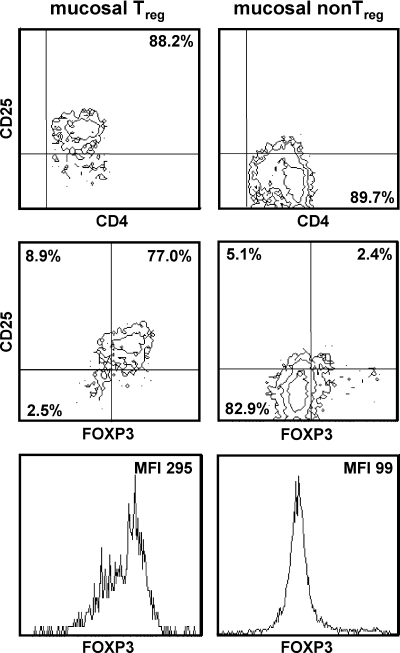
After isolation, mucosal CD4+ CD25+ and CD4+ CD25− cells were analyzed for CD4, CD25, and FOXP3 expression by flow cytometry. The purity of the CD4+ CD25+ cell subset and the CD4+ CD25− cell subset ranged from 87.8 to 97.4% and from 89.7 to 98.2%, respectively. The CD4+ CD25+ cell subset contained 77.0 to 93.3% FOXP3-expressing cells. Flow cytometric analysis of mucosal cell subsets isolated from colonic mucosa of one SIV-infected animal is shown in Fig. 1. In this animal, FOXP3 transcript expression analyzed by microarray was detectable with isolated CD4+ CD25+ cells but not with isolated CD4+ CD25− cells. Since the majority of the isolated CD4+ CD25+ cells expressed FOXP3, representing the key factor for the function of regulatory CD4+ T cells, we refer to these cells as the mucosal Treg subset. Isolated CD4+ CD25− cells are designated the mucosal non-Treg subset.
FIG. 1.
Isolation of mucosal Treg and non-Treg subsets. Treg and non-Treg were isolated from colonic lamina propria lymphocytes of chronically SIV-infected rhesus macaques and analyzed by flow cytometry. Cells were gated for lymphocytes by their characteristic forward-sideward-scatter profile and were analyzed for expression of CD4, CD25, and FOXP3. Mean fluorescence intensity (MFI) is given for FOXP3 in the lymphocyte gate.
Low efficiency of SIV RNA production in infected mucosal Treg.
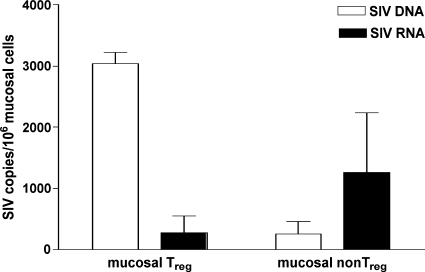
For the gut mucosal tissue of chronically HIV-infected individuals, an increase in the percentage of Treg within CD4+ T cells has been described, although absolute numbers of mucosal CD4+ T cells were decreased (15). The reason for this dichotomy remained unclear. One possible explanation might be that mucosal Treg are selectively spared from infection-mediated depletion. In order to assess the in vivo level of SIV infection in mucosal Treg and non-Treg, we quantified cell-associated SIV DNA and SIV RNA copy numbers in both mucosal CD4+ T-cell subsets, which were isolated in parallel from each of three chronically SIV-infected animals (Fig. 1). In one animal, viral sequences could not be detected in the mucosal Treg subset due to poor cell counts and the subsequently low recovery rate of viral DNA and RNA. Therefore, the mucosal Treg subset of this animal was not included in the analysis. Mucosal Treg contained SIV DNA copies that were 11.7-fold higher than those of non-Treg (Fig. 2). These cell-associated SIV DNA copies may reflect integrated proviruses and/or unintegrated reverse transcripts, which both are a record of infection events. Interestingly, despite being infected at a higher level, the mucosal Treg subset contained 4.5-fold fewer SIV RNA copies than the non-Treg subset (Fig. 2). These findings indicate that SIV RNA production is less efficient in mucosal Treg than in their nonregulatory counterpart, either by insufficient integration of SIV DNA or transcription of proviral DNA.
FIG. 2.
SIV RNA production is less efficient in mucosal Treg than in non-Treg. Cell-associated SIV DNA and SIV RNA in mucosal Treg and non-Treg isolated from chronically SIV-infected animals were quantified and are given as number of SIVgag DNA or SIVgag RNA copies per 106 mucosal cells. Data are given in medians, with error bars representing standard deviations.
Mucosal Treg are less affected by SIV-related cell death than mucosal non-Treg.
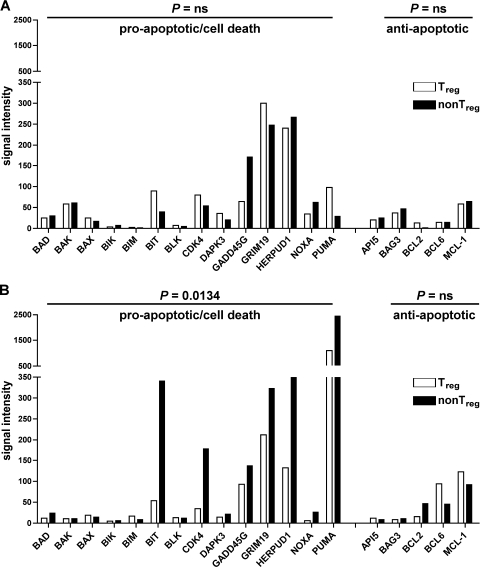
The observed differences in the efficiency levels of SIV RNA production suggest that virus replication is attenuated in Treg compared to non-Treg. As a consequence, mucosal Treg and non-Treg could be differentially affected by SIV-mediated cell death, either via virus-induced cytopathic effects or immune response-mediated cytolysis. To determine the level of SIV-related cell death-inducing pathways, mucosal Treg and non-Treg from a chronically SIV-infected animal and a healthy SIV-uninfected animal were compared for cell death-related gene expression by microarray experiments. Flow cytometric analysis of the mucosal cell subsets isolated from the SIV-infected animal is shown in Fig. 1. The purity was comparable to that of Treg and non-Treg subsets isolated from the SIV-uninfected animal. Normalized signal intensities for single genes were analyzed to identify mRNA expression levels of different genes in mucosal Treg and non-Treg. Genes whose normalized signal intensity values were less than 5 in both subsets of one animal were excluded from the analysis. In the SIV-uninfected animal, transcript expression levels of proapoptotic/cell death-related genes did not differ between mucosal Treg and non-Treg (Fig. 3A). This was different with the chronically SIV-infected animal, for which the transcript expression levels of genes with cell death-related function were significantly higher in the mucosal non-Treg subset than in the Treg subset (P = 0.0134) (Fig. 3B). These included genes previously demonstrated to be directly associated with HIV production, such as CDK4, GADD45G, and PUMA (3, 21, 33, 38, 42). Transcription of anti-apoptotic genes was found rarely with both animals (Fig. 3A and B). In both the SIV-infected animal and the SIV-uninfected animal, transcript levels of genes related to control and regulation of proliferation were comparable in mucosal Treg and non-Treg (data not shown). This provides additional support that the high level of cell death-related gene expression found in the non-Treg subset of the SIV-infected animal was mediated by direct viral infection and/or immune defense mechanisms rather than increased homeostatic or activation-induced proliferation of mucosal non-Treg over Treg. Taken together, these results suggest that SIV-related cell death-inducing mechanisms occurred preferentially in the mucosal non-Treg subset.
FIG. 3.
In chronic SIV infection, expression levels of cell death-related genes are higher in mucosal non-Treg than in Treg. Microarray analysis of apoptosis- and cell death-related genes in mucosal Treg and non-Treg isolated in parallel from a chronically SIV infected (A) and a healthy SIV-uninfected (B) rhesus macaque. Normalized signal intensities for single genes were analyzed to identify mRNA expression levels of different genes. Genes whose normalized signal intensity values were less than 5 for both cell subsets were excluded from the analysis. Statistical analysis was performed using the Wilcoxon matched-pairs test. ns, not significant.
Longitudinal study of mucosal CD4+ T cells.
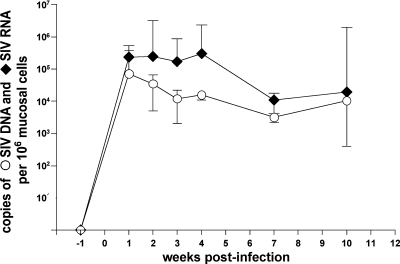
Longitudinal analysis of alterations in the mucosal CD4+ T-cell pool was performed with five rhesus macaques during the course of SIV infection (Table 1, group 2). Acute HIV/SIV infection is characterized by high viral replication and massive loss of CD4+ T cells, which is most pronounced in the gastrointestinal mucosa and persists during chronic infection (11, 35, 46). In the colonic mucosa of all animals, high viral replication occurred within the first 4 weeks of infection, as determined by comparing the cell-associated copy numbers of SIV RNA and SIV DNA (Fig. 4). Consequently, and consistent with previous observations (12), we refer to the first 4 weeks of infection as the acute phase and to the following weeks as the chronic phase of SIV infection.
FIG. 4.
Mucosal viral load in the colon of SIV-infected rhesus macaques. Five animals were infected with SIVmac251. SIV DNA and RNA were quantified longitudinally in colonic tissue during the course of SIV infection and are given as number of SIVgag DNA or SIVgag RNA copies per 106 mucosal cells. Data are given in medians, with error bars representing standard deviations.
SIV infection is associated with increased proliferation of mucosal CD4+ T cells, including Treg.
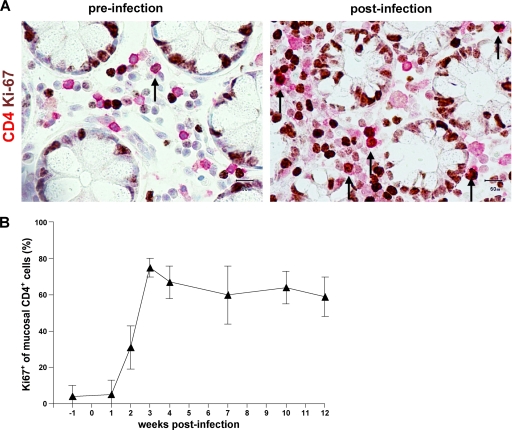
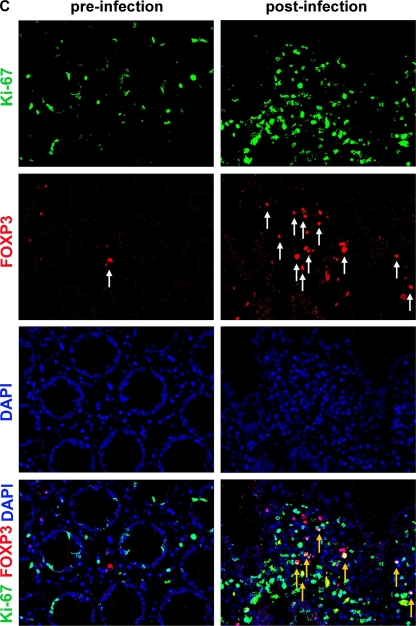
Given that mucosal Treg are spared from SIV-mediated cell death, proliferation of the mucosal CD4+ T-cell pool may, in the course of SIV infection, favor the accumulation of Treg. In order to assess this possibility, mucosal CD4+ T cells in colon biopsy specimens taken longitudinally during SIV infection were phenotypically analyzed in situ for coexpression of the proliferation marker Ki67. Immunohistochemical double staining revealed less than 10% of mucosal CD4+ T cells expressing Ki67 before infection (Fig. 5A and B). Ki67 expression of CD4+ T cells increased to 40.0% ± 11.9% by week 2 and to 74.5 ± 5.4% by week 3 after infection (Fig. 5B). This high number of CD4+ cells expressing Ki67 in mucosal CD4+ T cells was maintained during the follow-up, indicating a remarkably increased proliferation of mucosal CD4+ T cells in chronic SIV infection (Fig. 5A and B). To examine whether Treg proliferate within the mucosal CD4+ T-cell pool, we analyzed coexpression of FOXP3 and Ki67 in mucosal cells by immunofluorescence staining. We found coexpression of FOXP3 and Ki67 in the chronic phase of SIV infection, indicating proliferation of Treg within the mucosal CD4+ T-cell pool (Fig. 5C). Thus, in addition to preferential SIV-mediated cell death in non-Treg, local expansion may account for increased mucosal Treg in chronic SIV infection.
FIG. 5.
Proliferative response in mucosal CD4+ T cells after SIV infection includes Treg. (A) Representative immunohistochemical staining of proliferating CD4+ T cells (arrows) in colonic mucosa identified by double immunoenzymatic labeling for CD4 (red, membranous) and Ki67 (brown, nuclear). Original magnification, ×400. (B) Longitudinal examination of proliferation of colon mucosal CD4+ T cells in five rhesus macaques during the course of SIV infection. Frequency of mucosal CD4+ Ki67+ cells in relation to mucosal CD4+ T cells. Data are given in medians, with error bars representing standard deviations. (C) Representative immunofluorescence staining of proliferating mucosal Treg identified by double staining for FOXP3 (red, white arrows) and Ki67 (green). FOXP3- and Ki67-coexpressing mucosal cells are indicated by yellow arrows. Original magnification, ×400.
Increase of mucosal Treg is most pronounced after SIV-induced depletion of mucosal CD4+ T cells.
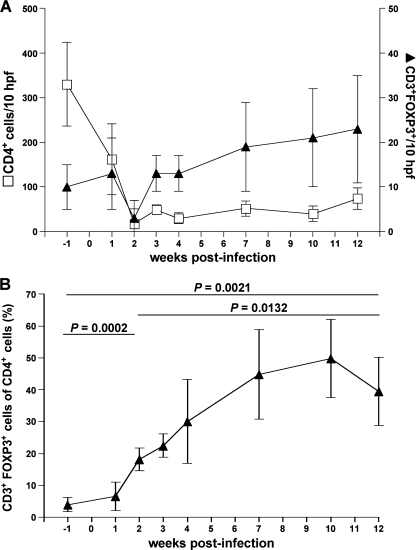
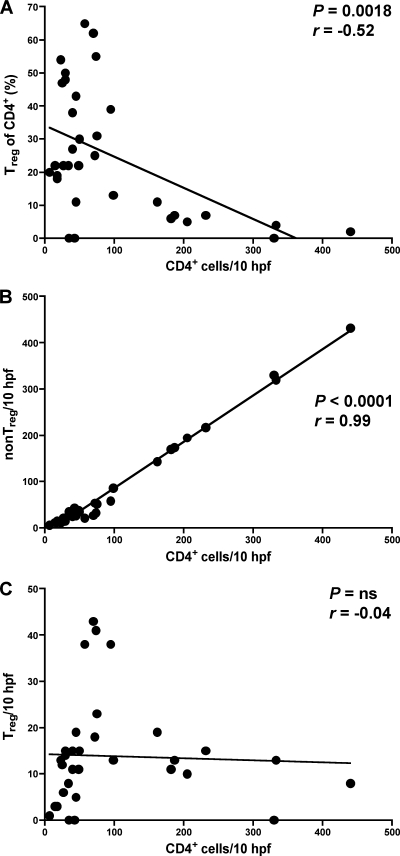
To determine the dynamics of mucosal Treg during the course of SIV infection, we immunohistochemically examined the changes in the absolute frequency of mucosal Treg in sequential biopsy specimens. In addition, we analyzed the changes of Treg relative to the number of CD4+ cells (relative frequency of mucosal Treg). As expected, the depletion of mucosal CD4+ T cells was almost complete within 2 weeks after infection, and mucosal CD4+ T cells remained low throughout the observation period (Fig. 6A). Mucosal Treg numbers remained unchanged at week 1 and declined below preinfection levels by week 2 postinfection (Fig. 6A). Relative frequencies of mucosal Treg increased compared to preinfection levels (P = 0.0002) (Fig. 6B), suggesting a preferential loss of non-Treg during the first 2 weeks of infection, which occurs probably due to SIV-mediated cell death. Thereafter, the relative frequencies of mucosal Treg increased remarkably to 44.9 ± 14.4% at week 7 after infection compared with only 4.0 ± 2.2% at preinfection (P = 0.0136) and remained high during the follow-up (Fig. 6B). During the course of infection, loss of mucosal CD4+ T cells correlated with a relative increase of mucosal Treg, indicating that mucosal Treg were spared from SIV-mediated depletion (P = 0.0018) (Fig. 7A). Consistent with this, we found a highly significant correlation between the number of CD4+ T cells and the number of mucosal non-Treg (P < 0.0001) but not of mucosal Treg (7B and C). These observations support our aforementioned findings that Treg are less susceptible to SIV-related cell death than non-Treg.
FIG. 6.
Mucosal Treg increase during the course of SIV infection in colon despite mucosal CD4+ T-cell depletion. Longitudinal examination of mucosal Treg in five animals following SIV infection. (A) Absolute numbers of mucosal CD4+ T cells and mucosal FOXP3+ Treg per 10 hpf in five rhesus macaques before and after SIV infection. (B) Frequency of mucosal Treg in relation to mucosal CD4+ T cells (relative frequency of mucosal Treg). Data are given in medians, with error bars representing standard deviations, and were analyzed by using the two-tailed Student t test.
FIG. 7.
Depletion of mucosal CD4+ T cells correlates with loss of mucosal non-Treg but not of Treg. Correlation between the number of mucosal CD4+ T cells and the relative frequency of mucosal Treg (A), the number of mucosal non-Treg (B), and the number of mucosal Treg (C), respectively. CD4+ T cells and Treg were quantified in colonic tissue, and the absolute number of non-Treg was calculated by subtracting the number of Treg from the number of CD4+ T cells. Pearson's test was used to determine correlation. ns, not significant.
After week 2 postinfection, mucosal Treg increased also in absolute numbers (Fig. 6A). Therefore, the high relative frequency of mucosal Treg in the chronic phase not only was attributed to a preferential loss of non-Treg but may also have resulted from an additional local expansion of Treg within the colonic mucosa. Consistent with this, an increase of Treg numbers after week 2 postinfection coincided with high proliferation of the mucosal CD4+ T-cell pool, which included Treg (Fig. 5).
Increase of mucosal Treg correlates with a reduction of perforin-expressing cells in colonic mucosa.
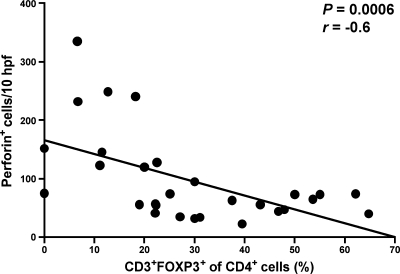
The accumulation of Treg in colonic tissue might impair mucosal immune responses. In order to assess the potential contribution of mucosal Treg to the counterregulation of the cytotoxic immune response, we examined in situ the expression of the cytotoxic molecule perforin after SIV infection and compared the numbers of perforin+ cells to the frequencies of mucosal Treg. Consistent with previous findings (43), perforin expression in the colonic tissue of all five animals increased during acute SIV infection and returned to preinfection levels afterward (data not shown). There was a significant inverse correlation between the relative frequency of Treg and the number of perforin-expressing cells (Fig. 8), which is consistent with the suggestion that mucosal Treg inhibit cytotoxic immune responses in chronic HIV/SIV infection.
FIG. 8.
Increase of Treg in colonic mucosa correlates with a local reduction of perforin-expressing cells. Correlation between the relative frequency of mucosal Treg and the number of perforin-expressing cells in SIV infection. Perforin+ cells were quantified in colonic tissue of SIV-infected rhesus macaques and compared with the corresponding relative frequencies of mucosal Treg (see Fig. 7A). Pearson's test was used to determine the correlation.
DISCUSSION
Although HIV/SIV infection causes a massive loss of CD4+ T cells in gut mucosal tissues (11, 23, 35, 36, 46, 54), the frequency of Treg among mucosal CD4+ T cells has been described to be increased in the duodenal and colonic tissue of infected humans and nonhuman primates (9, 15, 19). These findings suggest that mucosal Treg are selectively spared from HIV/SIV-induced CD4+ T-cell depletion. Conflicting in vitro data have been reported regarding the susceptibility of Treg to HIV infection. In some reports, the enhanced HIV gene expression and cell death of HIV-infected Treg are described (6, 25, 40), whereas others have observed repressed HIV transcription and improved survival of Treg (22, 39, 47). This apparent discrepancy indicates that experimental methods, cell sources, and cell culture conditions might influence the susceptibility of Treg to HIV infection. In vitro studies may thus be inadequate in assessing the in vivo susceptibility of Treg to HIV infection because in vitro conditions do not reflect the physiological situation. In mucosal tissues, the close cell-to-cell proximity, the cell subset distribution, and particularly the local immune activation status may have an impact on viral replication.
In this study, we examined the susceptibility of mucosal Treg to SIV infection in vivo by quantifying cell-associated viral DNA and viral RNA in mucosal Treg and non-Treg isolated from the colonic tissue of chronically SIV-infected rhesus macaques. With the mucosal Treg subset, we found an intracellular content of SIV DNA that was higher than that of its non-Treg counterpart. This finding indicates a level of infection in the mucosal Treg subset that is higher than that in its nonregulatory counterpart and is in line with previous studies of peripheral CD4+ T-cell subsets isolated from HIV-infected subjects (14, 53). Interestingly, despite the high frequency of SIV DNA in the mucosal Treg subset, viral RNA in mucosal Treg was present in copy numbers lower than those for non-Treg, indicating that in vivo SIV RNA production is diminished in infected mucosal Treg. This was evident, although a minor percentage of nonregulatory CD25+ FoxP3− CD4+ T cells was present in the isolated Treg subset, and thus, it could be possible that only residual or no SIV RNA production occurs in mucosal Treg in chronic SIV infection. In vitro studies have suggested that FOXP3 overexpression inhibits HIV long-terminal repeat transcriptional activity (22, 47). Therefore, repressing SIV DNA transcription by high FOXP3 expression level in mucosal Treg of SIV-infected animals may be one possible mechanism of the low susceptibility of mucosal Treg to productive SIV infection. Alternatively, intracellular factors might counterregulate the integration process of viral DNA. Although underlying mechanisms remain unclear in this study, our findings suggest that, in vivo, mucosal Treg have low susceptibility to productive SIV infection.
As a consequence of attenuated virus production, including synthesis, posttranslational processing, assembly, and release of viral proteins, mucosal Treg may experience only limited effects of SIV-mediated cell death. Correspondingly, chronic SIV infection was associated with expression levels of cell death-related genes in mucosal Treg that were lower than those in non-Treg, including those that have been demonstrated to be directly associated with HIV production (3, 21, 33, 38, 42). Therefore, the low susceptibility of mucosal Treg to productive SIV infection is likely to protect them, at least to some extent, from SIV-induced cytolysis and antiviral immune responses as well, thus contributing to an enrichment of Treg in the mucosal CD4+ T-cell pool.
Longitudinal examination revealed an increased percentage of Treg within the mucosal CD4+ T-cell subset by week 2 after SIV infection, although absolute numbers of mucosal CD4+ T cells decreased. This suggests a preferential loss of non-Treg, which is in accordance with our finding that Treg are spared from productive SIV infection. After mucosal CD4+ T-cell depletion, percentages of Treg within mucosal CD4+ T cells further increased and were maintained at markedly high levels during the chronic phase of infection. This was not due exclusively to the selective loss of non-Treg, because Treg increased also in absolute numbers, a result that is consistent with our previous study of HIV infection (15). We found that this accumulation of mucosal Treg was driven by a proliferative response in the mucosal CD4+ T-cell pool. This proliferation was induced during mucosal CD4+ T-cell depletion, remained continuously high after the depletion, and importantly, included mucosal Treg. Expression levels of genes related to proliferation and cell division were comparable between mucosal Treg and non-Treg, indicating similar responses in both subsets, with regard to their proliferative activity. These observations are in agreement with studies demonstrating that Treg proliferate in parallel to memory CD4+ T cells at sites of antigenic challenge as a mechanism of the immune system to prevent local hyperimmune activation (55). Even though limited to a small number of animals, the present study indicates that mucosal Treg are not only spared from productive SIV infection and subsequent cell death but also favored by the SIV-induced proliferation of the mucosal CD4+ T-cell pool. A relationship between the numbers of Treg and proliferating cells has also been demonstrated with the lymph node tissue of SIV-infected rhesus macaques (17). In addition to the improved survival and proliferation of mucosal Treg, a redistribution of Treg to tissue sites of SIV expression may occur as a reaction of the immune system to prevent immune hyperactivation and thus facilitate the accumulation of Treg in the gut mucosa.
The findings from our longitudinal study are contrary to a previous cross-sectional study of Chase et al. demonstrating a severe depletion of mucosal Treg in SIV infection (13). These differences may be due to the naturally occurring interindividual variations in cross-sectional studies. A further explanation for this conflict may be the use of the highly pathogenic SIV/Delta B670, SIV/17E-Fr/pigtailed macaque model employed in that study, which is associated with persistently high levels of viral replication and accelerated progression to AIDS-like disease compared to our SIVmac251/Chinese rhesus macaque model, for which the progression rate is variable, as is that for humans infected with HIV. The results obtained from the study of the rhesus macaque model presented here is in agreement with those for HIV-infected patients (15). Thus, our model appears to be suitable to the characterization of mucosal HIV-related immunopathogenesis. However, the findings of Chase et al. indicate that Treg are not completely spared from depletion under conditions of uncontrolled viral replication and thus might explain the loss of mucosal Treg in absolute numbers that we observed by week 2 after infection, when high levels of viral replication accounted for the massive depletion of mucosal CD4+ T cells. This is also supported by a limited study showing decreased mucosal Treg in SIV-infected rhesus macaques during end-state simian AIDS (41).
In summary, our findings suggest that during chronic SIV infection mucosal Treg have a susceptibility to productive SIV infection that is lower than that of non-Treg and thus are selectively spared from infection-mediated cell death. Furthermore, after mucosal CD4+ T-cell depletion, the residual mucosal CD4+ T-cell pool is maintained by a proliferative response, which not only provides new targets for SIV infection but also promotes local expansion of mucosal Treg. As a consequence, mucosal Treg increase remarkably within mucosal CD4+ T cells and also in absolute numbers. Even though viral production occurs predominantly in the non-Treg subset, mucosal Treg may indirectly contribute to sustained viral replication in gut mucosal tissue by impairing antiviral immune responses and thus prevent elimination of the virus in gut mucosal tissue.
Acknowledgments
We thank Diana Bösel, Simone Spieckermann, Heike Lerch, and Julian Lünig for excellent technical assistance. We also thank Annette Schrod and Thorsten Eggers for taking biopsies. We are grateful to Wolfgang Vess and Bernhard Gerstmayer from Miltenyi Biotech for their help with the microarray data analysis.
This work was supported by the German Research Foundation (DFG KFO grant 104/1-2).
Footnotes
Published ahead of print on 13 January 2010.
REFERENCES
- 1.Aandahl, E. M., J. Michaelsson, W. J. Moretto, F. M. Hecht, and D. F. Nixon. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allers, K., D. Kunkel, V. Moos, M. Eisenblatter, C. Stahl-Hennig, F. J. Kaup, R. Ignatius, and T. Schneider. 2008. Migration patterns of nonspecifically activated versus nonactivated nonhuman primate T lymphocytes: preferential homing of activated autologous CD8+ T cells in the rectal mucosa. J. Immunother. 31:334-344. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, J. L., E. S. Zimmerman, J. L. DeHart, S. Murala, O. Ardon, J. Blackett, J. Chen, and V. Planelles. 2005. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 12:326-334. [DOI] [PubMed] [Google Scholar]
- 4.Andersson, J., H. Behbahani, J. Lieberman, E. Connick, A. Landay, B. Patterson, A. Sonnerborg, K. Lore, S. Uccini, and T. E. Fehniger. 1999. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS 13:1295-1303. [DOI] [PubMed] [Google Scholar]
- 5.Andersson, J., S. Kinloch, A. Sonnerborg, J. Nilsson, T. E. Fehniger, A. L. Spetz, H. Behbahani, L. E. Goh, H. McDade, B. Gazzard, H. Stellbrink, D. Cooper, and L. Perrin. 2002. Low levels of perforin expression in CD8+ T lymphocyte granules in lymphoid tissue during acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 185:1355-1358. [DOI] [PubMed] [Google Scholar]
- 6.Antons, A. K., R. Wang, K. Oswald-Richter, M. Tseng, C. W. Arendt, S. A. Kalams, and D. Unutmaz. 2008. Naive precursors of human regulatory T cells require FoxP3 for suppression and are susceptible to HIV infection. J. Immunol. 180:764-773. [DOI] [PubMed] [Google Scholar]
- 7.Aziz, S., O. T. Fackler, A. Meyerhans, N. Muller-Lantzsch, M. Zeitz, and T. Schneider. 2005. Replication of M-tropic HIV-1 in activated human intestinal lamina propria lymphocytes is the main reason for increased virus load in the intestinal mucosa. J. Acquir. Immune Defic. Syndr. 38:23-30. [DOI] [PubMed] [Google Scholar]
- 8.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 9.Boasso, A., M. Vaccari, A. Hryniewicz, D. Fuchs, J. Nacsa, V. Cecchinato, J. Andersson, G. Franchini, G. M. Shearer, and C. Chougnet. 2007. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J. Virol. 81:11593-11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365-1371. [DOI] [PubMed] [Google Scholar]
- 11.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centlivre, M., M. Sala, S. Wain-Hobson, and B. Berkhout. 2007. In HIV-1 pathogenesis the die is cast during primary infection. AIDS 21:1-11. [DOI] [PubMed] [Google Scholar]
- 13.Chase, A. J., A. R. Sedaghat, J. R. German, L. Gama, M. C. Zink, J. E. Clements, and R. F. Siliciano. 2007. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J. Virol. 81:12748-12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunham, R. M., B. Cervasi, J. M. Brenchley, H. Albrecht, A. Weintrob, B. Sumpter, J. Engram, S. Gordon, N. R. Klatt, I. Frank, D. L. Sodora, D. C. Douek, M. Paiardini, and G. Silvestri. 2008. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J. Immunol. 180:5582-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epple, H. J., C. Loddenkemper, D. Kunkel, H. Troger, J. Maul, V. Moos, E. Berg, R. Ullrich, J. D. Schulzke, H. Stein, R. Duchmann, M. Zeitz, and T. Schneider. 2006. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood 108:3072-3078. [DOI] [PubMed] [Google Scholar]
- 16.Epple, H. J., T. Schneider, H. Troeger, D. Kunkel, K. Allers, V. Moos, M. Amasheh, C. Loddenkemper, M. Fromm, M. Zeitz, and J. D. Schulzke. 2009. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 58:220-227. [DOI] [PubMed] [Google Scholar]
- 17.Estes, J. D., Q. Li, M. R. Reynolds, S. Wietgrefe, L. Duan, T. Schacker, L. J. Picker, D. I. Watkins, J. D. Lifson, C. Reilly, J. Carlis, and A. T. Haase. 2006. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J. Infect. Dis. 193:703-712. [DOI] [PubMed] [Google Scholar]
- 18.Fackler, O. T., M. Schafer, W. Schmidt, T. Zippel, W. Heise, T. Schneider, M. Zeitz, E. O. Riecken, N. Mueller-Lantzsch, and R. Ullrich. 1998. HIV-1 p24 but not proviral load is increased in the intestinal mucosa compared with the peripheral blood in HIV-infected patients. AIDS 12:139-146. [DOI] [PubMed] [Google Scholar]
- 19.Favre, D., S. Lederer, B. Kanwar, Z. M. Ma, S. Proll, Z. Kasakow, J. Mold, L. Swainson, J. D. Barbour, C. R. Baskin, R. Palermo, I. Pandrea, C. J. Miller, M. G. Katze, and J. M. McCune. 2009. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 5:e1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontenot, J. D., M. A. Gavin, and A. Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330-336. [DOI] [PubMed] [Google Scholar]
- 21.Genini, D., D. Sheeter, S. Rought, J. J. Zaunders, S. A. Susin, G. Kroemer, D. D. Richman, D. A. Carson, J. Corbeil, and L. M. Leoni. 2001. HIV induces lymphocyte apoptosis by a p53-initiated, mitochondrial-mediated mechanism. FASEB J. 15:5-6. [DOI] [PubMed] [Google Scholar]
- 22.Grant, C., U. Oh, K. Fugo, N. Takenouchi, C. Griffith, K. Yao, T. E. Newhook, L. Ratner, and S. Jacobson. 2006. Foxp3 represses retroviral transcription by targeting both NF-kappaB and CREB pathways. PLoS Pathog. 2:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 17:1881-1888. [DOI] [PubMed] [Google Scholar]
- 25.Holmes, D., G. Knudsen, S. Mackey-Cushman, and L. Su. 2007. FoxP3 enhances HIV-1 gene expression by modulating NFkappaB occupancy at the long terminal repeat in human T cells. J. Biol. Chem. 282:15973-15980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057-1061. [DOI] [PubMed] [Google Scholar]
- 27.Hryniewicz, A., A. Boasso, Y. Edghill-Smith, M. Vaccari, D. Fuchs, D. Venzon, J. Nacsa, M. R. Betts, W. P. Tsai, J. M. Heraud, B. Beer, D. Blanset, C. Chougnet, I. Lowy, G. M. Shearer, and G. Franchini. 2006. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 108:3834-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kewenig, S., T. Schneider, K. Hohloch, K. Lampe-Dreyer, R. Ullrich, N. Stolte, C. Stahl-Hennig, F. J. Kaup, A. Stallmach, and M. Zeitz. 1999. Rapid mucosal CD4(+) T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology 116:1115-1123. [DOI] [PubMed] [Google Scholar]
- 29.Khattri, R., T. Cox, S. A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337-342. [DOI] [PubMed] [Google Scholar]
- 30.Kinter, A., J. McNally, L. Riggin, R. Jackson, G. Roby, and A. S. Fauci. 2007. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc. Natl. Acad. Sci. U. S. A. 104:3390-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinter, A. L., M. Hennessey, A. Bell, S. Kern, Y. Lin, M. Daucher, M. Planta, M. McGlaughlin, R. Jackson, S. F. Ziegler, and A. S. Fauci. 2004. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 200:331-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinter, A. L., R. Horak, M. Sion, L. Riggin, J. McNally, Y. Lin, R. Jackson, A. O'Shea, G. Roby, C. Kovacs, M. Connors, S. A. Migueles, and A. S. Fauci. 2007. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res. Hum. Retroviruses 23:438-450. [DOI] [PubMed] [Google Scholar]
- 33.Lee-Huang, S., L. Zhang, P. L. Huang, Y. T. Chang, and P. L. Huang. 2003. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem. Biophys. Res. Commun. 307:1029-1037. [DOI] [PubMed] [Google Scholar]
- 34.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 35.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 36.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno-Fernandez, M. E., W. Zapata, J. T. Blackard, G. Franchini, and C. A. Chougnet. 2009. Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J. Virol. 83:12925-12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nekhai, S., R. R. Shukla, A. Fernandez, A. Kumar, and N. J. Lamb. 2000. Cell cycle-dependent stimulation of the HIV-1 promoter by Tat-associated CAK activator. Virology 266:246-256. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson, J., A. Boasso, P. A. Velilla, R. Zhang, M. Vaccari, G. Franchini, G. M. Shearer, J. Andersson, and C. Chougnet. 2006. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108:3808-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oswald-Richter, K., S. M. Grill, N. Shariat, M. Leelawong, M. S. Sundrud, D. W. Haas, and D. Unutmaz. 2004. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2:E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira, L. E., F. Villinger, N. Onlamoon, P. Bryan, A. Cardona, K. Pattanapanysat, K. Mori, S. Hagen, L. Picker, and A. A. Ansari. 2007. Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys. J. Virol. 81:4445-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perfettini, J. L., T. Roumier, M. Castedo, N. Larochette, P. Boya, B. Raynal, V. Lazar, F. Ciccosanti, R. Nardacci, J. Penninger, M. Piacentini, and G. Kroemer. 2004. NF-kappaB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J. Exp. Med. 199:629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quigley, M. F., K. Abel, B. Zuber, C. J. Miller, J. K. Sandberg, and B. L. Shacklett. 2006. Perforin expression in the gastrointestinal mucosa is limited to acute simian immunodeficiency virus infection. J. Virol. 80:3083-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raffatellu, M., R. L. Santos, D. E. Verhoeven, M. D. George, R. P. Wilson, S. E. Winter, I. Godinez, S. Sankaran, T. A. Paixao, M. A. Gordon, J. K. Kolls, S. Dandekar, and A. J. Baumler. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14:421-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakaguchi, S., M. Ono, R. Setoguchi, H. Yagi, S. Hori, Z. Fehervari, J. Shimizu, T. Takahashi, and T. Nomura. 2006. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212:8-27. [DOI] [PubMed] [Google Scholar]
- 46.Schneider, T., H. U. Jahn, W. Schmidt, E. O. Riecken, M. Zeitz, and R. Ullrich. 1995. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut 37:524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selliah, N., M. Zhang, S. White, P. Zoltick, B. E. Sawaya, T. H. Finkel, and R. Q. Cron. 2008. FOXP3 inhibits HIV-1 infection of CD4 T-cells via inhibition of LTR transcriptional activity. Virology 381:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shacklett, B. L., C. A. Cox, M. F. Quigley, C. Kreis, N. H. Stollman, M. A. Jacobson, J. Andersson, J. K. Sandberg, and D. F. Nixon. 2004. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J. Immunol. 173:641-648. [DOI] [PubMed] [Google Scholar]
- 49.Solano-Aguilar, G. I., K. G. Vengroski, E. Beshah, and J. K. Lunney. 2000. Isolation and purification of lymphocyte subsets from gut-associated lymphoid tissue in neonatal swine. J. Immunol. Methods 241:185-199. [DOI] [PubMed] [Google Scholar]
- 50.Suri-Payer, E., A. Z. Amar, A. M. Thornton, and E. M. Shevach. 1998. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212-1218. [PubMed] [Google Scholar]
- 51.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toulza, F., A. Heaps, Y. Tanaka, G. P. Taylor, and C. R. Bangham. 2008. High frequency of CD4+FoxP3+ cells in HTLV-1 infection: inverse correlation with HTLV-1-specific CTL response. Blood 111:5047-5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran, T. A., M. G. de Goer de Herve, H. Hendel-Chavez, B. Dembele, E. Le Nevot, K. Abbed, C. Pallier, C. Goujard, J. Gasnault, J. F. Delfraissy, A. M. Balazuc, and Y. Taoufik. 2008. Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS One 3:e3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 55.Vukmanovic-Stejic, M., E. Agius, N. Booth, P. J. Dunne, K. E. Lacy, J. R. Reed, T. O. Sobande, S. Kissane, M. Salmon, M. H. Rustin, and A. N. Akbar. 2008. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J. Clin. Investig. 118:3639-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]