Abstract
The evolutionary demand for rapid nerve impulse conduction led to the process of myelination-dependent organization of axons into distinct molecular domains. These domains include the node of Ranvier flanked by highly specialized paranodal domains where myelin loops and axolemma orchestrate the axoglial septate junctions. These junctions are formed by interactions between a glial isoform of neurofascin (NfascNF155) and axonal Caspr and Cont. Here we report the generation of myelinating glia-specific NfascNF155 null mouse mutants. These mice exhibit severe ataxia, motor paresis, and death before the third postnatal week. In the absence of glial NfascNF155, paranodal axoglial junctions fail to form, axonal domains fail to segregate, and myelinated axons undergo degeneration. Electrophysiological measurements of peripheral nerves from NfascNF155 mutants revealed dramatic reductions in nerve conduction velocities. By using inducible PLP-CreER recombinase to ablate NfascNF155 in adult myelinating glia, we demonstrate that paranodal axoglial junctions disorganize gradually as the levels of NfascNF155 protein at the paranodes begin to drop. This coincides with the loss of the paranodal region and concomitant disorganization of the axonal domains. Our results provide the first direct evidence that the maintenance of axonal domains requires the fence function of the paranodal axoglial junctions. Together, our studies establish a central role for paranodal axoglial junctions in both the organization and the maintenance of axonal domains in myelinated axons.
Keywords: myelin, myelinated axons, axonal domains, paranodes, axoglial junctions
The anatomical organization of myelinated axons into distinct molecular domains is the basis for rapid propagation of action potentials in a saltatory manner (Hartline and Colman, 2007). Although the signal transduction mechanisms that underlie the axonal organization into specific domains (i.e., the node, the paranode, the juxtaparanode, and the internode) are poorly understood, considerable progress has been made in identifying key molecular components within these axonal domains. The paranodal region is unique in its organization and ultrastructural characteristics and contains specialized axoglial junctions referred to as the paranodal axoglial septate junctions, which resemble the ladder-like invertebrate septate junctions (Einheber et al., 1997; Pedraza et al., 2001; Banerjee et al., 2006a, b). Three major paranodal proteins have been identified: Caspr or paranodin (Einheber et al., 1997; Menegoz et al., 1997; Peles et al., 1997; Bhat et al., 2001), and a GPI-anchored neural cell adhesion molecule Contactin (Cont; Berglund et al., 1999; Boyle et al., 2001) on the axonal side, and the 155-kDa neurofascin isoform (NfascNF155) on the glial side (Tait et al., 2000; Charles et al., 2002). Although NfascNF155 is the only known glial paranodal protein, many proteins expressed in myelinating glia are required at some level in the formation and/or stability of the paranodal junctions (Coetzee et al., 1996; Griffiths et al., 1998; Ishibashi et al., 2002; Lappe-Siefke et al., 2003; Southwood et al., 2004; Rasband et al., 2005).
Mutational analysis of Caspr and Cont loci revealed their essential roles in the formation of paranodal axoglial junctions and segregation of Na+ and K+ channels at the nodes and juxtaparanodes, respectively (Bhat et al., 2001; Boyle et al., 2001). Myelinated axons of Caspr mutants displayed disorganized paranodal regions with axonal cytoskeletal disorganization, resulting in organelle accumulation and axonal degeneration (Bhat et al., 2001; Einheber et al., 2006; Garcia-Fresco et al., 2006; Pillai et al., 2007 On the axonal cytoskeletal side, the cytoplasmic domain of Caspr assembles a protein complex that includes band 4.1B, αII and βII spectrins, and ankyrin B (Denisenko-Nehrbass et al., 2003; Garcia-Fresco et al., 2006; Ogawa et al., 2006). The localization of these proteins at the paranodes in Caspr mutants is disrupted, suggesting that Caspr is required for the formation of this paranodal cytoskeletal complex (Garcia-Fresco et al., 2006).
Recently, Nfasc mutants lacking both the glial NfascNF155 and the neuronal NfascNF186 isoforms were generated, and transgenic rescue of the glial and neuronal Nfasc isoforms indicated specific function of these isoforms in nodal organization (Sherman et al., 2005; Zonta et al., 2008). The Nfasc mutants died at about postnatal day (P) 6, when paranodal and nodal organization has not been completed and myelination is still taking place. This early lethality combined with the loss of both NfascNF155 and NfascNF186 isoforms has hampered a detailed phenotypic analysis to determine the specific role of glial NfascNF155 in the formation and function of the axoglial junctions. In this study, using embryonic and adult gene disruption strategies, we have specifically addressed the role of myelinating glia-specific NfascNF155 in the formation and maintenance of the paranodal axoglial junctions, without affecting the function of neuronal NfascNF186. We show that embryonic loss of glial NfascNF155 in mice results in motor coordination defects, loss of paranodal axoglial junctions, a significant delay in nerve conduction velocity, and lethality at P17. We further demonstrate that ablation of NfascNF155 in adult myelinating glia results in a gradual loss of paranodal axoglial junctions and a concomitant disorganization of the axonal domains. Together our results establish that paranodal axoglial junctions are central to domain organization and maintenance in myelinated axons.
MATERIALS AND METHODS
Animals
All animal experiments were carried out according to UNC-IACUC-approved guidelines for ethical treatment of laboratory animals. Transgenic mice used in this study expressing Cre recombinase from the Cnp locus (Cnp-Cre; Lappe-Siefke et al., 2003), the P0 promoter (Feltri et al., 1999), and inducible Plp-CreER (Doerflinger et al., 2003) were generously provided by Drs. Klaus Nave (Max Planck Institute of Experimental Medicine), Laura Feltri (San Rafaele Scientific Institute, Italy), and Brian Popko (University of Chicago), respectively.
Conditional Targeting of Nfasc Gene
The neurofascin (Nfasc) gene spans ~171 kb of the genomic DNA and generates many alternative spliced mRNAs (refer http://www.ensemble.org/mouse). To ablate Nfasc gene expression and function conditionally, a targeting construct was generated in which loxP sites were targeted to flank exons 2 and 3 of the Nfasc gene. A BAC clone (bMQ228D3) from a 129 AB2.2 BAC library containing the targeted Nfasc exons was obtained from the Sanger Center, England. With BAC recombineering technology, a polymerase II promoter directed diphtheria toxin (DT) vector containing short PCR-generated arms homologous to those of the Nfasc DNA sequence was transformed into Escherichia coli DY380 recombinant bacterial strain (heat-induced RED and GAM recombinase complex) containing the Nfasc BAC. By this approach, an ~9.4-kb fragment from Nfasc BAC was successfully retrieved into the DT vector by means of gap repair. The first loxP site was inserted into intron 3 by transforming a PGK-neo cassette flanked with loxP sites. This confers kanamycin resistance to DT-Nfasc plasmid. The DT-Nfasc clone with proper neo cassette integration was transformed into strain EL350, and, upon arabinose-induced Cre recombination in transformed EL350, the neo cassette was removed, leaving the loxP site in intron 3. Similar steps were repeated for the insertion of another neo cassette flanked with FRT and FRT-loxP sites into intron 1. Prior to ES cell targeting, the functionality of the loxP sites as well as the expected restriction enzyme digestion patterns in the targeting vector was confirmed. The targeting construct was linearized with NotI, followed by electroporation into ES cells. The targeted ES cells were screened by PCR amplification and standard molecular biological methods (for details see Bhat et al., 2001).
Antibodies, Immunofluorescence, and Immunoblotting
Three antisera were generated against the two Nfasc protein isoforms as follows. Sequences corresponding to the NfascNF155-specific fibronectin III domain 3 (amino acids KIRV--ASFP), the NfascNF186-specific mucin domain (amino acids TVGT--VYSR), and the common cytoplasmic region (amino acids FIKR--YSLA) were used to immunize guinea pigs and rats as previously described (Bhat et al., 1999, 2001). Other primary antibodies used were guinea pig anti-Caspr antibodies (Bhat et al., 2001), rabbit anticalbindin, mouse pansodium channel, rabbit and mouse antipotassium channel (Kv1.1; Sigma, St. Louis, MO), mouse anti-β-tubulin (Chemicon, Temecula, CA), mouse anti-PLP (ABR Bioreagents), mouse anti-MBP (Sterberger Monoclonals, Lutherville, MD), rabbit anti-NrCAM (Abcam, Cambridge, MA), rat anti-PLP (A. Gow, Wayne State University), and rabbit anti-Cont (J. Salzer, NYU; Rios et al., 2000). The secondary antibodies conjugated to Alexa Fluors-488, -568 and -647 were obtained from Invitrogen Corporation (La Jolla, CA).
Preparation of Teased Sciatic Nerves and Cerebellar and Spinal Cord Sections
Fixed sciatic nerves were teased into individual fibers in ice-cold PBS, mounted on glass slides, and dried overnight at RT, followed by treatment with acetone at −20°C for 20 min. The slides were washed with PBS several times before immunostaining; in some cases, slides were stored at −80°C until processed further. For cerebellar and spinal cord sections, wild-type and mutant mice were deeply anesthetized and transcardially perfused with saline buffer followed by a solution of 4% paraformaldehyde in PBS. For immunofluorescence, cerebelli or spinal cords were sectioned on a Vibratome (Leica) at 30 µm thickness and processed as previously described (Garcia-Fresco et al., 2006).
Transmission Electron Microscopy
TEM was carried out essentially as described by Garcia-Fresco et al. (2006). After intracardiac perfusion, the whole animal was postfixed for 2 weeks at 4°C in the same fixation solution. After an overnight incubation in Millonig’s buffer, nerve fibers, spinal cords, or cerebellar tissue from each animal was postfixed for 1 hr in cacodylate-buffered 1% osmium tetroxide. The tissue was then rinsed, dehydrated in increasing concentrations of cold ethanol, and infiltrated and embedded in PolyBed (Polysciences, Warrington, PA). One-micrometer and ninety-nanometer sections were cut and stained with to-luidine blue or a combination of uranyl acetate and lead citrate, respectively. The 1-µm-thick sections were analyzed with a Nikon Eclipse 800, and the 90-nm-thick sections were analyzed with a JEOL JEM1230 transmission electron microscope equipped with a Gatan Ultrascan digital camera for acquiring images at high resolution.
Image Analysis and Quantitation
Confocal images were captured with a Bio-Rad Radiance 2000 laser scanning system attached to a Zeiss Axioplan2 microscope. The immunofluorescence images shown are Z stacks of five to eight sections with a scan step of 0.25 µm. All scanning parameters were optimized for the wild-type tissues, and the mutant tissues were scanned at identical settings. Quantitative measurements were performed on at least three independent tissue samples for immunofluorescence analysis and two independent samples for TEM analysis. For paranodal numbers and length estimation, only those paranodes were counted that looked morphologically normal and did not display structural distortions or stretching as a result of teasing of the fibers during preparation.
Tamoxifen-Induced Nuclear Translocation of CreER Recombination
The tamoxifen induction experiments were carried out essentially according to Doerflinger et al. (2003). Animals of the genotype Plp-CreER;NfascFlox/+ and Plp-CreER;NfascFlox were intraperitoneally injected with 100 µl of suspension (=1 mg tamoxifen) for 10 consecutive days beginning at P23. The animals were then sacrificed at various time points for phenotypic analysis by light and electron microscopy and for electrophysiological measurements.
Electrophysiological Studies
Age-matched wild-type, Cnp-Cre;NfascFlox/Flox, P0- Cre;NfascFlox/Flox, and Plp-CreER;NfascFlox/Flox mutant mice were deeply anesthetized with Avertin or sacrificed by cervical dislocation. Left tibial/plantar nerves (mean length 13.5 mm) were carefully dissected free, cleaned of excess connective tissue, and maintained in cooled, oxygenated, modified Bretag’s solution (123 mM NaCl, 3.5 mM KCl, 0.7 mM MgSO4, 2 mM CaCl2, 9.5 mM Na gluconate, 1.7 mM NaH2PO4, 5.5 mM glucose, 7.5 mM sucrose, 10 mM HEPES; pH 7.40, osmolarity 290) for 2 hr to permit recovery from the acute injury of the dissection. In a dual-compartment ex vivo recording chamber (Stampfli, 1954; Reeh, 1986), the nerves were superfused in oxygenated Bretag’s solution at 30°C. Rectangular wave pulses (0.01 msec) were delivered by an Ag/AgCl suction electrode to the proximal portion of the nerve. One millimeter of the distal portion of the nerve was pulled out of the solution, suspended in a layer of mineral oil, and positioned on a gold recording electrode. A reference electrode was placed in solution close to the gold recording electrode. Evoked compound action potentials (CAP) were amplified and stored as digitized signals with pClamp 10.2 software (Molecular Devices, Union City, CA). Amplitude of the stimulus pulses (10–30 V) was adjusted to ensure a near-maximal stimulation of the A-component of the CAP (Gasser and Grundfest, 1939). In other words, the nerve was stimulated at an intensity sufficient to elicit a stable A-component amplitude and latency. Conduction latency was measured as the time in milliseconds from the start of the stimulus artifact to the start of the first upward deflection of the A-component. Conduction velocity (m/sec) was calculated as the distance between the stimulating and recording electrodes divided by the conduction latency.
RESULTS
Generation and Phenotypic Characterization of Myelinating Glia-Specific Neurofascin (NfascNF155) Null Mutants
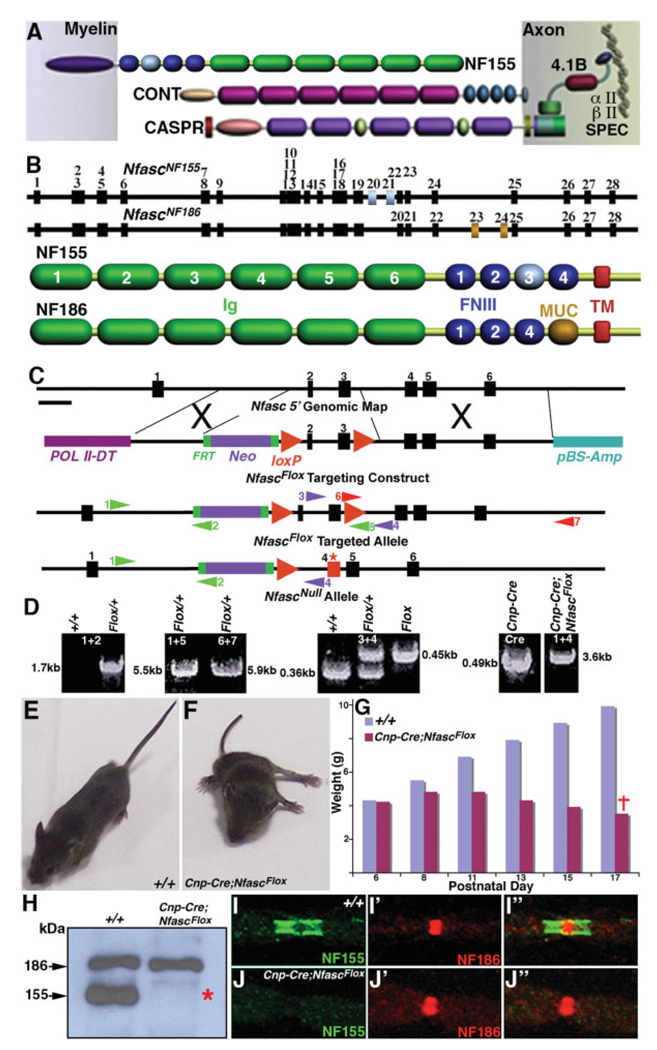
The main molecular components of the paranodal axoglial junctional complex include Caspr and its cis interacting partner Cont on the axonal side and the NfascNF155 on the glial side (Fig. 1A; Peles and Salzer, 2000; Pedraza et al., 2001; Bhat, 2003; Salzer, 2003). Recently, Nfasc mutants were generated revealing that Nfasc is required for the formation of paranodal axoglial junctions and organization of the node of Ranvier (Sherman et al., 2005). We first determined the molecular differences between the glial and the neuronal isoforms of Nfasc by analyzing the reported Nfasc cDNA sequences and the mouse genomic DNA databases (for details see http://www.ensembl.org/mouse). This analysis revealed that the major Nfasc isoforms contain 28 exons, with NfascNF155 containing exons 20 and 21, encoding the third fibronectin (FN) III domain (Fig. 1B, exons in gray). NfascNF186 instead contains exons 23 and 24, which are absent in NfascNF155, encoding the mucin (MUC) domain (Fig. 1B, exons in dark gold; Davis et al., 1996; Tait et al., 2000).
Fig. 1.
Conditional ablation of NfascNF155 in myelinating glia. A: Main molecular components of the paranodal axoglial junctional complex with NfascNF155 on the myelin side and Caspr and Cont on the axonal side. Additional paranode-enriched proteins that either interact or are associated with Caspr protein complex are also shown. B: Exon/intron structure of two major Nfasc transcripts expressed either in myelinating glia, NfascNF155, or in neurons, NfascNF186. The glial transcript contains unique exons numbered 20 and 21 encoding the third FNIII domain (colored in gray) in NfascNF155. The neuronal transcript contains unique exons numbered 23 and 24 that encode a mucin domain (colored in dark gold) in NfascNF186. C: Exon/intron structure in the 5′ region of Nfasc showing exons 1–6. The exon that encodes the putative initiation methionine, based on the reported protein sequences of NfascNF155 and NfascNF186, is referred to here as exon 1. To generate a null mutation in Nfasc, a targeting construct was engineered by using the BAC recombineering technology (Liu et al., 2003). A 1.9-kb fragment upstream of exon 2 was inserted between PolII-DT and FRT-Neo-FRT-loxP cassette, and a second loxP site was inserted downstream of exon 3. This targeting construct, NfascFlox, was used for ES cell transformation. The targeted ES cell clones were identified by PCR amplification with primer sets depicted on the map. Upon Cre-mediated recombination, ~2.1 kb of genomic DNA containing exons 2 and 3 was deleted, resulting in translation termination in exon 4 (red asterisk) that generated the NfascNull allele. D: PCR amplification strategies using color-coded primer sets with numbers to distinguish various genotypes. Wild-type (+/+), NfascFlox/+ (Flox/+), and NfascFlox/Flox (Flox) were distinguished by the primer sets 3 and 4. Upon Cre-mediated deletion of ~2.1 kb of the genomic DNA containing exons 2 and 3, the primer sets 1 and 4 amplified a 3.6-kb fragment in Cnp-Cre;NfascFlox. E: Appearance of a wild-type (+/+) mouse at P17 with normal walking posture. F: A littermate Cnp-Cre;NfascFlox mouse is less than half the weight (3.8 vs. 9.8 g) of the wild-type mouse and exhibits a characteristic wide base in the hind limbs. The mutant mice are severely hypomotile and are not able to walk forward. G: The chart illustrates the weights of wild-type (+/+) and Cnp-Cre;NfascFlox male littermates. Both genotypes at P6 have identical weight (4.2 g). The weight of the mutant mice progressively declines, and all die before reaching P18. The weights shown are mean of four animals in each age group. H: Immunoblot analysis of spinal cord protein lysates from wild-type (+/+) and Cnp-Cre;NfascFlox mice using NFCT antibodies. The wild-type lysates show two predominant protein bands migrating at 186 and 155 kDa corresponding to NfascNF186 and NfascNF155, respectively. The Cnp-Cre;NfascFlox mutant lysates contain the 186-kDa band, corresponding to NfascNF186, and lack the 155-kDa band, demonstrating specific loss of NfascNF155 in Cnp-Cre;Nfasc Flox mutant mice (red asterisk). I–I″: Immunofluorescence analysis of the sciatic nerves from wild-type (+/+) mice showing paranodal localization of NfascNF155 (I, green) and nodal localization of NfascNF186 (I′, red) and together in the merged image (I″). J–J″: Immunofluorescence analysis of the sciatic nerves from Cnp-Cre;NfascFlox mutants showing loss of paranodal NfascNF155 (J, green) but normal localization of nodal NfascNF186 (J′, red) and together in the merged image (J″) demonstrating specific loss of NfascNF155.
To ablate the function of NfascNF155 specifically without interfering with the function of NfascNF186, we first analyzed the structure of the 5′ region of the Nfasc locus. We generated a targeting construct (NfascFlox) such that the coding exons 2 and 3 were flanked by loxP sites. Myelinating glia-specific Cre-mediated recombination resulted in the deletion of exons 2/3, causing translation termination of NfascNF155 in exon 4 (Fig. 1C). A 30-amino-acid polypeptide from the putative initiation codon and 20 unrelated amino acids were produced as a result of frame shift without affecting neuronal NfascNF186 expression (Fig. 1C, red asterisk). A detailed strategy for the generation of the NfascFlox targeting construct is given in Materials and Methods. After germline transmission, heterozygous and homozygous mice were confirmed by PCR amplification across the targeted region. The primer combinations and the products of the PCR amplification from wild-type (+/+), NfascFlox/+, NfascFlox/Flox, and Cnp-Cre-mediated deletion of the floxed exons are shown in Figure 1D. The PCR amplification using primer sets 1 and 4 confirmed that both exons 2 and 3 were deleted in Cnp-cre/+;NfascFlox/Flox (Fig. 1D).
To ablate the expression of NfascNF155 specifically in the myelinating glial cells in the CNS and PNS, we used two well-established myelinating glia-specific Cre lines, Cnp-cre (Lappe-Siefke et al., 2003) and myelin P0-cre (Feltri et al., 1999). The mice of the genotype Cnpcre/+;NfascFlox/Flox, in which only NfascNF155 has been ablated in both the Schwann cells and the oligodendrocytes, are born at the expected Mendelian frequency from heterozygous intercrosses. The NfascNF155 mutant mice are indistinguishable from their wild-type littermates until approximately P8. Starting at about P12, the mice become identifiable by their smaller stature and progressive neurological defects that reach maximal severity toward the beginning of the third postnatal week, and all mutants die at about P16/17. A typical P16 Cnpcre/+;NfascFlox/Flox animal has a posture shown in Figure 1F and is easily differentiated from the wild type shown in Figure 1E. At P16, the defects include hypomotility and severe motor coordination defects. These mice fail to maintain their balance on a stationary beam and are nearly immobile in open field tests (data not shown). We monitored the weight of the Cnp-cre/+;NfascFlox/Flox animals and compared it with that of their wild-type littermates. As shown in Figure 1G, at about P6, the wild-type and Cnp-cre/+;NfascFlox/Flox littermates are of almost identical weight, but, from P8 onward, the Cnp-cre/+;NfascFlox/Flox animals begin to show progressive loss of weight, and, by P16/17, the weight of the Cnp-cre/+;NfascFlox/Flox animals falls below what they had weighed at P6. The Cnp-cre/+;NfascFlox/+ are indistinguishable from their wild-type littermates and live a normal life span without displaying any physical or behavioral phenotypes. In contrast to Nfasc mutants reported by Sherman et al. (2005), which die at P6 when paranodal and nodal organization is just taking place, our Cnpcre/+;NfascFlox/Flox mice die at P16/17, emphasizing the role of individual Nfasc isoforms in paranodal and nodal organization.
To demonstrate that we had specifically generated an NfascNF155 null allele in myelinating glia, we carried out Western blot analysis using antisera generated against the C-terminal domain of Nfasc (NFCT), which recognizes both NfascNF155 and NfascNF186. As shown in Figure 1H, the wild-type (+/+) spinal cord lysate shows both NfascNF155 and NfascNF186 protein isoforms (arrows), whereas Cnp-cre/+;NfascFlox/Flox animals show only the NfascNF186 isoform and lacks the NfascNF155 isoform (red asterisk). These results indicate specific loss of NfascNF155 in the myelinating glial cells. To establish that the NfascNF155 isoform is absent from the paranodes of the myelinated axons, we immunostained teased sciatic nerve fibers with isoform-specific antibodies against NfascNF155 and NfascNF186 (Fig. 1B). As shown in Figure 1I,J and in the merged image in Figure 1I″, wildtype (+/+) fibers showed specific paranodal localization of NfascNF155 (Fig. 1I, green), and nodal localization of NfascNF186 (Fig. 1I′, red). The Cnp-cre/+;NfascFlox/Flox mutant sciatic nerve fibers lacked immunoreactivity against NfascNF155 (Fig. 1J, green) but showed normal nodal localization of NfascNF186 (Fig. 1J′, red) in the merged image (Fig. 1J″). Similarly P0-cre/+;NfascFlox/Flox mice revealed loss of NfascNF155 specifically in the peripheral nerve fibers both by immunoblot and by immunohistochemical analyses and not in the central myelinated fibers (data not shown). Taken together, our data demonstrate that we have generated myelinating gliaspecific NfascNF155 mutants without altering the expression and localization of NfascNF186 at the node of Ranvier.
Axonal Domain Organization is Disrupted in NfascNF155 Mutant Myelinated Axons
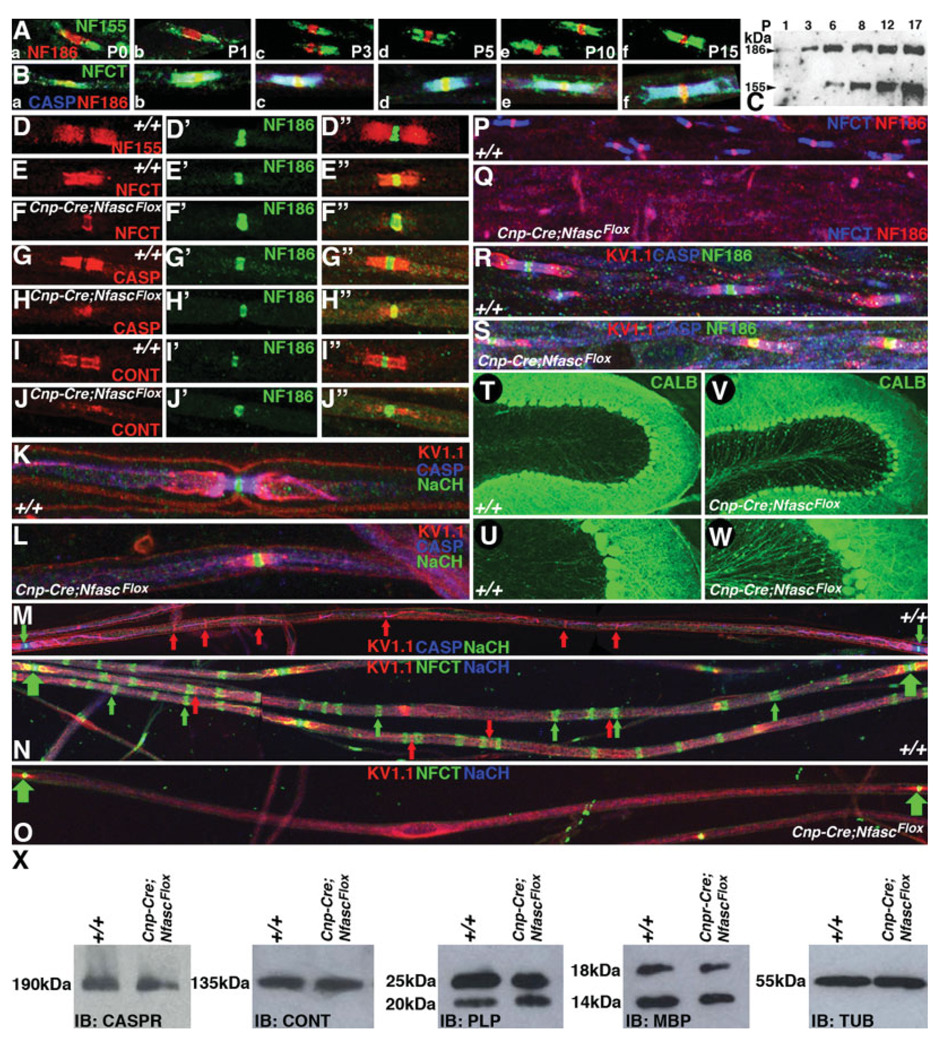
To determine the relationship between expression of the Nfasc isoforms with paranodal biogenesis, we first analyzed the developmental expression profile of NfascNF155 in myelinated sciatic nerve fibers by using immunohistochemistry. As shown in Figure 2A, at P0, NfascNF155 is expressed at the sites where paranodes are beginning to form. At this stage, NfascNF186 localizes to the node, which shows a broad morphology (Fig. 2Aa, red). Similarly, we analyzed P1, P3, P5, P10, and P15 sciatic nerves, which revealed that NfascNF155 is expressed at the paranodes during their formation. At P10 and P15, as paranode formation is completed, NfascNF186 localization becomes more concentrated at the node (compare Fig. 2Aa–f). We next analyzed the distribution of NfascNF155 along with the axonal paranodal protein Caspr and nodal NfascNF186 to determine their precise colocalization during paranode formation. As shown in Figure 2B, NfascNF155 (green) colocalizes with Caspr at the paranodes (blue) at all postnatal developmental stages beginning with P0 through P15. The immunoreactivity for Caspr at P0 is qualitatively lower compared with later stages. From P3 onward, NfascNF155 and Caspr showed complete colocalization (Fig. 2Bc–f). To establish the comparative expression levels of NfascNF155 and NfascNF186 during postnatal development, we carried out Western blot analysis of P1, P3, P6, P8, P12, and P15 spinal cords using NFCT antibodies that recognize both isoforms. As shown in Figure 2C, NfascNF186 protein levels are comparatively higher than NfascNF155 at early stages (Fig. 2C, compare P1 and P3). At later stages, NfascNF155 expression levels increase compared with earlier stages and with NfascNF186 (Schafer et al., 2006). These results are consistent with the increase in NfascNF155 fluorescence observed at later developmental stages in the immunohistochemical analysis shown in Figure 2A,B. Thus NfascNF155 expression pattern coincides with paranode formation during postnatal development.
Fig. 2.
NfascNF155 mutants display abnormal localization of the paranodal proteins and disorganization of axonal domains. A: Immunofluorescence of sciatic nerve fibers depicting the postnatal expression profile of two Nfasc encoded protein isoforms using antibodies generated against unique domains of NfascNF155 and NfascNF186. NfascNF155 localizes to developing paranodes (Aa–f, green), and NfascNF186 localizes to the nodes (Aa–f, red). B: Immunofluorescence of sciatic nerve fibers using antibodies against both NfascNF155 and NfascNF186 (Ba–f, NFCT, green), against Caspr (CASP, blue), and against NfascNF186 (red) during postnatal development, as in Aa–f. The paranodal colocalization of NfascNF155 and Caspr is seen throughout postnatal development. C: Immunoblot of spinal cord lysates for a postnatal expression profile of NfascNF155 (155 kDa) and NfascNF186 (186 kDa) using NFCT antibodies that recognize both isoforms. NfascNF186 levels are relatively higher than NfascNF155 levels until P3. However, longer exposures of the blots reveal the presence of NfascNF155 at P1 as well. Equal amounts of the total protein were loaded for these immunoblots and confirmed with antitubulin immunoblots (data not shown). D–D″: Wild-type sciatic nerve fibers immunostained against paranodal NfascNF155 (D, red) and NfascNF186 (D′, green) and in a merged image (D″). E–E″: Wild-type sciatic nerve fibers immunostained against NFCT antibodies (E, NfascNF155/NfascNF186, red) and nodal NfascNF186 (E′, green) and in a merged image (D″). Note that NFCT recognizes both NfascNF155 and NfascNF186. NfascNF186 immunoreactivity by the two antibodies appears yellow in the merged panel (E″). F–F″: Cnp-Cre;NfascFlox sciatic nerve fibers immunostained with NFCT antibodies (F, NfascNF155/NfascNF186, red) and nodal NfascNF186 (F′, green) and in a merged image (F″). Note that NfascNF155 is absent from the paranodes, whereas NfascNF186 immunoreactivity with two different antibodies remains unaffected. G–G″: Wild-type sciatic nerve fibers immunostained against paranodal Caspr (G, CASP, red) and nodal NfascNF186 (G′, green) and in a merged image (G″). H–H″: Cnp-Cre;NfascFlox sciatic nerve fibers immunostained against paranodal Caspr (red) and nodal NfascNF186 (F′, green) and in a merged image (H″). Note that Caspr fails to localize at the paranodes and is diffusely present around the nodal/paranodal region, occasionally showing overlap with NfascNF186 (H″). I–I″: Wild-type sciatic nerve fibers immunostained against paranodal Cont (I, red) and nodal NfascNF186 (I′, green) and in a merged image (I″). J–J″: Cnp-Cre;Nfasc Flox sciatic nerve fibers immunostained against paranodal Cont (J, red) and nodal NfascNF186 (J′, green) and in a merged image (J″). Note that Cont fails to localize at the paranodes and is diffusely present around the nodal/paranodal region. K: Wild-type sciatic nerve fibers triply immunostained against the juxtaparanodal potassium channels (Kv1.1, red), Caspr (blue), and nodal sodium channels (NaCH, green). Note that these proteins localize to distinct domains. L: Cnp-Cre;NfascFlox sciatic nerve fibers triply immunostained against the juxtaparanodal potassium channels (red), Caspr (blue), and nodal sodium channels (green). Note that the juxtaparanodal K+ channels fail to segregate from the nodal Na+ channels. Caspr (blue) immunoreactivity is diffusely present along the axon. M: Wild-type sciatic nerve fibers triply immunostained against Na+ channels to highlight nodes (green arrows) flanking the entire internodal region. Caspr (blue) highlights the paranodes. Note the K+ channel localization at the Schmidt-Lanterman incisures and the inner mesaxon (red arrows). N: Wild-type sciatic nerve fibers triply immunostained against Na+ channels (blue) and NFCT highlight two contiguous nodes (green arrows). Note that NfascNF155 labels the Schmidt-Lanterman incisures (NFCT, green arrows) as well as Kv1.1 (red arrows). NfascNF155 and Kv1.1 immunoreactivity is always observed either overlapping with or in close proximity to each other. O: Cnp-Cre;NfascFlox sciatic nerve fibers triply immunostained against Na+ channels (NaCH, blue) and NfascNF186 (green) highlight two nodes (green arrows). Between the two nodes, the internodal region shows no NfascNF155 immunoreactivity. Kv1.1 (red) highlights juxtaparanodal regions and is also diffusely present along the axon. P: Wild-type spinal cord sections against NFCT (blue) and NfascNF186 (red). NfascNF155 is in blue and the nodal NfascNF186 is in pink, as both antibodies recognize NfascNF186. Q: Cnp-Cre;NfascFlox spinal cord sections stained against NFCT (blue) and NfascNF186 (red). NfascNF155 (blue) is absent, but the nodal NfascNF186 is present and indistinguishable from the wild-type NfascNF186 (as in Q). R: Wild-type spinal cord sections stained against axonal domain markers Kv1.1 (red), Caspr (blue), and NfascNF186 (green) show proper domain organization. S: Cnp-Cre;NfascFlox spinal cord sections stained against axonal domain markers Kv1.1 (red), Caspr (blue), and NfascNF186 (green) show abnormal domain organization. The paranodal domain is compromised and Kv1.1 is almost overlapping with nodal NfascNF186, which is never observed in the wild-type animals. T,U: A wild-type cerebellar loop immunostained against calbindin (green) to reveal anatomical organization of various layers and cerebellar white matter at low magnification (T) and at a higher magnification (U) with a focus on the Purkinje axons. V,W: A Cnp-Cre;NfascFlox cerebellar loop immunostained against calbindin (green) to reveal the anatomical organization of various layers and cerebellar white matter at low magnification (V) and at a higher magnification (W) with a focus on the Purkinje axons. Note the Purkinje axonal swellings as green dots on the axons, which are never observed in the wild-type animals. X: Immunoblots of protein lysates from wild-type (+/+) and Cnp-Cre;NfascFlox brains against paranodal proteins (Caspr, Cont) and myelin proteins (PLP, MBP) did not shown any significant differences in the levels of the these proteins. Identical blots were probed with mouse antitubulin as a loading control.
To establish the consequence of loss of NfascNF155 in the Schwann cells in Cnp-cre/+;NfascFlox/Flox animals, we analyzed mutant sciatic nerve fibers by coimmunostaining against Caspr and Cont paranodal proteins along with nodal NfascNF186. Wild-type nerve fibers from P17 animals show the expected localization of NfascNF155 at the paranodes (Fig. 2D, red), and NfascNF186 localization at the node (Fig. 2D′, green, see also merged image in Fig. 2D″). Similarly, immunostaining with NFCT antibodies reveals proper paranodal localization of NfascNF155 (Fig. 2E–E″). In Cnp-cre/+;NfascFlox/Flox animals, NFCT immunoreactivity indicates specific loss of NfascNF155 at the paranodes without affecting NfascNF186 (Fig. 2F, red), as is seen with NfascNF186 specific immunoreactivity (Fig. 2F′, green; also shown in the merged image in Fig. 2F″,F″′). Immunostaining against Caspr in the wild-type (Fig. 2G, red) and Cnp-cre/+;NfascFlox/Flox animals (Fig. 2H, red) and against Cont in the wild-type (Fig. 2I, red) and Cnp-cre/+;NfascFlox/Flox animals (Fig. 2J, red) shows that both Caspr and Cont fail to localize at the paranodes in the Cnp-cre/+;NfascFlox/Flox animals. These results indicate that glial NfascNF155 is essential for paranodal Caspr and Cont localization and that the localization of nodal NfascNF186 is not affected in Cnp-cre/+;NfascFlox/Flox mutant fibers.
A hallmark of myelinated axons is the localization of voltage-gated Na+ channels at the nodes and the delayed rectifier Shaker-type K+ channels at the juxtaparanodes and to regions of the internode that oppose the noncompacted myelin membranes, e.g., the Schmidt-Lanterman incisures and the internal mesaxon (Arroyo et al., 1999). To determine whether loss of NfascNF155 at the paranodal junctions in Cnp-cre/+;NfascFlox/Flox mice affects the distribution of these channels, we carried out immunofluorescence staining of sciatic nerves. In both wild-type (Fig. 2K) and Cnp-cre/+;NfascFlox/Flox (Fig. 2L) mice, Na+ channels (Fig. 2K,L, green) were restricted to the nodes of Ranvier. Unlike the localization of Na+ channels, the distribution of the K+ channels (Fig. 2K,L, Kv1.1, red) was markedly different between the wild-type and the Cnp-cre/+;NfascFlox/Flox mice. In wild-type nerves (Fig. 2K), K+ channels were completely separated from the Na+ channels by the paranodal region, as expected. In contrast, Cnp-cre/+;NfascFlox/Flox mice showed mislocalized K+ channels in the paranodal region, immediately adjacent to and, in some cases, slightly overlapping with Na+ channels (note the areas of overlap appear as yellow in the merged images; Fig. 2L). These data indicate that glial NfascNF155 at the paranodal axoglial junctions is required for the segregation of Na+ and K+ channels into distinct domains.
To characterize further the role of NfascNF155 in myelinated axons, we coimmunostained sciatic nerve fibers from wild-type (Fig. 2M,N) and Cnp-cre/+;NfascFlox/Flox (Fig. 2O) mice and analyzed them at low magnification. As shown in Figure 2M, an entire internodal segment flanked with two nodes was immunostained against Na+ channels (green arrows), and paranodes were immunostained against Caspr (blue) and K+ channels (Kv1.1, red) with distinct localization at the juxtaparanodal region and Schmidt-Lanterman incisures (red arrows) and inner mesaxon spanning the internodal region. Immunostaining of the wild-type sciatic nerves with antibodies against NFCT (Fig. 2N, green), Na+ channels (Fig. 2N, blue), and K+ channels (Fig. 2N, Kv1.1, red) revealed that NfascNF155 is strongly expressed in the internodal region at Schmidt-Lanterman incisures (green arrows). The NfascNF155 immunoreactivity either overlaps or is present adjacent to K+ channel immunoreactivity (Fig. 2N, red and green arrows). Immunostaining of sciatic nerve fibers from Cnp-cre/+;NfascFlox/Flox mice revealed that NfascNF155 immunoreactivity along the internodes at the Schmidt-Lanterman incisures is absent (Fig. 2O, green) and that the localization of K+ channels at or near the incisures is compromised (Fig. 2O, red). These data suggest that NfascNF155 expressed by myelinating Schwann cells may be required for the formation of internodal specializations.
To establish that loss of NfascNF155 also affected central myelinated axons, we carried out immunofluorescence analysis of the spinal cords from wild-type and Cnp-cre/+;NfascFlox/Flox mice. As shown in Figure 2P, wild-type spinal cord white matter immunostained against NFCT (blue) and NF186 (red) showed paranodal NfascNF155 (blue) and nodal NfascNF186 localization (pink, because both antibodies recognize NfascNF186). The spinal cord white matter from Cnp-cre/+;NfascFlox/Flox mice, immunostained with the same antibodies as in Figure 2P, showed no immunoreactivity against NfascNF155 but showed normal localization of NfascNF186 (Fig. 2Q, pink), indicating specific loss of NfascNF155 in the oligodendrocytes. We also analyzed the domain organization in the spinal cord white matter by using coimmunostaining against Caspr (Fig. 2R,S, blue), NF186 (green) and Kv1.1 (red). As observed in the PNS, the spinal cord also revealed loss of the paranodal domain and absence of segregation between the juxtaparanodal and the nodal components (Fig. 2S).
To determine further whether loss of glial NfascNF155 phenocopied the presence of axonal swellings in the cerebellar Purkinje neurons, as was observed in Caspr (Garcia-Fresco et al., 2006; Pillai et al., 2007), Cont (Boyle et al., 2001), and CGT (Garcia-Fresco et al., 2006) mutants, we carried out immunostaining of the cerebella from wild-type and Cnp-cre/+;NfascFlox/Flox mice with anticalbindin antibodies. As shown in Figure 2T,U, wild-type Purkinje neuron axons displayed normal axonal morphology. In contrast, Purkinje axons from Cnp-cre/+;NfascFlox/Flox mice displayed extensive presence of axonal swellings (Fig. 2V,W). These swellings are reminiscent of those observed in Caspr and CGT mutants, which we had previously shown to be caused by cytoskeletal abnormalities and accumulation of the cellular organelles at the disrupted paranodal regions (Garcia-Fresco et al., 2006). To establish further that loss of glial NfascNF155 did not alter the protein expression levels of myelin or paranodal proteins, we carried out Western blot analysis of brain lysates from wild-type and Cnp-cre/+;NfascFlox/Flox mice. As shown in Figure 2X, the protein levels of the paranodal proteins Caspr and Cont and the myelin proteins proteolipid protein (PLP) and myelin basic protein (MBP) between the wild-type and Cnp-cre/+;NfascFlox/Flox mice revealed no significant differences. These results indicate that loss of glial NfascNF155 does not alter the steady-state levels of paranodal or myelin proteins. Taken together, our data demonstrate that loss of glial NfascNF155 results in the disruption of the paranodal region and failure to segregate the axonal domains in the myelinated axons.
NfascNF155 Mutants Fail To Establish Paranodal Axoglial Junctions
As established above, myelinating glia-specific loss of NfascNF155 did not alter myelin protein levels; therefore, we wanted to determine whether histological organization of the nervous system, including myelination, was affected. Light microscopy did not reveal any obvious abnormalities in the histological organization or the extent of myelination (data not shown). As shown in Figure 3, the myelinated sciatic nerve fibers from the wild-type (Fig. 3A) and Cnp-cre/+;NfascFlox/Flox (Fig. 3B) mice were indistinguishable, and morphometric analysis of the myelinated fibers in the PNS showed no significant differences in axon and nerve fiber diameters, myelin thickness, or the g value (ratio of axon to fiber diameter; data not shown). These results, together with the normal myelin protein levels (Fig. 2X), suggest that lack of NfascNF155 does not result in any significant alterations in the myelination process.
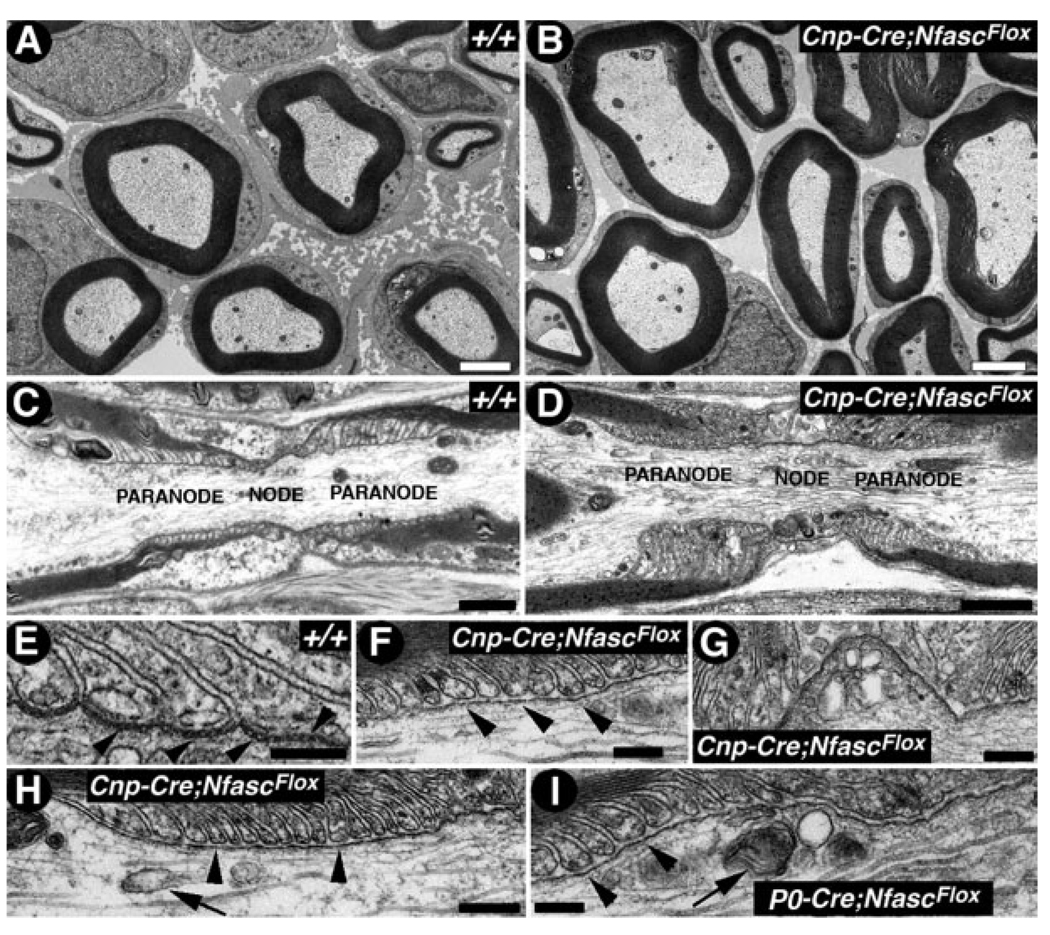
Fig. 3.
NfascNF155 mutants fail to form paranodal axoglial junctions. A,B: Electron micrographs of cross-sections of sciatic nerves from P17 littermates wild-type (A, +/+) and Cnp-Cre;NfascFlox (B) mice. No significant changes in myelin thickness or axon diameter were noticed between these genotypes. C,D: Electron micrographs of longitudinal sections through the nodal/paranodal region of sciatic nerves from P17 wild-type (C, +/+) and Cnp-Cre;NfascFlox mutants (D). At low magnification, the paranodal myelin loops in the mutant fibers are properly arrayed and show normal profiles as in the wild-type. E: Electron micrograph of a longitudinal section through the paranodal region of a sciatic nerve myelinated axon from a P17 wild-type (+/+) mouse shows paranodal loops tightly apposed to and indenting the axon, the characteristic transverse bands are also apparent (black arrowheads). F,H: Electron micrographs of longitudinal sections through the paranodal region of sciatic nerve myelinated axons from a P17 Cnp-Cre;Nfasc Flox mutant mouse. Note that the paranodal loops come in close apposition and face the axon but fail to form the transverse bands or septa with the axolemma. G: Electron micrograph of the nodal region from a Cnp-Cre;NfascFlox mutant sciatic nerve fiber showing organelle build up, forming nodal pockets. Such abnormalities have been previously observed in Caspr mutants with disrupted axoglial junctions (Einheber et al., 2006). I: Electron micrograph of a longitudinal section through the paranodal region of a sciatic nerve myelinated axon from a P17 P0-Cre;NfascFlox mutant mouse. These mutant fibers display same phenotypes in the PNS as the Cnp-Cre;NfascFlox mutant fibers. Scale bars = 2 µm in A, B; 1 µm in C, D; 0.2 µm in E–I.
Because immunofluorescence analysis established that NfascNF155 was localized to the myelin side of the paranodal region, and loss of NfascNF155 specifically in myelinating glia resulted in mislocalization of Caspr and Cont, we examined the ultrastructure of the paranodal region in the PNS and CNS. As shown in Figure 3, low-magnification images of the sciatic nerve nodal and paranodal regions from wild-type (Fig. 3C) and Cnp-cre/+;NfascFlox/Flox (Fig. 3D) mice reveal no major anatomical or organizational abnormalities. At higher magnification, the paranodal region in the wild-type showed myelin loops arrayed sequentially and in close apposition to the axonal membrane (Fig. 3E). Between the loops and the axon, periodic densities corresponding to the septate-like transverse bands were readily apparent (Fig. 3E, black arrowheads). In the Cnp-cre/+;NfascFlox/Flox sciatic nerve fibers, the organization of the paranodal loops was preserved; however, the characteristic transverse bands were consistently absent (Fig. 3F,G, black arrowheads). In addition, the spacing between the paranodal loops and the axolemma was often abnormally wide, and the normal indentation of the axolemma by the paranodal loops was often absent (compare Fig. 3F–H with E). A consistent abnormality observed was the absence of the regular array of transverse bands between the paranodal loops and the axon. This was further confirmed by analyzing the Schwann cell-specific P0-cre/+; NfascFlox/Flox sciatic nerve fibers (Fig. 3I). Interestingly, the P0-cre/+; NfascFlox/Flox mice do not show any significant peripheral neurological deficits until P90. However, it remains to be seen whether older P0-cre/+;NfascFlox/Flox mice will develop any severe peripheral nerve functional abnormalities. Furthermore, the Cnp-cre/+;NfascFlox/Flox sciatic nerve fibers showed accumulation of organelles, such as mitochondria, and nodal membrane folds resulting from cytoskeletal abnormalities at the nodal/paranodal region (Fig. 3G). Together these data reveal that loss of NfascNF155 in Schwann cells results in the loss of paranodal axoglial septate junctions.
Aside from the NfascNF155 localization to paranodes in the PNS, it also localizes to CNS paranodal junctions; therefore, we examined the ultrastructure of this region in the spinal cord and cerebella of Cnp-cre/+;NfascFlox/Flox mice. As shown in Figure 4A, in the wild-type spinal cord white matter, the paranodal loops are arrayed in close apposition to the axonal membrane, which establishes the septate-like transverse bands (black arrowheads). In contrast, the paranodal loops in Cnp-cre/+;NfascFlox/Flox spinal cord white matter come in close apposition with the axolemma, but the regular array of transverse bands between paranodal loops and the axon is absent (Fig. 4B,C, black arrowheads). The most striking paranodal abnormalities often observed in Cnp-cre/+;NfascFlox/Flox are the overlapping myelin loops of two adjacent myelinating glia that obscure the nodal regions (Fig. 4D, OL). In the example shown in Figure 4D, paranodal loops from the myelin glial segment on the left (labeled OL) overlap the loops of the myelin segment on the right. Additional defects observed in Cnp-cre/+;NfascFlox/Flox included astrocytic processes that were interposed between the paranodal loops and the axon (Fig. 4D, black arrows) and everted and disorganized paranodal loops that are rarely observed at the paranodes in the wild-type spinal cords (data not shown). These data demonstrate that loss of NfascNF155 in oligodendrocytes results in paranodal abnormalities and the loss of paranodal axoglial junctions.
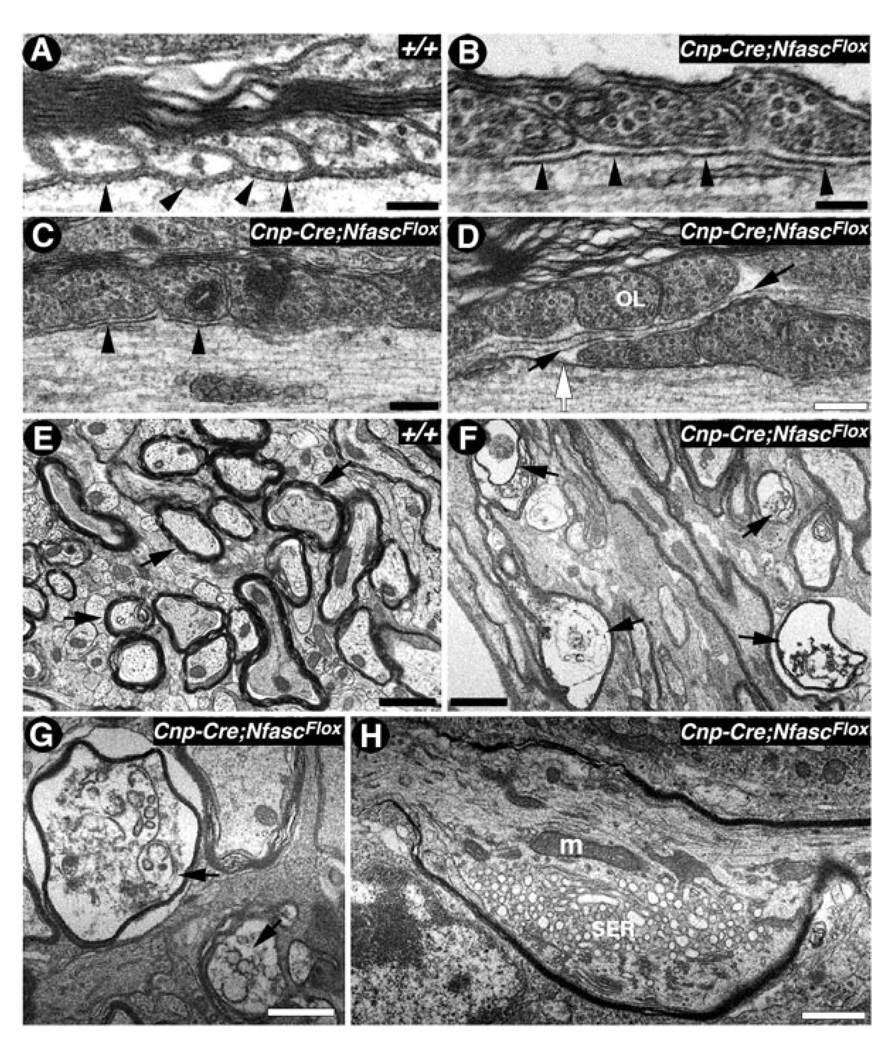
Fig. 4.
NfascNF155 mutants display organelle accumulation and Purkinje neuron axonal degeneration. A: Electron micrograph of the paranodal region of a spinal cord myelinated fiber from P17 wild-type (+/+) mouse shows paranodal loops tightly apposed to and indenting the axon; the characteristic transverse bands are apparent (black arrowheads). B–D: Electron micrographs of the paranodal regions of spinal cord myelinated fibers from P17 Cnp-Cre;NfascFlox mutants show closely apposed paranodal loops but a lack of transverse bands (B,C, black arrowheads). In D, paranodal loops from one myelin segment override loops (OL) from another segment, thereby occluding the node (white arrow). An astrocytic process has entered the gap between the overriding myelin loops (black arrows). E: Electron micrographs of cerebellar white matter from P17 wild-type (+/+) mice show normal morphology of the myelinated axons in cross-section (black arrows). F–H: Electron micrographs of the cerebellar white matter from P17 Cnp-Cre;NfascFlox mutants show severe axonal pathology and degeneration (F, black arrows). A myelinated axon displays vacuolated and deformed axonal cytoskeleton (G, black arrows). A section through a Purkinje axonal swelling (H) shows accumulation of mitochondria (m) and smooth endoplasmic reticulum (SER) caused by the disorganized paranodal axonal cytoskeleton. Scale bars = 0.2 µm in A, B; 0.1 µm in C,D; 1 µm in E–H.
Myelinating Glia-Specific NfascNF155 Mutants Display Organelle Accumulation and Degeneration of Cerebellar Purkinje Axons
Our immunofluorescence analysis of the cerebella from Cnp-cre/+;NfascFlox/Flox mice revealed Purkinje neuron axonal swellings as previously observed in Cont (Boyle et al., 2001), Caspr, and CGT mutants (Garcia-Fresco et al., 2006). To establish whether Purkinje axons from the Cnp-cre/+;NfascFlox/Flox mice also undergo axonal degeneration and organelle accumulation, similarly to Caspr, Cont, and CGT mutants, we carried out cross-sections of the wild-type cerebellar white matter and found normal morphology of the myelinated axons with no signs of any vacuolation or degeneration (Fig. 4E, black arrows). In contrast, in the Cnp-cre/+;NfascFlox/Flox cerebellar white matter, axonal degeneration was very apparent (Fig. 4F, black arrows). Higher magnifications revealed severe structural abnormalities and vacuolation in the degenerating axons (Fig. 4G, black arrows). These characteristics are displayed by axons that are in the terminal stages of degeneration (Palay and Palay, 1974). In an effort to determine the effect of NfascNF155 deletion on the axonal structure, we performed TEM analysis of the Purkinje axon swellings in Cnp-cre/+;NfascFlox/Flox mice. We observed large axonal accumulations of organelles, that included mitochondria (m) and smooth endoplasmic reticulum (SER; Fig. 4H). These axonal accumulations dramatically altered the axonal structure and may ultimately lead to axonal degeneration as observed in Figure 4F,G. Consistently with our immunofluorescence data, we found that the axonal swellings were frequently formed within or in close proximity to the paranodal region, as previously observed in Caspr mutants (Einheber et al., 2006; Garcia-Fresco et al., 2006). Taken together, the immunofluorescence and ultrastructural analyses show that loss of NfascNF155 at the paranodal axoglial junctions leads to paranodal cytoskeletal disorganization and the eventual degeneration of Purkinje axons.
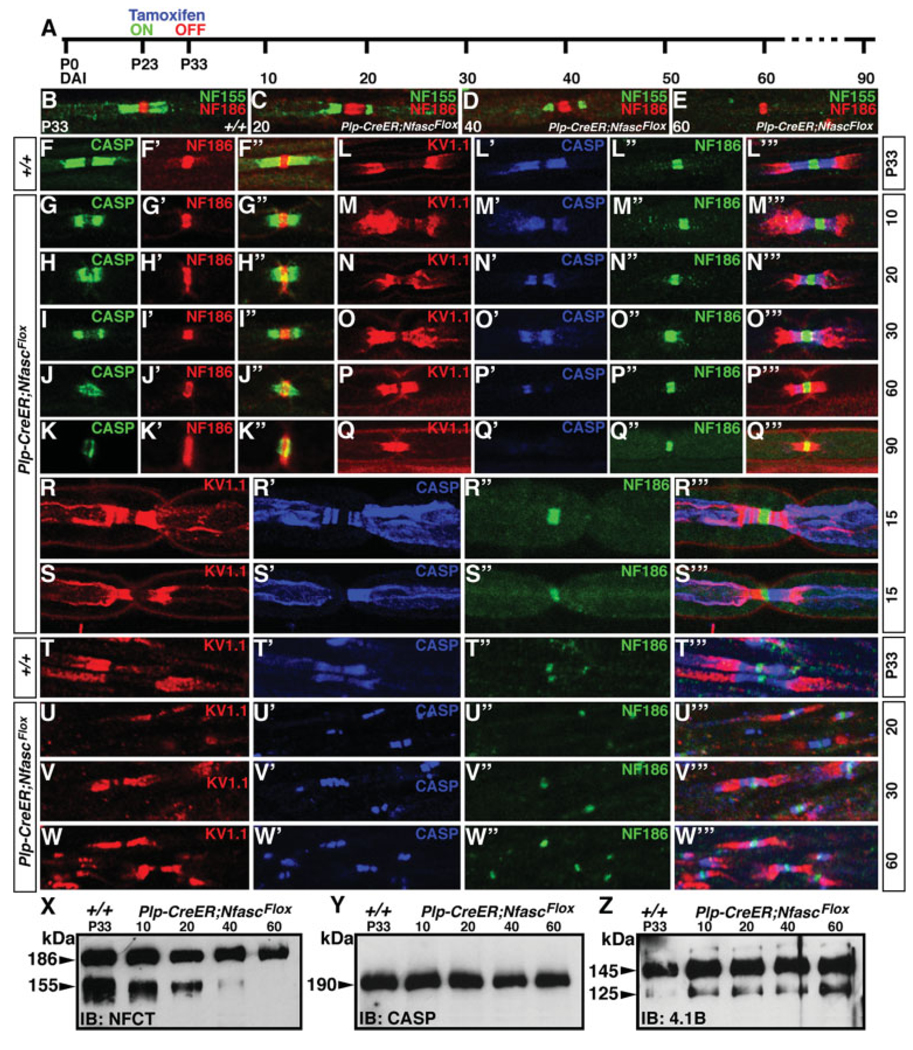
Ablation of NfascNF155 in Adult Myelinating Glia (NfascNF155AD) Reveals Gradual Disorganization of Axonal Domains
The ablation of NfascNF155 in myelinating glia during early development showed that NfascNF155 is required for the formation of the paranodal axoglial junctions but not myelination per se. However, these studies did not allow us to address how the axoglial junctions and axonal domains are maintained throughout adult life in myelinated axons. To address this question, we took advantage of a tamoxifen inducible-Cre line, developed by Doerflinger et al. (2003), which is specifically expressed in myelinating glia (Plp-CreER) to ablate NfascNF155 during postnatal development, when axoglial junctions and axonal domains have been well formed and established. P23 Plp-CreER;NfascFlox/Flox mice and age-matched or littermate wild-types were injected with tamoxifen for the next 10 days, until P33 (Fig. 5A, for details see Materials and Methods). The myelinated sciatic nerve fibers and spinal cords of the injected mice were analyzed by immunofluorescence over a period of 10–90 days postinjection for alterations in NfascNF155 paranodal localization and the domain structure at the nodal/paranodal regions. As shown in Figure 5B, at P33, Plp–CreER;NfascFlox/Flox mice showed normal paranodal localization of NfascNF155 (green) and NfascNF186 (red). After 20 days postinjection, NfascNF155 levels at the paranodes began to decline (Fig. 5C, green). By 40 days postinjection, NfascNF155 levels were drastically reduced (Fig. 5D, green), and, by postinjection day 60, NfascNF155 immunoreactivity at the paranodes was essentially absent (Fig. 5E, green). In control mice, which also received tamoxifen, NfascNF155 localized normally to the paranodes and was indistinguishable from that in the untreated wild types (data not shown). Next, we followed the localization and stability of the axonal paranodal protein Caspr in control and Plp–CreER;-NfascFlox/Flox treated mice. As shown in Figure 5F, at P33, wild-type, control treated mice showed normal localization of Caspr (Fig. 5F, green) and nodal NfascNF186 (Fig. 5F′, red; also seen in the merged image, Fig. 5F″). In Plp–CreER;NfascFlox/Flox fibers, Caspr immunoreactivity began to decline gradually, to the point at which only traces of paranodal Caspr were seen by 90 days after tamoxifen injection (compare Caspr localization in Fig. 5G–K with F). In contrast, the localization of NfascNF186 was not affected in Plp-CreER;NfascFlox/Flox fibers (compare Fig. 5G′–K′ with F′). The Caspr immunoreactivity present after 60 and 90 days postinjection was also observed in the nodal region overlapping with NfascNF186 (Fig. 5J″,K″), suggesting that NfascNF155 is required to maintain Caspr localization at the paranodes.
Fig. 5.
Maintenance of axonal domains requires intact paranodal axoglial junctions. A: Experimental strategy to create myelinating gliaspecific NfascNF155 null adult mutants. Tamoxifen was injected into mice of desired genotypes from P23 to P33, and mice were analyzed at various time points by immunostaining and immunoblotting. B–E: Sciatic nerves from wild-type P33 (B, +/+) and tamoxifen-injected P33 Plp-CreER;NfascFlox mice after 20 (C), 40 (D), and 60 (E) days of injection immunostained with NfascNF155 (green) and NfascNF186 (red). The levels of NfascNF155 at paranodes begin to decrease, and it cannot be detected at 60 days after injection. NfascNF186 levels are not affected. F–K″: Sciatic nerves from P33 wild-type (F–F″, +/+) and tamoxifen-injected P33 Plp-CreER;NfascFlox mice after 10 (G– G″), 20 (H–H″), 30 (I–I″), 60 (J–J″), and 90 (K–K″) days of injection immunostained with Caspr (F–K, green) and NfascNF186 (F′–K′, red). The levels of Caspr at the paranodes begin to fall gradually and could barely be detected at 90 days after injection. At 90 days, the remaining Caspr protein is occasionally seen overlapping with the nodal NfascNF186 (see merged image in K′). L–Q″′: Sciatic nerves from P33 wild-type (L–L″′, +/+) and tamoxifen-injected P33 Plp-CreER;NfascFlox mice after 10 (M–M″′), 20 (N–N″′), 30 (O–O″′), 60 (P–P″′), and 90 (Q–Q″′) days of injection immunostained with juxtaparanodal K+ channel (L–Q, red), paranodal Caspr (L′–Q′, blue), and nodal NfascNF186 (L″–Q″, green). As NfascNF155 levels begin to decrease (C–E), Caspr localization at the paranodes also decreases, and concomitantly Kv1.1 starts to move toward the node as the paranodal region is getting lost. By 90 days, all of the Caspr is absent at the paranodes, and the domain organization is lost. R–S″′: Sciatic nerves from tamoxifen-injected P33 Plp-CreER;NfascFlox mice after 15 of injection immunostained with juxtaparanodal K+ channel (R,S, red), paranodal Caspr (R′–S′, blue), and nodal NfascNF186 (R″–S″, green) and in merged images (R″′, S″′). These fibers reveal midstages of overlap between juxtaparanodal and paranodal components as axoglial junctions are being compromised. T–W″′: Spinal cord sections from P33 wild-type (T–T″′, +/+) and tamoxifen-injected P33 Plp-CreER;NfascFlox mice after 20 (U–U″′), 30 (V–V″′) and 60 (W–W″′) days of injection immunostained with juxtaparanodal K+ channel (T–W, red), paranodal Caspr (T″′–W′, blue), and nodal NfascNF186 (T″–W″, green) and in merged images (T″′–W″′). Note that, even in the presence of remaining Caspr at the paranodes, Kv1.1 has already moved next to the nodal region (W″′), indicating that intact axoglial junctions are required to maintain axonal domains. X–Z: Immunoblots of spinal cord lysates showing the time course of changes in the levels of NfascNF155 in Plp-CreER;NfascFlox mice after 10, 20, 40, and 60 days of injection. Note that NfascNF155 levels begin to fall within 10 days and that by 40 days most of the NfascNF155 protein is lost (X). On the other hand, protein levels of Caspr (Y) and 4.1B (Z) are not affected by loss of NfascNF155.
We then wanted to determine consequences of loss of NfascNF155 in adult myelinated fibers on the maintenance of axonal domains, being the juxtaparanodes, the paranodes, and the nodes. As shown in Figure 5L–L″′, wild-type control mice showed distinct localization of Kv1.1 at the juxtaparanodes (Fig. 5L, red), Caspr at the paranodes (Fig. 5L′, blue), NfascNF186 at the nodes (green), and together in the merged image (Fig. 5L″′). In Plp-CreER;NfascFlox/Flox fibers, within 10 days after injection, the juxtaparanodal Kv1.1 channels (Fig. 5M, red) began to migrate toward the nodal region. By 30 days postinjection, Kv1.1 channels (Fig. 5O, red) had reached closer to the nodal region and began to overlap with the remaining Caspr (Fig. 5O′, blue) at the paranodes. By 60 days after injection, no discrete paranodal region was observed; Caspr immunoreactivity was low, and Kv1.1 channels had almost reached the node (merged image in Fig. 5P″′). By 90 days after injections, Kv1.1 channels essentially abutted nodal NfascNF186, and the paranodal domain was essentially nonexistent (compare Fig. 5M″′–Q″′ with L″′). A quantitative analysis of the paranodal and other domain changes after tamoxifen injection is presented in Table I. Together these data indicate that glial NfascNF155 is required for the maintenance of paranodal axoglial junctions and that axoglial junctions are critical for the maintenance of the paranodal region and axonal domains in myelinated axons.
TABLE I.
Quantitative Analysis of Loss of Paranodal Region and Disorganization of Axonal Domains in Sciatic Nerves of NfascNF155AD Mutants*
| Genotype | Days after injectiona | Postnatal ageb | Paranodes analyzedc | Number and Percent of Paranodesd Paranodal Lengthe |
|||
|---|---|---|---|---|---|---|---|
| 100% | 50% | 25% | >10% | ||||
| +/+ | 10 | P43 | 200 | 194 (97) | 6 (3) | 0 | 0 |
| +/+ | 20 | P53 | 190 | 186 (98) | 4 (2) | 0 | 0 |
| +/+ | 90 | P123 | 210 | 206 (98) | 4 (2) | 0 | 0 |
| Plp-CreER;NfascFlox/Flox | 10 | P43 | 220 | 154 (70) | 37 (18) | 18 (8) | 9 (4) |
| Plp-CreER;NfascFlox/Flox | 20 | P53 | 200 | 90 (45) | 60 (30) | 30 (15) | 20 (10) |
| Plp-CreER;NfascFlox/Flox | 90 | P123 | 210 | 10 (5) | 31 (15) | 95 (45) | 74 (35) |
| Plp-CreER;NfascFlox/Flox | 180 | P213 | 200 | 6 (3) | 10 (5) | 10 (5) | 174 (87) |
Animals were injected with tamoxifen at P23 for 10 days. Injected animals were analyzed by immunofluorescence at various time points.
Postnatal age at the time of tissue analysis after tamoxifen injection (see Fig. 5, Fig 6 and Fig 7 for details).
Total number of paranodes analyzed based on the immunoreactivity against paranodal Caspr and nodal NF186.
The average length of the paranodal region was based on Caspr immunoreactivity compared to average wild-type length (generally 4–5 µm using calibrated preetched micrometer-grid slides from Zeiss). If the variation between paranodes was less than 10%, they were grouped with the next lower percentage. Variations in length that exceeded 11% were grouped in the next higher percentage. Paranodes that appeared too stretched from mechanical teasing during preparation were not included in the estimates.
Paranodal lengths were estimated as 100%, if they measured 4–5 µm, 50% for 2–2.5 µm, 25% for 1–1.25 µm, and <10% at 0.5 µm and lower.
As observed in Figure 5CH″,N″′, 20 days after injection, NfascNF155 and Caspr already show reduced protein levels; therefore, we wanted to determine whether we could identify fibers that were in the process of losing axonal domains, by showing partial overlap of domain markers. As shown in Figure 5R–R″′,S–S″′, as soon as Caspr localization at the paranodes becomes aberrant (Fig. 5R′, blue), Kv1.1 begins to migrate toward the paranodes (Fig. 5R′, red). When Caspr localization is intact, for example, in the right paranode (Fig. 5S′, blue), Kv1.1 remains at the juxtaparanodes (Fig. 5S, red, and S″′, merged image). The localization of NfascNF186 (Fig. 5R″,S′, green) is not affected by loss of NfascNF155. These data suggest that, as soon as axoglial junctions begin to disassemble, axonal domain disorganization ensues, and juxtaparanodal components begin their migration toward the nodal domain.
To determine whether central myelinated axons also follow the same pattern as the peripheral fibers, we analyzed the spinal cord white matter in Plp-CreER;N-fascFlox/Flox mice. As shown in Figure 5T–T″′, wild-type controls show normal domain organization, whereas the adult animals that lose NfascNF155 show gradual disorganization of the axonal domains (Fig. 5U–W″′). One noteworthy feature of the central fibers is that, even in the presence of robust Caspr immunoreactivity at the paranodes (Fig. 5V′,W′, blue), the Kv1.1 channels from the juxtaparanodes (Fig. 5V,W, red) persisted in shifting closer to the node (merged images in Fig. 5V″′,W″′). These results suggest that it is the axoglial junctions, not the mere presence of Caspr, that maintains the axonal domains in the myelinated axons. To establish further the time line of loss of NfascNF155 in adults after injection and the consequences of this on other paranodal proteins, we carried out immunoblot analysis of the spinal cords from age-matched P33 wild-type and Plp-CreER;NfascFlox/Flox tamoxifen-injected animals. As shown in Figure 5X, the levels of NfascNF155 began to decline steadily by 10 days after injection. By 20 days postinjection, nearly half of the NfascNF155 protein remained, and nearly none of NfascNF155 protein could be detected on the immunoblots by postinjection day 60, which is consistent with our immunofluorescence observations (Fig. 5C–E). However, the protein levels of Caspr (Fig. 5Y) and the paranode-enriched protein 4.1B (Fig. 5Z) were not significantly affected. Taken together, these data demonstrate that paranodal axoglial junctions are critical for the maintenance of axonal domains in myelinated axons.
NfascNF155AD Mutants Display Gradual Loss of Paranodal Axoglial Junctions
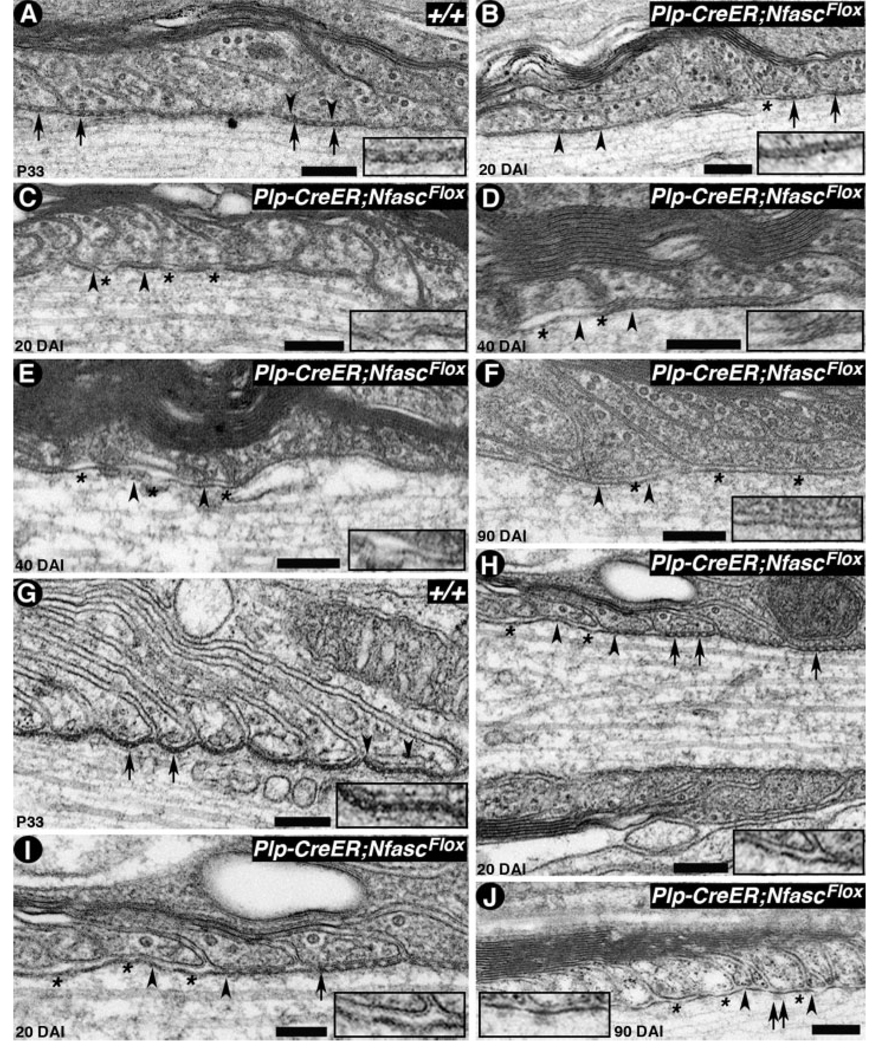
Because the light microscopic immunofluorescence analyses in tamoxifen-injected Plp-CreER;NfascFlox/Flox mice revealed disorganization of axonal domains, we wanted to determine the structural changes that occur in the electron-dense transverse bands, or septa, at the para-nodal axoglial junctions. To address this, we carried out electron microscopic examination of spinal cord white matter and sciatic nerves of tamoxifen-injected wild-type and Plp-CreER;NfascFlox/Flox mice. As shown in Figure 6A, P33 wild-type nerves showed normal apposition of paranodal myelin loops with the axolemma, with distinct septa at the interface (Fig. 6, black arrows). The region between the black arrowheads is shown at a higher magnification in Figure 6A, inset. In contrast, the myelinated fibers from Plp-CreER;NfascFlox/Flox 20 days after injection (Fig. 6B), showed atypical transverse bands (septa) from the juxtaparanodal side (left in Fig. 6B), with a loosening of the junctions and diffuse electron-dense structures in the cleft (Fig. 6B, see inset), whereas the paranodal loops toward the nodal side (right in Fig. 6B) maintained distinct transverse bands as seen in the wild type (Fig. 6A). After 20 days of injection, the mutant fibers showed progressive disorganization of the septal morphology (see Fig. 6, inset) and the axolemma detaching from the myelin loops (Fig. 6C, asterisks). Further analysis of Plp-CreER;NfascFlox/Flox mice after 40 (Fig. 6D,E) and 90 (Fig. 6F) days of injection, revealed that, by 40 days, some electron density is still present in the myelin/axon cleft at the paranodes, but no presence of intact transverse bands is observed (insets in Fig. 6D,E). By 90 days postinjection, no transverse bands were visible between the myelin loops and the axolemma, and the electron densities noticed at earlier stages had diminished (Fig. 6F, inset). Similarly, we analyzed the sciatic nerves from wild-type and Plp-CreER;NfascFlox/Flox injected mice. As shown in Figure 6G, wild-type fibers displayed typical morphology of the axoglial transverse bands (Fig. 6G, inset), whereas mutant fibers after 20 days of injection revealed disorganization of axoglial junctions (Fig. 6H). Accordingly, the junctions appeared to disassemble from the juxtaparanodal side (left in Fig. 6H) and progressed gradually toward the node. As shown in Figure 6I at higher magnification, the paranodal loops toward the nodal region side still maintain intact axoglial junctions with their typical morphology. By 90 days after injection, all the axoglial junctions were lost (Fig. 6J, see inset); however, electron densities on the paranodal axonal side were still observed. The presence of these densities indicates that the remnants of the paranodal axonal scaffold that links axoglial junctions to axonal cytoskeleton may remain intact well beyond the disruption of axoglial junctions (Garcia-Fresco et al., 2006). Taken together, the ultrastructural analysis reveals that the paranodal axoglial junctions disorganize gradually, and this disorganization begins from the juxtaparanodal side and progresses gradually toward the nodal side. This conclusion is strengthened by the observations that the juxtaparanodal components, like Kv1.1, begin to shift toward the nodal area immediately after axoglial junctions begin to disappear.
Fig. 6.
Axoglial junctions disorganize gradually in NfascNF155AD adult mutants. A: Electron micrograph of a longitudinal section through the paranodal region of a spinal cord fiber from P33 wild-type (+/+) mouse shows paranodal loops tightly apposed to and indenting the axon, forming transverse bands (arrows). The region between the black arrowheads is shown at a higher magnification in the inset. B–F: Electron micrographs of longitudinal sections through the paranodal regions of spinal cord nerve fibers from tamoxifen-injected P33 Plp-CreER;NfascFlox mutants after 20 (B,C), 40 (D,E), and 90 (F) days. At 20 days, transverse band morphology on the right side is still intact (B, black arrows), but on the left side septa have become abnormal and diffuse (B, black arrowheads). Other fibers show diffuse transverse bands and axolemma detaching from the myelin membrane (C, black arrows, inset). At 40 days, transverse bands remain as unpatterned electron densities (D,E, black arrowheads, insets) and are essentially absent by 90 days (F, black arrowheads, inset). Axolemmal detachments from the myelin membrane are also observed during disorganization of the axoglial junctions (C–F, asterisks). G: Electron micrograph of a longitudinal section through the paranodal region of a sciatic nerve fiber from P33 wild-type (+/+) mouse shows paranodal loops forming transverse bands (black arrows). The region between black arrowheads is shown at a higher magnification in the inset. H–J: Electron micrographs of longitudinal sections through the paranodal regions of sciatic nerve fibers from tamoxifen-injected Cnp-Cre;NfascFlox mutants after 20 (H,I) and 90 (J) days. At 20 days, transverse band morphology on the right side is still intact (H, black arrows), but on the left side the transverse bands have already disappeared (H, black arrowheads, inset). At a higher magnification (I), absence of transverse bands (asterisks), diffuse transverse bands, and intact transverse bands are all observed in this fiber. At 90 days, the transverse bands are essentially absent (J, black arrowheads, inset, asterisks). Note that some electron densities on the axolemmal side are still present, which may represent the remaining axonal cytoskeletal complexes at the disorganized paranodes (J, black arrows). Scale bars = 0.2 µm.
Peripheral Nerve Conduction Is Severely Affected in NfascNF155 and Modestly Affected in NfascNF155AD Mutants
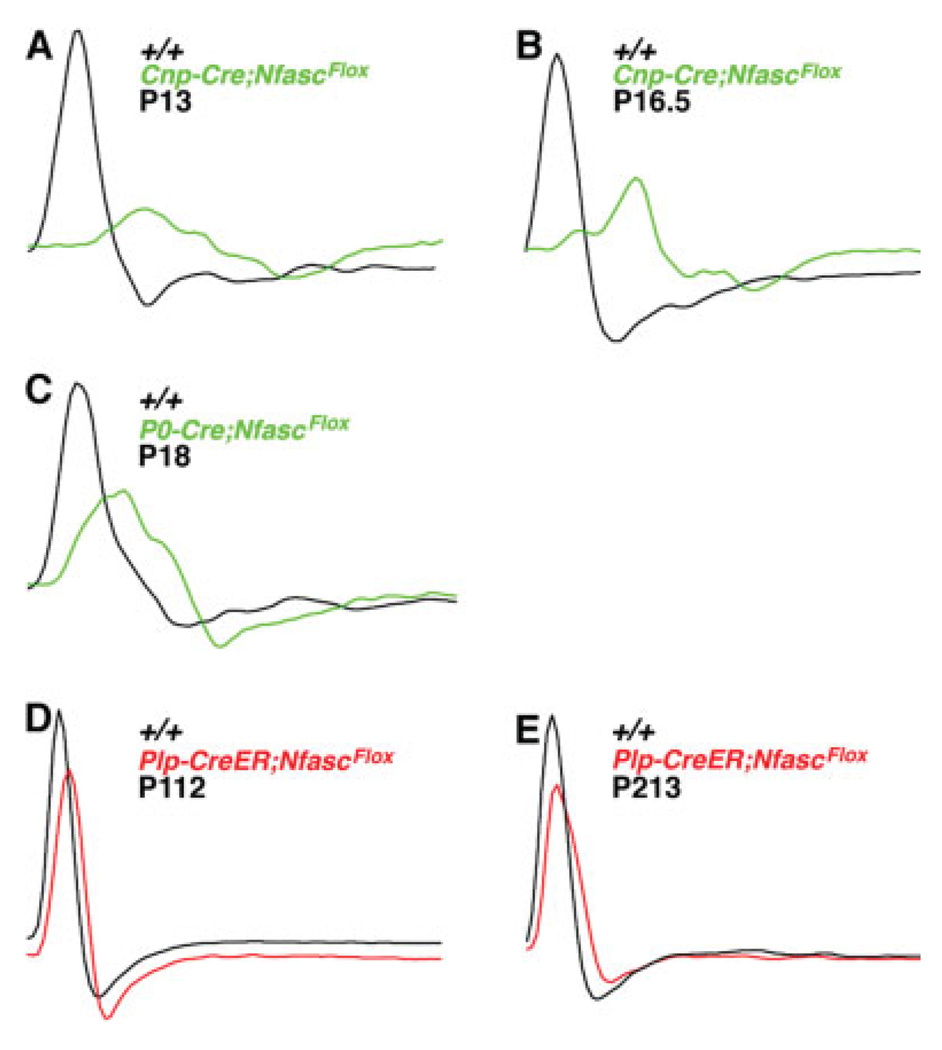
To determine whether the loss of the paranodal axoglial junctions and/or the altered distribution of ion channels observed in the Cnp-Cre;NfascFlox/Flox, P0-Cre;NfascFlox/Flox, and Plp-CreER;NfascFlox/Flox affected the electrophysiological properties of peripheral nerves, we performed extracellular recordings from tibial/plantar nerves of litter- or age-matched wild-type (+/+) and mutant mice. Nerves were stimulated at an intensity and duration sufficient to elicit a stable A-component amplitude and latency (for details see Materials and Methods). The electrophysiological measurements from various genotypes at different ages are given in Table II, and representative plots are presented in Figure 7. In Cnp-Cre;NfascFlox/Flox nerves, conduction velocity (CV) was reduced by 50% at P13 (8.47 m/sec compared with wild-type at 16.83 m/sec; Fig. 7A). At P16.5, Cnp-Cre;NfascFlox/Flox fibers revealed a CV of 9.5 m/sec compared with a wild-type CV of 17 m/sec (Fig. 7B). At P18, P0-Cre/+;NfascFlox/Flox nerves had a 41% reduction in CV compared with wild-type (+/+, 17.9; P0-Cre, 12.6; Fig. 7C). In addition to the substantial delay in conduction, Cnp-cre;NfascFlox/Flox nerves exhibited altered action potential amplitude and duration. Cnp-Cre;NfascFlox/Flox demonstrated a greater than 50% decrease in amplitude and 50% increase in duration (Fig. 7A,B). This was also observed in P0-Cre/+;NfascFlox/Flox nerves (Fig. 7C, Table II). In Plp-CreER;NfascFlox/Flox fibers, the CV was modestly reduced, with a 24% decrease compared with wild-type (Fig. 7D). A further reduction in CV was observed in older Plp-CreER;NfascFlox/Flox nerves (Fig. 7E, Table II). Taken together, the electrophysiological measurements indicate an important role for paranodal axoglial junctions in ensuring normal action potential propagation in myelinated axons.
TABLE II.
Electrophysiological Measurements of Tibial/Plantar Nerve Conduction Velocities
| Nfasc allele | Genotype | CV (m/sec mean ± SEM) |
Postnatal age (mean days) |
n |
|---|---|---|---|---|
| WT | +/+ | 16.9 ± 0.09 | 13 | 4 |
| NfascNF155 |
Cnp-Cre/+; NfascFlox/Flox |
9.5 ± 0.97 | 13 | 4 |
| WT | +/+ | 21.75 ± 4.12 | 21 | 4 |
| NfascNF155 |
P0-Cre; NfascFlox/Flox |
12.83 ± 2.86 | 21 | 4 |
| WT | +/+ | 33.50 ± 4.30 | 86a | 2 |
| NfascNF155AD |
Plp-CreER; NfascFlox/Flox |
25.60 ± 5.60 | 86a | 2 |
Animals were injected at P23 for 10 days and allowed to age to specified time points for electrophysiological measurements.
Fig. 7.
Peripheral nerve conduction velocities are severely disrupted in NfascNF155 mutants. A,B: Representative electrophysiological profiles showing A-component CAPs from P13 wild-type (A, +/+, black) and Cnp-Cre;NfascFlox (A, green) and P16.5 wild-type (B, +/+, black) and Cnp-Cre;NfascFlox (B, green) mice show severe conduction delay, decrease in amplitude, and increase in the duration of the action potentials. C: Representative electrophysiological profiles A-component CAPs from P18 wild-type (+/+, black) and P0-Cre;N-fascFlox (green) mice show severe conduction delay, decrease in amplitude, and increase in the duration of the action potentials. Thus Cnp- and P0-Cre-mediated deletion of NfascNF155 independently produce similar electrophysiological deficits, eliminating any major contributions from the genetic backgrounds of these animals. D,E: Representative electrophysiological profiles A-component of CAP from P112 wild-type (E, +/+, black) and tamoxifen-injected Plp-CreER;NfascFlox/Flox (E, red) and P213 wild-type and tamoxifen-injected Plp-CreER;NfascFlox/Flox (F, red) mice show a modest delay in conduction and a minimal decrease in amplitude.
DISCUSSION
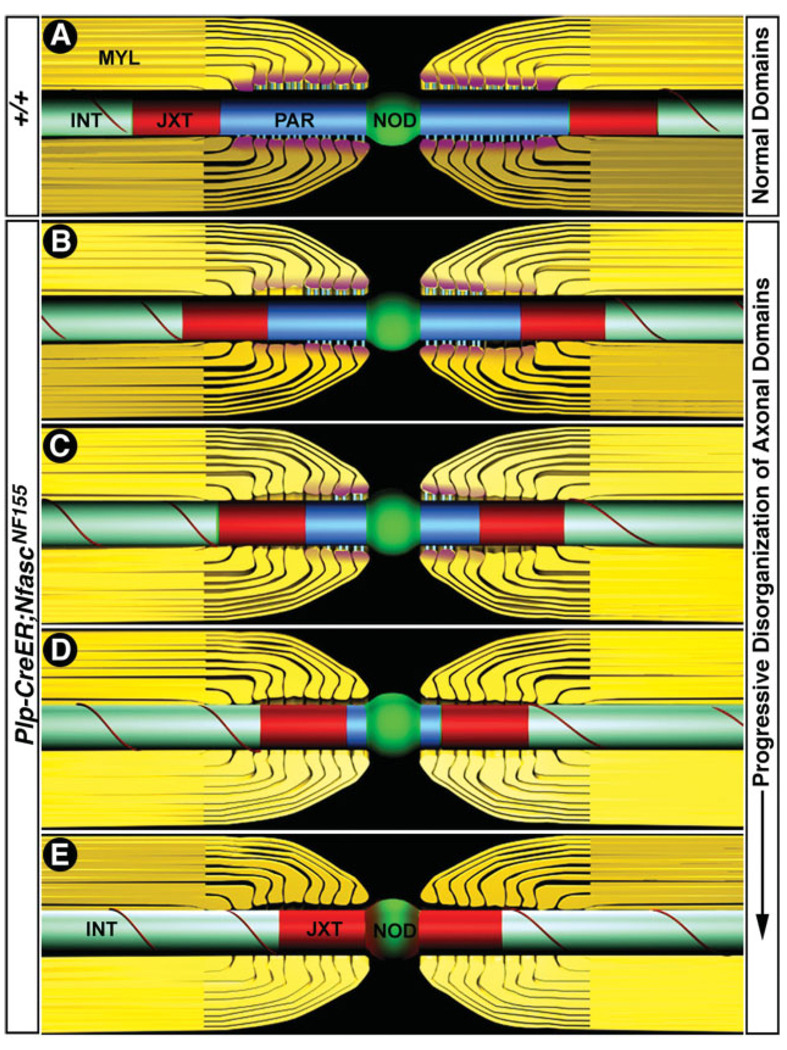
The process of myelination and its ultrastructural attributes have long fascinated biologists (Robertson, 1957). Understanding these fundamental processes, and the proteins required for the association of glia with neurons is critical for our understanding of myelin-related neuropathies and how they manifest. Recent work has focused on the molecular composition of axonal domains by biochemical methods, but the role of individual components in axonal domain organization remains elusive (Coetzee et al., 1996; Bhat et al., 2001; Boyle et al., 2001; Sherman et al., 2005; Zonta et al., 2008). Here, by using a glial-specific conditional knockout, we have addressed the role of NfascNF155 in the organization and maintenance of paranodal axoglial junctions and axonal domains. Our data reveal that NfascNF155 is a critical component of paranodal junctions and that loss of NfascNF155 in adult myelinating glia results in the gradual loss of axoglial junctions and concomitant failure to maintain segregated axonal domains. These mutant mice therefore provide a unique animal model for analyzing the role of glial paranodal components in the formation, stabilization, and maintenance of axonal domains in myelinated axons and may further contribute to our understanding of myelin-related diseases (Fig. 8).
Fig. 8.
Schematic illustrations of the loss of axoglial junctions and failure to maintain axonal domains in the peripheral myelinated axons. A: Schematic illustration (not to scale) of the axonal domains in wild-type adult myelinated fibers showing node of Ranvier (green), paranodes (blue), and juxtaparanodes (red) and internode (aqua). The internodal region contains compact myelin, and the paranodal region contains loops of noncompact myelin. Molecular interactions between glial NfascNF155 (pink) and axonal Caspr/contactin (blue) create ladder-like junctions at the paranodal axon–glia interface. The nodal region where Na+ channels are clustered (green) is well separated from the K+ channels at the juxtaparanodal region by the paranodes. B–E: Various stages of domain disorganization after tamoxifen-induced NfascNF155 disruption. The levels of paranodal NfascNF155 begin to decrease (B, pink), and axoglial junction disorganization starts from the juxtaparanodal region, and K+ channels begin to move into the paranodal region, which begins to get smaller. Axoglial junctions near the nodal region are still intact. NfascNF155 levels continue to decrease at the paranodes, and more axoglial junctions have been lost, and paranodal region has become smaller (C). K+ channels continue to move toward the node. NfascNF155 is undetectable at the paranodes (D), and close to 75% of the paranode has been lost. K+ channels continue to move toward the node. Paranodes have disappeared, and K+ channels have reached the nodal region and almost overlap with the nodal Na+ channels. At all stages, the nodal Na1 channels remain clustered and do not seem to diffuse into the paranodal area or the axons. (Note that the illustrations are not to scale and reflect approximate changes in the domain disorganization of the peripheral myelinated axons).
NfascNF155 Mutants Display Severe Motor Coordination Defects
Previous work from our laboratory has shown the importance of Caspr/paranodin to paranodal axoglial junction formation (Bhat et al., 2001). Circumstantial evidence suggests that glial NfascNF155 can associate with Caspr and its axonal partner Cont, but no phenotypic analysis of a glial-specific knockout of NfascNF155 has been performed, and there is much debate over the exact mechanism(s) of how these proteins associate (Charles et al., 2002; Gollan et al., 2003; Bonnon et al., 2007). Here we find, with glial-specific Cnp-Cre, that NfascNF155 mutant mice exhibit severe tremors, motor paresis, ataxia, and immobility and die at postnatal day 16 or 17, similarly to both Caspr and Cont mutant mice (Berglund et al., 1999; Bhat et al., 2001; Boyle et al., 2001). We believe that these neurological defects reflect the function of NfascNF155 in the myelinating glial cells and not the neuronal NfascNF186, because Cnp-Cre expression has been shown to be restricted to glia within the CNS and PNS (Lappe-Siefke et al., 2003). Additionally, we have shown that NfascNF186 proteins levels remain unchanged and that the nodal complex remains intact in these animals. These findings indicate for the first time the importance of glial-specific NfascNF155 in the formation of paranodal axoglial junctions and in coordination of motor function.
Axon–Glial Interactions at the Nodes and Paranodes of NfascNF155 Mutants
The proposed role of glial NfascNF155 in the organization of the axoglial junctions was suggested by the mutant phenotypes observed in Nfasc mutants that lack both NfascNF155 and NfascNF186 (Sherman et al., 2005). These mutant mice have severe amyelination and dismyelination as well as a reduction in the levels of myelin-specific proteins, such as MBP and MAG (Zonta et al., 2008). In the current study, we found that glia-specific loss of NfascNF155 did not alter the myelin ultra-structure but instead resulted in the absence of transverse bands, the hallmark of axoglial junctions. These findings provide formal evidence that NfascNF155 is a key glial component of axoglial junctions and is consistent with its localization to the paranode (Tait et al., 2000; Charles et al., 2002; Sherman et al., 2005). Interestingly, other paranodal/nodal abnormalities observed in NfascNF155 mutants are also seen in Caspr mutants, suggesting further that loss of either of the paranodal components generates identical phenotypes. Furthermore, the similarities in phenotype between these mutant may be attributed to cytoskeletal abnormalities created by the disruption of the axoglial junctions as seen previously (Einheber et al., 2006; Garcia-Fresco et al., 2006; Sousa and Bhat, 2007).
In the CNS of NfascNF155 mutants, the morphological organization of the paranodal region is severely affected, as evidenced by the appearance of everting and overriding glial paranodal loops. These organizational defects can be attributed to a failure of the paranodal loops to make physical adhesions with the axon in the NfascNF155 mutant mice. Although the paranodal loops of these mutant mice fan outward, the structure of myelin remains unchanged and suggests that the formation and stabilization of the paranode is independent of myelin stabilization. Upon further examination, it was seen that the overriding loops often occluded the node (Fig. 4D), and, although nodal proteins remain localized, such as NfascNF186, Na channels, and AnkG (data not shown), the effect of such an obstruction on nodal function remains unknown. The change in the paranodal structure in NfascNF155 mutants may be a result of a change in the trapezoidal geometry of the myelin sheath, as is observed in Caspr mutants (Bhat et al., 2001). Other striking abnormalities observed in NfascNF155 mutants are the axonal swellings and degeneration of cerebellar Purkinje axons (Fig. 2V, W, Fig. 4F–H). In earlier studies, we found that Caspr mutant mice exhibited similar abnormalities. We proposed then that such phenotypes were a result of the disorganization of the paranodal axonal cytoskeleton that results in organelle transport defects at the paranodes and eventual axonal degeneration (Einheber et al., 2006; Garcia-Fresco et al., 2006; Sousa and Bhat, 2007). Our findings indicate further that axoglial junction disruption leads to axonal transport defects and degeneration and indicate a role for the glial cytoskeleton in the maintenance and stabilization of the septate-like junctions as an anchor for the association of glia with axons.
Keeping Neighbors Apart: Paranodal Junctions and Maintenance of Axonal Domains
A major observation of this study is that the organization of axonal domains in myelinated axons requires myelinating glia-specific expression of NfascNF155 and that, along with Caspr and Cont, it is able to establish the transverse bands/septa required at the paranodal axon–glial interface. Recent work has suggested that intact paranodal axoglial domains alone, formed by reintroducing NfascNF155 into a complete Nfasc null background, may act to cluster and form the node independent of the neuronal-specific NfascNF186 (Zonta et al., 2008). Interestingly, these mutant mice were not able to survive past P6, the same expiry as the complete null mice lacking both NfascNF155 and NfascNF186. Here we find that Na+ and K+ channels are able to cluster in the absence of the glial NfascNF155 and intact paranodal junctions. This suggests that these junctions are not required for the clustering of ion channel domains and nodal proteins but instead are likely necessary for their continued segregation (see below). This is consistent with previous observations demonstrating that clustering of nodal components occurs prior to mature paranodal junction formation in the developing PNS (Melendez-Vasquez et al., 2001) and in CGT mutant mice that fail to form paranodal axoglial junctions (Coetzee et al., 1996; Dupree et al., 1999). Accordingly, in the absence of the paranodal axoglial junctions in NfascNF155, Caspr, Cont, and CGT mutants, the nodal Na+ and juxtapara-nodal K+ channel domains remain largely segregated, with only occasional overlaps into the nodal region. These phenotypes indicate that distinct molecular interactions, possibly involving specific axonal and glial scaffolding proteins, maintain nodal protein complexes to their respective domains (Peles and Salzer, 2000; Pedraza et al., 2001; Bhat, 2003; Sherman and Brophy, 2005).
To address the importance of axoglial domain stabilization to the segregation of axonal domains, i.e., the node, paranode, and juxtaparanode, in adults we utilized a tamoxifen-inducible Plp-CreER to knock out NfascNF155 after stablilization of these domains was complete. Upon observation, we found that, 20 days posttamoxifen injection, NfascNF155 levels were reduced by 50% and that only after 90 days of injection is near-complete loss of NfascNF155 seen. These findings indicate that NfascNF155 is quite stable once it is incorporated into axoglial junctions. In addition, we found that, with increasing time after injection, Caspr staining is gradually reduced and that the juxtaparanodal K+ channels migrated toward the nodal region. The concomitant shift of the clustered juxtaparanodal K+ channels toward the node suggests that these complexes are mobile and are not anchored by, or within, the axonal/glial cytoskeleton. However, it is unclear why the K+ channels move toward the nodal area instead of diffusing throughout the internode; if it was a simple diffusion in the membrane, then one would expect that they should diffuse in either direction. Interestingly, these new findings are in direct contrast to findings from the Cnp-Cre;NfascFlox mutant mice shown here, which fail to form paranodal axoglial junctions but still maintain segregated nodal and juxtaparanodal domains. These results indicate that two independent functions exist to segregate and maintain axonal domains during development and in mature animals. During development, each axonal domain is assembled and stabilized independently of the other and thus retains its anchorage at the respective domain, whereas, in the adult, maintenance of the domains requires the paranode to act as a fence to segregate the domains. This suggests that, with time, as these animals progressively lose the paranodal axoglial junctions, that both the juxtaparanodal complex and the nodal complex may diffuse throughout the internodes, which may lead to severe depolarization and abnormal saltatory conduction.
Conduction Velocity and the Role of Paranodal Axoglial Junctions
Electrophysiological data on NfascNF155 mutant mice generated during embryonic development reveal that population nerve CVs are severely reduced in peripheral nerve fibers. These defects may reflect changes in the properties of fast-conducting myelinated fibers and may also be caused by a conduction block in a subset of fibers and/or slower propagation in the remaining fibers. Another possibility for the weakened CVs observed in NfascNF155 mutant mice is that the disorganized paranodal region may cause current leakage into the periaxonal space. Leakage of the current may in turn increase the capacitance of the axonal membrane, leading to slow action potential propagation (Chiu and Ritchie, 1980; Boyle et al., 2001). Interestingly, it might be expected that in the adult NfascNF155 mutant mice CVs would be slowed concomitantly with the degradation of axoglial junctions; however, we found that both the CV and the CAP peak-to-peak amplitudes were not dramatically altered in the mutant mice compared with control mice of the same age. These results were seen even in mice examined 6 months postinjection that had near-complete loss of NfascNF155 protein levels and disassembled paranodal junctions (Fig. 7). It is not clear why the loss of axoglial junctions during adult stages has a less dramatic effect on the electrophysiological properties of the peripheral nerve fibers than loss in early developmental stages. One possibility is that many structural features are maintained in adult mutant nerves that allow the conduction of action potentials at near-normal or at a slightly reduced velocity. Alternatively, it could be that different classes of fibers in these adult nerves conduct differently and what we observed is a summation of all the fibers. It is unlikely that the reduced change in CVs is due to the injection and variability of its penetration to all glial cells, insofar as our quantitative measurements revealed that greater than 90–95% of the Schwann cells lost NfascNF155 after 10 days of tamoxifen treatment (Table I), so the modest changes in CV and amplitude in adult fibers may be due to the structural integrity of the nerve fibers and slow disorganization of the axonal domains over time. It would be interesting to see how the NfascNF155 mutant mice CVs faired through extended time, in that it has been shown that human demyelinating neuropathies, where axonal domains are compromised, such as multiple sclerosis and Charcot-Marie-Tooth disease, CVs are severely reduced (Nicholson, 1999; Wolswijk and Balesar, 2003; Coman et al., 2006). Further studies on NfascNF155 mutant mice may reveal more similarities to human neurological disorders and provide a system for studying human disease. In summary, we have demonstrated that glial NfascNF155 is critical for the formation of the paranodal axoglial junctions and that these junctions are central to the organization and maintenance of the axonal domains in myelinated axons.
ACKNOWLEDGMENTS
We are immensely grateful to Drs. Brian Popko (University of Chicago), Klaus Nave (Max Planck Institute of Experimental Medicine), and Laura Feltri (San Rafaele Scientific Institute, Italy) for generously sharing their Cre-expressing mouse strains. Our special thanks go to Dr. Edward Perl for his generous support and advice and for making his electrophysiology facility available to us to perform nerve conduction studies. We also thank Dr. G. Garcia-Fresco for drawing the schematics in Figure 8; Drs. W. Snider, J. Zhong, J. Salzer, and A. Gow for reagents; Drs. A. Fanning and S. Banerjee for comments on the manuscript; and members of the Bhat laboratory for valuable discussions. The electron microscopy was performed at the VCU and supported, in part, with funding from NIH-NINDS Center core grant 5P30NS047463.
Contract grant sponsor: National Institutes of Health; Contract grant number: GM63074; Contract grant number: NS050356; Contract grant sponsor: National MS Society; Contract grant sponsor: State of North Carolina (to M.A.B.).
REFERENCES
- Arroyo EJ, Xu YT, Zhou L, Messing A, Peles E, Chiu SY, Scherer SS. Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvbeta2, and Caspr. J Neurocytol. 1999;28:333–347. doi: 10.1023/a:1007009613484. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Pillai AM, Paik R, Li J, Bhat MA. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J Neurosci. 2006a;26:3319–3329. doi: 10.1523/JNEUROSCI.5383-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006b;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- Berglund EO, Murai KK, Fredette B, Sekerkova G, Marturano B, Weber L, Mugnaini E, Ranscht B. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 1999;24:739–750. doi: 10.1016/s0896-6273(00)81126-5. [DOI] [PubMed] [Google Scholar]
- Bhat MA. Molecular organization of axoglial junctions. Curr Opin Neurobiol. 2003;13:552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Izaddoost S, Lu Y, Cho KO, Choi KW, Bellen HJ. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St. Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon–glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Bonnon C, Bel C, Goutebroze L, Maigret B, Girault JA, Faivre-Sarrailh C. PGY repeats and N-glycans govern the trafficking of paranodin and its selective association with contactin and neurofascin-155. Mol Biol Cell. 2007;18:229–241. doi: 10.1091/mbc.E06-06-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc AM, Girault JA, Brophy PJ, Lubetzki C. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol. 2002;12:217–220. doi: 10.1016/s0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Ritchie JM. Potassium channels in nodal and internodal axonal membrane of mammalian myelinated fibres. Nature. 1980;284:170–171. doi: 10.1038/284170a0. [DOI] [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Coman I, Aigrot MS, Seilhean D, Reynolds R, Girault JA, Zalc B, Lubetzki C. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3186–3195. doi: 10.1093/brain/awl144. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain−) and NrCAM at nodal axon segments. J Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, Yamakawa H, Ohara O, Carnaud M, Girault JA. Protein 4.1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres. Eur J Neurosci. 2003;17:411–416. doi: 10.1046/j.1460-9568.2003.02441.x. [DOI] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. Axoglial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147:1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Bhat MA, Salzer JL. Disrupted axoglial junctions result in accumulation of abnormal mitochondria at nodes of Ranvier. Neuron Glia Biol. 2006;2:165–174. doi: 10.1017/S1740925X06000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, D’Antonio M, Previtali S, Fasolini M, Messing A, Wrabetz L. P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann N Y Acad Sci. 1999;883:116–123. [PubMed] [Google Scholar]
- Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, Dupree JL, Bhat MA. Disruption of axoglial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proc Natl Acad Sci USA. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser HS, Grundfest H. Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian A fibers. Am J Physiol. 1939;127:22. [Google Scholar]
- Gollan L, Salomon D, Salzer JL, Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. J Cell Biol. 2003;163:1213–1218. doi: 10.1083/jcb.200309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave KA. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17:R29–R35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dupree JL, Ikenaka K, Hirahara Y, Honke K, Peles E, Popko B, Suzuki K, Nishino H, Baba H. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J Neurosci. 2002;22:6507–6514. doi: 10.1523/JNEUROSCI.22-15-06507.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Vasquez CV, Rios JC, Zanazzi G, Lambert S, Bretscher A, Salzer JL. Nodes of Ranvier form in association with ezrin-radixin-moesin (ERM)-positive Schwann cell processes. Proc Natl Acad Sci USA. 2001;98:1235–1240. doi: 10.1073/pnas.98.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, Ezan P, Arnos F, Girault JA. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Nicholson GA. Mutation testing in Charcot-Marie-Tooth neuropathy. Ann N Y Acad Sci. 1999;883:383–388. [PubMed] [Google Scholar]
- Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband MN. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci. 2006;26:5230–5239. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Palay VC. Cerebellar cortex. New York: Springer-Verlag; 1974. [Google Scholar]
- Pedraza L, Huang JK, Colman DR. Organizing principles of the axoglial apparatus. Neuron. 2001;30:335–344. doi: 10.1016/s0896-6273(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Peles E, Salzer JL. Molecular domains of myelinated axons. Curr Opin Neurobiol. 2000;10:558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein–protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AM, Garcia-Fresco GP, Sousa AD, Dupree JL, Philpot BD, Bhat MA. No effect of genetic deletion of contactin-associated protein (CASPR) on axonal orientation and synaptic plasticity. J Neurosci Res. 2007;85:2318–2331. doi: 10.1002/jnr.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Tayler J, Kaga Y, Yang Y, Lappe-Siefke C, Nave KA, Bansal R. CNP is required for maintenance of axon–glia interactions at nodes of Ranvier in the CNS. Glia. 2005;50:86–90. doi: 10.1002/glia.20165. [DOI] [PubMed] [Google Scholar]
- Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett. 1986;66:141–146. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JD. The ultrastructure of nodes of Ranvier in frog nerve fibres. J Physiol. 1957;137:8P–9P. [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Custer AW, Shrager P, Rasband MN. Early events in node of Ranvier formation during myelination and remyelination in the PNS. Neuron Glia Biol. 2006;2:69–79. doi: 10.1017/S1740925X06000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF, Brophy PJ. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Sousa AD, Bhat MA. Cytoskeletal transition at the paranodes: the Achilles’ heel of myelinated axons. Neuron Glia Biol. 2007;3:169–178. doi: 10.1017/S1740925X07000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwood C, He C, Garbern J, Kamholz J, Arroyo E, Gow A. CNS myelin paranodes require Nkx6–2 homeoprotein transcriptional activity for normal structure. J Neurosci. 2004;24:11215–11225. doi: 10.1523/JNEUROSCI.3479-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfli R. A new method for measuring membrane potentials with external electrodes. Experientia. 1954;10:508–509. doi: 10.1007/BF02166189. [DOI] [PubMed] [Google Scholar]
- Tait S, Gunn-Moore F, Collinson JM, Huang J, Lubetzki C, Pedraza L, Sherman DL, Colman DR, Brophy PJ. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axoglial junction. J Cell Biol. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 2003;126:1638–1649. doi: 10.1093/brain/awg151. [DOI] [PubMed] [Google Scholar]
- Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, Brophy PJ. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J Cell Biol. 2008;181:1169–1177. doi: 10.1083/jcb.200712154. [DOI] [PMC free article] [PubMed] [Google Scholar]