Abstract
The overview covers the discovery of N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers, initial studies on their synthesis, evaluation of biological properties, and explorations of their potential as carriers of biologically active compounds in general and anticancer drugs in particular. The focus is on the research in the authors’ laboratory – the development of macromolecular therapeutics for the treatment of cancer and musculoskeletal diseases. In addition, the evaluation of HPMA (co)polymers as building blocks of mod and new biomaterials is presented: the utilization of semitelechelic poly(HPMA) and HPMA copolymers for the modification of biomaterial and protein surfaces and the design of hybrid block and graft HPMA copolymers that self-assemble into smart hydrogels. Finally, suggestions for the design of second-generation macromolecular therapeutics are portrayed.
Keywords: N-(2-hydroxypropyl)methacrylamide, Cancer, Cleavable spacers, Bioconjugates, Hydrogels, Self-assembly
1. Introduction
Water-soluble polymers have a distinguished record of clinical relevance. They have been used in the clinics and/or clinical trials for the modification of proteins, modification of liposomes, surface modification of biomaterials, and as carriers of drugs, genes, and oligonucleotides.
The concept of water-soluble macromolecular carriers of (anticancer) drugs has evolved continuously over the last century. In 1906, Ehrlich coined the phrase “magic bullet”, and recognized the importance of biorecognition for successful drug delivery [1]. DeDuve discovered the lysosomotropism of macromolecules and the high enzymatic activity localized in the lysosomal compartment [2]. The conjugation of drugs to synthetic and natural macromolecules was initiated more than 50 years ago. Jatzkewitz used a dipeptide spacer to attach a drug (mescaline) to polyvinylpyrrolidone in the early fifties [3]. Ushakov’s group in St. Petersburg (Leningrad) synthesized numerous water-soluble polymer–drug conjugates in the sixties and seventies [4–6]. Mathé et al. pioneered conjugation of drugs to immunoglobulins, setting the stage for targeted delivery [7]. Ringsdorf presented a clear summary of the field in 1975 [8].
The research in our laboratory, first in Prague, later in Utah, concentrated on hydrophilic polymers as biomaterials and drug carriers. The early program in Prague on the biocompatibility of hydrophilic polymers resulted in clinical application of hydrogels as implants [9] and in the design of water-soluble polymers as carriers of biologically active compounds (drugs) [10].
This special volume is devoted to N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers. It is an opportunity to review what was done and identify directions for future research. The HPMA development and data presented will be related mostly to the authors’ laboratory, not to overlap with other author’s contributions in this volume. The work done with HPMA copolymers as drug carriers, protein, and surface modifiers, and as synthetic components in smart hybrid biomaterials design has been summarized. More details and work from other laboratories may be found in the other chapters in this volume that cover more focused topics.
2. Origins
2.1. Early history
Our research in the sixties was concentrated on the hydrophilic biomedical polymers based on hydrophilic esters and N-substituted amides of (meth)acrylic acid. The focus was on four tasks [10]: a) selection of a suitable group of polymers and the study of their chemical properties – polymerization kinetics, stability, mechanical properties, permeability, possibility of introduction of functional (reactive) groups; b) study of the relationship between the chemical and physical structure of crosslinked polymers (hydrogels) and their biocompatibility; c) study of the interaction of water-soluble polymers with living organisms – using evaluation criteria from blood plasma expanders; and d) modification of soluble polymers by attachment of biologically active compounds (drugs, hormones, enzymes) as well as the modification of the polymer backbone and side-chains with enzymatically degradable sequences.
The choice of HPMA for development as drug carrier was not random. Based on the detailed studies of the relationship between the structure of hydrophilic polymers and their biocompatibility [11–21], we have chosen N-substituted methacrylamides as our target because the α-carbon substitution and the N-substituted amide bond ensured hydrolytic stability of the side-chains. We synthesized a series of compounds trying to identify a crystalline monomer for easy purification and reproducible synthesis. The first crystalline N-substituted methacrylamide we succeeded to synthesize, HPMA, was chosen for future development [22,23].
2.2. First HPMA copolymer drug and/or protein conjugates
The research on the use of HPMA copolymers as drug carriers commenced in the early 70s. In April 1974 we filed two patent applications [24,25] which covered the synthesis of N-substituted (meth)acrylamides containing oligopeptide sequences and their application as drug (and other biologically active compounds) carriers.
The first HPMA copolymer–drug (N-(4-aminobenzensulfonyl)-N′-butylurea) conjugate (Fig. 1) was presented at the Prague Microsymposiumon Polymers in Medicine in 1977 [26] and published [27]. At the same time studies in the modification of proteins were initiated – HPMA copolymer–insulin [28] and HPMA copolymer–chymotrypsin [29,30] conjugates.
Fig. 1.
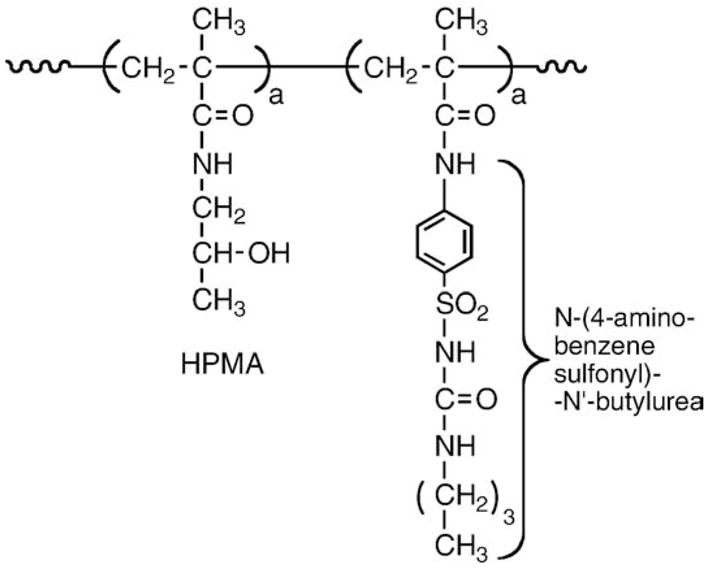
First HPMA copolymer–drug conjugate; HPMA copolymer–N-(4-aminobenzenesulfonyl)- N′-butylurea [26,27].
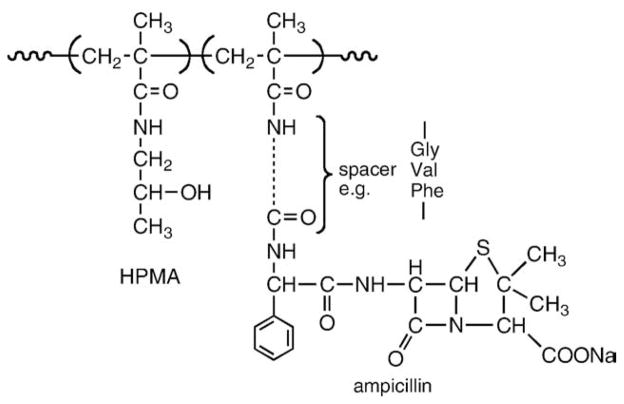
Two major synthetic routes were used for the synthesis of HPMA copolymer–drug conjugates, copolymerization and polymer-analogous attachment [10,21]. Copolymerization of HPMA with a polymerizable derivative of a drug, e.g., N-(4-aminobenzensulfonyl)-N′-butylurea [27] is the example of the first route. Insulin [28], chymotrypsin [29,30] and ampicillin [31] (Fig. 2) were attached to HPMA copolymers by aminolysis [32,33] of reactive polymeric precursors. The development of HPMA copolymers is summarized in Table 1.
Fig. 2.
HPMA copolymer–ampicillin conjugate [31].
Table 1.
Milestones in HPMA (co)polymer research.
| Year | Study | Publication |
|---|---|---|
| 1973 | First synthesis | J. Kopeček, H. Bažilová, Poly[N-(2-Hydroxypropyl)methacrylamide]. 1. Radical polymerization and copolymerization. Europ. Polym. J. 9 (1973) 7–14. |
| 1974 | Characterization of PHPMA solution properties | M. Bohdanecký, H. Bažilová, J. Kopeček, Poly[N-(2-Hydroxypropyl)methacrylamide]. II. Hydrodynamic properties diluted polymer solutions. Europ. Polym. J. 10 (1974) 405–410. |
| 1974 | First hydrogels | J. Kopeček, H. Bažilová, Poly[N-(2-Hydroxypropyl)methacrylamide]. III. Crosslinking copolymerization. Europ. Polym. J. 10 (1974) 465–470. |
| 1976 | First enzymatic release of ligand from a polymer conjugate in vitro | J. Drobník, J. Kopeček, J. Labský, P. Rejmanová, J. Exner, V. Saudek, J. Kálal, Enzymatic cleavage of side-chains synthetic water-soluble polymers. Makromol. Chem. 177 (1976) 2833–2848. |
| 1978 | First HPMA modified protein | V. Chytrý, A. Vrána, J. Kopeček, Synthesis and activity of a polymer which contains insulin covalently bound on a copolymer of N-(2-hydroxypropyl)methacrylamide and N-methacryloylglycylglycine 4-nitrophenyl ester. Makromol. Chem. 179 (1978) 329–336. |
| 1979 | First HPMA–drug conjugate | B. Obereigner, M. Burešová, A. Vrána, J. Kopeček, Preparation of polymerizable derivatives of N-(4-aminobenzenesulfonyl)-N′-butylurea. J. Polym. Sci. Polym. Symp. 66 (1979) 41–52. |
| 1981 | First enzymatic release of ligand from a polymeric substrate by a polymer-modified enzyme | J. Kopeček, P. Rejmanová, V. Chytrý, Polymers containing enzymatically degradable bonds 1. Chymotrypsincatalyzed hydrolysis of p-nitroanilides of phenylalanine and tyrosine attached to side-chains of copolymers of N-(2-hydroxypropyl)methacrylamide. Makromol. Chem. 182 (1981) 799–809. |
| 1981 | First enzymatic release of ligand from a polymer conjugate in vivo | J. Kopeček, I. Cífková, P. Rejmanová, J. Strohalm, B. Obereigner, K. Ulbrich, Polymers containing enzymatically degradable bonds. 4. Preliminary experiments in vivo. Makromol. Chem. 182 (1981) 2941–2949. |
| 1982 | First degradable hydrogels | K. Ulbrich, J. Strohalm, J. Kopeček, Polymers containing enzymatically degradable bonds. VI. Hydrophilic gels cleavable by chymotrypsin. Biomaterials 3 (1982) 150–154. |
| 1985 | First HPMA–drug–antibody conjugate | B. Říhová, J. Kopeček, Biological Properties of targetable poly[N-(2-hydroxypropy)methacrylamide]–antibody conjugates. J. Controlled Release 2 (1985) 289–310. |
| 1994 | First combination therapy using polymer-bound drugs | N.L. Krinick, Y. Sun, D. Jonyer, J.D. Spikes, R.C. Straight, J. Kopeček, A polymeric drug delivery system for the simultaneous delivery of drugs activatable by enzymes and/or light. J. Biomat. Sci. Polym. Ed. 5 (1994) 211–222. |
| 1995 | First semitelechelic HPMA | S. Kamei, J. Kopeček, Prolonged blood circulation in rats of nanospheres surface-modified with semitelechelic [N-(2-hydroxypropyl)methacrylamide]. Pharmaceutical Res. 12 (1995) 663–668. |
| 1999 | First clinical trials | P.A. Vasey, S.B. Kaye, R. Morrison, C. Twelves, P. Wilson, R. Duncan, A.H. Thomson, L.S. Murray, T.E. Hilditch, T. Murray, S. Burtles, D. Fraier, E. Frigerio, J. Cassidy, and on behalf of the Cancer Research Campaign Phase I/II Committee, Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents–drug–polymer conjugates. Clin. Cancer Res. (1999) 83–94. |
| 1999 | First ATRP polymerization | M. Teodorescu, K. Matyjaszewski, Atom transfer radical polymerization of (meth)acrylamides. Macromolecules (1999) 4826–4831. |
| 1999 | First self-assembly into hybrid hydrogels | C. Wang, R.J. Stewart, J. Kopeček, Hybrid hydrogels assembled from synthetic polymers and coiled-coil protein domains. Nature 397 (1999) 417–420. |
| 2005 | First RAFT polymerization | C.W. Scales, Y.A. Vasilieva, A.J. Convertine, A.B. Lowe, C.L. McCormick, Direct, controlled synthesis of the nonimmunogenic, hydrophilic polymer, poly(N-(2-hydroxypropyl)methacrylamide) via RAFT in aqueous media. Biomacromolecules 6 (2005) 1846–1850. |
| 2009 | First self-assembly at cell surface | K. Wu, J. Liu, R.N. Johnson, J. Yang, J. Kopeček, Drug-free macromolecular therapeutics: induction of apoptosis coiled-coil mediated crosslinking of antigens at cell surface. Submitted |
2.3. Development of oligopeptide spacers
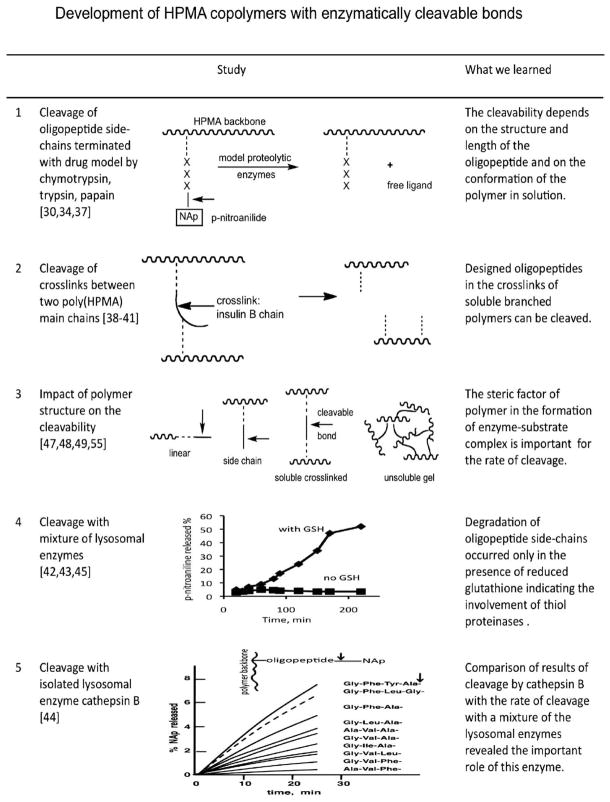
Macromolecules are internalized by cells via endocytosis and ultimately localize in the (enzyme rich) lysosomal compartment. Consequently, we developed HPMA copolymers containing enzymatically degradable bonds (Fig. 3) [34]. Oligopeptide side-chains were designed as drug attachment/release sites [35] and shown to be degradable in vivo [36].
Fig. 3.
HPMA copolymers containing enzymatically cleavable bonds [30,34,37–45,47–49,55].
The relationship between the structure of oligopeptide sequences and the rate of enzymatically catalyzed release of a drug or drug model was studied thoroughly. First, model enzymes, chymotrypsin [30,37–39], trypsin [40], and papain [41] were evaluated, followed by intracellular (lysosomal) enzymes. A study with a mixture of lysosomal enzymes revealed that cysteine (thiol) proteinases were responsible for the cleavage of these polymer–drug conjugates [42,43]. Subsequently, polymers were designed to match the specificity of individual cysteine proteinases: cathepsins B [44], L, H, and artificial mixtures of lysosomal enzymes [45]. The stability of the oligopeptide side-chains in blood plasma and serum was verified [46]. Based on these results it was possible to control the degradability of HPMA copolymers by a particular enzyme. Early in vivo validation of the design was achieved – the drug model (p-nitroaniline) was released in in vivo following intravenous (i.v.) administration in Wistar rats [36].
Later, oligopeptide spacers were combined with self-immolative groups. For bone-specific delivery of prostaglandins a cathepsin K sensitive spacer combined with a 1,6-elimination unit was designed [50]; for oral delivery of 9-aminocamptothecin a combination of a reducible aromatic azo bond with a 1,6-elimination unit was employed [51].
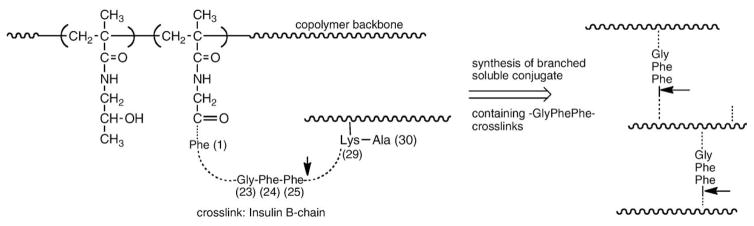
The first degradable polymer carriers based on HPMA were also reported at the Polymers in Medicine Microsymposium in the Prague in 1977 [52] and at conferences in Varna [53] and Tashkent [54]. We used the oxidized insulin B chain (it contains two amino groups at positions 1 and 29) to prepare branched, water-soluble HPMA copolymers by reacting insulin B-chain with HPMA copolymers containing side-chains terminated in p-nitrophenyl esters. The polymers were cleavable (Fig. 4), so we chose the sequence 23–25 (Gly–Phe–Phe) from the insulin B-chain (the bond originating at amino acid 25 is cleavable by chymotrypsin) and synthesized branched, soluble high molecular weight enzymatically degradable copolymers containing the Gly–Phe–Phe segments in crosslinks connecting primary chains [38]. The latter type of polymer carrier was evaluated in vivo in rats and it was shown that the branched polymer carrier is degradable and its molecular weight distribution decreases with time following i.v. administration [36]. These experiments demonstrated the possibility to manipulate the intravascular half-life of polymeric carriers based on HPMA.
Fig. 4.
Branched HPMA copolymers containing the GFF degradable sequence in crosslinks; this sequence mimics the amino acid residues 23–25 of the insulin B chain [38,52].
2.4. Validation of the targetability of HPMA copolymer–drug conjugates
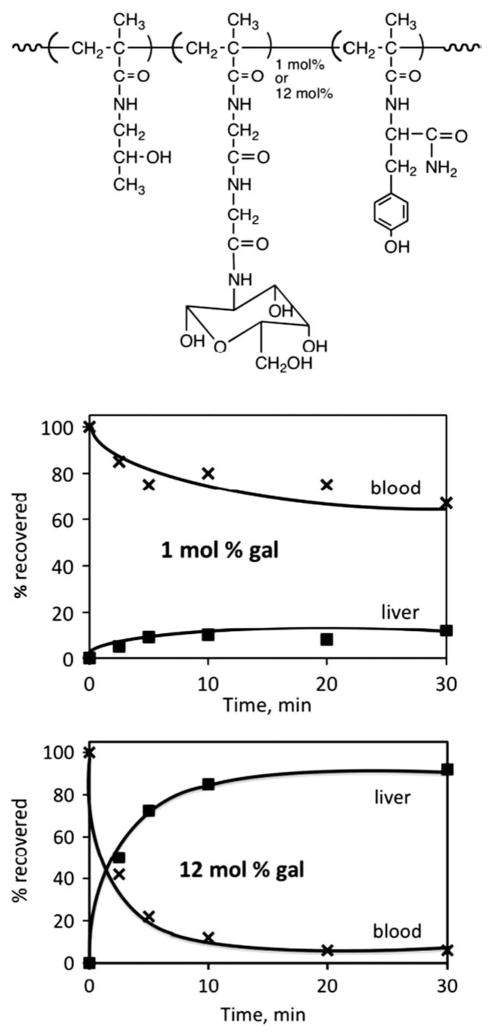
The choice and design of a targeting system has to be based on a sound biological rationale. The design of the first targetable HPMA copolymer was based on the observation [56] that small changes in the structure of glycoproteins lead to dramatic changes in the fate of the modified glycoprotein in the organism. When a glycoprotein (ceruloplasmin) was administered into rats, a long intravascular half-life was observed. However, when the terminal sialic acid was removed from ceruloplasmin, the asialoglycoprotein (asialoceruloplasmin) formed contains side-chains exposing the penultimate galactose units. The intravascular half-life of the latter was dramatically shortened due to the biorecognition of the molecule by the asialoglycoprotein receptor on the hepatocytes. This receptor recognizes galactose and N-acetylgalactosamine moieties [56]. To determine if one can mimic this process with a synthetic macromolecule, we synthesized HPMA copolymers with N-methacryloylglycylglycine p-nitrophenyl ester and attached galactosamine by aminolysis [57]. These copolymers behaved similarly to the glycoproteins and were biorecognizable in vivo (Fig. 5). Their clearance from the bloodstream was related to the N-acylated galactosamine content (1–11 mol%) of the HPMA copolymer [57–59]. Separation of the rat liver into hepatocytes and non-parenchymal cells indicated that the polymer is largely associated with hepatocytes, and density-gradient subcellular fractionation of the liver confirmed that the HPMA copolymers were internalized by liver cells and transported, with time, into the secondary lysosomes [59,60]. It was very important to find that HPMA copolymers containing side-chains terminated in galactosamine and anticancer drug adriamycin also preferentially accumulated in the liver, i.e., it appeared that non-specific hydrophobic interactions with cell membranes did not interfere with the biorecognition by hepatocytes [61].
Fig. 5.
Validations of the targetability of HPMA copolymers. N-acylated galactosamine as the targeting moiety was chosen to mimic the glycoprotein–asialoglycoprotein system [57–59].
In parallel, efforts on the targetability of HPMA copolymer–antibody conjugates started. First HPMA copolymer conjugates with polyclonal and monoclonal anti-Thy-1.2 antibodies and anti-FITC (fluorescein isothiocyanate) antibodies were evaluated. Targetable conjugates containing daunomycin were synthesized and in vitro experiments have shown two orders of magnitude enhanced cytotoxicity of the targeted conjugate (when compared to the nontargeted one) [62]. The targetability and activity of anti-Thy1.2 conjugates with HPMA copolymer–daunomycin conjugates was proven in vivo on a mouse model [63]. Anti-Thy1.2 antibodies were also efficient in targeting HPMA copolymer–photosensitizer (chlorin e6) conjugates [64].
2.5. Early interdisciplinary collaborations
At the beginning of the eighties, we started collaborations with coworkers from the biological field: John Lloyd and Ruth Duncan from the University of Keele in United Kingdom, and Blanka Říhová from the Institute of Microbiology in Prague. The collaboration with the Keele group was initiated by Helmuth Ringsdorf who gave a lecture at the 1977 Prague symposium (where Kopecek presented first HPMA copolymer–drug conjugates and biodegradable carriers based on HPMA). After the meeting Ringsdorf suggested to Lloyd to contact Kopecek because he thought that the collaboration would be beneficial for both. Kopecek met Lloyd in Dresden in July 1978 and they agreed on the evaluation of HPMA copolymer conjugates. First samples were synthesized (different side-chains terminated in p-nitroanilide as drug model) and evaluated at Keele for their cleavability by lysosomal enzymes [42,65] and their stability in blood plasma and serum [46]. More than 300 different polymer structures containing oligopeptide sequences were synthesized in the Prague laboratory [24,25,35,47], and biological properties of a number of them evaluated at Keele within a 10 year period [66,67]. The collaboration with Vladimír Kostka and coworkers from the Institute of Organic Chemistry and Biochemistry in Prague on the cleavability of peptide sequences in HPMA copolymers by cathepsin B [44, Fig. 4], the most important lysosomal cysteine proteinase, resulted in the identification of GFLG sequence, which is incorporated in all conjugates used in clinical trials. From the two fastest cleaving oligopeptides, GFLG and GFTA (see Fig. 3, example 5), we have chosen the GFLG sequence over the GFTA to avoid T; at that time we were worried about the potential immunogenicity.
In 1978 Kopecek gave a lecture at the Institute of Microbiology in Prague. After the lecture he discussed with Říhová and the collaboration with her group on the immunogenicity/biocompatibility [69–72] and biorecognition (targeting) [62–64] of HPMA conjugates commenced.
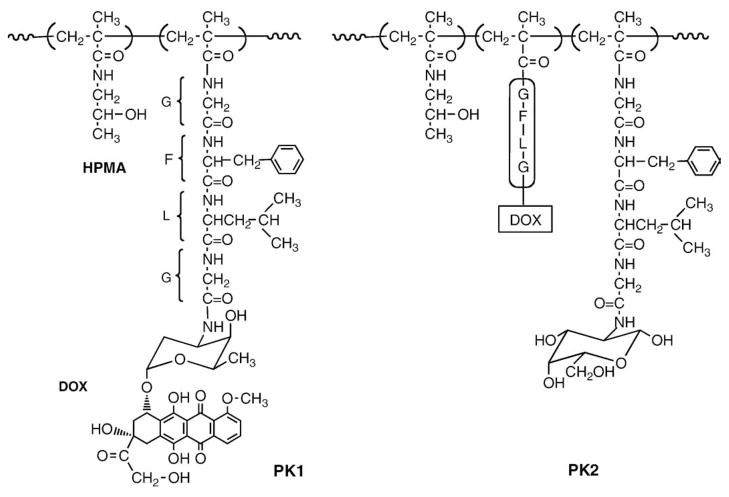
These collaborations resulted in the filing of “Polymeric drugs” patent application in 1985 [68]. Kopecek coined the name for the HPMA copolymers evaluated in clinical trials as PK1 and PK2(P for Prague, K for Keele) (Fig. 6).
Fig. 6.
Structures of PK1 and PK2, first HPMA copolymers evaluated in clinical trials [68]. Conjugate PK1 contains doxorubicin bound to HPMA copolymer via a tetrapeptide sequence stable in the blood stream but susceptible to enzymatically catalyzed hydrolysis in the lysosomes. Conjugate PK2 contains in addition side-chains terminated in N-acylated galactosamine complementary to the asialoglycoprotein receptor on hepatocytes.
3. HPMA copolymer–drug conjugates
The early experiments provided the foundation for the development of HPMA copolymers as drug carriers. As in the majority of new scientific areas, the research initially focused on the accumulation of basic data on the structure–properties relationship. The summary of research in areas we consider important for the development of clinically relevant HPMA copolymer conjugates follows:
Rationale
HPMA copolymer–drug conjugates are nanosized (5–20 nm) water-soluble constructs. Their unique structural, physicochemical, and biological properties are advantageous when compared to low molecular weight drugs. The concept of targeted polymer–drug conjugates was developed to address the lack of specificity of low molecular weight drugs for cancer cells. This approach was based on the work of DeDuve, who realized that the endocytic pathway is suitable for lysosomotropic drug delivery [2]. The features needed to design an effective conjugate [10,21,35,73–75] comprise a polymer–drug linker that is stable during transport [46] and able to release the drug in the lysosomal compartment of the target cell at a predetermined rate [34,47,75], adequate physicochemical properties of the conjugate (solubility, conformation in the biological environment) [48,76–78], and the capability to target the diseased cell or tissue by an active (receptor–ligand) [75] or a passive (pathophysiological) mechanism [79]. Since the activity of many drugs depends on their subcellular location, a mechanism for the manipulation of the subcellular drug location would be beneficial.
The advantages of polymer-bound drugs (when compared to low molecular weight drugs) are (reviewed in [10,47,67,73–75,80,81]): a) active uptake by fluid-phase pinocytosis (non-targeted polymer-bound drug) or receptor-mediated endocytosis (targeted polymer-bound drug), b) increased active accumulation of the drug at the tumor site by targeting [82–84], c) increased passive accumulation of the drug at the tumor site by the enhanced permeability and retention (EPR) effect [85], d) long-lasting circulation in the bloodstream [86], e) decreased non-specific toxicity of the conjugated drug [82], f) decreased immunogenicity of the targeting moiety [82], f) immunoprotecting and immunomobilizing activities [87], and g) modulation of the cell signaling and apoptotic pathways [88–94].
3.1. Enhanced permeability and retention effect
The enhanced permeability and retention (EPR) effect is the predominant mechanism by which soluble macromolecular anticancer drugs exhibit their therapeutic effect on solid tumors. The phenomenon is attributed to high vascular density of the tumor, increased permeability of tumor vessels, defective tumor vasculature, and malfunctioning or suppressed lymphatic drainage in the tumor interstitium [79]. Other factors, however, may have an opposite effect. For example, a high interstitial pressure may result in a convective fluid flow from the center of the tumor to the periphery, which might carry macromolecules [95]. Nevertheless, a number of studies showed increased accumulation of macromolecules in tumors as compared to that in normal tissue [85,96]. The degree of accumulation was dependent on molecular weight [85,86], charge [97,98], and their overall hydrophobic –hydrophilic character. The tumor type and microenvironment may influence its transport characteristics (pore cutoff size) [99].
The efficiency of extravasation into solid tumors depends on the concentration gradient between the vasculature and tumor tissue and time. Consequently, high molecular weight (long-circulating) polymer conjugates accumulate efficiently in tumor tissue [85] due to the EPR effect [79,100]. However, if they possess a non-degradable backbone, they may deposit and accumulate in various organs [18]. We have previously synthesized high molecular weight carriers by connecting HPMA chains via lysosomally degradable oligopeptide sequences [34] to form water-soluble branched conjugates [36,38–41,101–103]. Following intravenous (i.v.) administration to rats, the oligopeptide crosslinks were cleaved and the resulting lower molecular weight polymer chains were excreted into the urine [36]. These water-soluble copolymers were synthesized by crosslinking (short of gel point) of HPMA copolymer precursors (containing oligopeptide side-chains terminated in a reactive ester group) with diamines.
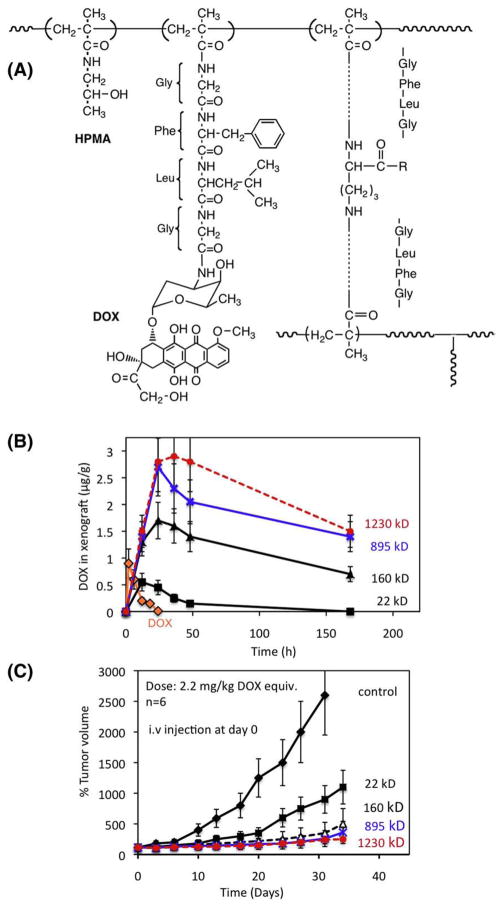
Later, we designed a new, reproducible synthetic pathway for long-circulating HPMA copolymers [85,104]. New crosslinking agents were synthesized and high molecular weight copolymers prepared by crosslinking copolymerization. The composition of the monomer mixture, however, has to be such that at the end of the polymerization the system is short of the gel point (water-soluble). This method [104] is also suitable for the synthesis of HPMA copolymers, which contain, in addition to oligopeptide crosslinks, oligopeptide side-chains terminated in doxorubicin (DOX) (or other anticancer drugs).
The influence of the molecular weight of such conjugates on their biological activity was evaluated [85]. Copolymerization of HPMA, a polymerizable derivative of DOX (N-methacryloylglycylphenylalanylleucylglycyl doxorubicin) and a crosslinking agent, N2,N5-bis(N-methacryloylglycylphenylalanylleucylglycyl) ornithine resulted in high molecular weight, branched, water-soluble HPMA copolymers containing lysosomally degradable oligopeptide sequences in the crosslinks as well as in side-chains terminated in DOX. Four conjugates with Mw of 22, 160, 895, 1230 kDa were prepared. Biodistribution of the conjugates and their treatment efficacy in nu/nu mice bearing s.c. human ovarian OVCAR-3 carcinoma xenografts were determined (Fig. 7). The half-life of conjugates in the blood was up to 5 times longer and the elimination rate from the tumor was up to 25 times slower as the Mw of conjugates increased from 22 to 1230 kDa. The treatment with HPMA copolymer-bound DOX possessing an Mw higher than 160 kDa inhibited the tumor growth more efficiently than that of 22 kDa or free DOX(p<0.02). The data clearly indicated that the higher the molecular weight of the conjugate the higher the treatment efficacy of human ovarian xenografts in nu/nu mice [85].
Fig. 7.
Long-circulating HPMA copolymer–DOX (P-DOX) conjugates of different molecular weight (Mw). (A) Chemical structure of HPMA copolymer–doxorubicin conjugate containing glycylphenylalanylleucylglycine side-chains and N2,N5-bis(N-methacryloylglycylphenylalanylleucylglycyl)ornithine crosslinker [104]; (B) concentration of DOX in OVCAR-3 carcinoma xenografts in nu/nu mice after i.v. bolus of free DOX or P-DOX of different Mw; (C) growth inhibition of s.c. human ovarian OVCAR-3 carcinoma xenografts in nu/nu mice by long-circulating P-DOX conjugates. The mice received i.v. injection of 2.2 mg/kg DOX equivalent dose as P-DOX of different Mw [85].
3.2. Multidrug resistance
The acquired resistance of malignant tumors to chemotherapeutic agents remains the major cause of cancer therapy failure [105]. A membrane glycoprotein, termed P-glycoprotein, has been shown to be responsible for cross-resistance to a broad range of structurally and functionally distinct cytotoxic agents. This glycoprotein, encoded in humans by the mdr1 gene, functions as an energy-dependent efflux pump and removes cytotoxic agents from the resistant cells. The elucidation of the function of P-glycoprotein [106], other ATP-driven efflux pumps [107], as well as other mechanisms of multidrug resistance [108] has had a major impact on the understanding of multidrug resistance in human tumors. The exclusion of the polymer–drug conjugate from the cytoplasmof the cell, through intracellular trafficking in membrane-limited organelles, renders the efflux pumps ineffective. Furthermore, subcellular trafficking along the endocytic pathway from the plasmamembrane to the perinuclear region changes the gradient of distribution of drugs inside cells [109,110]. The concentration gradient of free drugs is directed from the plasma membrane to the perinuclear region, in contrast to polymer-bound drugs, which have a gradient in exactly the opposite direction. The drug, released from the polymeric carrier in the lysosomal compartment, enters the cytoplasm in the perinuclear region. Consequently, the probability of its interaction with nuclear DNA and/or topoisomerase II is higher than the probability of its recognition by the P-glycoprotein efflux pump [109].
We hypothesized that HPMA copolymer-bound DOX [P(GFLG)- DOX] (P is the HPMA copolymer backbone) would behave differently than free DOX during long term incubation with cancer cells. To verify the hypothesis, we have studied the effect of free DOX and P(GFLG)- DOX on the induction of multidrug resistance and changes in metabolism in human ovarian carcinoma A2780 cells during repeated cyclic (chronic) exposure [111]. Such experiments are of therapeutic relevance. The development of multidrug resistance during adaptation of sensitive human ovarian carcinoma A2780 cells to free DOX and P(GFLG)-DOX was analyzed. Adaptation of sensitive A2780 cells to repeated action of free DOX augmented cellular resistance to DOX and finally led to the over-expression of the MDR1 gene. On the other hand, P(GFLG)-DOX induced neither the multidrug resistance with or without MDR1 gene expression, nor the adaptation of the sensitive A2780 cells to free DOX [111].
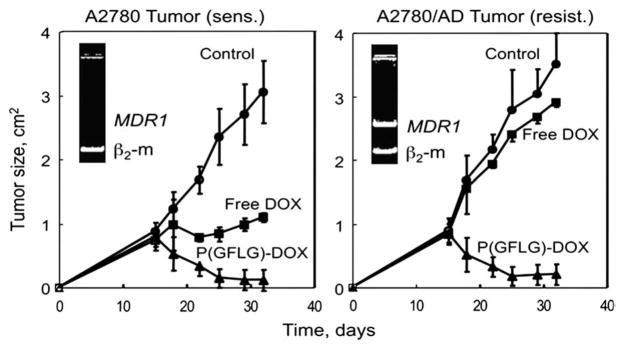
The in vivo comparison of the efficiency of DOX and P(GFLG)-DOX in solid tumor mice models of DOX sensitive (A2780) and resistant (A2780/AD) human ovarian carcinoma [89] demonstrated the advantages of polymer conjugation. Free DOX was effective only in sensitive tumors decreasing the tumor size about three times, while P(GFLG)-DOX decreased the tumor size 28 and 18 times in the sensitive and resistant tumors, respectively (Fig. 8). An enhanced accumulation of P(GFLG)- DOX in the tumor was observed, whereas only low concentrations of DOX were detected in other organs (brain, liver, kidney, lung, spleen, and heart) following P(GFLG)-DOX administration [89].
Fig. 8.
Effect of free DOX (squares) and HPMA copolymer-bound DOX (triangles) on the growth of sensitive A2780 and multidrug resistant A2780/AD human ovarian carcinoma xenografts in female nu/nu mice. Mice were treated i.p. 6 times over 3 weeks (1st and 4th day of each week) with the maximum tolerated dose of free DOX (5 mg/kg) and P(GFLG)- DOX (25 mg/kg). Circles – control tumor. Means±SE are shown [89].
3.3. Combination therapy with polymer-bound drugs
Photodynamic therapy is a newer paradigm in anticancer therapy that involves activation of specific compounds called photosensitizers with specific wavelengths of light to induce cell death. Illumination of these compounds results in the generation of free radicals and singlet oxygen, which cause cell damage and ultimately cell death. A combination of chemotherapy and photodynamic therapy may result in a synergistic response, resulting in a better cure rate than monotherapy. On two cancer models, Neuro 2A neuroblastoma [112] and human ovarian carcinoma heterotransplanted in nude mice [113], we have shown that combination therapy with HPMA copolymer–anticancer drug [DOX and meso chlorin e6 mono(N-2-aminoethylamide) {Mce6}] conjugates showed tumor cures that could not be obtained with either chemotherapy or photodynamic therapy alone. Cooperativity of the action of both drugs contributed to the observed effect [114]. Based on biodistribution data [115], we hypothesized that combination therapy of s.c. human ovarian carcinoma OVCAR-3 xenografts in nude mice using multiple doses/irradiation of P(GFLG)–Mce6 and P(GFLG)–DOX may acquire low effective doses without sacrificing the therapeutic efficacy. Indeed, 10 out of 12 tumors exhibited complete responses in the group of mice receiving multiple photodynamic therapy (PDT) plus multiple chemotherapy [116].
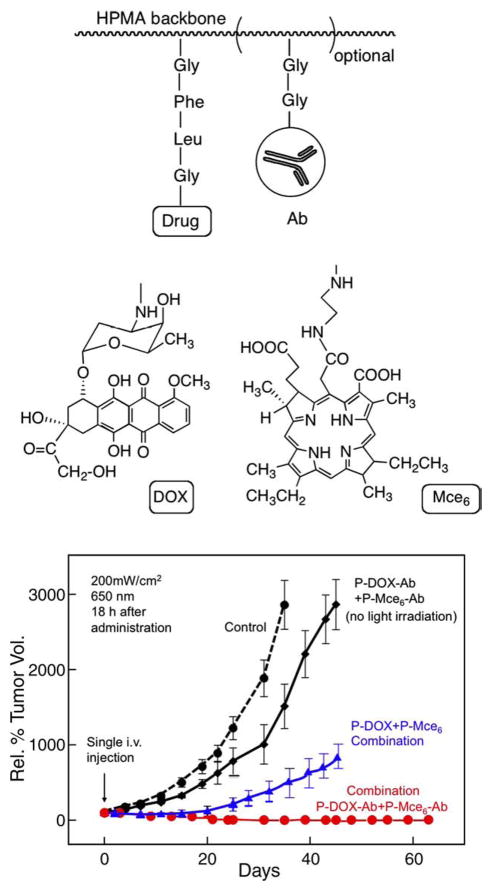
Finally, we have demonstrated the advantages of targeted combination chemotherapy and photodynamic therapy using OV-TL16- targeted HPMA copolymer–DOX and HPMA copolymer–mesochlorin e6 conjugates. OV-TL16 antibodies are complementary to the OA-3 antigen (CD47) present on the majority of ovarian cancers. The immunoconjugates (Fig. 9) preferentially accumulated in human ovarian carcinoma OVCAR-3 xenografts in nude mice with a concomitant increase in therapeutic efficacy when compared with non-targeted conjugates [83]. The targeted conjugates suppressed tumor growth for the entire length of the experiment (>60 days; unpublished data).
Fig. 9.
Efficacy of combination chemotherapy and photodynamic therapy of OVCAR-3 xenografts in nude mice with non-targeted and OV-TL16 antibody-targeted HPMA copolymer conjugates. Therapeutic efficacy of combination therapy of HPMA copolymer-bound Mce6 (P(GFLG)–Mce6) and DOX (P(GFLG)–DOX) targeted with OV-TL 16 antibodies toward OVCAR-3 xenografts was compared to non-treated xenografts and non-targeted combination chemotherapy and photodynamic therapy. Equivalent doses of targeted combination therapy enhanced the tumor-suppressive effect as compared to non-targeted combination therapy. Dose administered: 2.2 mg/kg DOX equivalent and 1.5 mg/kg Mce6 equivalent. Irradiation for photodynamic therapy: 650 nm, 200 mW/cm2 18 h after administration [83, unpublished].
The combination index (CI) analysis was used to quantify the synergism, antagonism, and additive effects of binary combinations of free and HPMA copolymer-bound anticancer drugs, 2,5-bis(5-hydroxymethyl- 2-thienyl)furan (SOS), DOX, and mesochlorin e6 mono-ethylenediamine (Mce6) in anticancer effect toward human renal carcinoma A498 cells. The combination of SOS+DOX proved to be synergistic over all cell growth inhibition levels. All other combinations exhibited synergism in a wide range of drug effect levels [117]. Similarly, the targeted (using Fab′ of OV-TL16 antibody) and nontargeted targeted HPMA copolymer–drug conjugates, P(GFLG)–Mce6 and P(GFLG)–SOS, were evaluated against human ovarian carcinoma OVCAR-3 cells. The observations that most combinations produced synergistic effects will be important for clinical translation [118].
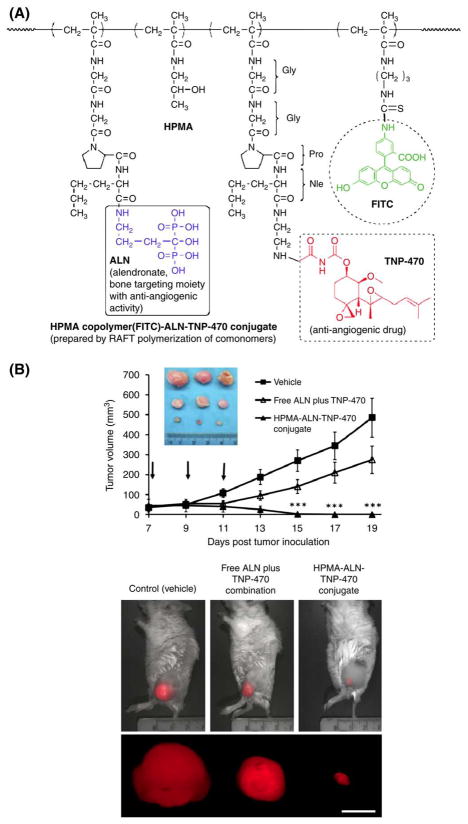
In collaboration with Satchi-Fainaro’s laboratory at the University of Tel Aviv a new therapeutic strategy for bone neoplasms using combined targeted polymer-bound angiogenesis inhibitors was developed [119]. The aminobisphosphonate alendronate (ALN), and the potent anti-angiogenic agent TNP-470 were conjugated with HPMA copolymer. Using reversible addition–fragmentation chain transfer (RAFT) polymerization, we synthesized a HPMA copolymer–ALN-TNP-470 conjugate bearing a cathepsin K-cleavable linker, a protease overexpressed in bone tissues. Free and conjugated ALNTNP- 470 demonstrated their synergistic anti-angiogenic and antitumor activity by inhibiting proliferation, migration and capillary-like tube formation of endothelial and osteosarcoma cells. The bi-specific HPMA copolymer conjugate reduced vascular hyperpermeability and remarkably inhibited human osteosarcoma growth in mice by 96%. These findings indicate that HPMA copolymer–ALN-TNP-470 is the first narrowly dispersed anti-angiogenic conjugate synthesized by RAFT polymerization that targets both the tumor epithelial and endothelial compartments warranting its use on osteosarcomas and bone metastases (Fig. 10) [119].
Fig. 10.
Inhibition of MG-63-Ras human osteosarcoma growth in mice by HPMA copolymer–ALN-TNP470 conjugate. (A) Structure of the conjugate; (B) effects of free (open triangles) or conjugated (closed triangles) ALN and TNP-470 on MG-63-Ras human osteosarcoma tumor growth compared to vehicle-treated group (closed squares) and dissected tumors images. Scale bar represents 10 mm. Data represent mean±S.E. (n=5 mice per group). Adapted from [119].
3.4. Novel targeting strategies
As discussed in 3.1, HPMA copolymer–drug conjugates accumulate passively in solid tumors as a result of the (molecular weight dependent) enhanced permeation and retention (EPR) effect [85]. Active targeting of HPMA copolymer–drug conjugates can be achieved with the incorporation of cancer cell-specific ligands, such as carbohydrates, lectins, antibodies, antibody fragments, and peptides, resulting in enhanced uptake of conjugates by cancer cells through receptor-mediated endocytosis with concomitant improvement of therapeutic efficacy [120,121].
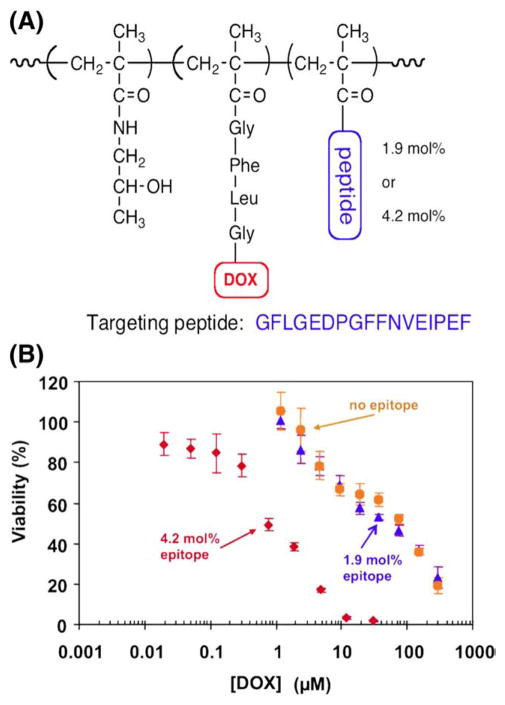
Among different cancer targeting molecules, peptides are of particular interest. Enhanced peptide targeting efficiency can be achieved through multivalent interactions [122] between targets and HPMA copolymer–peptide conjugates containing multiple copies of peptides within a single polymer chain (Fig. 11) [123].
Fig. 11.
Multivalency effect in the biorecognition of HPMA copolymer–peptide–DOX conjugates. Inhibition of Raji B cell growth by exposure to HPMA copolymer–DOX (P (GFLG)–DOX) conjugate containing varying amount of targeting peptide, EDPGFFN-VEIPEF, per macromolecule. (A) Structure of conjugate; (B) inhibition of Raji B cell growth by P(GFLG)–DOX (no targeting peptide), P(GFLG)–DOX containing 1.9 mol% targeting peptide, and P(GFLG)–DOX containing 3.9 mol% targeting peptide. Adapted from [123].
Combinatorial approaches, such as phage display or synthetic peptide libraries, are suitable for the identification of targeting peptides. Overexpression of the CD21 receptor was found on lymphoblastoid cell lines such as Raji cells; consequently, we have used these techniques to identify targeting moieties for lymphomas [124,125]. With phage display, five distinctive peptides (RMWPSSTVNLSAGRR, PNLDFSPTCSFRFGC, GRVPSMFGGHFFFSR, RLAYWCFSGLFLLVC, and PVAAVSFVPYLVKTY) were identified as ligands of CD21 receptor. The dissociation constants of selected peptides were determined to be in the micromolar range [124]. Using a synthetic chemical combinatorial technique, one-bead one-compound (OBOC) method, we identified four heptapeptides (YILIHRN, PTLDPLP, LVLLTRE, and IVFLLVQ) as ligands for the CD21 receptor [125]. The dissociation constants were found to be similar to peptides selected by phage display. Importantly, the peptides retained their biorecognizability towards CD21 receptor after they were conjugated to HPMA copolymers and demonstrated a multivalency effect [125]. Several peptide-targeted HPMA copolymer– drug conjugates displayed anticancer activity [123,126,127]. The combinatorial chemistry approach (OBOC), when combined with a high-stringency screening method, is able to identify peptides with a picomolar affinity [128,129].
3.4.1. Oral, colon-specific delivery of drugs
The development of drug delivery systems capable of selective release of drug in the colon has received much attention. Site-specific delivery to the colon can be achieved by the exploitation of the microbial enzyme activities present predominantly in the colon. The colon has a concentration of microorganisms 5 orders of magnitude greater than the small intestine or stomach. Some of the enzymatic activity produced by microorganisms in the colon, e.g., azoreductase and glycosidase activities do not overlap with the enzymatic activities in the upper GI tract. The azoreductase activities have been studied in detail and used to convert low molecular weight prodrugs into active metabolites in the colon as well as to release active species from water-soluble polymeric carriers [130]. To achieve colon-specific delivery, a (aromatic amino group-containing) drug may be attached to HPMA copolymer side-chains via an aromatic azo bond cleavable by the azoreductase activities present in the colon [51,131–138]. For example, the release of 5-aminosalicylic acid bound to HPMA copolymers via an aromatic azo bond was demonstrated using Streptococcus faecium, an isolated strain of bacteria commonly found in the colon [131], the cecum contents of rats, guinea pigs, and rabbits [133], and in human feces [133].
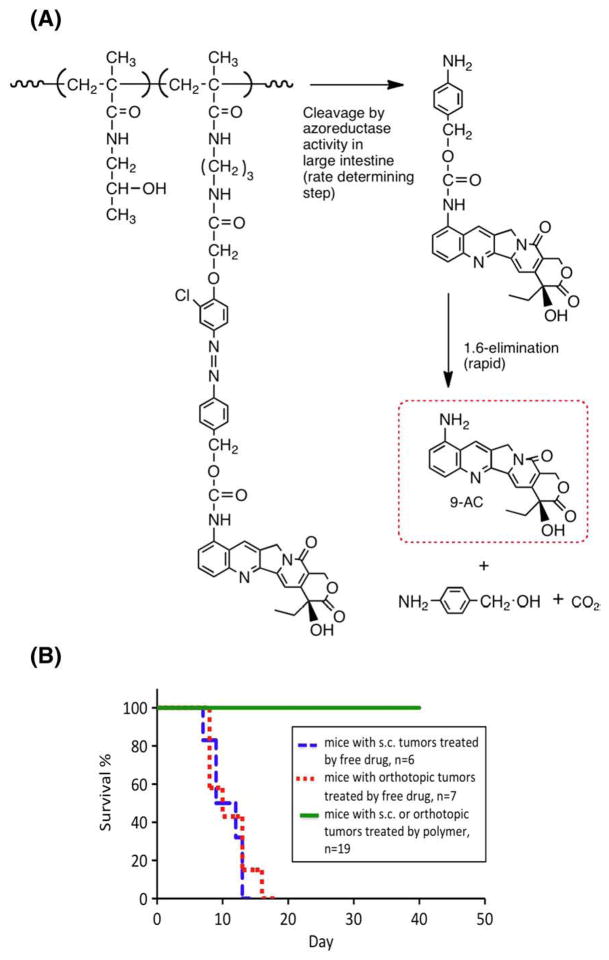
Recently, we concentrated on the oral delivery of 9-aminocamptothecin (9-AC). First, we attached 9-AC to HPMA copolymers through a spacer containing an aromatic azo bond and amino acid residues [134,135]. It was shown that the aromatic azo bond was cleaved first in vitro [134] and in vivo [135], followed by peptidase-catalyzed cleavage of the amino acid (dipeptide) drug derivative resulting in the release of free 9-AC. However, the cleavage of the peptide drug derivative was not fast enough to achieve high concentrations of free 9-AC in the colon. These results indicated that conjugates containing a spacer with a faster 9-AC release rate need to be designed. To this end, a monomer containing 9-AC, an aromatic azo bond and a 1,6- elimination spacer was designed and synthesized [51]. The combination of the colon-specific aromatic azo bond cleavage and 1,6- elimination reaction resulted in a fast and highly efficient release of unmodified 9-AC from the HPMA copolymer conjugate by cecal contents in vitro, with concomitant stability in simulated upper GI tract conditions. The conjugate possessed a favorable pharmacokinetics [136,137] and was effective in colon cancer models (Fig. 12) [138].
Fig. 12.
HPMA copolymer–9-aminocamptothecin conjugate. (A) Structure and scheme of release of unmodified 9-AC from HPMA copolymer–9-AC conjugates by a two-step process – rate controlling aromatic azo bond cleavage, followed by fast 1,6-elimination [51]; (B) survival curves of mice bearing human colon carcinoma xenografts treated by 9-AC and P-9-AC at a dose of 3 mg/kg of 9-AC or 9-AC equivalent [138].
3.4.1.1. Targeting in the gastrointestinal tract
Cell-surface glycoproteins reflect the stage of differentiation and maturity of colon epithelial cells. Diseased tissues, carcinomas and pre-cancerous conditions such as inflammatory bowel disease, have altered glycoprotein expression when compared to healthy ones. Consequently, lectins may be used as targeting moieties for polymer-bound drugs [139–141]. Whereas WGA (wheat germ agglutinin) binds to healthy tissues, PNA (peanut agglutinin) binds to diseased tissues. We hypothesized that HPMA copolymer–lectin–drug conjugates could deliver therapeutic agents to diseased tissues by targeting colonic glycoproteins. We examined biorecognition of free and HPMA copolymer-conjugated WGA and PNA and anti-Thomsen-Friedenreich (TF) antigen antibody binding in normal neonatal, adult and diseased rodent tissues, human specimens of inflammation and Barrett’s esophagus. Neonatal WGA binding was comparable to the adult, with additional luminal columnar cell binding. PNA binding was more prevalent; luminal columnar cell binding existed during the first 2 1/2 weeks of life. WGA binding was strong in both normal and diseased adult tissues; a slight decrease was noted in disease. PNA binding was minimal in normal tissues; increases were seen in disease. Anti-TF antigen antibody studies showed that PNA was not binding to the antigen. The results suggest that HPMA copolymer–lectin–drug conjugates may provide site-specific treatment of conditions like colitis or Barrett’s esophagus [141].
3.5. Subcellular fate and targeting
As discussed above, macromolecular therapeutics are internalized by endocytosis with an ultimate location in the lysosomes. Numerous conjugates were synthesized and evaluated based on this biological rationale. Recently, however, research has been focusing on the identification of different routes of cell entry with the aim to deliver drugs into subcellular compartments different from lysosomes [142– 146]. This direction was mainly driven by attempts to deliver genes or oligonucleotides, i.e., compounds, which may degrade in the lysosomes; however, other rationales may be important: a) the activity of many drugs depends on their subcellular location; and b) the mechanism of action of polymer-bound drugs may be different than that of the free drug. Consequently, manipulation of the subcellular fate of macromolecular therapeutics may result in more effective conjugates.
3.5.1. Mitochondrial targeting
A wide variety of therapeutic agents may benefit by specifically directing them to the mitochondria in tumor cells. To design delivery systems that would enable a combination of tumor and mitochondrial targeting, novel HPMA copolymer-based delivery systems that employ triphenylphosphonium ions as mitochondriotropic agents [147] were developed [142]. Constructs were initially synthesized with fluorescent labels substituting for drug and were used for validation experiments. Microinjection and incubation experiments performed using these fluorescently-labeled constructs confirmed the mitochondrial targeting ability [148]. Subsequently, HPMA copolymer–drug conjugates were synthesized using a photosensitizer mesochlorin e6 (Mce6). Mitochondrial targeting of HPMA copolymer-bound Mce6 enhanced cytotoxicity as compared to non-targeted HPMA copolymer–Mce6 conjugates [142]. Minor modifications may be required to adapt the current design and allow for tumor site-specific mitochondrial targeting of other therapeutic agents.
3.5.2. Hormone-mediated nuclear delivery
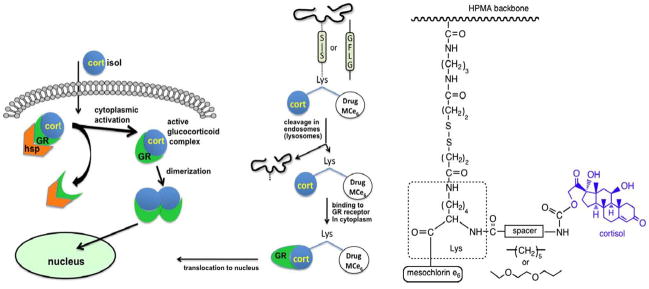
Steroid hormone receptors (SHRs) are known to shuttle between the cytoplasm and nucleus of cells. The rationale for using SHRs as vehicles for transporting drugs from the cytoplasm into the nucleus is based upon the binding of the steroid ligand to receptors such as glucocorticoid receptor (GR); the ligand–receptor complex actively migrates to the nucleus [149]. Analysis of the structure of SHRs [150] indicated that hormone structure might be modified without impairing binding to the receptor. Indeed, Rebuffat et al. used steroid receptors as shuttles to facilitate the uptake of transfected DNA into the nucleus of GR-positive cells [151]. Using a similar strategy, we synthesized a hormone-modified photosensitizer (cortisol-modified mesochlorin e6, Cort-Mce6) capable of binding to GR in the cytoplasm and then localizing to the nucleus.
Novel HPMA copolymer-based delivery systems of this derivative were also synthesized [143]. After internalization of a HPMA copolymer–Cort-Mce6 conjugate (via lysosomally degradable GFLG spacer) by endocytosis, Cort-Mce6 was cleaved, translocated to the cytoplasm, bound to the GR, and translocated to the nucleus [143]. To verify that coupling of cortisol to Mce6 maintains the capacity to form a complex with the cytosolic GR resulting in nuclear localization, we investigated the subcellular fate of the modified drug. Cort-Mce6 was monitored in 1471.1 cells transfected with plasmid that expresses green fluorescent protein labeled glucocorticoid receptor (GFP-GR). Cortisol and Mce6 served as positive and negative controls, respectively. GR translocated to the nucleus after attachment of a glucocorticoid analog (e.g., cortisol). The fluorescent GFP label permits the movement of the GR to be monitored in real time. The data (Fig. 13) clearly indicated the time- and concentration-dependent nuclear localization of cortisol–Lys–Mce6 and cortisol. In contrast, cells incubated with Mce6 did not show any alteration in receptor localization following treatment [143].
Fig. 13.
Design of a polymeric construct for targeting to the nucleus. Nuclear targeting ability of cortisol linked to photosensitizer Mce6 was verified using human ovarian SKOV-3 cells transfected with green fluorescence protein (GFP)-tagged glucocorticoid receptor (GR) [143].
3.5.3. Nuclear entry of macromolecules
Once macromolecules are delivered to the cytosol, a number of methods may be employed to traffic them to specific subcellular compartments, such as the nucleus, in order to enhance their therapeutic efficacy [152]. Nuclear localization of macromolecules has been mediated by targeting peptides [145,146].
However, macromolecules (without subcellular targeting moieties) are typically excluded from entering membrane-limited organelles, with the exception of nucleus whose membrane possesses channels that allow the passive uptake of intermediate-sized macromolecules. The NPC (nuclear pore complex) of the nuclear envelope is composed of about 30 different nucleoporin proteins and is the conduit for both nuclear import and export of macromolecules, such as proteins and nucleic acids. In active transport, cargo as large as 40 nm possessing NLS (nuclear localization sequence) or NES (nuclear export sequence) signaling peptides are guided through the channel after binding to nuclear transport receptor proteins [153]. For smaller macromolecules below 10 nm, however, NPCs have been shown to act as non-specific pores that allow exchange between the nucleus and cytoplasm by diffusion [154]. As a conduit for non-biological macromolecules, the NPCs have been shown to transmit PEG-coated gold colloid particles 4–7 nm in diameter [155].
Recently, using fluorescently-labeled HPMA copolymers, we characterized the basic physicochemical properties that determine the distribution and fate of synthetic macromolecules in living cells [152]. Twelve different classes of water-soluble copolymers were created by incorporating eight different functionalized comonomers. These comonomers possessed functional groups with positive or negative charges, or contained short hydrophobic peptides. The copolymers were fractionated to create parallel “ladders” consisting of 10 fractions of narrow polydispersity with molecular weights ranging from 10 to 200 kDa. The intracellular distributions were characterized for copolymer solutions microinjected into the cytoplasm of cultured ovarian carcinoma cells. Even the highest molecular weight HPMA copolymers were shown to quickly and evenly diffuse throughout the cytoplasm and remain excluded from membrane-bound organelles, regardless of composition (Fig. 14). The exceptions were the strongly cationic copolymers, which demonstrated a pronounced localization to microtubules [152]. For all copolymers, nuclear entry was consistent with passive transport through the nuclear pore complex (NPC). Nuclear uptake was shown to be largely dictated by the molecular weight of the copolymers, however, detailed kinetic analyses showed that nuclear import rates were moderately, but significantly, affected by differences in comonomer composition. HPMA copolymers containing amide-terminated phenylalanine –glycine (FG) sequences, analogous to those found in the NPC channel proteins, demonstrated a potential to regulate import to the nuclear compartment. Kinetic analyses showed that 15 kDa copolymers containing GGFG, but not those containing GGLFG, peptide pendant groups altered the size-exclusion characteristics of NPC-mediated nuclear import [152]. One possible explanation is that the GGFG moieties were able to weakly bind to FG-domain crosslinks in a way that altered the dynamics of a putative Nup hydrogel structure, whereas GGLFG peptides would be expected to bind more strongly and not allow a rapid transfer of crosslinks in the hydrogel-like structure of the nucleopore proteins [156].
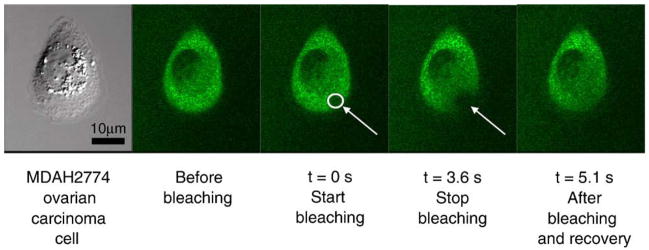
Fig. 14.
Example of time-lapse imaging from FRAP (fluorescence recovery after photobleaching). Here, MDAH2774 ovarian cancer cells were microinjected with HPMA copolymer containing 5 mol% of methacrylic acid (Mw=96 kDa) 2 h before experiment. Area was bleached as indicated and cytosolic copolymer re-diffused and restored equilibrium concentration within 3 s [152].
3.6. Mechanism of action and signaling
The hypothesis that free and polymer-bound drugs activate different signaling pathways is based on their internalization mechanisms. Free drug may initiate signaling pathways by interaction with membrane-bound proteins. In contrast, a polymer-bound drug may be hidden in the hydrophilic corona of the random polymer coil and prevented from interaction with membrane proteins during internalization. It may interact with proteins and DNA after being released from the carrier in the lysosomes and translocated into the cytoplasm [87,91]. We shall demonstrate results supporting this hypothesis using two examples: HPMA copolymer conjugates with geldanamycin and DOX.
3.6.1. HPMA copolymer–geldanamycin conjugates
Geldanamycin (GDM), a benzoquinone ansamycin antibiotic, is a heat shock protein inhibitor. It inhibits the capacity of heat shock proteins such as Hsp-90 to form complexes with client oncoproteins. This results in the ubiqitination of cytoplasmic proteins and subsequent proteosomal degradation [157]. However, the development of GDM as a new anticancer drug has been impaired due to its severe toxicity. Structure–activity studies of a large number of GDM derivatives revealed that modification at the 17-position might improve chemical and physicochemical properties, such as stability in serum and water solubility, while maintaining Hsp90-inhibitory activity. Recently, 17-allylamino-17-demethoxy-geldanamycin was evaluated in a phase I clinical study. Significant hepatotoxicity was observed due to lack of tumor cell selectivity [157]. These limitations may be overcome by the use of a drug delivery system.
We developed a novel method for the substitution of the 17-methoxy group of GDM to introduce a primary amino group that is useful for conjugation with targeting moieties and HPMA copolymer-based drug carriers [158]. HPMA copolymers containing different AR-GDM (AR=3-aminopropyl (AP), 6-aminohexyl (AH), and 3-amino-2-hydroxypropyl (AP(OH)), attached via a lysosomally degradable GFLG spacer, were synthesized and characterized [159]. The cytotoxic efficacy of HPMA copolymer–AR-GDM conjugates depended on the structure of AR-GDM [160].
To verify the hypothesis that P(AP-GDM) [HPMA copolymer–17-(3-aminopropylamino)-17-demethoxy-geldanamycin conjugate] may change the gene expression profiles of low molecular weight GDM derivatives, 32P-macroarray analysis (Clonetech) was employed to evaluate the gene expression profiles in human ovarian carcinoma A2780 cells treated with GDM, AP-GDM and P(AP-GDM) at 2 times 50% cell growth inhibitory concentration (IC50). About 1200 genes related to cancer were evaluated at 6 h and 12 h and three-fold changes in expression were considered significant. Considerable similarities in gene expression profiles were found after AP-GDM and P(AP-GDM) treatments as demonstrated by the hierarchical clustering of the gene expression ratios [91]. However, the outcome was different when individual genes relevant to the mechanism of action of geldanamycin were analyzed. P(AP-GDM)-treated cells showed lower expression of HSP70 and HSP27 compared with AP-GDM up to 12 h. Possibly, internalization pathways and subcellular drug localization of P(AP-GDM), different from low molecular AP-GDM, may modulate the cell stress responses induced by AP-GDM. The results of 32P-macroarray were confirmed by RT-PCR and Western blotting [91]. It is possible that internalization of HPMA copolymer–AP-GDM conjugate via endocytosis may circumvent interactions with external components of the cell, such as plasma membrane, which may be sensitive to stressors and environmental changes (Fig. 15). Similarly, we previously observed that A2780 cells treated with HPMA copolymer–DOX conjugate showed a down-regulation of the HSP70 gene more pronounced than that observed in the cells treated with free DOX [89]. These findings may suggest that conjugation of AP-GDM to HPMA copolymer may be able to modulate the cell stress responses induced by AP-GDM due to differences in its internalization mechanism, subcellular localization, and intracellular concentration gradients [91].
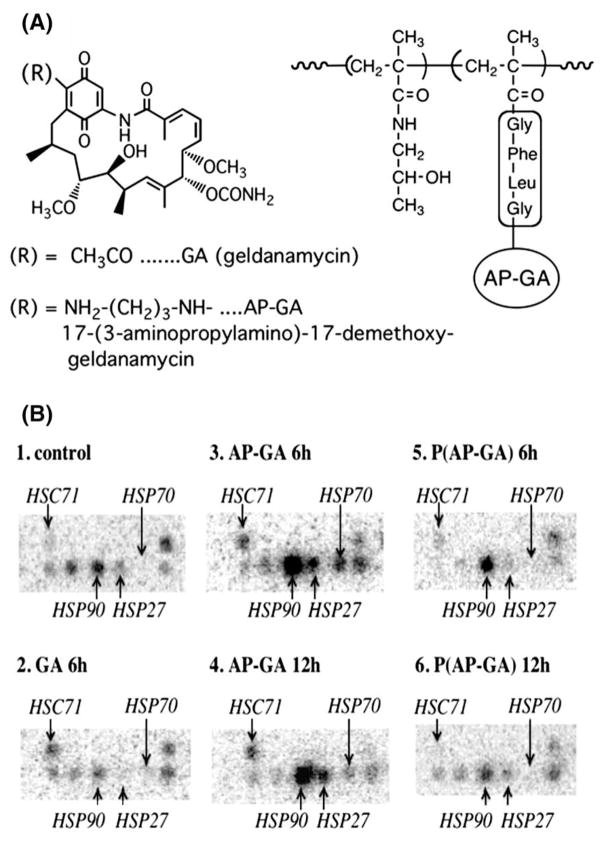
Fig. 15.
(A) Structures of geldanamycin (GA), 17-(3-aminopropylamino)-17-demethoxy-geldanamycin (AP-GA), and P(AP-GA) (AP-GA bound to HPMA copolymer via GFLG spacers); (B) gene expression of cell stress response-related proteins in A2780 ovarian carcinoma cells treated with GA, AP-GA, and P(AP-GA) at 2×IC50 concentration for 6 or 12 h on the Atlas human 1.2 array [91].
3.6.2. HPMA copolymer–DOX conjugates
A detailed evaluation of the mechanism of cell death was performed on A2780 human ovarian carcinoma cells treated with free doxorubicin (DOX) and HPMA copolymer-bound DOX (P(GFLG)–DOX). Using flow cytometry and Western blot analysis it was shown that P(GFLG)–DOX could induce programmed cell death in A2780 cells. The apoptosis was marked by changes in plasma membrane structure, mitochondrial function, caspases activation, and expression level of apoptosis-related proteins [92,93].
For the first time, it was shown that HPMA copolymer–bound DOX induces apoptosis in ovarian cancer cells by simultaneous activation of both caspase-dependent and caspase-independent pathways of DNA damage. This was determined by monitoring the translocation of the mitochondrial proteins cytochrome c and apoptosis-inducing factor to cytosol [93]. The altered balance between anti-apoptotic and pro-apoptotic members of the Bcl-2 family of proteins was responsible for the mitochondrial function distraction. HPMA copolymer-bound doxorubicin induced time-dependent decrease in the expression of the anti-apoptotic Bcl-2 and Bcl-xL proteins, which control cell survival. At the same time, the expression level of pro-apoptotic members (Bax, Bad) of the Bcl-2 family was increased under the chosen experimental conditions. Altogether, these results indicate that HPMA copolymer-bound doxorubicin induced apoptosis in ovarian cancer cells through the mitochondrial pathway [92,93].
Both forms of DOX up-regulated Fas receptor expression on the cell membrane in a time-dependent manner. The peak of the extent of the Fas protein expression on the A2780 cell membrane was observed at 16–24 h with free DOX and at 36–48 h with P(GFLG)–DOX (Fig. 16). The enhanced up-regulation of Fas with P(GFLG)–DOX (when compared with free DOX) may sensitize the cells toward the attack of immunocompetent cells and result in enhanced therapeutic efficacy [161].
Fig. 16.
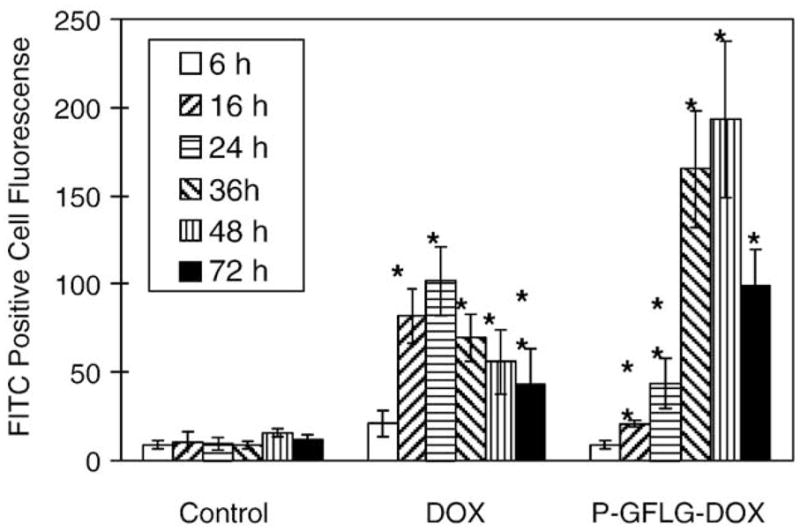
FAS receptor expression on human ovarian A2780 cells after exposure to 2×IC50 concentration of DOX and P(GFLG)–DOX for 24 h. After incubation with drugs, cells sequentially were washed, incubated with monoclonal anti-Fas, washed, and stained with FITC-conjugated goat anti-mouse IgG antibody. Cell fluorescence was analyzed on a FAC Scan flow cytometer. Data are presented as the mean±S.D. Statistical comparisons were made between cells treated with drug and untreated cells at representative time points (*p<0.01; **p<0.05) [92].
Generally, the mechanism of cell death following exposure to polymer–drug conjugates is a complex phenomenon and certainly comprises both apoptosis and necrosis. Numerous pathways might be activated depending on the structure of the polymer–drug conjugate, cell line, concentration of drug, time, conformation of the conjugate (this impacts the possible interaction with the plasma membrane), etc. In addition, secondary necrosis following apoptosis may occur [75,162].
3.7. Cancer: clinical trials
HPMA copolymer-based macromolecular therapeutics have been developed considerably in the last 20 years – numerous conjugates have entered clinical trials for therapeutic validation in the last decade. These include HPMA copolymer–DOX [163–165], HPMA copolymer–DOX–galactosamine [166], HPMA copolymer–camptothecin [167], HPMA copolymer–paclitaxel [168], and HPMA copolymer–platinates [169]. Results from testing of some of these conjugates are promising; hopefully the FDA approval of a first macromolecular therapeutics will occur soon. In Section 4.1 we summarized our ideas on the design principles of second-generation conjugates with enhanced therapeutic potential.
3.8. HPMA copolymer conjugates in the treatment of non-cancerous diseases
HPMA copolymer–drug conjugates may be used also for the treatment of diseases other than cancer. We designed bone-targeted HPMA copolymer-conjugated with a well-established bone anabolic agent (prostaglandin E1; PGE1) for the treatment of osteoporosis and other musculoskeletal diseases [50,170–175]. The biorecognition of the conjugates by the skeleton was mediated by an octapeptide of D-aspartic acid (D-Asp8) or alendronate [170,172].
This system has the potential to deliver the bone anabolic agent, PGE1, specifically to the hard tissues after systemic administration. Once bound to bone, the PGE1 will be preferentially released at the sites of higher turnover rate (greater osteoclasts activity) via cathepsin K (osteoclast specific) catalyzed hydrolysis of a specific peptide spacer and subsequent 1,6-elimination [50,176]. When given in anabolic dosing range, the released PGE1 will activate corresponding EP receptors on bone cells surface to achieve net bone formation. The main features of the design are HPMA copolymer backbone containing cathepsin K-cleavable oligopeptide side-chains (Gly–Gly–Pro–Nle) terminating in either D-Asp8 or in p-aminoben-zyloxycarbonyl- 1-prostaglandin E1, a PGE1 prodrug (Fig. 17A).
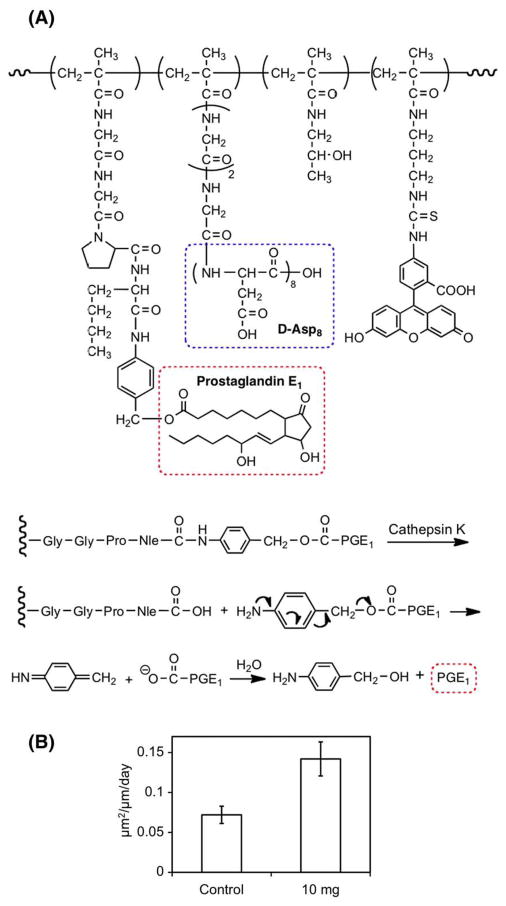
Fig. 17.
Structure of HPMA copolymer–prostaglandin E1-Asp8 conjugate and mechanism of its cleavage by cathepsin K followed by 1,6-elimination (A) [50]; Bone formation measured in cancellous bone from the lumbar vertebral bodies in ovariectomized rats 4 weeks after a single injection of 10 mg of the conjugate (n=8) (B) [174].
This novel delivery system has several distinct advantages. First of all, it is a double-targeted delivery system, which contains a bone-binding moiety (D-Asp8) and a cathepsin K (osteoclast specific enzyme) specific releasing mechanism. By directing PGE1 specifically to the skeleton, the side effects of systemic administration of the drug would be greatly reduced. Secondly, E-series prostaglandins (PGEs) are powerful anabolic agents in bone, and this delivery system will better target these molecules to sites in the skeleton with a high turnover rate, where new bone formation would be more beneficial. Thirdly, the system permits improved control of drug concentration at the target (bone) site after systemic administration [171,173]. Fourthly, the polymeric carrier can be eliminated from hard tissues and, subsequently, cleared from the body via kidney glomerular filtration. It also offers proper protection of the conjugated PGE1 from metabolism before it reaches bone tissue. Most recently, we observed the preferential deposition of the proposed delivery system to the bone resorption sites in ovariectomized rats; this strongly supports our higher turnover sites/drug-release hypothesis [172]. In vivo experiments on ovariectomized rats have proven the concept. Following a single i.v. administration of the HPMA copolymer–Asp8–PGE1 conjugate to aged, ovariectomized rats, bone formation rates were substantially greater than controls when measured 28 days later (Fig. 17B) [173].
HPMA copolymer conjugates have been also successful in the treatment of rheumatoid arthritis [175,177].
3.9. Methods of synthesis of HPMA copolymer conjugates
The topic of conjugate synthesis will be extensively covered throughout this volume. We have demonstrated several approaches through this chapter. Consequently, we shall just mention some of the important points, which were not covered.
3.9.1. Hydrolytically cleavable bond between drug and HPMA copolymer
Due to the decreased pH in the endosomes and lysosomes, pH-sensitive bonds are suitable for intracellular drug delivery. We have synthesized HPMA copolymer–adriamycin (ADR=DOX) conjugates where ADR was bound via cis-aconityl bond [177] (Fig. 18). The determination of the cytotoxicity of P(aconityl)-ADR toward A2780 sensitive and A2780/AD resistant human ovarian carcinoma cells indicated that the polymer conjugate could overcome the P-glycoprotein efflux pump expressed in A2780/AD cells [178].
Fig. 18.
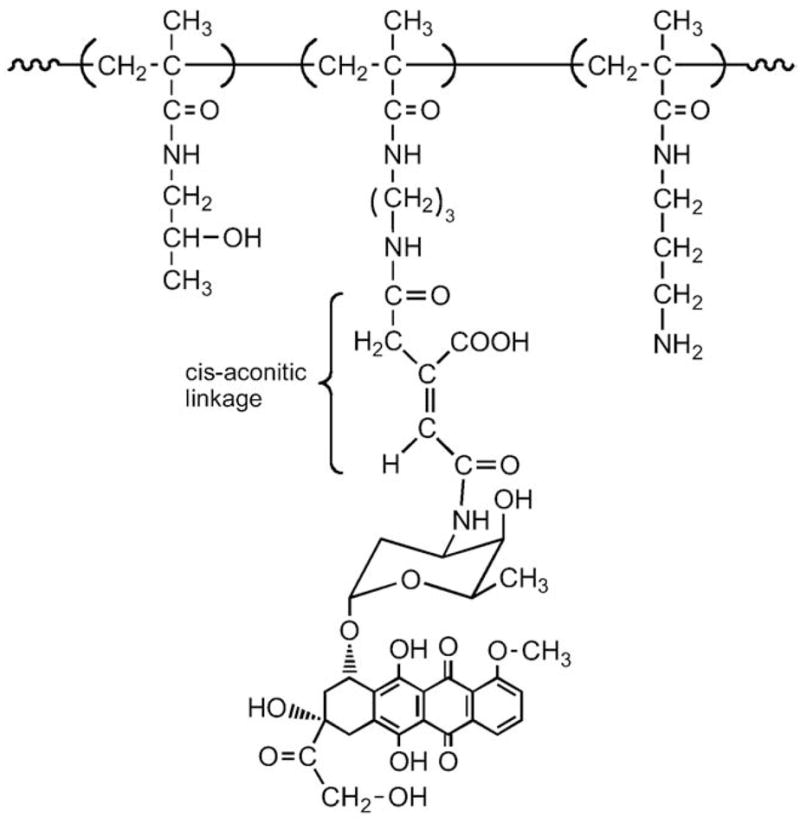
HPMA copolymer–DOX conjugate with hydrolytically cleavable cis-aconitic acid bonds [178].
3.9.2. Disulfide-linked HPMA copolymer–mesochlorin e6 conjugates
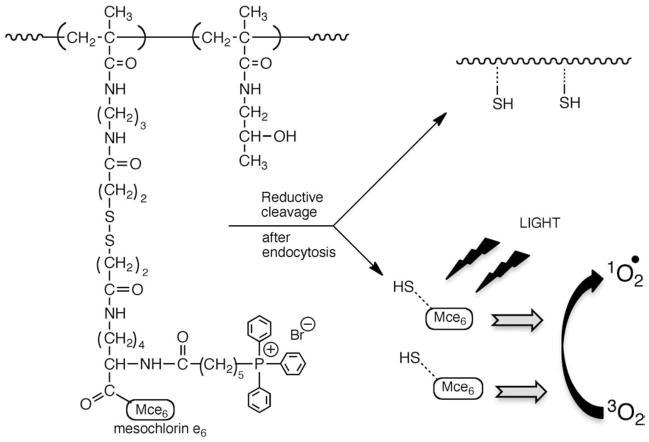
Novel polymeric delivery systems for the photosensitizer mesochlorin e6 (Mce6) were synthesized to overcome problems of systemic toxicity. A disulfide bond was included to allow for quick release ofMce6 from the HPMA copolymer backbone once internalized in tumor tissue (Fig. 19). Synthesized conjugates demonstrated a time-dependent reductive cleavage with an accompanying increase in the quantum yield of singlet oxygen generation on exposure to dithiothreitol. Faster release kinetics and a higher cytotoxicity in SKOV-3 human ovarian carcinoma cells were obtained as compared to polymer conjugate with a proteolytically cleavable glycylphenylalanylleucylglycyl spacer. These novel conjugates hold promise as clinically relevant drug delivery systems for photodynamic therapy of cancer [179].
Fig. 19.
Design and structure of disulfide-linked HPMA copolymer–Mce6 conjugate [179].
3.9.3. Attachment of targeting moieties
The chemistry used for attachment of targeting moieties has an impact on the biorecognition of the conjugate. We compared several methods of antibody attachment (see below), investigated how the attachment of antibodies to HPMA copolymers impacts the mechanism of internalization and subcellular trafficking [180] and designed polymerizable antibody fragments [84].
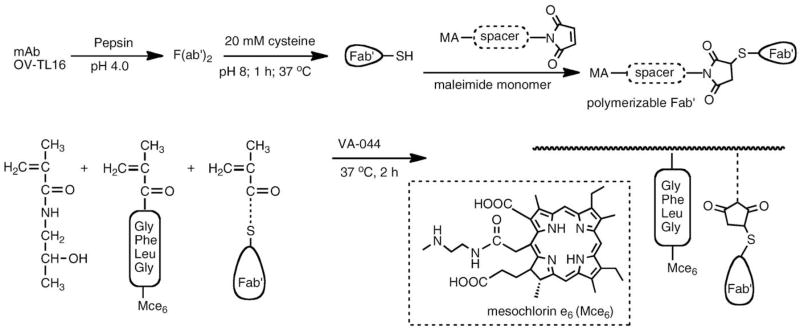
3.9.4. Polymerizable antibody fragments
An innovative pathway for the synthesis of targeted polymeric drug delivery systems using polymerizable antibody fragments was designed [84]. A new macromonomer, a polymerizable antibody Fab′ fragment (MA-Fab′) of the OV-TL 16 antibody (IgG1) has been synthesized and copolymerized with HPMA to produce poly(HPMA-co- MA-Fab′) (Fig. 20). The concept of using polymerizable Fab′ fragments as macromonomers provides a new paradigm for the synthesis of targeted polymeric drug delivery systems, and may have unique applications in other areas, such as immunoassays, biosensor technology and affinity chromatography [84].
Fig. 20.
Synthesis of polymerizable antibody Fab′ fragment and its copolymerization to produce a targeted HPMA copolymer–Mce6 conjugate [84].
3.9.5. Impact of chemistry of attachment of antibodies to HPMA copolymers on the binding affinity of the conjugates
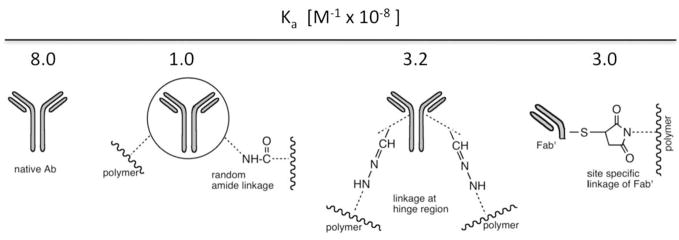
The influence of different methods of coupling the OV-TL16 antibody and its Fab′ fragment to HPMA copolymer–drug (ADR, Mce6) carriers on the binding affinity of the conjugates to the CD47 antigen associated with ovarian carcinoma (OVCAR-3) cells was studied. Three different methods of covalently binding the Ab or Fab′ to polymers were used [181]. Method A: binding via amide bonds formed by aminolysis of active ester groups on the HPMA copolymer–drug (ADR or Mce6) conjugates by amino groups on the antibody; Method B: binding via hydrazone bonds formed by the reaction of aldehyde groups on the oxidized antibody with hydrazo groups on the HPMA copolymer–Mce6 conjugates; Method C: binding via thioether bonds formed by the reaction of sulfhydryl groups of Fab′ fragments with maleimido groups on the side-chain termini of the HPMA copolymer–Mce6 conjugate. Differences in Ka were observed as shown in Fig. 21.
Fig. 21.
The influence of different methods of binding the OV-TL16 antibody and its Fab′ fragment to HPMA copolymer–drug (adriamycin {ADR} or meso chlorin e6 mono(N-2-aminoethylamide) {Mce6}] conjugates on the affinity of conjugates to an ovarian carcinoma OVCAR-3 cell associated antigen. Affinity constants Ka (M−1×10−8) are shown for free antibody; antibody bound via amino groups, antibody bound via oxidized saccharide units in the hinge region; and Fab′ fragment bound via thioether bonds [181].
3.9.6. Living radical polymerization
The synthesis of HPMA copolymer conjugates, especially their molecular weight distribution, can be controlled by methods of living radical polymerization, RAFT (reversible addition–fragmentation chain transfer) [182] and ATRP (atom transfer radical) polymerizations [183]. For example, HPMA copolymer conjugates containing two drugs and one fluorescent label per macromolecule using RAFT copolymerization were recently synthesized [119; Fig. 10].
3.9.7. Attachment of oligonucleotides
Aminolysis of HPMA copolymer precursors can be used for attachment of oligonucleotides to HPMA copolymers. We have attached a 21-mer phosphorothioate oligonucleotide (5′-TTTATAAGGGTCGATGTCCXX-3′) to HPMA copolymers containing GG-ONp and GFLG-ONp (ONp is p-nitrophenoxy) side-chains [184]. The oligonucleotide had a primary amine on the 5′-end and a fluorescein on the 3′-end. The subcellular fate and activity in inhibiting the hepatitis B virus of the HPMA copolymer–phosphorothioate oligonucleotides was studied. Covalently attaching the oligonucleotides to the HPMA copolymers via non-degradable dipeptide GG spacers resulted in sequestering the oligonucleotide in vesicles after internalization. Conjugation of the oligonucleotides to an HPMA copolymer via a lysosomally cleavable tetrapeptide GFLG spacer resulted in release of the oligonucleotide in the lysosome and subsequent translocation into the cytoplasm and nucleus of the cells. The degradable HPMA copolymer–oligonucleotide conjugate possessed antiviral activity indicating that phosphorothioate oligonucleotides released from the carrier in the lysosome were able to escape into the cytoplasm and nucleus and remain active. The Hep G2 cells appeared to actively internalize the phosphorothioate oligonucleotides as oligonucleotide –HPMA copolymer conjugates were internalized to a greater extent than unconjugated polymers [184].
3.9.8. Attachment of cell penetrating peptides (CPP)
These peptides, such as the Tat peptide (48GRKKRRQRRR57) which originates from HIV-1 Tat protein, a potent activator of HIV-1 transcription, have been attached using various chemical designs. One approach is to add several amino acid residues, e.g. to produce 48GRKKRRQRRR57YK(FITC)C. The latter may be attached to HPMA copolymer side-chains terminated in maleimide via thioether bonds [185]. We have recently reviewed the biological implications of CPP attachment [144], so we shall not discuss it here.
4. Modification of proteins and vesicular carriers with HPMA (co)polymers
4.1. Modification with semitelechelic poly(HPMA)
Semitelechelic (ST) polymers are linear macromolecules having a reactive functional group at one end of the polymer chain [186]. The single functional group provides the opportunity to conjugate or graft the macromolecule to other species or surfaces. Davis and coworkers have shown that attachment of ST poly(ethylene glycol) (PEG) to therapeutical proteins results in an increase of their resistance to proteolysis, reduction of their antigenicity, and prolongation of intravascular half-life [187]. The extent of protein property changes depends on the degree of PEG substitution and PEG molecular weight [188]. Modification of biomedical surfaces with PEG reduces their biorecognizability and prevents protein adsorption [186].
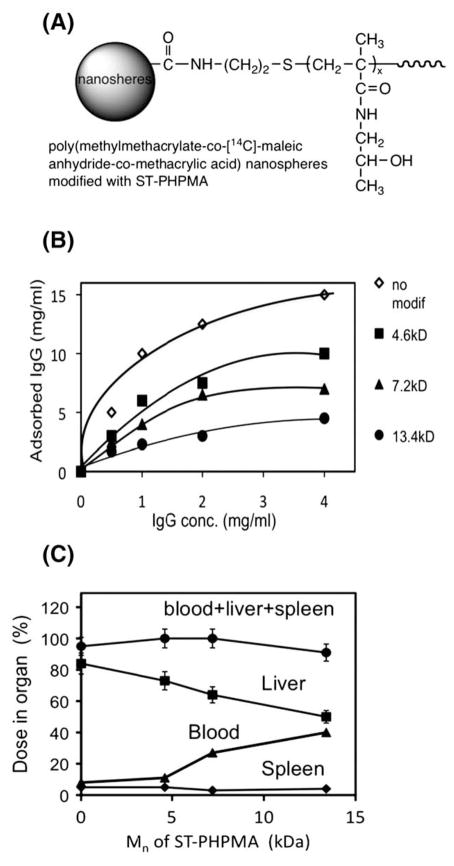
Poly(HPMA) [189] exhibits similar properties as poly(ethylene glycol) [190] when used for modification of enzymes or vesicular carriers. The first report on semitelechelic poly(HPMA) (ST-PHPMA) was published in 1995 [191]. Modification of nanospheres, based on a copolymer of methyl methacrylate, maleic anhydride, and methacrylic acid, with ST-PHPMA resulted in decreased protein adsorption in vitro and increased intravascular half-life, as well as decreased accumulation in the liver, after intravenous administration into rats. The higher the molecular weight of ST-PHPMA, the more pronounced the changes in these properties (Fig. 22). These data seem to indicate the influence of the hydrodynamic thickness of the coating layer on the process of opsonization and capture by Kupffer cells of the liver and macrophages of the spleen [191].
Fig. 22.
First synthesis and application of semitelechelic poly(HPMA) to modify surface of nanospheres. (A) Structure; (B) adsorption isotherms of IgG onto surface-modified P (MMA-MA-MAA) nanospheres in 1/15 M saline at 25 °C for 3 h. Each point represents the mean±SD (n=3); (C) body distribution profiles for unmodified and modified [14C]-P(MMA-MA-MAA) nanospheres in rats 24 h after i.v. administration and the correlation with Mn of ST-PHPMA. Each point represents the mean±SD (n=5) [191].
Similarly, carboxyl and amino group modification of chymotrypsin with ST-PHPMA-CONHNH2 and ST-PHPMA-COOSu (N-hydroxysuccinimide ester) produced conjugates [189] with comparable properties to PEG-modified chymotrypsin [187]. Ulbrich et al. used ST-PHPMA to modify ribonuclease, chymotrypsin [192], and superoxide dismutase [193]. The proteolytic stability of modified protein increased and their immunogenicity decreased [192,193].
4.2. Modification with HPMA copolymers containing multiple reactive side-chains
First protein modified with HPMA copolymers was prepared by the reaction of the copolymer of HPMA with N-methacryloylglycylglycine p-nitrophenyl ester with insulin [28]. The unreacted protein was separated on Sephadex 75; the HPMA copolymer–insulin conjugate exhibited a slower onset and a slight prolongation of hypoglycemic effect in rats when compared to free insulin [28]. Chymotrypsin [29,30] and cobra venom acetylcholinesterase [194] followed.
To obtain a better insight into the steric hindrance of the polymer chains on the formation of enzyme–substrate complex, we have studied the hydrolysis of polymeric substrates (HPMA copolymers with oligopeptide side-chains terminated in p-nitroanilide) catalyzed by HPMA copolymer-bound chymotrypsin. The kinetic analysis showed that the hydrolysis of polymer substrates with polymer-bound chymotrypsin led to a decrease in both kcat and kcat/KM, but the relationship between the individual substrates remained intact. Apparently, the steric effects of two independent polymer chains (one bound to substrate, the other to enzyme) were roughly additive [30].
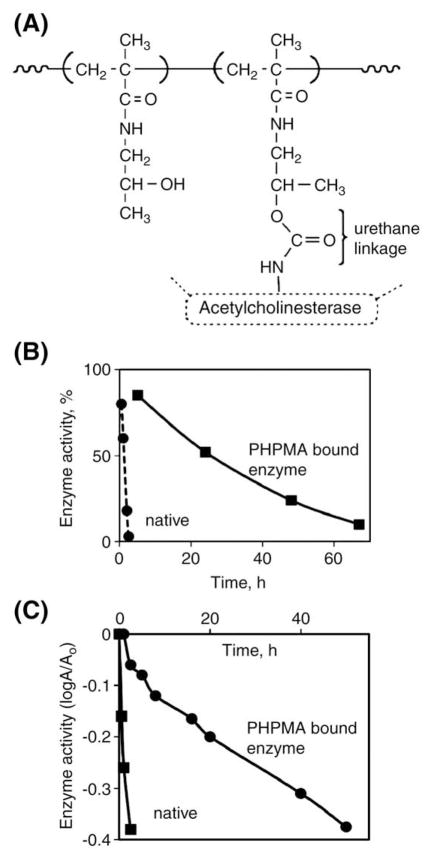
Different chemistry was used for the modification of cobra venom acetylcholinesterase [194]. The secondary OH groups of poly(HPMA) (Mw 25–30 kDa) were activated with 4-nitrophenyl chloroformate in dimethylformamide followed by attachment of acetylcholinesterase in borate buffer. The poly(HPMA)-modified acetylcholinesterase demonstrated a 70-fold prolongation of enzyme activity in blood after intravenous injection into mice when compared to unmodified enzyme. The thermoinactivation rate of the polyHPMA–acetylcholinesterase conjugate was 74 times smaller than that of native enzyme (Fig. 23) [194].
Fig. 23.
HPMA copolymer-modified acetylcholinesterase. (A) Structure; (B) enhancement of intravascular half-life in mice after i.v. administration of HPMA copolymer-modified enzyme; (C) augmentation of thermal stability of HPMA copolymer-modified enzyme [194].
HPMA copolymer with N-methacryloylglycylphenylanylleucylglycine p-nitrophenylester was used for the modification of ribonuclease [195] and superoxide dismutase [193]. No difference in biological activity of conjugates prepared using ST-PHPMA and HPMA copolymers with reactive side-chains was detected [193].
There is considerable activity in using HPMA copolymers with reactive side-chains to stabilize complexes of DNA with viruses. This research is covered in the chapter by Seymour in this volume and in our review [196].
5. HPMA in biomaterials design
5.1. HPMA-based hydrogels
First HPMA-based hydrogels were synthesized by crosslinking copolymerization of HPMA and methylene-bis-acrylamide or ethylene- bis-methacrylamide in the early 70s [197]. The kinetic course of copolymerization, interaction parameter polymer–water, the modulus of elasticity, and concentration of elastically effective chains were characterized.
Degradable hydrogels containing oligopeptide crosslinks susceptible to chymotrypsin-catalyzed hydrolysis were synthesized by crosslinking HPMA copolymers containing reactive side-chains (terminated in p-nitrophenoxy groups) with oligopeptide (GGY, GFY, GLF, AGVY, and AGFY)-containing diamines [49]. The degradability of hydrogels was dependent on the length and detailed structure of the oligopeptide sequence and on the network density, and thus the equilibrium degree of swelling (Fig. 24). The higher the degree of swelling, the faster the rate of degradation. The degree of swelling also has an impact on surface vs. bulk degradation of the hydrogel. If the enzyme cannot diffuse into the hydrogel interior, only surface degradation takes place. This was demonstrated by a comparison of the degradation of HPMA copolymer crosslinked with AGVY-containing sequences catalyzed by chymotrypsin and HPMA copolymer-bound chymotrypsin. Only surface degradation of the hydrogel was observed in the latter case due to the larger size of polymer-modified enzyme [49]. HPMA-based hydrogels containing the sequence GFYAA in the crosslinks were cleavable with cathepsin B, a lysosomal thiol proteinase [44]. In further experiments, HPMA-based hydrogels with degradable crosslinks were shown to release FITC-dextran and daunomycin during incubation with a mixture of lysosomal enzymes (Tritosomes) or chymotrypsin [198].
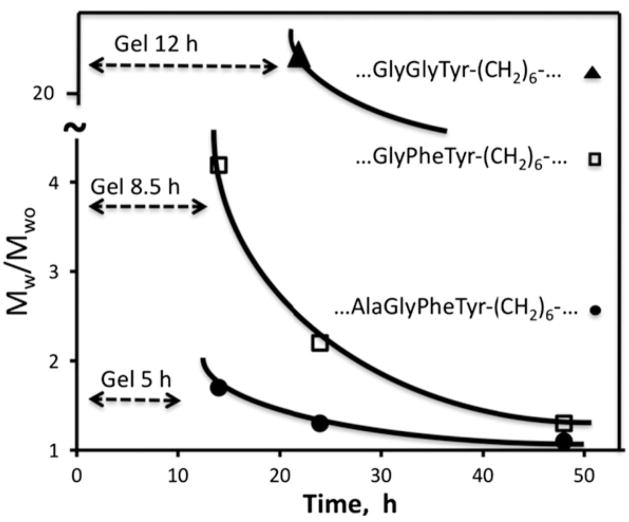
Fig. 24.
HPMA copolymer-based degradable hydrogels. Degradation of hydrogels containing different oligopeptides in crosslinks by chymotrypsin. The dotted line shows the time needed for hydrogel dissolution; the curve shows the change of molecular weight of the soluble fraction with time [49].
Crosslinking copolymerization of HPMA with N,O-dimethacryloyl-hydroxylamine produced hydrolytically degradable hydrogels [199]. These hydrogels were used as a depo for anticancer drug (DOX); results of combination therapy using DOX release from hydrogels followed by antibody-targeted therapy has been effective in the treatment of terminal stages of Bcl1 leukemia in mice [200].
Interpenetrating network (IPN) hydrogels, composed of a pH-sensitive, aromatic azo group-containing network as one component (A), and a hydrolyzable network as the other (B), were designed to refine their swelling profile in the gastrointestinal tract (A is a copolymer of N,N-dimethylacrylamide, acrylic acid, N-tert-butylacrylamide, and N-methacryloylglycylglycine p-nitrophenyl ester crosslinked with an aromatic azo group-containing diamine; B is a copolymer of HPMA with N,O-dimethacryloylhydroxylamine) [201].
Hennink et al. synthesized ABA triblock copolymers, where block A is thermosensitive poly(HPMA lactate) and block B is PEG. Heating of the copolymer results in the formation of a viscoelastic material, which may be stabilized (crosslinked) by photopolymerization [202]. These materials are suitable for protein delivery [203].
5.2. HPMA-based hybrid hydrogels
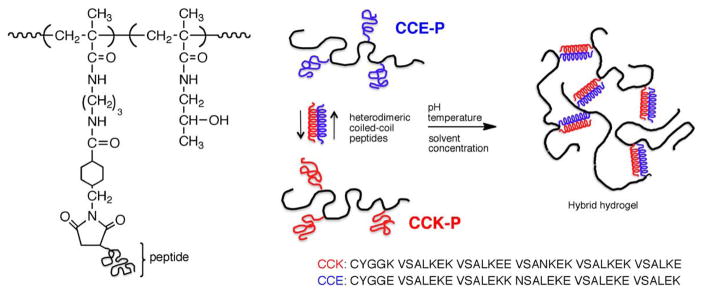
Traditional synthetic methods, such as crosslinking copolymerization, crosslinking of polymeric precursors, and polymer–polymer reactions, have produced numerous hydrogel materials with excellent properties [9,204,205]. However, these approaches do not permit a precise control of chain length, sequence, and 3D structure. On the contrary, self-assembly of (macro)molecules into physical hydrogels has the potential to produce precisely defined, hierarchical 3D structures [206–209]. Self-assembly is highly specific and frequently found in biology both in the peptide/protein and DNA worlds.
Hybrid hydrogels are usually referred to as hydrogel systems that possess components from at least two distinct classes of molecules, for example, synthetic polymers and biological macromolecules, interconnected either covalently or non-covalently [210]. Conjugation of peptide domains and synthetic polymers may lead to novel materials with properties superior to those of individual components [206–218].
5.2.1. Self-assembly of hybrid graft HPMA copolymers into hydrogels mediated by coiled-coil formation
Our research has focused on the factors controlling the self-assembly of graft copolymers composed of a synthetic polymer backbone and a pair of oppositely charged peptide grafts. Two distinct pentaheptad coiled-coil forming peptides (CCE and CCK) were designed to create a dimerization motif and serve as physical crosslinkers [211]. We hypothesized that the antiparallel orientation of heterodimers will contribute to the homogeneity of the self-assembled hybrid hydrogels due to unique interchain dimer formation and decreased steric hindrance of the (synthetic) polymer backbone on the “in-register” alignment of heterodimers. Indeed, equimolar mixtures of the graft copolymers, CCE-P/CCK-P (P is the HPMA copolymer backbone), have been observed to self-assemble into hydrogels in PBS (phosphate buffered saline) solution at neutral pH at concentrations as low as 0.1 wt.% (Fig. 25). Circular dichroism spectroscopy, sedimentation equilibrium experiments, and microrheology data revealed that the self-assembly process corresponded to the two-stranded α-helical coiled-coil formation between CCE and CCK. Moreover, the formation of hybrid hydrogels was reversible. Denaturation of the coiled-coil domains with guanidine hydrochloride (GdnHCl) solutions resulted in disassembly of the hydrogels. Removal of GdnHCl by dialysis caused coiled-coil refolding and hydrogel re-assembly. Similarly, dynamic light scattering data indicated that exposure of the self-assembled hydrogel to a competing peptide (CCK) resulted in a decrease of crosslinking density and partial disassembly of the hydrogel [212]. As expected, concentration of graft copolymers had a significant impact on the kinetics of self-assembly [212] and on the structure and morphology of self-assembled hydrogels [211].
Fig. 25.
Self-assembly of HPMA graft copolymers, CCK-P and CCE-P, into hybrid hydrogels mediated by antiparallel heterodimer coiled-coil formation. Two distinct pentaheptad peptides (CCE and CCK) were designed to create a dimerization motif and serve as physical crosslinkers (P is the HPMA copolymer backbone). Aqueous solutions of CCE-P or CCK-P did not form gels. In contrast, gel-like materials were formed from equimolar mixtures of CCE-P/CCK-P at low concentrations [211].
5.2.2. Self-assembly of hybrid graft HPMA copolymers into hydrogels mediated by β-sheet formation
The self-assembly of hybrid block- [213] and graft- [214] copolymers composed of polyHPMA and β-sheet motifs was also investigated. β-sheet forming peptides were conjugated to polyHPMA via thiol-maleimide coupling reaction. ST-PHPMA-SH was synthesized via RAFT polymerization of HPMA followed by post-polymerization end-group modification by aminolysis. The diblock copolymer was obtained by the polymer-analogous reaction with N-terminal maleimide-modified β-sheet peptide [213]. The graft copolymer was prepared by attachment of N-terminal cysteine modified β-sheet peptide to poly(HPMA) precursor, which contained pendant maleimide groups [214]. Circular dichroism spectra showed that the strong tendency of the peptide to self-assemble into β-sheets was retained in the copolymers. In addition, β-sheet sensitivity to temperature and pH variations decreased due to poly (HPMA) shielding effect. Atomic force microscopy and small-angle X-ray scattering showed that copolymer had the ability to self-assemble into fibrils. Moreover, transmission electron microscopy showed that poly(HPMA-g-CGGBeta11) fibrils formed matrices with minimal lateral aggregation, dramatically different from the highly aggregated peptide fibrils [214]. This observation coincided with the FTIR results, which suggested poly(HPMA) hindered the twisting of the fibrils formed in copolymer through the antiparallel arrangement of the β-strand peptides. Such materials may be useful in a broad range of biomedical applications, from depots for drug delivery, to scaffolds for cell delivery and tissue engineering.
5.2.3. Protein containing HPMA copolymer-based hydrogels
Enzyme-based hybrid hydrogels that translate substrate recognition into mechanical motion were devised [215]. The design employed the substrate–enzyme interactions of adenylate kinase with ATP to induce a conformational change with concomitant decrease of the hydrogel volume.
Escherichia coli adenylate kinase (AKe; EC 2.7.4.3) is a 214 residue, three domain bacterial enzyme (transferase), which catalyzes the phosphoryl transfer reaction Mg2+·ATP+AMP↔Mg2+·ADP+ADP. Upon binding of a substrate (or inhibitor), AKe undergoes a large conformational change. The bulky lid domain closes over the active site to shield it from water, avoid substrate hydrolysis, and facilitate the transfer of phosphate group.
AKe was incorporated into defined hydrogel structures through a thiol-maleimide reaction. HPMA copolymers with pendant maleimide groups were chosen as the backbone. To create two attachment points, a triple mutant of adenylate kinase, AKtm (C77S, A55C, V169C) was designed, engineered and purified [215]. The wild-type enzyme has one cysteine at position 77. The distance between the Cα-atoms of residues 55 and 169 decreases from 29.5 Å in the apo-enzyme to 12.4 Å when forming the enzyme–substrate complex. The SH groups at positions 55 and 169 are easily accessible as shown by reactivity studies involving 26 AK double mutants [219]. The enzymatic activity of AKtm was examined and shown to be similar to AKe.
Hybrid HPMA-based hydrogels were synthesized in a mold by crosslinking the HPMA copolymer with AKtm or AKtm and additional crosslinking agent dithiothreitol (DTT). As we hypothesized, when the hydrogels were exposed to ATP (substrate), a conformational transition from an “open” to “closed” conformation occurred, resulting in hydrogel volume change. The collapse of about 5–17% in the hydrogel volumes was proportional to the AKtm content. Control gel, crosslinked with DTT only, did not collapse [215]. The degree of hydrogel deswelling increased with the concentration of the substrate. Repeated exposure of hydrogel to ATP buffer followed by a washing step in ATP-free buffer demonstrated the reproducibility of hydrogel swelling alterations and of the AKtm conformational changes.
The novelty of our design (Fig. 26) is the combination of biorecognition with a catalyzed chemical reaction–the transfer of a phosphate from ATP to AMP. There are numerous enzymes that undergo conformational changes following the binding of a substrate to the enzyme’s active site. Thus, this is a new paradigm for hybrid hydrogel design, where a variety of chemical reactions can be combined with biorecognition to transform nano-scale conformational changes into macroscopic motion.
Fig. 26.
Hydrogels containing a triple mutant of adenylate kinase, AKtm (C77S, A55C, V169C), as a crosslinker are able to translate the enzyme conformation change upon binding a substrate into mechanical motion. A) Ribbon diagram of the structure of adenylate kinase (AKe) in two conformational states: open state (a); and closed state (b). B) HPMA-based hydrogels crosslinked with AKtm and dithiothreitol (DTT) and the macroscopic motion of hydrogels triggered by substrate recognition. The deswelling ratios of gel containing variable amounts of AKtm as crosslinker (0–100%). C) Three cycles of deswelling of hydrogel crosslinked with 100% of AKtm. Adapted from reference [215].
6. Conclusions and future prospects
The HPMA copolymer research achieved many milestones in the last 30 years. Numerous advantages of HPMA copolymer-bound drugs over free drugs have been identified. The water-soluble HPMA copolymer carriers are biocompatible as shown in clinical trials, HPMA copolymer–drug conjugates are active to numerous cancer models, and the targetability of the conjugates has been demonstrated. However, the translation of laboratory data into the clinics has been slow. To enhance the speed of translation and/or to design new, more efficient second-generation conjugates research in numerous areas is needed:
6.1. Design of biocompatible long-circulating conjugates for passive and active targeting
The extravasation of polymer–drug conjugates into a solid tumor depends on the concentration gradient and time of circulation. The renal threshold limited the molecular weight of the first generation of polymeric carriers to well below 40 kDa; this lowered the retention time of the conjugate in the circulation with concomitant decrease in pharmaceutical efficiency. The design, synthesis, and evaluation of new second-generation anticancer nanomedicines based on high molecular-weight polymeric drug carriers containing degradable bonds in the main chain (polymer backbone) are needed. Recent advances in living radical polymerization [182,183] and in bioconjugate chemistry [220] provide a solid scientific basis for further developments.
6.2. Targetability and multivalency
The targetability of HPMA copolymer conjugates was demonstrated using numerous targeting moieties in vitro, in vivo, and in clinical trials. The synthesis of high molecular weight conjugates will permit binding of several targeting moieties per macromolecule, enhancing the avidity of the conjugate [221]; concomitantly, the efficacy should increase.
6.3. Conformation of multifunctional conjugates
Polymeric drug carriers containing multiple components, such as targeting modules, drug releasing modules, and endosome disruptive modules, have showed the potential to perform multiple functions within a single structure. Ideally, each component within the delivery system should function independently, without affecting the functionality of the other components. However, the physical and biological properties of multifunctional conjugates can be expected to exert some influence on the other components [48,50,78]. For example, poly(HPMA) assumes a random coil conformation in aqueous solutions [23]. Incorporation of hydrophobic side-chains into HPMA copolymers may result in intra- and/or intermolecular association between the hydrophobic moieties. Therefore, awareness of the complexities caused by the introduction of each component (like polymer self-association) is needed to design multi-component drug conjugates. For example, intra- or intermolecular hydrophobic interactions of a targeting peptide [78] or a hydrophobic drug [48,50] can result in the formation of unimolecular micelles with concomitant decrease in the rate of (enzymatically catalyzed) drug release [78]. Combination of physicochemical methods, such as FRET (fluorescence energy transfer) [78], light scattering [76,77], determination (for photosensitizers) of the quantum yield of singlet oxygen formation [76,77], and determination of biological activity can guide the optimal design of particular conjugates. The preference for either inter-polymer association or intra-polymer association is intrinsically determined by polymer concentration and structural parameters such as the nature and the size of the polymer chain, or the type, content, and arrangement of hydrophobes in the polymer. It can also be influenced by extrinsic factors, such as temperature, solvent, ionic strength, and pH [78 and references therein].
6.4. Mechanism of internalization and subcellular trafficking
Studies have shown that multiple endocytic pathways can concurrently take action when a macromolecular drug conjugate is exposed to a single type of cell. Consequently, it is important to study the mechanisms of internalization and subcellular fate when developing a macromolecular therapeutic for a particular disease [180,222]. As shown above, methods to manipulate the subcellular fate have a great potential to enhance the efficiency of macromolecular therapeutics; this line of research should be pursued.
6.5. Further studies on combination therapy
Quantitative in vitro and in vivo evaluation of combination therapy using polymer-bound drugs needs to be undertaken to optimize the design, structure, and dosing of the conjugates. It is important to address the question on the impact of the mechanism of drug action and of pharmacokinetics/pharmacodynamics on the design selection in combination therapy: two drugs per one macromolecule [119] or a combination of two polymer conjugates [112,113,117,118] containing one drug type per macromolecule?
6.6. Dream conjugate
The research summarized above should result in optimized structures of macromolecular therapeutics. An efficient conjugate for the future must have an increased intravascular half-life. Consequently, for the long-circulating conjugate to remain biocompatible and eliminate from the organism, labile bonds, cleavable either enzymatically or pH-sensitive, should be incorporated into the polymer backbone. Two targeting moieties should be attached: one for cellular targeting to direct the conjugate to a subset of cells, the other for subcellular targeting to direct the released drug into a particular organelle. In addition, two biologically active moieties might be attached to the one macromolecule (Fig. 27).
Fig. 27.
Dream conjugate.
6.7. New design paradigms
Finally, new designs based not on systematic improvements of known concepts, but on very new ideas are needed. For example, inspired by the high level of biorecognition of peptides self-assembling into antiparallel heterodimers (described in Section 5.2.1), we designed drug-free macromolecular therapeutics. Their activity is based on the crosslinking of receptors at the cell surface mediated by coiled-coil formation and concomitant induction of apoptosis [223].
Acknowledgments
The research in the authors’ laboratory was supported in part by the Czechoslovak Academy of Sciences, the National Institutes of Health (recently NIH grants CA51578, CA132831, GM069847, EB005288, and TW006260), Department of Defense grant W81XWH-04-1-0900, and the University of Utah Research Foundation. The HPMA journey since 1973 was really enjoyable thanks to our coworkers and numerous collaborators. We are truly indebted to all of them; their scientific contributions are reflected in the references.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “HPMA Copolymers: 30 Years of Advances”.
References
- 1.Ehrlich P. Studies in Immunity. Plenum Press; New York: 1906. [Google Scholar]
- 2.De Duve C, De Barsy T, Poole B, Trouet A, Tulkens P, van Hoof F. Lysosomotropic agents. Biochem Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 3.Jatzkewitz H. Peptamin (glycyl-L-leucyl-mescaline) bound to blood plasma expander (polyvinylpyrrolidone) as a new depot form of a biologically active primary amine (mescaline) Z Naturforsch. 1955;10b:27–31. [Google Scholar]
- 4.Panarin EF, Ushakov SN. Synthesis of polymer salts and amidopenicillines (in Russian) Khim Pharm Zhur. 1968;2:28–31. [Google Scholar]
- 5.Givetal NI, Ushakov SN, Panarin EF, Popova GO. Experimantal studies on penicillin polymer derivatives (in Russian) Antibiotiki. 1965;10:701–706. [PubMed] [Google Scholar]
- 6.Shumikina KI, Panarin EF, Ushakov SN. Experimental study of polymer salts of penicillins (in Russian) Antibiotiki. 1966;11:767–770. [PubMed] [Google Scholar]
- 7.Mathé G, Loc TB, Bernard J. Effect on mouse leukemia 1210 of a combination by diazo-reaction of a methopterin and gamma-globulins from hamsters inoculated with such leukemia by heterografts (in French) CR Hebd Seances Acad Sci. 1958;246:1626–1628. [PubMed] [Google Scholar]
- 8.Ringsdorf H. Structure and properties of pharmacologically active polymers. J Polym Sci, Polym Symp. 1975;51:135–153. [Google Scholar]
- 9.Voldřich Z, Tománek Z, Vacík J, Kopeček J. Long-term experience with the poly (glycol monomethacrylate) gel in plastic operations of the nose. J Biomed Mater Res. 1975;9:675–685. doi: 10.1002/jbm.820090612. [DOI] [PubMed] [Google Scholar]
- 10.Kopeček J. Soluble biomedical polymers. Polim Med. 1977;7:191–221. [PubMed] [Google Scholar]
- 11.Šprincl L, Vacík J, Kopeček J, Lím D. Biological tolerance of poly(N-substituted methacrylamides) J Biomed Mater Res. 1971;5:197–205. doi: 10.1002/jbm.820050307. [DOI] [PubMed] [Google Scholar]
- 12.Kopeček J, Šprincl L, Bažilová H, Vacík J. Biological tolerance of poly(N-substituted acrylamides) J Biomed Mater Res. 1973;7:111–121. doi: 10.1002/jbm.820070109. [DOI] [PubMed] [Google Scholar]
- 13.Šprincl L, Kopeček J, Lím D. Effect of porosity of heterogeneous poly(glycol monomethacrylate) gels on the healing-in of test implants. J Biomed Mater Res. 1971;5:447–458. doi: 10.1002/jbm.820050503. [DOI] [PubMed] [Google Scholar]
- 14.Šprincl L, Vacík J, Kopeček J, Lím D. Biological tolerance of ionogenic hydrophilic gels. J Biomed Mater Res. 1973;7:123–136. doi: 10.1002/jbm.820070110. [DOI] [PubMed] [Google Scholar]
- 15.Ulbrich K, Šprincl L, Kopeček J. Biocompatibility of poly(2, 4-pentadiene-1ol) J Biomed Mater Res. 1974;8:155–161. doi: 10.1002/jbm.820080205. [DOI] [PubMed] [Google Scholar]
- 16.Kopeček J, Šprincl L. Relationship between the structure and biocompatibility of hydrophilic gels. Polim Med. 1974;4:109–117. [PubMed] [Google Scholar]
- 17.Kopeček J, Šprincl L, Lím D. New types of synthetic infusion solutions. I. Investigation of the effect of solutions of some hydrophilic polymers on blood. J Biomed Mater Res. 1973;7:179–191. doi: 10.1002/jbm.820070206. [DOI] [PubMed] [Google Scholar]
- 18.Šprincl L, Exner J, Štěrba O, Kopeček J. New types of synthetic infusion solutions. III. Elimination and retention of poly[N-(2-hydroxypropyl)methacrylamide] in a test organism. J Biomed Mater Res. 1976;10:953–963. doi: 10.1002/jbm.820100612. [DOI] [PubMed] [Google Scholar]
- 19.Paluska E, JČinátl, Korčáková L, Štěrba O, Kopeček J, Hrubá A, Nezvalová J, Staněk R. Immunosuppressive effect of a synthetic polymer – poly[N-(2-hydroxypropyl) methacrylamide] (Duxon) Folia Biologica. 1980;26:304–311. [PubMed] [Google Scholar]
- 20.Paluska E, Hrubá A, Štěrba O, Kopeček J. Effect of a synthetic poly[N-(2-hydroxypropyl)methacrylamide] (Duxon) on haemopoiesis and graft versus host reaction. Folia Biol. 1986;32:91–102. [PubMed] [Google Scholar]
- 21.Kopeček J. Soluble polymers in medicine. In: Williams DF, editor. Systemic Aspects of Biocompatibility. II. CRC Press; Boca Raton, Florida: 1981. pp. 159–180. [Google Scholar]
- 22.Kopeček J, Bažilová H. Poly[N-(2-hydroxypropyl)methacrylamide]. 1. Radical polymerization and copolymerization. Europ Polym J. 1973;9:7–14. [Google Scholar]
- 23.Bohdanecký M, Bažilová H, Kopeček J. Poly[N-(2-hydroxypropyl)methacrylamide]. II. Hydrodynamic properties of diluted polymer solutions. Europ Polym J. 1974;10:405–410. [Google Scholar]
- 24.Kopeček J, Ulbrich K, Vacík J, Strohalm J, Chytrý V, Drobník J, Kálal J. Copolymers based on N-substituted acrylamides, N-substituted methacrylamides and N, N-disubstituted acrylamides and the method of their manufacturing. 4, 062, 831. US Patent. 1977 Dec 13;
- 25.Drobník J, Kopeček J, Labský J, Rejmanová P, Exner J, Kálal J. Preparation of biologically active substances bearing NH2 groups in a form releasable by enzymatic cleavage. 4, 097. US Patent. 1978 June 27;:470.
- 26.Obereigner B, Burešová M, Vrána A, Kopeček J. Preparation of polymerizable derivatives of N-(4-aminobenzenesulfonyl)-N′-butylurea. 17th Prague Micro-symposium on Macromolecules; Institute of Macromolecular Chemistry Prague. July 1977; Abstract C64. [Google Scholar]
- 27.Obereigner B, Burešová M, Vrána A, Kopeček J. Preparation of polymerizable derivatives of N-(4-aminobenzenesulfonyl)-N′-butylurea. J Polym Sci Polym Symp. 1979;66:41–52. [Google Scholar]
- 28.Chytrý V, Vrána A, Kopeček J. Synthesis and activity of a polymer which contains insulin covalently bound on a copolymer of N-(2-hydroxypropyl) methacrylamide and N-methacryloylglycylglycine 4-nitrophenyl ester. Makromol Chem. 1978;179:329–336. [Google Scholar]
- 29.Lääne A, Chytrý V, Haga M, Sikk P, Aaviksaar A, Kopeček J. Covalent attachment of chymotrypsin to poly[N-(2-hydroxypropyl)methacrylamide] Collection Czechoslov Chem Commun. 1981;46:1466–1473. [Google Scholar]
- 30.Kopeček J, Rejmanová P, Chytrý V. Polymers containing enzymatically degradable bonds 1. Chymotrypsin catalyzed hydrolysis of p-nitroanilides of phenylalanine and tyrosine attached to side-chains of copolymers of N-(2-hydroxypropyl)methacrylamide. Makromol Chem. 1981;182:799–809. [Google Scholar]
- 31.Solovskij MV, Ulbrich K, Kopeček J. Synthesis of N-(2-hydroxypropyl)methacrylamide copolymers with antimicrobial activity. Biomaterials. 1983;4:44–48. doi: 10.1016/0142-9612(83)90069-8. [DOI] [PubMed] [Google Scholar]
- 32.Rejmanová P, Labský J, Kopeček J. Aminolyses of monomeric and polymeric p-nitrophenyl esters of methacryloylated amino acids. Makromol Chem. 1977;178:2159–2168. [Google Scholar]
- 33.Kopeček J. Reactive copolymers of N-(2-hydroxypropyl)methacrylamide with N-methacryloylated derivatives of L-leucine and L-phenylalanine. I. Preparation, characterization and reaction with diamines. Makromol Chem. 1977;178:2169–2183. [Google Scholar]
- 34.Kopeček J, Rejmanová P. Enzymatically degradable bonds in synthetic polymers. In: Bruck SD, editor. Controlled Drug Delivery. I. CRC Press; Boca Raton, Florida: 1983. pp. 81–124. [Google Scholar]
- 35.Kopeček J. Biodegradation of polymers for biomedical use. In: Benoit H, Rempp P, editors. IUPAC Macromolecules. Pergamon; Oxford: 1982. pp. 305–320. [Google Scholar]
- 36.Kopeček J, Cífková I, Rejmanová P, Strohalm J, Obereigner B, Ulbrich K. Polymers containing enzymatically degradable bonds. 4. Preliminary experiments in vivo. Makromol Chem. 1981;182:2941–2949. [Google Scholar]
- 37.Drobník J, Kopeček J, Labský J, Rejmanová P, Exner J, Saudek V, Kálal J. Enzymatic cleavage of side-chains of synthetic water-soluble polymers. Makromol Chem. 1976;177:2833–2848. [Google Scholar]
- 38.Kopeček J, Rejmanová P. Reactive copolymers of N-(2-hydroxypropyl)methacrylamide with N-methacryloylated derivatives of L-leucine and L-phenylalanine. II. Reaction with the polymeric amine and stability of crosslinks towards chymotrypsin in vitro. J Polym Sci Polym Symp. 1979;66:15–32. [Google Scholar]
- 39.Rejmanová P, Obereigner B, Kopeček J. Polymers containing enzymatically degradable bonds. 2. Poly[N-(2-hydroxypropyl)methacrylamide] chains connected by oligopeptide sequences cleavable by chymotrypsin. Makromol Chem. 1981;182:1899–1915. [Google Scholar]
- 40.Ulbrich K, Strohalm J, Kopeček J. Polymers containing enzymatically degradable bonds. 3. Poly[N-(2-hydroxypropyl)methacrylamide] chains connected by oligo-peptide sequences cleavable by trypsin. Makromol Chem. 1981;182:1917–1928. [Google Scholar]
- 41.Ulbrich K, Zacharieva EI, Obereigner B, Kopeček J. Polymers containing enzymatically degradable bonds. 5. Hydrophilic polymers degradable by papain. Biomaterials. 1980;1:199–204. doi: 10.1016/0142-9612(80)90017-4. [DOI] [PubMed] [Google Scholar]
- 42.Duncan R, Cable HC, Lloyd JB, Rejmanová P, Kopeček J. Degradation of side-chains of N-(2-hydroxypropyl)methacrylamide copolymers by lysosomal thiol proteinases. Biosci Rep. 1982;2:1041–1046. doi: 10.1007/BF01122173. [DOI] [PubMed] [Google Scholar]
- 43.Duncan R, Cable HC, Lloyd JB, Rejmanová P, Kopeček J. Polymers containing enzymatically degradable bonds. 7. Design of oligopeptide side-chains in poly[N-(2-hydroxypropyl)methacrylamide] copolymers to promote efficient degradation by lysosomal enzymes. Makromol Chem. 1983;184:1997–2008. [Google Scholar]
- 44.Rejmanová P, Pohl J, Baudyš M, Kostka V, Kopeček J. Polymers containing enzymatically degradable bonds. 8. Degradation of oligopeptide sequences in N- (2-hydroxypropyl)methacrylamide copolymers by bovine spleen cathepsin B. Makromol Chem. 1983;184:2009–2020. [Google Scholar]
- 45.Šubr V, Kopeček J, Pohl J, Baudyš M, Kostka V. Cleavage of oligopeptide side-chains in N-(2-hydroxypropyl)methacrylamide copolymers by mixtures of lysosomal enzymes. J Control Release. 1988;8:133–140. [Google Scholar]
- 46.Rejmanová P, Kopeček J, Duncan R, Lloyd JB. Stability in rat plasma and serum of lysosomally degradable oligopeptide sequences in N-(2-hydroxypropyl) methacrylamide copolymers. Biomaterials. 1985;6:45–48. doi: 10.1016/0142-9612(85)90037-7. [DOI] [PubMed] [Google Scholar]
- 47.Kopeček J. Controlled degradability of polymers – a key to drug delivery systems. Biomaterials. 1984;5:19–25. doi: 10.1016/0142-9612(84)90062-0. [DOI] [PubMed] [Google Scholar]
- 48.Ulbrich K, Koňák Č, Tuzar Z, Kopeček J. Solution properties of drug carriers based on poly[N-(2-hydroxypropyl)methacrylamide] containing biodegradable bonds. Makromol Chem. 1987;188:1261–1272. [Google Scholar]
- 49.Ulbrich K, Strohalm J, Kopeček J. Polymers containing enzymatically degradable bonds. 6. Hydrophilic gels cleavable by chymotrypsin. Biomaterials. 1982;3:150–154. doi: 10.1016/0142-9612(82)90004-7. [DOI] [PubMed] [Google Scholar]
- 50.Pan H, Kopečková P, Wang D, Yang J, Miller S, Kopeček J. Water-soluble HPMA copolymer–prostaglandin conjugates containing a cathepsin K sensitive spacer. J Drug Targeting. 2006;14:425–435. doi: 10.1080/10611860600834219. [DOI] [PubMed] [Google Scholar]
- 51.Gao SQ, Lu ZR, Petri B, Kopečková P, Kopeček J. Colon-specific 9-aminocamptothecin–HPMA copolymer conjugates containing a 1, 6-elimination spacer. J Control Release. 2006;110:323–331. doi: 10.1016/j.jconrel.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Kopeček J, Rejmanová P. Reactive copolymers of N-(2-hydroxypropyl)methacrylamide with N-methacryloylated derivatives of L-leucine and L-phenylalanine. II. Reaction with the polymeric amine, 17th Prague Microsymposium on Macromolecules; Institute of Macromolecular Chemistry Prague. July 1977; Abstract C45. [Google Scholar]
- 53.Kopeček J. Poly[N-(2-hydroxypropyl)methacrylamide] as a carrier of biologically active compounds. Symposium “Polymers 77”; Varna, Bulgaria. October, 1977. [Google Scholar]
- 54.Kopeček J. Polymeranalogous reactions leading to enzymatically degradable polymers. IUPAC International Symposium on Macromolecular Chemistry; Tashkent, Soviet Union. October 1978; Abstract 4–29. [Google Scholar]
- 55.Ulbrich K, Strohalm J, Kopeček J. Poly(ethylene glycol)s containing enzymatically degradable bonds. Makromol Chem. 1986;187:1131–1141. [Google Scholar]
- 56.Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- 57.Duncan R, Kopeček J, Rejmanová P, Lloyd JB. Targeting of N-(2-hydroxypropyl) methacrylamide copolymers to liver by incorporation of galactose residues. Biochim Biophys Acta. 1983;755:518–521. doi: 10.1016/0304-4165(83)90258-1. [DOI] [PubMed] [Google Scholar]
- 58.Duncan R, Lloyd JB, Rejmanová P, Kopeček J. Methods of targeting N-(2-hydroxypropyl)methacrylamide copolymers to particular cell types. Makromol Chem Suppl. 1985;9:3–12. [Google Scholar]
- 59.Duncan R, Seymour LCW, Scarlett L, Lloyd JB, Rejmanová P, Kopeček J. Fate of N-(2-hydroxypropyl)methacrylamide copolymers with pendent galactosamine residues after intravenous administration to rats. Biochim Biophys Acta. 1986;880:62–71. doi: 10.1016/0304-4165(86)90120-0. [DOI] [PubMed] [Google Scholar]
- 60.Wedge SR, Duncan R, Kopečková P. Comparison of the liver subcellular distribution of free daunomycin and that bound to galactosamine targeted N-(2- hydroxypropyl)methacrylamide copolymers, following intravenous administration in the rat. Br J Cancer. 1991;63:546–549. doi: 10.1038/bjc.1991.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duncan R, Hume IC, Kopečková P, Ulbrich K, Strohalm J, Kopeček J. Anticancer agents coupled to N-(2-hydroxypropyl)methacrylamide copolymers. 3. Evaluation of adriamycin conjugates against mouse leukemia L1210 in vivo. J Control Release. 1989;9:21–32. [Google Scholar]
- 62.Říhová B, Kopeček J. Biological properties of targetable poly[N-(2-hydroxypropyl) methacrylamide]–antibody conjugates. J Control Release. 1985;2:289–310. [Google Scholar]
- 63.Říhová B, Kopeček J, Kopečková-Rejmanová P, Strohalm J, Plocová D, Semorádová H. Bioaffinity therapy with antibodies and drugs bound to synthetic polymers. J Chromatogr Biomed Appl. 1986;376:221–233. [PubMed] [Google Scholar]
- 64.Říhová B, Krinick NL, Kopeček J. Targetable photoactivatable drugs. 3. Specific in vitro destruction of a human hepatocarcinoma cell line (PLC/PRF/5) or mouse splenocytes by polymer bound chlorin e6, targeted by galactosamine or anti-Thy-1.2 antibodies, respectively. J Control Release. 1993;25:71–87. [Google Scholar]
- 65.Duncan R, Lloyd JB, Kopeček J. Degradation of side-chains of N-(2-hydroxypropyl) methacrylamide copolymers by lysosomal enzymes. Biochem Biophys Res Commun. 1980;94:284–290. doi: 10.1016/s0006-291x(80)80218-x. [DOI] [PubMed] [Google Scholar]
- 66.Duncan R, Kopeček J. Soluble synthetic polymers as potential drug carriers. Adv Polym Sci. 1984;57:51–101. [Google Scholar]
- 67.Kopeček J, Duncan R. Targetable polymeric drugs. J Control Release. 1987;6:315–327. [Google Scholar]
- 68.Kopeček J, Rejmanová P, Strohalm J, Ulbrich K, Říhová B, Chytrý V, Lloyd JB, Duncan R. Synthetic polymeric drugs. 5, 037. US Patent. 1991 Aug 6;:883.
- 69.Říhová B, Ulbrich K, Kopeček J, Mančal P. Immunogenicity of N-(2-hydroxypropyl)methacrylamide copolymers – potential hapten and drug carriers. Folia microbiologica. 1983;28:217–227. doi: 10.1007/BF02884085. [DOI] [PubMed] [Google Scholar]
- 70.Říhová B, Kopeček J, Ulbrich K, Pospíšil J, Mančal P. Effect of chemical structure of N-(2-hydroxypropyl)methacrylamide copolymers on their ability to induce antibody formation in inbred strains of mice. Biomaterials. 1984;5:143–148. doi: 10.1016/0142-9612(84)90048-6. [DOI] [PubMed] [Google Scholar]
- 71.Říhová B, Kopeček J, Ulbrich K, Chytrý V. Immunogenicity of N-(2-hydroxypropyl) methacrylamide copolymers. Makromol Chem Suppl. 1985;9:13–24. [Google Scholar]
- 72.Šimečková B, Plocová D, Říhová B, Kopeček J. Activity of complement in the presence of N-(2-hydroxypropyl)methacrylamide copolymers. J Bioact Comp Polym. 1986;1:20–31. [Google Scholar]
- 73.Krinick NL, Kopeček J. Soluble polymers as targetable drug carriers. In: Juliano RL, editor. Handbook of Experimental Pharmacology, Targeted Drug Delivery. Vol. 100. Springer; 1991. pp. 105–179. [Google Scholar]
- 74.Putnam D, Kopeček J. Polymer conjugates with anticancer activity. Adv Polym Sci. 1995;122:55–123. [Google Scholar]
- 75.Kopeček J, Kopečková P, Minko T, Lu ZR. HPMA copolymer–anticancer drug conjugates: design, activity, and mechanism of action. Eur J Pharmaceutics Biopharm. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 76.Shiah JG, Koňák Č, Spikes JD, Kopeček J. Solution and photoproperties of N-(2-hydroxypropyl)methacrylamide copolymer–meso-chlorin e6 conjugates. J Phys Chem B. 1997;101:6803–6809. doi: 10.3109/10717549809031387. [DOI] [PubMed] [Google Scholar]
- 77.Shiah JG, Koňák Č, Spikes JD, Kopeček J. Influence of pH on aggregation and photoproperties of N-(2-hydroxypropyl)methacrylamide copolymer–mesochlorin e6 conjugates. Drug Delivery. 1998;5:119–126. doi: 10.3109/10717549809031387. [DOI] [PubMed] [Google Scholar]
- 78.Ding H, Kopečková P, Kopeček J. Self-association properties of HPMA copolymers containing an amphipatic heptapeptide. J Drug Targeting. 2007;15:465–474. doi: 10.1080/10611860701500016. [DOI] [PubMed] [Google Scholar]
- 79.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer therapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 80.Duncan R. The dawning era of polymer therapeutics. Nature Rev Drug Disc. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 81.Říhová B. Immunomodulating activities of soluble synthetic polymer-bound drugs. Adv Drug Delivery Rev. 2002;54:653–674. doi: 10.1016/s0169-409x(02)00043-1. [DOI] [PubMed] [Google Scholar]
- 82.Říhová B, Kopečková P, Strohalm J, Rossmann P, Větvička V, Kopeček J. Antibody directed affinity therapy applied to the immune system: in vivo effectiveness and limited toxicity of daunomycin conjugates to HPMA copolymers and targeting antibody. Clin Immunol Immunopathol. 1988;46:100–114. doi: 10.1016/0090-1229(88)90010-4. [DOI] [PubMed] [Google Scholar]
- 83.Shiah JG, Sun Y, Kopečková P, Peterson CM, Straight RC, Kopeček J. Combination chemotherapy and photodynamic therapy of targetable N-(2-hydroxypropyl) methacrylamide copolymer–doxorubicin/mesochlorin e6–OV-TL16 antibody immunoconjugates. J Control Release. 2001;74:249–253. doi: 10.1016/s0168-3659(01)00325-x. [DOI] [PubMed] [Google Scholar]
- 84.Lu ZR, Kopečková P, Kopeček J. Polymerizable Fab′ antibody fragments for targeting of anticancer drugs. Nat Biotechnol. 1999;17:1101–1104. doi: 10.1038/15085. [DOI] [PubMed] [Google Scholar]
- 85.Shiah JG, Dvořák M, Kopečková P, Sun Y, Peterson CM, Kopeček J. Biodistribution and antitumor efficacy of long-circulating N-(2-hydroxypropyl) methacrylamide copolymer–doxorubicin conjugates in nude mice. Eur J Cancer. 2001;37:131–139. doi: 10.1016/s0959-8049(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 86.Seymour LW, Duncan R, Strohalm J, Kopeček J. Effect of molecular weight of N-(2-hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal and intravenous administration. J Biomed Mater Res. 1987;21:1341–1358. doi: 10.1002/jbm.820211106. [DOI] [PubMed] [Google Scholar]
- 87.Říhová B, Kovář L, Kovář M, Hovorka O. Cytotoxicity and immunostimulation: double attack on cancer cells with polymeric therapeutics. Trends Biotechnol. 2009;27:11–17. doi: 10.1016/j.tibtech.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Minko T, Kopečková P, Kopeček J. Comparison of the anticancer effect of free and HPMA copolymer-bound adriamycin in human ovarian carcinoma cells. Pharmaceutical Res. 1999;16:986–996. doi: 10.1023/a:1018959029186. [DOI] [PubMed] [Google Scholar]
- 89.Minko T, Kopečková P, Kopeček J. Efficacy of chemotherapeutic action of HPMA copolymer-bound doxorubicin in a solid tumor model of ovarian carcinoma. Int J Cancer. 2000;86:108–117. doi: 10.1002/(sici)1097-0215(20000401)86:1<108::aid-ijc17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 90.Minko T, Kopečková P, Kopeček J. Preliminary evaluation of caspases-dependent apoptosis signaling pathways of free and HPMA copolymer-bound doxorubicin in human ovarian carcinoma cells. J Control Release. 2001;71:227–237. doi: 10.1016/s0168-3659(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 91.Nishiyama A, Nori A, Malugin A, Kasuya Y, Kopečková P, Kopeček J. Free and N-(2-hydroxypropyl)methacrylamide copolymer-bound geldanamycin derivative induce different stress responses in A2780 human ovarian carcinoma cells. Cancer Res. 2003;63:7876–7882. [PubMed] [Google Scholar]
- 92.Malugin A, Kopečková P, Kopeček J. HPMA copolymer-bound doxorubicin induces apoptosis in human ovarian carcinoma cells by a Fas independent pathway. Mol Pharmaceutics. 2004;1:174–182. doi: 10.1021/mp049967q. [DOI] [PubMed] [Google Scholar]
- 93.Malugin A, Kopečková P, Kopeček J. HPMA copolymer-bound doxorubicin induces apoptosis in ovarian carcinoma cells by the disruption of mitochondrial function. Mol Pharmaceutics. 2006;3:351–361. doi: 10.1021/mp050065e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malugin A, Kopečková P, Kopeček J. Liberalization of doxorubicin from HPMA copolymer conjugate is essential for the indiction of cell cycle arrest and nuclear fragmentation in ovarian carcinoma cells. J Control Release. 2007;124:6–10. doi: 10.1016/j.jconrel.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jain RK. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989;81:570–576. doi: 10.1093/jnci/81.8.570. [DOI] [PubMed] [Google Scholar]
- 96.Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, Maeda H. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn J Cancer Res. 1998;89:307–314. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takakura Y, Fujita T, Hashida M, Sezaki H. Disposition of macromolecules in tumor-bearing mice. Pharm Res. 1990;7:339–346. doi: 10.1023/a:1015807119753. [DOI] [PubMed] [Google Scholar]
- 98.Tabata Y, Kawai T, Murakami Y, Ikada Y. Electric charge influence of dextran derivatives on their tumor accumulation after intravenous injection. Drug Deliv. 1997;4:213–221. [Google Scholar]
- 99.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maeda H, Seymour LM, Miyamoto Y. Conjugates of anticancer agents and polymers: advantages of macromolecular therapeutics in vivo. Bioconjug Chem. 1992;3:351–362. doi: 10.1021/bc00017a001. [DOI] [PubMed] [Google Scholar]
- 101.Cartlidge SA, Duncan R, Lloyd JB, Rejmanová P, Kopeček J. Soluble, crosslinked N-(2-hydroxypropyl)methacrylamide copolymers as potential drug carriers. 1. Pinocytosis by rat visceral yolk sacs and rat intestine cultured in vitro. Effect of molecular weight on uptake and intracellular degradation. J Control Release. 1986;3:55–66. [Google Scholar]
- 102.Cartlidge SA, Duncan R, Lloyd JB, Kopečková-Rejmanová P, Kopeček J. Soluble, crosslinked N-(2-hydroxypropyl)methacrylamide copolymers as potential drug carriers. 2. Effect of molecular weight on blood clearance and body distribution in the rat after intraveneous administration. Distribution of unfractionated copolymer after intraperitoneal, subcutaneous or oral administration. J Control Release. 1987;4:253–264. [Google Scholar]
- 103.Cartlidge SA, Duncan R, Lloyd JB, Kopečková-Rejmanová P, Kopeček J. Soluble, crosslinked N-(2-hydroxypropyl)methacrylamide copolymers as potential drug carriers. 3. Targeting by incorporation of galactosamine residues. Effect of route of administration. J Control Release. 1987;4:265–278. [Google Scholar]
- 104.Dvořák M, Kopečková P, Kopeček J. High-molecular weight HPMA copolymer–adriamycin conjugates. J Control Release. 1999;60:321–332. doi: 10.1016/s0168-3659(99)00087-5. [DOI] [PubMed] [Google Scholar]
- 105.Persidis A. Cancer multidrug resistance. Nat Biotechnol. 1999;17:94–95. doi: 10.1038/5289. [DOI] [PubMed] [Google Scholar]
- 106.German UA. P-glycoprotein: a mediator of multidrug resistance in tumour cells. Eur J Cancer. 1996;32A:927–944. doi: 10.1016/0959-8049(96)00057-3. [DOI] [PubMed] [Google Scholar]
- 107.Loe DW, Decley RG, Cole SPC. Biology of the multidrug resistance associated protein, MRP. Eur J Cancer. 1996;32A:945–957. doi: 10.1016/0959-8049(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 108.Tew KD. Gluthatione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 109.Omelyanenko V, Kopečková P, Gentry C, Kopeček J. Targetable HPMA copolymer–adriamycin conjugates. Recognition, internalization, and subcellular fate. J Control Release. 1998;53:25–37. doi: 10.1016/s0168-3659(97)00235-6. [DOI] [PubMed] [Google Scholar]
- 110.Omelyanenko V, Gentry C, Kopečková P, Kopeček J. HPMA copolymer–anticancer drug–OV-TL16 antibody conjugates. 2. Processing in epithelial ovarian carcinoma cells in vitro. Int J Cancer. 1998;75:600–608. doi: 10.1002/(sici)1097-0215(19980209)75:4<600::aid-ijc18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 111.Minko T, Kopečková P, Kopeček J. Chronic exposure to HPMA copolymer-bound adriamycin does not induce multidrug resistance in a human ovarian carcinoma cell line. J Control Release. 1999;59:133–148. doi: 10.1016/s0168-3659(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 112.Krinick NL, Sun Y, Joyner D, Spikes JD, Straight RC, Kopeček J. A polymeric drug delivery system for the simultaneous delivery of drugs activatable by enzymes and/or light. J Biomat Sci Polym Ed. 1994;5:303–324. doi: 10.1163/156856294x00040. [DOI] [PubMed] [Google Scholar]
- 113.Peterson CM, Lu JM, Sun Y, Peterson CA, Shiah JG, Straight RC, Kopeček J. Combination chemotherapy and photodynamic therapy with N-(2-hydroxypropyl) methacrylamide copolymer-bound anticancer drugs inhibit human ovarian carcinoma heterotransplanted in nude mice. Cancer Res. 1996;56:3980–3985. [PubMed] [Google Scholar]
- 114.Lu M, Peterson CM, Shiah JG, Gu ZW, Peterson CA, Straight RC, Kopeček J. Cooperativity between free and N-(2-hydroxypropyl)methacrylamide copolymer bound adriamycin and mesochlorin e6 monoethylene diamine induced photodynamic therapy in human epithelial ovarian carcinoma in vivo. Int J Oncol. 1999;15:5–16. doi: 10.3892/ijo.15.1.5. [DOI] [PubMed] [Google Scholar]
- 115.Shiah JG, Sun Y, Peterson CM, Kopeček J. Biodistribution of free and N-(2- hydroxypropyl)methacrylamide copolymer-bound meso chlorin e6 and adriamycin in nude mice bearing human ovarian carcinoma OVCAR-3 xenografts. J Control Release. 1999;61:145–157. doi: 10.1016/s0168-3659(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 116.Shiah JG, Sun Y, Peterson CM, Straight RC, Kopeček J. Antitumor activity of HPMA copolymer–meso chlorin e6 and adriamycin conjugates in combination treatments. Clin Cancer Res. 2000;6:1008–1015. [PubMed] [Google Scholar]
- 117.Hongrapipat J, Kopečková P, Prakongpan S, Kopeček J. Enhanced antitumor activity of combinations of free and HPMA copolymer-bound drugs. Int J Pharm. 2008;351:259–270. doi: 10.1016/j.ijpharm.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hongrapipat J, Kopečková P, Liu J, Prakongpan S, Kopeček J. Combination chemotherapy and photodynamic therapy with Fab′ fragment targeted HPMA copolymer conjugates in human ovarian carcinoma cells. Mol Pharm. 2008;5:696–709. doi: 10.1021/mp800006e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Segal E, Pan H, Ofek P, Ugadawa T, Kopečková P, Kopeček J, Satchi-Fainaro R. Targeting angiogenesis-dependent calcified neoplasms using combined polymer therapeutics. PLoS ONE. 2009;4(4):e5233. doi: 10.1371/journal.pone.0005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kopeček J, Kopečková P. Macromolecular therapeutics: state-of-the-art and future potential. Bull Tech Gattefossé. 2003;96:9–21. [Google Scholar]
- 121.Cuchelkar V, Kopeček J. Polymer–drug conjugates. In: Uchegbu IF, Schätzlein AG, editors. Polymers in Drug Delivery; CRC Press; 2006. pp. 155–182. [Google Scholar]
- 122.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems. Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;3:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 123.Tang A, Kopečková P, Kopeček J. Binding and cytotoxicity of HPMA copolymers to lymphocytes mediated by receptor-binding epitopes. Pharm Res. 2003;20:360–367. doi: 10.1023/a:1022639701388. [DOI] [PubMed] [Google Scholar]
- 124.Ding H, Prodinger WM, Kopeček J. Identification of CD21-binding peptides with phage display and investigation of binding properties of HPMA copolymer–peptide conjugates. Bioconjug Chem. 2006;17:514–523. doi: 10.1021/bc0503162. [DOI] [PubMed] [Google Scholar]
- 125.Ding H, Prodinger WM, Kopeček J. Two-step fluorescence screening of CD21-binding peptides with one-bead one-compound library and investigation of binding of HPMA copolymer–peptide conjugates. Biomacromolecules. 2006;7:3037–3046. doi: 10.1021/bm060508f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Omelyanenko V, Kopečková P, Prakash RK, Ebert CD, Kopeček J. Biorecognition of HPMA copolymer–adriamycin conjugates by lymphocytes mediated by synthetic receptor binding epitopes. Pharm Res. 1999;16:1010–1019. doi: 10.1023/a:1018975414165. [DOI] [PubMed] [Google Scholar]
- 127.Line BR, Mitra A, Nan A, Ghandehari H. Targeting tumor angiogenesis: comparison of peptide and polymer–peptide conjugates. J Nucl Med. 2005;46:1552–1560. [PubMed] [Google Scholar]
- 128.Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha4beta1 integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2:381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- 129.Chen X, Gambhir SS. Significance of one-bead-one-compound combinatorial chemistry. Nat Chem Biol. 2006;2:351–352. doi: 10.1038/nchembio0706-351. [DOI] [PubMed] [Google Scholar]
- 130.Yeh PY, Kopečková P, Kopeček J. Biodegradable and pH-sensitive hydrogels: synthesis by crosslinking of N.N-dimethylacrylamide copolymer precursors. J Polym Sci Part A: Polym Chem. 1994;32:1627–1637. [Google Scholar]
- 131.Grim Y, Kopeček J. Bioadhesive water-soluble polymeric drug carriers for site-specific oral drug delivery. Synthesis, characterization and release of 5-aminosalicylic acid by Streptococcum faecium in vitro. New Polym Mater. 1991;3:49–59. [Google Scholar]
- 132.Kopeček J. Polymers for colon-specific drug delivery. J Control Release. 1992;19:121–130. [Google Scholar]
- 133.Kopečková P, Rathi R, Takada S, Říhová B, Berenson MM, Kopeček J. Bioadhesive N-(2-hydroxypropyl)methacrylamide copolymers for colon-specific drug delivery. J Control Release. 1994;28:211–222. [Google Scholar]
- 134.Sakuma S, Lu ZR, Kopečková P, Kopeček J. Biorecognizable HPMA copolymer–drug conjugates for colon-specific delivery of 9-aminocamptothecin. J Control Release. 2001;75:365–379. doi: 10.1016/s0168-3659(01)00405-9. [DOI] [PubMed] [Google Scholar]
- 135.Sakuma S, Lu ZR, Pecharová B, Kopečková P, Kopeček J. N-(2-hydroxypropyl)- methacrylamide copolymer–9-aminocamptothecin conjugate: colon-specific delivery in rats. J Bioact Comp Polym. 2002;17:305–319. [Google Scholar]
- 136.Gao SQ, Lu ZR, Kopečková P, Kopeček J. Biodistribution and pharmacokinetics of colon-specific HPMA copolymer–9-aminocamptothecin conjugate in mice. J Control Release. 2007;117:179–185. doi: 10.1016/j.jconrel.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gao SQ, Sun Y, Kopečková P, Peterson CM, Kopeček J. Pharmacokinetic modeling of absorption behavior of 9-aminocamptothecin (9-AC) released from colon-specific HPMA copolymer–9-AC conjugate in rats. Pharm Res. 2008;25:218–226. doi: 10.1007/s11095-007-9465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao S-Q, Sun Y, Kopečková P, Peterson CM, Kopeček J. Antitumor efficacy of colon-specific HPMA copolymer–9-aminocamptothecin conjugates in mice bearing human colon carcinoma xenografts. Macromol Biosci. 2009;9:1135–1142. doi: 10.1002/mabi.200900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu ZR, Gao SQ, Kopečková P, Kopeček J. Synthesis of bioactive lectin–HPMA copolymer–cyclosporine conjugates. Bioconjug Chem. 2000;11:3–7. doi: 10.1021/bc990098a. [DOI] [PubMed] [Google Scholar]
- 140.Wróblewski S, Říhová B, Rossmann P, Hudcovicz T, Řeháková Z, Kopečková P, Kopeček J. The influence of a colonic microbiota on HPMA copolymer–lectin conjugates binding in rodent intestine. J Drug Target. 2001;9:85–94. doi: 10.3109/10611860108997920. [DOI] [PubMed] [Google Scholar]
- 141.Wróblewski S, Berenson M, Kopečková P, Kopeček J. Biorecognition of HPMA copolymer–lectin conjugates as an indicator of differentiation of cell surface glycoproteins in development, maturation and diseases of human and rodent gastrointestinal tissues. J Biomed Mater Res. 2000;51:329–342. doi: 10.1002/1097-4636(20000905)51:3<329::aid-jbm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 142.Cuchelkar V, Kopečková P, Kopeček J. Novel HPMA copolymer-bound constructs for combined tumor and mitochondrial targeting. Mol Pharm. 2008;5:696–709. doi: 10.1021/mp800019g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cuchelkar V, Kopečková P, Lim C, Kopeček J. unpublished data [Google Scholar]
- 144.Nori A, Kopeček J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv Drug Deliv Rev. 2005;57:609–636. doi: 10.1016/j.addr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 145.Tijerina M, Kopečková P, Kopeček J. Correlation of subcellular compartmentalization of HPMA copolymer–Mce6 conjugates with chemotherapeutic activity in human ovarian carcinoma cells. Pharm Res. 2003;20:728–737. doi: 10.1023/a:1023425300829. [DOI] [PubMed] [Google Scholar]
- 146.Tijerina M, Kopečková P, Kopeček J. Mechanism of cytotoxicity in human ovarian carcinoma cells exposed to free Mce6 or HPMA copolymer–Mce6 conjugates. Photochem Photobiol. 2003;77:645–652. doi: 10.1562/0031-8655(2003)077<0645:mociho>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 147.Muratovska A, Lightowlers RN, Taylor RW, Turnbull DM, Smith RA, Wilce JA, Martin SW, Murphy MP. Targeting peptide nucleic acid (PNA) oligomers to mitochondria within cells by conjugation to lipophilic cations: implications for mitochondrial DNA replication, expression and disease. Nucleic Acids Res. 2001;29:1852–1863. doi: 10.1093/nar/29.9.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Callahan J, Kopeček J. Semitelechelic HPMA copolymers functionalized with triphenylphosphonium as drug carriers for membrane transduction and mitochondrial localization. Biomacromolecules. 2006;7:2347–2356. doi: 10.1021/bm060336m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Williams SP, Sigler PB. Atomic structure of progesterone complexed with its receptor. Nature. 1998;393:392–396. doi: 10.1038/30775. [DOI] [PubMed] [Google Scholar]
- 151.Rebuffat A, Bernasconi A, Ceppi M, Wehrli H, Brenz Verca S, Ibrahim M, Frey BM, Frey FJ, Rusconi S. Selective enhancement of gene transfer by steroid-mediated gene delivery. Nat Biotechnol. 2001;19:1155–1161. doi: 10.1038/nbt1201-1155. [DOI] [PubMed] [Google Scholar]
- 152.Callahan J, Kopečková P, Kopeček J. The intracellular trafficking and subcellular distribution of a large array of HPMA copolymer conjugates. Biomacromolecules. 2009;10:1704–1714. doi: 10.1021/bm801514x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Panté N, Kann M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol Biol Cell. 2002;13:425–434. doi: 10.1091/mbc.01-06-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Paine PL, Moore LC, Horowitz SB. Nuclear envelope permeability. Nature. 1975;254:109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- 155.Feldherr CM, Akin D. The location of the transport gate in the nuclear pore complex. J Cell Sci. 1997;110:3065–3070. doi: 10.1242/jcs.110.24.3065. [DOI] [PubMed] [Google Scholar]
- 156.Frey S, Richter RP, Görlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314:815–817. doi: 10.1126/science.1132516. [DOI] [PubMed] [Google Scholar]
- 157.Blagosklonny MV. HSP-90-associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia (Baltimore) 2002;16:455–462. doi: 10.1038/sj.leu.2402415. [DOI] [PubMed] [Google Scholar]
- 158.Kasuya Y, Lu ZR, Kopečková P, Kopeček J. Improved synthesis and evaluation of 17-substituted aminogeldanamycin derivatives applicable to drug delivery systems. Bioorg Med Chem Lett. 2001;11:2089–2091. doi: 10.1016/s0960-894x(01)00374-2. [DOI] [PubMed] [Google Scholar]
- 159.Kasuya Y, Lu ZR, Kopečková P, Minko T, Tabibi SE, Kopeček J. Synthesis and characterization of HPMA copolymer–aminopropylgeldanamycin conjugates. J Control Release. 2001;74:203–211. doi: 10.1016/s0168-3659(01)00318-2. [DOI] [PubMed] [Google Scholar]
- 160.Kasuya Y, Lu ZR, Kopečková P, Tabibi SE, Kopeček J. Influence of the structure of drug moieties on the in vitro efficacy of HPMA copolymer–geldanamycin derivative conjugates. Pharm Res. 2002;19:115–123. doi: 10.1023/a:1014216712820. [DOI] [PubMed] [Google Scholar]
- 161.Říhová B, Strohalm J, Hoste K, Jelínková M, Hovorka O, Kovář M, Plocová D, Šírová M, Štastný M, Schacht E, Ulbrich K. Immunoprotective therapy with targeted anticancer drugs. Macromol Symp. 2001;172:21–28. [Google Scholar]
- 162.Demoy M, Minko T, Kopečková P, Kopeček J. Time- and concentration-dependent apoptosis and necrosis induced by free and HPMA copolymer-bound doxorubicin in human ovarian carcinoma cells. J Control Release. 2000;69:185–196. doi: 10.1016/s0168-3659(00)00301-1. [DOI] [PubMed] [Google Scholar]
- 163.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T, Burtles S, Fraier D, Frigerio E, Cassidy J on behalf of the Cancer Research Campaign Phase I/II Committee. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents—drug–polymer conjugates. Clin Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 164.Thomson AH, Vassey PA, Murray LS, Cassidy J, Fraier D, Frigerio E, Twelves C. Population pharmacokinetics in phase I drug development: a phase I study of PK1 in patients with solid tumors. Br J Cancer. 1999;81:99–107. doi: 10.1038/sj.bjc.6690657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seymour LW, Ferry DR, Kerr DJ, Rea D, Whitlock M, Ponyer R, Boivin C, Hesslewood S, Twelves C, Blackie R, Schätzlein A, Jodrell D, Bissett D, Calvert H, Lind M, Robbins A, Burtless S, Duncan R, Cassidy J. Phase II studies of polymer–doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal carcinoma. Int J Oncol. 2009;34:1629–1636. doi: 10.3892/ijo_00000293. [DOI] [PubMed] [Google Scholar]
- 166.Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Poyner R, Doran J, Young AM, Burtles S, Kerr DJ. Hepatic drug targeting: phase I evaluation of polymer-bound doxorubicin. J Clin Oncol. 2002;20:1668–1676. doi: 10.1200/JCO.2002.20.6.1668. [DOI] [PubMed] [Google Scholar]
- 167.Shoemaker NE, van Kesteren C, Rosing H, Jansen S, Swart M, Lieverst J, Fraier D, Breda M, Pellizzoni C, Spinelli R, Grazia Porro M, Beijnen JH, Schellens JHM, ten Bokkel Huinink WW. A phase I and pharmacokinetic study of MAG-CPT, a water-soluble polymer conjugate of camptothecin. Br J Cancer. 2002;87:608–614. doi: 10.1038/sj.bjc.6600516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meerum Terwogt JM, ten Bokkel Huinink WW, Schellens JH, Schot M, Mandjes IA, Zurlo MG, Rocchetti M, Rosing H, Koopman FJ, Beijnen JH. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anticancer Drugs. 2001;12:315–323. doi: 10.1097/00001813-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 169.Rademaker-Lakhai JM, Terret C, Howell SB, Baud CM, De Boer RF, Pluim D, Beijnen JH, Schellens JH, Droz JP. A phase I and pharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin Cancer Res. 2004;10:3386–3395. doi: 10.1158/1078-0432.CCR-03-0315. [DOI] [PubMed] [Google Scholar]
- 170.Wang D, Miller S, Sima M, Kopečková P, Kopeček J. Synthesis and evaluation of water-soluble polymeric bone-targeted drug delivery systems. Bioconjug Chem. 2003;14:853–859. doi: 10.1021/bc034090j. [DOI] [PubMed] [Google Scholar]
- 171.Wang D, Sima M, Mosley RL, Davda JP, Tietze N, Miller SC, Gwilt PR, Kopečková P, Kopeček J. Pharmacokinetic and biodistribution studies of bone-targeting drug delivery system based on N-(2-hydroxypropyl)methacrylamide) copolymers. Mol Pharm. 2006;3:717–725. doi: 10.1021/mp0600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang D, Miller SC, Shlyakhtenko LS, Portillo AM, Liu XM, Papngkorn K, Kopečková P, Lyubchenko Y, Higuchi WI, Kopeček J. Osteotropic peptide that differentiates functional domains of the skeleton. Bioconjug Chem. 2007;18:1375–1378. doi: 10.1021/bc7002132. [DOI] [PubMed] [Google Scholar]
- 173.Pan HZ, Sima M, Kopečková P, Wu K, Gao SQ, Liu J, Wang D, Miller SC, Kopeček J. Biodistribution and pharmacokinetic studies of bone-targeting N-(2-hydroxypropyl)methacrylamide copolymer–alendronate conjugates. Mol Pharmaceutics. 2008;5:548–558. doi: 10.1021/mp800003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Miller SC, Pan H, Wang D, Bowman BM, Kopečková P, Kopeček J. Feasibility of using a bone-targeted, macromolecular delivery system coupled with prostaglandin E1 to promote bone formation in aged, estrogen-deficient rats. Pharm Res. 2008;25:2889–2895. doi: 10.1007/s11095-008-9706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang D, Miller SC, Sima M, Parker D, Buswell H, Goodrich KC, Kopečková P, Kopeček J. The artrotropism of macromolecules in adjuvant-induced arthritis rat model: a preliminary study. Pharm Res. 2004;21:1741–1749. doi: 10.1023/b:pham.0000045232.18134.e9. [DOI] [PubMed] [Google Scholar]
- 176.Pan H, Liu J, Dong Y, Sima M, Kopečková P, Brandi ML, Kopeček J. Release of prostaglandin E1 from N-(2-hydroxypropyl)methacrylamide copolymer conjugates by bone cells. Macromol Biosci. 2008;8:599–605. doi: 10.1002/mabi.200700338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu XM, Quan LD, Tian J, Alnouti Y, Fu K, Thiele GM, Wang D. Synthesis and evaluation of a well-defined HPMA copolymer–dexamethasone conjugate for effective treatment of rheumatoid arthritis. Pharm Res. 2008;25:2910–2919. doi: 10.1007/s11095-008-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Choi WM, Kopečková P, Minko T, Kopeček J. Synthesis of HPMA copolymer containing adriamycin bound via an acid-labile spacer and its activity toward human ovarian carcinoma cells. J Bioact Comp Polym. 1999;14:447–456. [Google Scholar]
- 179.Cuchelkar V, Kopečková P, Kopeček J. Synthesis and biological evaluation of disulfide-linked HPMA copolymer–mesochlorin e6 conjugates. Macromol Biosci. 2008;8:375–383. doi: 10.1002/mabi.200700240. [DOI] [PubMed] [Google Scholar]
- 180.Liu J, Kopečková P, Bühler P, Wolf P, Pan H, Bauer H, Elsässer-Beile U, Kopeček J. Biorecognition and subcellular trafficking of HPMA copolymer–anti-PMSA antibody conjugates by prostate cancer cells. Mol Pharmaceutics. 2009;6:959–970. doi: 10.1021/mp8002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Omelyanenko V, Kopečková P, Gentry C, Shiah JG, Kopeček J. HPMA copolymer–anticancer drug–OV-TL16 antibody conjugates. 1. Influence of the method of synthesis on the binding affinity to OVCAR-3 ovarian carcinoma cells in vitro. J Drug Targeting. 1996;3:357–373. doi: 10.3109/10611869608996827. [DOI] [PubMed] [Google Scholar]
- 182.Scales CW, Vasilieva YA, Convertine AJ, Lowe AB, McCormick CL. Direct, controlled synthesis of the nonimmunogenic, hydrophilic polymer, poly(N-(2-hydroxypropyl)methacrylamide) via RAFT in aqueous media. Biomacromolecules. 2005;6:1846–1850. doi: 10.1021/bm0503017. [DOI] [PubMed] [Google Scholar]
- 183.Teodorescu M, Matyjaszewski K. Atom transfer radical polymerization of (meth)acrylamides. Macromolecules. 1999;32:4826–4831. [Google Scholar]
- 184.Jensen K, Kopečková P, Kopeček J. Antisense oligonucleotides delivered to the lysosome escape and actively inhibit the hepatitis B virus. Bioconjug Chem. 2002;13:975–984. doi: 10.1021/bc025559y. [DOI] [PubMed] [Google Scholar]
- 185.Nori A, Jensen KD, Tijerina M, Kopečková P, Kopeček J. Tat-conjugated synthetic macromolecules facilitate cytoplasmic drug delivery to human ovarian carcinoma cells. Bioconjug Chem. 2003;14:44–50. doi: 10.1021/bc0255900. [DOI] [PubMed] [Google Scholar]
- 186.Lu Z-R, Kopečková P, Kopeček J. Semitelechelic Poly[N-(2-hydroxypropyl) methacrylamide] for biomedical applications. In: Ottenbrite RM, Kim SW, editors. Polymeric Drugs & Delivery Systems. Technomics Publishing Co; Lancaster, PA: 2001. pp. 1–14. [Google Scholar]
- 187.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulation time of bovine liver catalase. J Biol Chem. 1977;252:3582–3586. [PubMed] [Google Scholar]
- 188.Chiu HC, Zalipski S, Kopečková P, Kopeček J. Enzymatic activity of chymotrypsin and its poly(ethylene glycol) conjugates toward low and high molecular weight substrates. Bioconjug Chem. 1993;4:290–295. doi: 10.1021/bc00022a007. [DOI] [PubMed] [Google Scholar]
- 189.Lu ZR, Kopečková P, Wu Z, Kopeček J. Functionalized semitelechelic poly[N-(2-hydroxypropyl)methacrylamide] for protein modification. Bioconjug Chem. 1998;9:793–804. doi: 10.1021/bc980058r. [DOI] [PubMed] [Google Scholar]
- 190.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Disc. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 191.Kamei S, Kopeček J. Prolonged blood circulation in rats of nanospheres surface-modified with semitelechelic poly[N-(2-hydroxypropyl)methacrylamide] Pharm Res. 1995;12:663–668. doi: 10.1023/a:1016247206531. [DOI] [PubMed] [Google Scholar]
- 192.Oupický D, Ulbrich K, Říhová B. Conjugates of semitelechelic poly[N-(2-hydroxypropyl)methacrylamide] with enzymes for protein delivery. J Bioactive Compatible Polym. 1999;14:213–231. [Google Scholar]
- 193.Šubr V, Etrych T, Ulbrich K, Hirano T, Kondo T, Todoroki T, Jelínková M, Říhová B. Synthesis and properties of poly[N-(2-hydroxypropyl)methacrylamide] conjugates of superoxide dismutase. J Bioactive Compatible Polym. 2002;17:105–121. [Google Scholar]
- 194.Lääne A, Aaviksaar A, Haga M, Chytrý V, Kopeček J. Preparation of polymer-modified enzymes of prolonged circulation times. Poly[N-(2-hydroxypropyl)methacrylamide] bound acetylcholinesterase. Makromol Chem Suppl. 1985;9:35–42. [Google Scholar]
- 195.Ulbrich K, Strohalm J, Plocová D, Oupický D, Šubr V, Souček J, Poučková P, Matoušek J. Poly[N-(2-hydroxypropyl)methacrylamide] conjugates of bovine seminal ribonuclease. Synthesis, physicochemical, and preliminary biological evaluation. J Bioactive Compatible Polym. 2000;15:4–26. [Google Scholar]
- 196.Merdan T, Kopeček J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Delivery Rev. 2002;54:715–758. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 197.Kopeček J, Bažilová H. Poly[N-(2-hydroxypropyl)methacrylamide]. III. Cross-linking copolymerization. Europ Polym J. 1974;10:465–470. [Google Scholar]
- 198.Šubr V, Duncan R, Kopeček J. Release of macromolecules and daunomycin from hydrophilic gels containing enzymatically degradable bonds. J Biomat Sci, Polym Ed. 1990;1:261–278. doi: 10.1163/156856289x00145. [DOI] [PubMed] [Google Scholar]
- 199.Ulbrich K, Šubr V, Podpěrová P, Burešová M. Synthesis of novel hydrolytically degradable hydrogels for controlled drug release. J Control Release. 1995;34:155–165. [Google Scholar]
- 200.Št’astný M, Plocová D, Etrych T, Kovář M, Ulbrich K, Říhová B. HPMA-hydrogels containing cytostatic drugs. Kinetics of the drug release and in vivo efficacy. J Control Release. 2002;81:101–111. doi: 10.1016/s0168-3659(02)00047-0. [DOI] [PubMed] [Google Scholar]
- 201.Chivukula P, Dušek K, Wang D, Dušková-Smrčková M, Kopečková P, Kopeček J. Synthesis and characterization of novel aromatic azo bond-containing pH-sensitive and hydrolytically cleavable IPN hydrogels. Biomaterials. 2006;27:1140–1151. doi: 10.1016/j.biomaterials.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 202.Vermonden T, Fedorovich NE, van Geemen D, Ablas J, van Nostrum CF, Dhert WJ, Hennink WE. Photopolymerized thermosensitive hydrogels: synthesis, degradation, and cytocompatibility. Biomacromolecules. 2008;9:919–926. doi: 10.1021/bm7013075. [DOI] [PubMed] [Google Scholar]
- 203.Censi R, Vermonden T, van Steenbergen MJ, Deschout H, Braeckmans K, De Smedt SC, van Nostrum CF, di Martino P, Hennink WE. Photo-polymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J Control Release. 2009 doi: 10.1016/j.jconrel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 204.Wichterle O, Lím D. Hydrophilic gels for biological use. Nature. 1960;185:117–118. [Google Scholar]
- 205.Wichterle O. Method for centrifugal casting a contact lens. 3, 408. US Patent. 1968 Oct 29;:429.
- 206.Kopeček J, Yang J. Hydrogels as smart biomaterials. Polym Int. 2007;56:1078–1098. [Google Scholar]
- 207.Kopeček J. Hydrogel biomaterials: a smart future? Biomaterials. 2007;28:5185–5192. doi: 10.1016/j.biomaterials.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kopeček J. Smart and genetically engineered biomaterials and drug delivery systems. Eur J Pharmaceutical Sci. 2003;20:1–16. doi: 10.1016/s0928-0987(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 209.Kopeček J, Yang J. Peptide-directed self-assembly of hydrogels. Acta Biomaterialia. 2009;5:805–816. doi: 10.1016/j.actbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kopeček J, Tang A, Wang C, Stewart RJ. De novo design of biomedical polymers. Hybrids from synthetic macromolecules and genetically engineered protein domains. Macomol Symp. 2001;174:31–42. [Google Scholar]
- 211.Yang J, Xu C, Wang C, Kopeček J. Refolding hydrogels self-assembled from HPMA graft copolymers by antiparallel coiled-coil formation. Biomacromolecules. 2006;7:1187–1195. doi: 10.1021/bm051002k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yang J, Wu K, Koňák C, Kopeček J. Dynamic light scattering study of self-assembly of HPMA hybrid graft copolymers. Biomacromolecules. 2008;9:510–517. doi: 10.1021/bm701001f. [DOI] [PubMed] [Google Scholar]
- 213.Radu L, Yang J, Kopeček J. Self-assembling block copolymers of poly[N-(2-hydroxypropyl)methacrylamide] and a β-sheet peptide. Macromol Biosci. 2009;9:36–44. doi: 10.1002/mabi.200800193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Radu-Wu L, Yang J, Wu K, Kopeček J. Self-assembled hydrogels from poly[N-(2- hydroxypropyl)methacrylamide] grafted with β-sheet peptides. Biomacromolecules. 2009;10:2319–2327. doi: 10.1021/bm9005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yuan W, Yang J, Kopečková P, Kopeček J. Smart hydrogels containing adenylate kinase: translating substrate recognition into macroscopic motion. J Am Chem Soc. 2008;130:15760–15761. doi: 10.1021/ja805634x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang C, Stewart RJ, Kopeček J. Hybrid hydrogels assembled from synthetic polymers and coiled-coil protein domains. Nature. 1999;397:417–420. doi: 10.1038/17092. [DOI] [PubMed] [Google Scholar]
- 217.Wu K, Yang J, Koňák Č, Kopečková P, Kopeček J. Novel synthesis of HPMA copolymers containing peptide grafts and their self-assembly into hybrid hydrogels. Macromol Chem Phys. 2008;209:467–475. [Google Scholar]
- 218.Chen L, Kopeček J, Stewart RJ. Responsive hybrid hydrogels with volume transitions modulated by a titin immunoglobulin module. Bioconjug Chem. 2000;11:734–740. doi: 10.1021/bc000046h. [DOI] [PubMed] [Google Scholar]
- 219.Jacob MH, Amir D, Ratner V, Gussakowsky E, Haas E. Predicting reactivities of protein surface cysteines as part of a strategy for selective multiple labeling. Biochemistry. 2005;44:13664–13672. doi: 10.1021/bi051205t. [DOI] [PubMed] [Google Scholar]
- 220.Remzi Becer C, Hoogenboom R, Schubert US. Click chemistry beyond metal-catalyzed cycloaddition. Angew Chem Int Ed. 2009;48:4900–4908. doi: 10.1002/anie.200900755. [DOI] [PubMed] [Google Scholar]
- 221.Johnson RN, Kopečková P, Kopeček J. Synthesis and evaluation of multivalent branched HPMA copolymer–Fab′ conjugates targeted to the B cell antigen. Bioconjug Chem. 2009;20:129–137. doi: 10.1021/bc800351m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu J, Bauer H, Callahan J, Kopečková P, Pan H, Kopeček J. Endocytic uptake of a large array of HPMA copolymers: Elucidation into the dependence on the physicochemical characteristics. doi: 10.1016/j.jconrel.2009.12.022. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wu K, Liu J, Johnson RN, Yang J, Kopeček J. Drug-free macromolecular therapeutics: Induction of apoptosis by coiled-coil mediated crosslinking of antigens on cell surface. doi: 10.1002/anie.200906232. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]