Abstract
Adoptive transfer of antigen-specific cytotoxic T lymphocytes (CTLs) can induce objective clinical responses in patients with malignant diseases. The option of providing a proliferative and survival advantage to adoptively transferred CTLs remains a challenge to improve their efficacy. Host lymphodepletion and administration of recombinant interleukin-2 (IL-2) are currently used to improve CTL survival and expansion after adoptive transfer, but these approaches are frequently associated with significant side effects and may increase proliferation of T regulatory cells. IL-7 is a crucial homeostatic cytokine that has been safely administered as a recombinant protein. However, while IL-7 induces robust expansion of naive and memory T lymphocytes, the lack of expression of the IL-7 receptor α chain (IL-7Rα) by CTLs precludes their response to this cytokine. We found that CTLs can be genetically modified to re-express IL-7Rα, and that this manipulation restores the response of these cells to IL-7 without apparent modification of their antigen specificity or dependency, and without changing their response to other common γ (γc) chain cytokines. This approach may allow selective expansion of CTLs without the unwanted effects associated with IL-2.
Introduction
Adoptive transfer of antigen-specific cytotoxic T lymphocytes (CTLs) and tumor infiltrating T lymphocytes can provide objective clinical responses in patients with malignant diseases.12,3,4,5,6,7 These clinical responses appear to require the in vivo expansion and persistence of the infused CTLs2,6,8,9 so that significant efforts have been made to maintain T cell survival in vivo.
Cytokines such as interleukin (IL)-2 promote both T cell expansion and survival,10 and IL-2 has frequently been used to support adoptive T cell therapies.1,6,11 Although often effective, the prolonged administration of IL-2 is associated with significant toxicity that includes severe mucositis, nausea, diarrhea, edema, respiratory distress, liver and renal dysfunctions,11,12,13 and expansion of regulatory T cells that impair the function of CTLs.14 Infusion of IL-7 may offer an alternative approach.
IL-7 is a common γ (γc) chain cytokine that is essential for homeostatic expansion of naive T cells10,15 and for maintaining memory T cells.16,17 Administration of IL-7 accelerates immunoreconstitution in murine models,18 and the cytokine has been well tolerated in early phase clinical trials.19,20 In these human studies, IL-7 produced polyclonal expansion of circulating naive CD4+ and CD8+ T lymphocytes without measurable expansion of regulatory T cells.19,20 Unfortunately, IL-7 administration can have minimal effects on adoptively transferred CTLs, as few of these cells express the IL-7Rα at the time of infusion. We have, therefore, determined whether forced expression of IL-7Rα in CTLs can restore their response to IL-7, or whether the downstream response to ligation of this receptor is lost at the same time as the receptor itself ceases to be expressed. Using our model of Epstein–Barr-Virus-specific CTLs (EBV-CTLs) that target post-transplant lymphomas, Hodgkin's lymphoma and nasopharyngeal carcinoma,2,4,5,21 we found that transgenic expression of IL-7Rα consistently promotes CTL proliferation in response to IL-7 both in vitro and in vivo, and provides antitumor activity. These effects occur without modification of CTL antigen specificity or removal of their antigen dependency. Hence, IL-7 can induce equivalent antitumor activity to IL-2 when CTLs express a transgenic IL-7Rα.
Results
Downregulation of IL-7Rα on antigen-specific CTLs
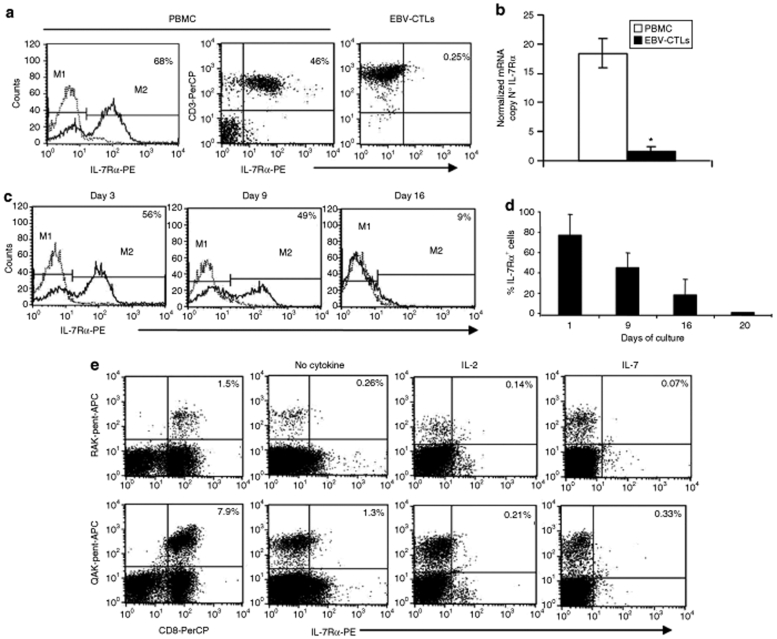
We used fluorescent flow cytometry to analyze IL-7Rα expression by established EBV-CTL lines made from five healthy EBV-seropositive donors. As shown in (Figure 1a), IL-7Rα was expressed on almost half of the freshly isolated peripheral blood mononuclear cells, but on only 2.4 ± 0.5% of the EBV-CTL lines derived therefrom. This low expression of IL-7Rα by EBV-CTLs correlated with a low expression of IL-7Rα specific messenger RNA transcripts measured by quantitative RT-PCR (Q-PCR) (Figure 1b).
Figure 1.
IL-7Rα is downregulated in EBV-CTLs. (a) illustrates the expression of IL-7Rα, as assessed by fluorescence-activated cell sorter (FACS) analysis, in freshly isolated PBMC and in a representative EBV-CTL line obtained after three stimulations with EBV-LCLs and IL-2. Solid lines and dashed lines represent the profile of the IL-7Rα and isotype control, respectively. (b) mRNA copy number for IL-7Rα detected by Q-PCR in unmanipulated PBMC and in EBV-CTL lines. PBMC and EBV-CTLs contained 18 ± 2.5 and 1.6 ± 0.8 copies of IL-7Rα mRNA, respectively (*P = 0.007). Data represent the results obtained in three different donors. (c) Downregulation of the IL-7Rα by day 16–20 in PBMC stimulated with EBV-LCLs on day 1 (PBMC:EBV-LCLs ratio 40:1) and on day 10 (CTLs: EBV-LCLs ratio 4:1). Data show histograms for a representative experiment in which solid lines and dotted lines represent the profile of IL-7Rα and isotype control, respectively. (d) Expression of IL-7Rα in PBMC measured at different time points during the generation of EBV-CTL lines in three different experiments. (e) Expression of IL-7Rα in EBV-CTLs detected using pentamer staining for two EBV-derived peptides (HLA-B8-RAKFKQLL-BZLF1 and HLA-B8-QAKWRLQTL-EBNA3A). Staining was performed on day 16 of culture. The expression of IL-7Rα was almost undetectable in both RAK-pentamer- and QAK-pentamer-positive cells. The addition of IL-2 (50 U/ml) or IL-7 (5 ng/ml) to the culture on day 1 and on day 10 or only on day 10 of culture did not affect the downregulation of IL-7Rα in EBV-CTLs. Data are representative of three different EBV-CTL lines. HLA, human leukocyte antigen.
The generation of CTLs for adoptive T cell transfer therapies requires multiple rounds of stimulation with antigen expressed by antigen-presenting cells.2 T cell receptor stimulation, whether by antigen or mitogen (OKT3 antibody), downregulates expression of IL-7Rα (data not shown). We determined the kinetics of this down modulation during the preparation of EBV-CTLs from primary T lymphocytes. As shown in (Figure 1c,d), the expression of IL-7Rα by the cultured T cells rapidly declined after each stimulation with EBV-lymphoblastoid cell lines (EBV-LCLs), so that by day 16 the overall level of IL-7Rα+ cells had fallen to <10%. EBV-CTLs detected within the cultured T cells by their binding of human leukocyte antigen–pentamers were entirely IL-7Rα− (Figure 1e). The kinetics of IL-7Rα down modulation in EBV-CTLs was not modified by the addition of IL-2 or IL-7 to the CTL cultures (Figure 1e).
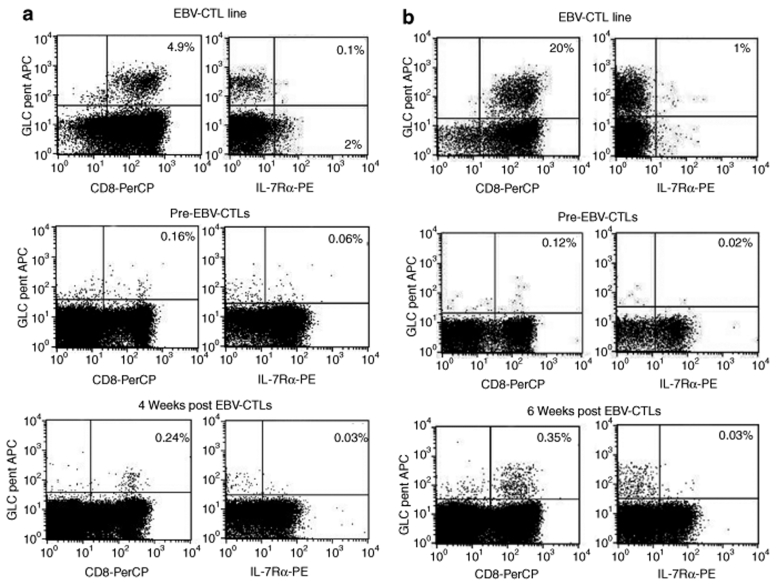
To discover whether down regulation of IL-7Rα during antigen-specific CTL induction and expansion was uniquely associated with EBV-CTLs or a phenomenon encountered during generation of human antigen-specific CTLs in general, we measured the expression of IL-7Rα by CTL lines specific for two other viral antigens such as pp65 Cytomegalovirus22 and the hexon capsid protein of AdV,23,24 and for cancer-testis antigens25 As shown in Supplementary Figure S1a,b, the lack of IL-7Rα expression was also documented in other virus-specific CTLs and cancer-testis antigen–specific CTL lines/clones. Finally, in order to evaluate whether IL-7Rα can be re-expressed by EBV-CTLs after adoptive transfer as previously described for tumor infiltrating T lymphocytes in melanoma patients,26 we collected peripheral blood samples, at different time points, from patients who received autologous EBV-CTLs as treatment/prevention of EBV-associated diseases.27,28 In two patients, we documented an increase of EBV-CTL precursors 4–6 weeks after CTL infusion using tetramer staining. As shown in (Figure 2a,b), EBV-tetramer+ cells detected in vivo after adoptive transfer remained largely IL-7Rα−.
Figure 2.
IL-7Rα remains downregulated in EBV-CTLs detected in vivo after adoptive transfer in patients with EBV-related diseases. Samples of peripheral blood were collected from patients treated with autologous EBV-CTLs and enrolled in previously reported clinical trials.27,28 (a,b) Data for two patients. Upper plots show the staining for GLC-tetramer of the EBV-CTL lines infused into the patients. GLC-tetramer+ cells lacked the expression of IL-7Rα. For the first patient (a), the frequency of GLC-tetramer+ CTLs increased from 0.1% pre-CTL infusion (middle plots) to 0.24% (lower plots) four weeks after CTL infusion. For the second patient (b), the frequency of GLC-tetramer+ CTLs raised from 0.12% pre-CTL infusion (middle plots) to 0.35% (lower plots) 6 weeks after CTL infusion. In both cases, the great majority of tetramer+ cells detected post-CTL infusion remained IL-7Rα negative (lower plots).
Functional IL-7Rα can be expressed by EBV-CTLs after γ-retroviral gene transfer
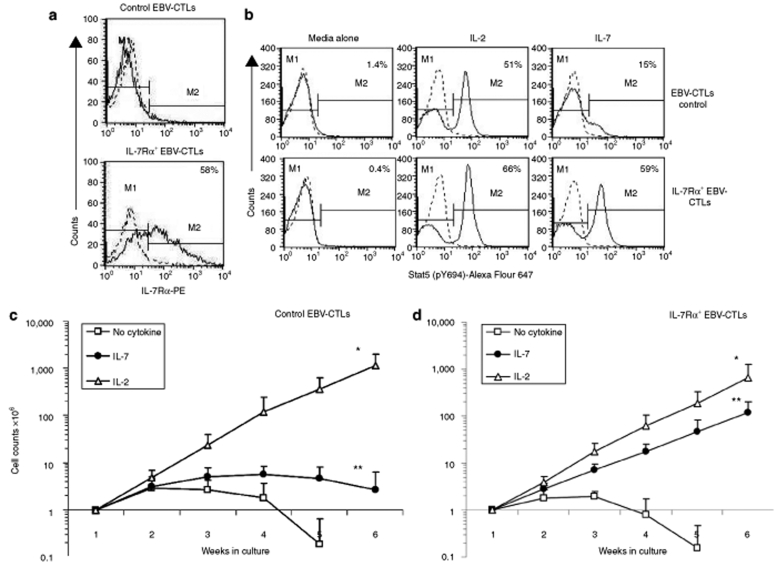
Since antigen-specific CTLs lack IL-7Rα expression, we used gene transfer to force expression of this molecule. EBV-CTL lines established from five healthy EBV-seropositive donors were transduced with SFG.IL-7Rα γ-retroviral vector. IL-7Rα expression, measured by fluorescence-activated cell sorter analysis, increased from 2.4 ± 0.5% to 50 ± 20% in IL-7Rα+EBV-CTLs (Figure 3a), while remaining almost undetectable in nontransduced CTLs or in CTLs transduced with a control vector (control EBV-CTLs). Q-PCR showed an 11 ± 3-fold increase in the copy number of IL-7Rα mRNA in IL-7Rα+EBV-CTLs as compared to controls (data not shown). To discover whether the transgenic IL-7Rα was functional in EBV-CTLs, we measured Stat5 phosphorylation in response to IL-2 and IL-7.29 Incubation for 15 minute with IL-7 (5 ng/ml) induced Stat5 phosphorylation to Tyr-694 in IL-7Rα+EBV-CTLs, but not in control EBV-CTLs (Figure 3b). In contrast, IL-2 (50 U/ml) induced Stat5 phosphorylation in controls and IL-7Rα+EBV-CTLs (Figure 3b) suggesting that physiologically expressed IL-2 receptor complex on EBV-CTLs remains functional in IL-7Rα+EBV-CTLs.
Figure 3.
Forced expression of IL-7Rα in EBV-CTLs provides responsiveness to IL-7. (a) Expression of IL-7Rα in control and IL-7Rα+EBV-CTLs. Solid and dashed lines represent the profile of IL-7Rα and isotype control, respectively. Data show a representative phenotypic analysis of five donors. (b) IL-2 induces Stat5 phosphorylation in both control and IL-7Rα+EBV-CTLs, while IL-7 induces Stat5 phosphorylation only in IL-7Rα+EBV-CTLs. Solid and dashed lines represent the profile of phosphorylated Stat5 and isotype control, respectively. (c,d) Expansion of control and IL-7Rα+EBV-CTLs after repeated weekly stimulations with EBV-LCLs and IL-2 (50 U/ml) or IL-7 (5 ng/ml). Control and IL-7Rα+EBV-CTLs equally expanded in response to the antigens and IL-2 (open triangle of c and d; *P = 0.196). In contrast, IL-7Rα+EBV-CTLs but not control EBV-CTLs significantly expanded when stimulated with EBV-LCLs and IL-7 (closed circles of d and c, respectively; ** P < 0.001). Control and IL-7Rα+EBV-CTLs equally declined in viable cell counts when exposed to EBV-LCLs alone.
To assess the expansion of IL-7Rα+EBV-CTLs in response to IL-7, both control and IL-7Rα+EBV-CTLs were stimulated once a week with EBV-LCLs alone, or with IL-2 (50 U/ml), or IL-7 (5 ng/ml). Viable cells were counted using Trypan blue exclusion. As shown in (Figure 3c,d), both control and IL-7Rα+EBV-CTLs expanded equally well in response to EBV-LCLs and IL-2 over 6 weeks of culture [from 1 × 106 cells to 1.1 × 109 (range 0.1 × 109 to 1.3 × 109) for control EBV-CTLs and from 1 × 106 cells to 0.7 × 109 (range 0.7 × 107 to 1.6 × 109) for IL-7Rα+EBV-CTLs (P = 0.196)]. In contrast, only IL-7Rα+EBV-CTLs expanded significantly when stimulated with EBV-LCLs and IL-7 [from 1 × 106 cells to 0.1 × 109 (range 0.6 × 108 to 0.3 × 109)], while viable cell numbers in control EBV-CTL lines progressively declined (P < 0.001). Both control and IL-7Rα+EBV-CTLs equally declined in viable cells when stimulated with EBV-LCLs without addition of cytokines.
IL-7Rα+ EBV-CTLs have a selective growth advantage in the presence of IL-7, but retain responsiveness to other γc chain cytokines, antigen specificity and effector function
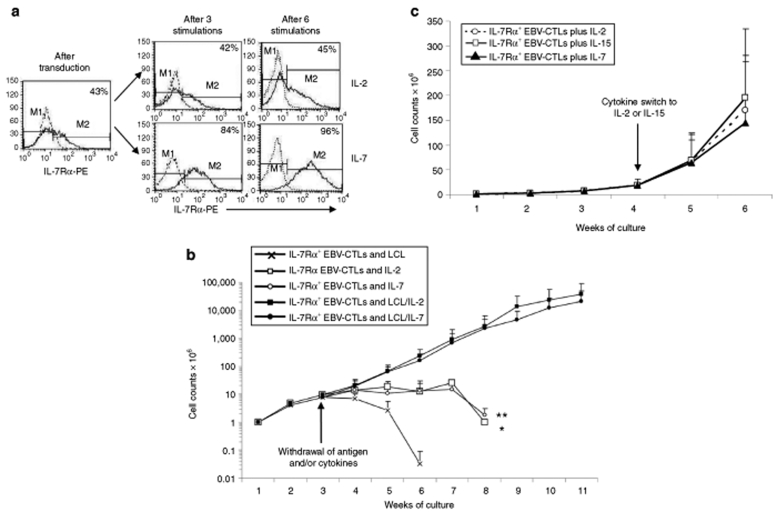
As anticipated, stimulation with EBV-LCLs and IL-7 produced a growth advantage for IL-7Rα+EBV-CTLs, since the percentage of IL-7Rα+ cells increased from 50 ± 20%, immediately after transduction, to 73 ± 23% (P = 0.0027) within one week of culture in the presence of IL-7 (Figure 4a). IL-7Rα expression was then sustained overtime. In contrast, the percentage of IL-7Rα+ cells was stable on culture in IL-2 (from 50 ± 22% to 48 ± 21%) indicating equal expansion of control and IL-7Rα+EBV-CTLs (Figure 4a). Despite the observed positive selection in the presence of IL-7, IL-7Rα+EBV-CTLs did not develop autonomous growth, because removal of antigen stimulation led to loss of growth in response to either IL-2 or IL-7 (Figure 4b). Finally, transgenic expression of IL-7Rα and progressive selection by culture with IL-7 did not impair the capacity of EBV-CTLs to respond to other γc chain cytokines. As shown in (Figure 4c), IL-7Rα+EBV-CTLs that had been expanded and selected with EBV-LCLs and IL-7 for >2 weeks continued to expand when exposed to EBV-LCLs and either IL-2 (50 U/ml) or IL-15 (10 ng/ml).
Figure 4.
Transgenic IL-7Rα does not sustain antigen independent growth of EBV-CTLs and does not impair responsiveness to other γc chain cytokines. (a) Percentage of IL-7Rα+ cells remained unchanged when IL-7Rα+EBV-CTLs were weekly stimulated with EBV-LCLs and IL-2 (50 U/ml), but it significantly increased when IL-7 (5 ng/ml) was used. The growth of IL-7Rα+EBV-CTLs remained, however, antigen dependent, as removal of EBV-LCLs (last stimulation with LCL on week 2) halted their expansion even after the addition of IL-2 (*P < 0.0001) or IL-7 (**P < 0.0001). (b) Data represent mean ± SD of five EBV-CTL lines. (c) IL-7Rα+EBV-CTLs expanded in vitro with EBV-LCLs, and IL-7 remained capable to respond to other γc chain cytokines as they continued to expand when exposed to the antigens and IL-2 (50 U/ml) or IL-15 (10 ng/ml). Data represent mean ± SD of three EBV-CTL lines.
To characterize the phenotype and function of EBV-CTLs expressing IL-7Rα, we examined IL-7Rα+EBV-CTLs cultured in IL-7 or IL-2 and compared them with control EBV-CTLs expanded in IL-2. After 6 weeks of culture, the majority of IL-7Rα+EBV-CTLs that grew either with IL-7 or IL-2 remained mainly CD3+/CD8+ (90 ± 8% and 91 ± 5%, respectively) similar to that of control EBV-CTLs cultured with IL-2 (91 ± 5%) (Supplementary Figure S2a), and maintained a memory-effector phenotype (Supplementary Table S1). Cytotoxic activity, measured in a 51Cr release assay, remained specific for autologous EBV-LCLs confirming the retention of Major histocompatibility complex–restriction (Supplementary Figure S2b). We also quantified the epitope specificity of each EBV-CTL population, using multimer and flow cytometry analysis to measure binding, and IFN-γ enzyme-linked immuno (ELI) spots to measure CTL responsiveness. As shown in Supplementary Figure S2c,d and Supplementary Table S2, the frequency, range, and function of tetramer/pentamer positive EBV-CTLs was retained. IL-7Rα+EBV-CTLs remained polyclonal as assessed by measuring the distribution of Vβ T cell receptor repertoire (Supplementary Table S3a,b).
IL-7Rα+ EBV-CTLs expand in vivo in response to IL-7
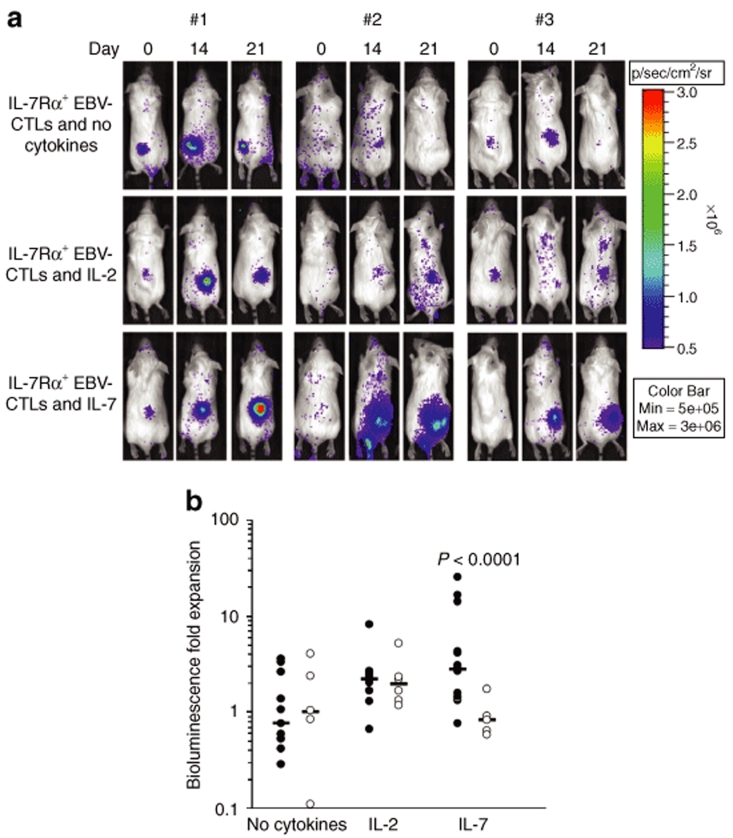
To assess the in vivo function of IL-7Rα+EBV-CTLs we used a severe combined immunodeficiency (SCID) mouse model of EBV+ human lymphoma (EBV-LCLs) and an in vivo imaging system.30,31 In this model the expansion of EBV-CTLs in response to EBV-LCLs and cytokines was measured as bioluminescence signal from EBV-CTLs cotransduced with the γ-retroviral vector encoding for eGFP-Firefly luciferase (eGFP-FFLuc).30 The percentages of EBV-CTLs expressing eGFP-FFLuc were equal among control and IL-7Rα+EBV-CTLs, and was ranging from 30 to 40% (data not shown). We have also previously shown that increases in light emission from a given site are directly proportional to increases in EBV-CTL numbers.30 Mice were engrafted subcutaneously with EBV-LCLs (10 × 106/mouse), and when the tumor was palpable, a single dose of 10 × 106 control or IL-7Rα+EBV-CTLs was injected intravenously. Mice were then divided into three groups: (i) No cytokines, (ii) IL-2 (1,000 U/mice × 3 times a week), and (iii) IL-7 (200 ng × 3 times a week). Localization and expansion of the EBV-CTLs in the tumor area was measured using the Xenogen-In Vivo Imaging System.30 As illustrated in (Figure 5a,b), mice receiving control or IL-7Rα+EBV-CTLs showed CTL homing to the tumor site. By day 15–21, in mice receiving either control or IL-7Rα+EBV-CTLs, CTL signals increased equally in response to IL-2. CTL signal increased 1.9-fold (range 1.17–5.19) in mice receiving control EBV-CTLs and IL-2 when compared with mice that received the same CTLs but no IL-2 (1.05-fold, range 0.11–4.06) (P = 0.154). Similarly, CTL signal increased 2.2-fold (range 0.67–8.16) in mice receiving IL-7Rα+EBV-CTLs and IL-2 when compared with mice that did not received the cytokine (0.76-fold, range 0.29–3.56) (P = 0.003). In contrast, only IL-7Rα+EBV-CTLs expanded in vivo in response to IL-7. By day 15–21, CTL signal increased 2.8-fold (range 0.77–25.2) in mice receiving IL-7Rα+EBV-CTLs and IL-7, while expansion was minimal in mice treated with control EBV-CTLs and IL-7 (0.83-fold, range 0.58–1.74) (P < 0.0001).
Figure 5.
In vivo expansion of IL-7Rα+EBV-CTLs. SCID mice engrafted subcutaneously with EBV-LCLs (10 × 106 cells) were injected intravenously either with control or IL-7Rα+EBV-CTLs (10 × 106 cells/mouse). To track their homing and in vivo expansion, EBV-CTLs were transduced with a γ-retroviral vector encoding for eGFP-FFLuc. CTL localization and expansion was monitored using an in vivo imaging system. For these experiments, we used EBV-CTL lines generated from three healthy EBV-seropositive donors. Mice were then divided in three groups and received intraperitoneal injection of IL-2, IL-7, or no cytokines after CTL transfer. (a) Images of representative mice receiving IL-7Rα+EBV-CTLs. The CTL signal intensity increased by day 15–21 in mice treated either with IL-2 or IL-7 as compared to mice receiving no cytokines. (b) The bioluminescence data of all mice engrafted with EBV-LCLs and treated either with control (open symbols) or IL-7Rα+EBV-CTLs (closed symbols), and receiving no cytokines or intraperitoneal injection of IL-2 or IL-7, after CTL transfer. The graph summarizes the fold expansion in bioluminescence obtained by day 15 after CTL transfer. Control and IL-7Rα+EBV-CTLs localized at the tumor site and equally expanded in mice treated with IL-2 compared to mice receiving CTLs without cytokines. On the contrary, when mice were treated with IL-7, CTL signals increased in mice infused with IL-7Rα+EBV-CTLs but not in those infused with control EBV-CTLs (P < 0.0001).
IL-7Rα+EBV-CTLs have antitumor activity in vivo in response to either IL-2 or IL-7
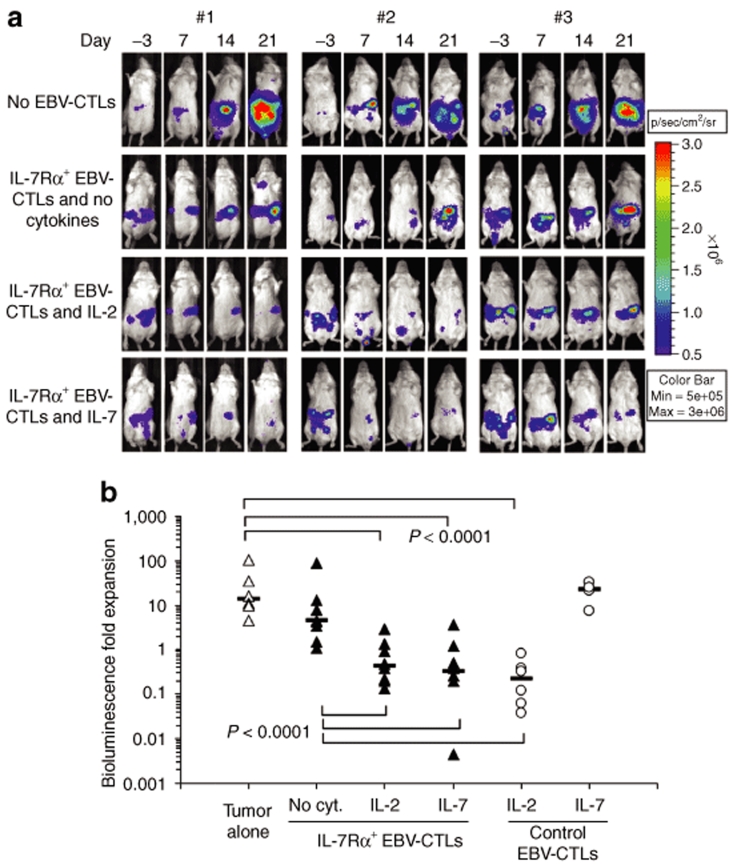
We have previously reported that adoptive transfer of EBV-CTLs and administration of IL-2 can control the growth of EBV+ lymphomas in SCID mice.30 To establish whether IL-7Rα+EBV-CTLs retained similar activity in response to IL-7, SCID mice were engrafted intraperitoneally with EBV-LCLs transduced with the vector encoding for FFLuc (3 × 106 cells/mouse). Once tumor signals were consistently detectable and increasing in at least two consecutive luminescence analyses (usually 7–10 days after tumor inoculation), mice were divided into a control group (no EBV-CTLs) and three experimental groups, receiving IL-7Rα+EBV-CTLs (10 × 106 per dose for three doses) either alone, or with IL-2 (1,000 U/mice 3 times a week), or with IL-7 (1,000 ng/mice three times a week). Two other groups received control EBV-CTLs and either IL-2 or IL-7. After treatment, tumor growth was measured using the bioluminescence system. As shown in (Figure 6a,b), tumor signal rapidly increased in mice that did not receive EBV-CTLs (13.4-fold, range 4.4–103). These animals were euthanized by day 20–25 after EBV-LCL injection. Tumor growth was also observed in mice receiving IL-7Rα+EBV-CTLs but no cytokines (4.47-fold, range 1–90). On the contrary, in mice receiving IL-7Rα+EBV-CTLs and either IL-2 or IL-7, the tumor signal significantly declined for at least 2 weeks (0.42-fold, range 0.13–2.90; and 0.32, range 0.004–3.57, respectively) (P < 0.0001 compared to those without cytokines). As expected, in mice treated with control EBV-CTLs and IL-2, tumor signals declined (0.22-fold, range 0.03–0.8) as observed for mice receiving IL-7Rα+EBV-CTLs and either IL-2 or IL-7 (P = 0.16). However, in mice treated with control EBV-CTLs and receiving IL-7, we did not observe control of the tumor growth (22.9-fold, range 7.35–32.6). Hence, IL-7 induces equivalent antitumor activity to IL-2 when EBV-CTLs express a transgenic IL-7Rα.
Figure 6.
In vivo antitumor effect of IL-7Rα+EBV-CTLs. To evaluate the antitumor effect, SCID mice were engrafted in the peritoneum with EBV-LCLs (3 × 106 cells/mouse) labeled with FFLuc, and then treated either with control or IL-7Rα+EBV-CTLs 7–10 days later. For these experiments, we used EBV-CTL lines generated from three healthy EBV-seropositive donors. Mice were divided in three groups that received no cytokines, or IL-2, or IL-7. An additional control group received no EBV-CTLs. Tumor growth was monitored using an in vivo imaging system. (a) Tumor growth in representative mice receiving IL-7Rα+EBV-CTLs. Tumor signals progressively increased in mice that did not receive CTLs or were treated with CTLs but no cytokines. These mice were euthanized by day 20–25 after CTL infusion for excessive tumor growth. On the contrary, significant control of tumor growth was observed in mice receiving IL-7Rα+EBV-CTLs that were treated either with IL-2 or IL-7. (b) Bioluminescence signal as a measurement of tumor growth by day 14 after CTL infusions in mice receiving control or IL-7Rα+EBV-CTLs. Tumor bioluminescence rapidly increased in mice that did not receive CTLs, or received IL-7Rα+EBV-CTLs but no cytokines. On the contrary, tumor growth was controlled for at least 2 weeks when mice received IL-7Rα+EBV-CTLs and either IL-2 or IL-7 (P < 0.0001). Similar tumor control was observed in mice receiving control EBV-CTLs and IL-2, but not in mice that received control EBV-CTLs and IL-7.
Discussion
Providing adoptively transferred antigen-specific CTLs with improved expansion and long-term survival in vivo should benefit their effectiveness as cancer immunotherapy.11,13,32,33 At this point, we show that this effect can be achieved by engineering EBV-CTLs to efficiently respond to IL-7, a cytokine that physiologically maintains T lymphocyte homeostasis15 and can be safely administered as a recombinant protein.19,20 Thus, the forced expression of the IL-7Rα subunit in EBV-CTLs restores their responsiveness to IL-7, and sustains their activity against a model human tumor in vitro and in vivo without affecting their antigen specificity or dependency.
T lymphocyte expansion and persistence requires, among other things, the cytokines IL-2, IL-7, and IL-15.10 Although the receptors for these cytokines share the γc chain component, each plays a distinct role at different phases of T cell development and each has a distinct signaling pathway.10,16,29 IL-2Rα and IL-15Rα specific subunits and the shared IL-2/15Rβ subunit are highly expressed after T cell receptor engagement, so that signaling through both pathways supports activation induced expansion of T lymphocytes.10 Expression of IL-7Rα, by contrast, is limited to naive and memory T cells, and its dominant role is in sustaining or restoring lymphoid homeostasis.15,18 Once T lymphocytes have encountered their cognate antigen and differentiated to effector T cells, IL-7Rα is downregulated in all but a small subset of effector CD8+ T lymphocytes that may form long-term memory T cells.16
These differentiation-associated changes in IL-7Rα expression have significant implications for the adoptive transfer of antigen-specific CTLs, as the in vitro stimulation received by peripheral blood T lymphocytes from antigens and cytokines can successfully expand the desired CTL populations, but would lead to a concomitant loss of IL-7Rα expression. It is presently evident that the in vivo expansion of CTLs after adoptive transfer is best accomplished in the lympho-depleted host, since in these individuals there is an increased production of IL-7 and IL-15 in an effort to restore the physiologic size of the lymphoid pool.13,32,34,35 Although CTLs generated ex vivo respond to IL-15, their lack of IL-7Rα will render them unresponsive in vivo not only to the endogenous released IL-7 but also to the administered recombinant protein. Downregulation of IL-7Rα during effector T cell differentiation is a general phenomenon. Although it has been reported that tumor-infiltrating T lymphocytes isolated from melanoma re-express IL-7Rα after adoptive transfer in vivo and acquire a less differentiated phenotype,26 these cells are more heterogeneous than highly selected antigen-specific CTLs, which do not physiologically re-express IL-7Rα in vivo. Our data showing that forced expression of IL-7Rα restores EBV-CTL response to IL-7 demonstrates that the signaling pathway downstream of the receptor remains functional in antigen-specific CTLs and that at this point the cells can expand and survive in response to IL-7 as well as to IL-2 and IL-15. We also found that IL-7Rα+ EBV-CTLs responding to IL-7 retain antitumor effects both in vitro and in vivo against EBV+ lymphomas and that the potency of the IL-7 induced effect was equivalent to IL-2, a cytokine that may induce significant toxicity and unwanted expansion of regulatory T cells. This restoration of IL-7 responsiveness is not exclusive to EBV-CTLs because we have found that re-expression of IL-7Rα by CTLs specific for other tumor-associated antigens has equivalent effects (Supplementary Figure S3).
In view of the fact that IL-7Rα is constitutively expressed predominantly by naive T cells, its forced re-expression in antigen-specific CTLs raises the concern that cytokine stimulation will not only promote cell survival and expansion, but will also produce unwanted alterations of function, such as diminished cytotoxic activity or shifts in the polarity of the cytokines released. Because CTLs are polyclonal, it is also possible that some subsets would respond with greater proliferation than others following cytokine stimulation, leading to selective outgrowth of CTLs with a more restricted range of specificities. IL-7 appears to have none of these undesired effects, unlike other γc chain cytokines such as IL-4, which drastically diminish cytolytic activity of mature CD8+ T lymphocytes.36,37 Forced expression of IL-7Rα by EBV-CTLs did not alter their cytotoxicity, specificity, or capacity to respond to other γc cytokines that provide proliferative signals, such as IL-2 and IL-15. Forced expression of IL-7Rα and IL-7 exposure in vitro also had no effect on the phenotype of the EBV-CTLs, which retained the effector-memory profile that has been associated with long-term persistence in our previous studies.8
From a safety perspective, it is encouraging to know that the growth of IL-7Rα+EBV-CTLs remained strictly antigen dependent and that expansion ceased after antigen withdrawal, even in the presence of cytokines. Although γ-retroviral gene transfer of the γc chain to the hematopoietic stem cells of X-SCID patients has led to five cases of T cell leukemia,38,39 the fact that we introduced the α-chain of one cytokine receptor rather than the γc chain shared by the receptors for multiple cytokines, and the fact that our target cells are fully differentiated T cells rather than hematopoietic stem cells reinforce the safety of this approach for a clinical application. However, whether the inclusion of a suicide gene is necessary, IL-7Rα could be coexpressed with one of them as we previously described for antigen-specific CTL genetically modified to express cytokines.30
We conclude that forced expression of the IL-7Rα by CTLs can be used to recapitulate the response of these cells to this cytokine and thereby promote their in vivo antitumor activity after an adoptive transfer, avoiding the detrimental effects that can be encountered using the administration of recombinant IL-2 to sustain the expansion of adoptively transferred tumor-specific CTLs.
Materials and Methods
Plasmid construction and retrovirus production. Full-length human IL-7Rα (NCBI NM_002185) was cloned into the SFG γ-retroviral vector (SFG.IL-7Rα).30 The γ-retroviral vectors encoding the fusion protein (eGFP-FFLuc) and FFLuc were previously described.30 γ-Retroviral supernatants were prepared using transient transfection of 293T cells, as previously described.40
Generation and transduction of antigen specific CTLs. EBV-CTLs were prepared using peripheral blood mononuclear cells, obtained from healthy donors (protocol approved by the Baylor College of Medicine's IRB), EBV-transformed lymphoblastoid cells (EBV-LCLs), and IL-2 (50 U/ml) (Proleukin; Chiron, Emeryville, CA) as previously described.2 EBV-CTL lines obtained after three stimulations were transduced with γ-retroviral supernatants, as previously described.30 In brief, 3 days after the stimulation with EBV-LCLs, CTLs were plated in 24-well plates precoated with a recombinant fibronectin fragment (FN CH-296; Retronectin; Takara Shuzo, Otsu, Japan). After the addition of γ-retroviral supernatant and IL-2 (100 U/ml), CTLs were spun and incubated at 37 °C in 5% CO2. Three days later EBV-CTLs were collected and then maintained in culture by weekly stimulation with EBV-LCLs and IL-2 (50 U/ml) or IL-7 (5 ng/ml) (PeproTech; Rocky Hill, NJ). The procedures to generate CTLs specific for Cytomegalovirus, adenovirus, and cancer-testis antigens were previously described.24,25
Immunophenotyping. Cells were stained with CD3, CD4, CD8, CD56, CD127, CD62L, CD28, CD27, CCR7, CD45RO, CD45RA monoclonal antibodies from Becton-Dickinson (Mountain View, CA) and with monoclonal antibodies specific for the T cell receptor -Vβ repertoire (IOTest βMark kit; Immunotech, Emeryville, CA). To detect the phosphorylation of Stat5, we used a monoclonal antibody binding to Tyr-694 and conjugated with Alexa Fluor 647 (from Becton-Dickinson). Human leukocyte antigen–tetramers and pentamers were provided by Baylor College of Medicine core facility and by Proimmune (Bradenton, FL), respectively. Cells were analyzed by a FACScan (Becton-Dickinson).
Chromium release assay. We evaluated the cytotoxic activity of EBV-CTLs using a standard 4-hour 51Cr release assay, as previously described.2 As target cells we used autologous and mismatched EBV-LCLs and K562 cell line to, respectively, measure major histocompatibility complex restricted, major histocompatibility complex unrestricted, and natural killer activity.
Real-time Q-PCR. IL-7Rα transcripts were detected by Q-PCR. Total RNA extraction and amplification were performed as previously described.25 Primer and probe sequences were provided by Applied Biosystems (Foster City, CA).
Enzyme-linked immunospot assay. IFN-γ enzyme-linked immunospot assay was performed, as previously described.28 T cells were plated in triplicate and serially diluted from 1 × 105 to 1 × 104 cells/well and then peptide (5 µmol/l) was added to each. T cells incubated with an irrelevant peptide and T cells stimulated with 25 ng/ml phorbol myristate acetate and 1 µg/ml ionomycin (Sigma, St Louis, MO) were used as negative and positive control, respectively.
Expansion and antitumor activity in a xenogenic SCID mouse model. To assess the expansion, persistence, and antitumor effect of EBV-CTLs in vivo, we used a SCID mouse model and an in vivo imaging system as previously described.30,31 CB17 SCID mice 8–10 week old were purchased from Harland Sprague Dawley (Indianapolis, IN). Mouse experiments were performed in accordance with Baylor College of Medicine's Animal Husbandry guidelines.
To evaluate the in vivo expansion, control and IL-7Rα+EBV-CTLs were transduced with a second γ-retroviral vector encoding for eGFP-FFLuc.30 SCID mice were sublethally irradiated (250 rad) and injected subcutaneously with 10 × 106 EBV-LCLs suspended in Matrigel (Becton-Dickinson). When the tumor was palpable (15–20 days after inoculation), one single dose of EBV-CTLs (10 × 106) was injected intravenously. After CTL transfer, mice received IL-2 (1,000 U) or IL-7 (200 ng) intraperitoneally three times per week. Control groups received no cytokines after CTL transfer. To in vivo image EBV-CTLs expressing eGFP-FFLuc, mice were injected intraperitoneally with D-luciferin (150 mg/kg), and analyzed using the Xenogen-In Vivo Imaging System, as previously described.30 The intensity of the signal was measured as total photon/s/cm2/sr (p/s/cm2/sr).41
To evaluate the antitumor effects, EBV-LCLs were transduced with the γ-retroviral vector encoding for FFluc and then selected in puromicin for two weeks. SCID mice were engrafted intraperitoneally with EBV-LCLs (3 × 106 cells/mouse), and tumor growth was monitored using the bioluminescence system. When the signal was consistently increasing, usually by day 7–10, mice were treated intraperitoneally with three doses of control or IL-7Rα+EBV-CTLs (10 × 106 CTLs/mouse). The animals were divided in three groups that received either IL-2 (1,000 U) or IL-7 (1,000 ng) intraperitoneally three times a week or no cytokines. One additional control group received only tumor cells.
Statistical analysis. All in vitro data are presented as mean ± 1 SD. After log transformation, ANOVA and Student's t test were used to compare the cell count data between treatment groups with multiple comparisons adjusted by Tukey method. When P-value < 0.05, a mean difference was accepted as statistically significant. To compare the growth and expansion trend over time, cell count data for every time points were analyzed by the generalized linear model. For the bioluminescence experiments, intensity signals were log-transformed and summarized using mean ± SD at baseline and multiple subsequent time points for each group of mice. Changes in intensity of signal from baseline at each time point were summarized. The response profiles over time were analyzed by the robust generalized estimating equations method for the repeated measurements.
Supplementary Material Figure S1. IL-7Rα is down regulated in CTLs specific for cytomegalovirus, adenovirus, and cancer testis antigens. Multivirus-specific CTLs were generated as previously described(25). Briefly PBMC obtained from healthy seropositive donors were stimulated with autologous monocytes and EBV-LCLs transduced with an adenovirus vectors encoding the CMV pp65 protein (AdF35pp65-I-GFP)(29) as a source of CMV and Adenovirus antigens. IL-2 (50 U/mL) was added to the culture at day 14. The expression of IL-7Rα was monitored by FACS analysis during culture. Panel a shows that IL-7Rα expression was down regulated in T lymphocytes during the generation of virus-specific CTLs. IL-7Rα was undetectable in CMV- and Ad-specific CTLs quantified using NLV- and TDL-specific tetramer staining, respectively (Panel b). Panels c-f illustrate the functional characterization of multivirus-specific CTL lines using IFNγ Elispot assay (panels c and e) and cytotoxicity assay (panels d and f). Figure S2. Growth in IL-7 does not impair the antigen specificity of IL-7Rα+EBV-CTLs. CTL clones specific for tumor-associated antigens such as PRAME were generated as previously described(24). Briefly, polyclonal T-cell lines obtained after 3 stimulations with artificial antigen presenting cells loaded with peptides derived from PRAME antigen were cloned by limiting dilution assay in 96 well plates, in the presence of IL-2 (100 U/mL), CD3 antibody (50 ng/mL OKT3; Ortho Biotech, Bridgewater, NJ) and irradiated (4,000 rad) allogeneic PBMC as feeder cells. Panel a show that these clones lack the expression of IL-7Rα by FACS analysis. Panels b and c illustrate that these clones are specific for the ALY-PRAME peptides using IFNγ Elispot assay (panel b) and cytotoxicity assay (panels c) against PHA-blasts loaded with ALY-peptide or an irrelevant peptide as target cells. Figure S3. Expression of IL-7Rα in MART-1 specific CTLs restores responsiveness to IL-7. Panel a. MART-1 specific CTLs were generated as previously described(24). Control MART-1 CTLs lacking the expression of IL-7Rα were transduced either with the SFG.IL-7Rα gamma retroviral vector or with the control vector ΔCD34. After transduction, IL-7Rα was re-expressed by CTLs transduced with SFG.IL7Rα but not by CTLs transduced with the control vector. Panel b illustrates that, after transduction, IL-7Rα was expressed by MART-1 tetramer+ CTLs. Panel c illustrates that transgenic IL-7Rα in MART-1 CTLs is functional as Stat5 is phosphorylated after incubation with IL-7. Panel d illustrates that after transduction the percentage of IL-7Rα+ MART-1 CTLs increased when the cells were stimulated once a week with antigen-presenting cells loaded with the MART-1 peptide and in presence of IL-7. Panel e shows that IL-7Rα+ MART-1 CTLs proliferate in response to IL-2 (50 U/ml) and IL-7 (5 ng/mL), while control MART-1 CTLs proliferate only in response to IL-2. Data represent two different MART-1 CTL lines. Cells were stimulated with antigen-presenting cells loaded with MART-1 peptide. CTL proliferation was quantified using the H3Thymidine incorporation assay. Table S1. Phenotypic analysis for memory markers of EBV-CTL lines. Table S2. Tetramer analysis of EBV-CTL lines. Table S3. Repertoire analysis of EBV-CTL lines generated from 4 representative donors.
Supplementary Material
IL-7Rα is down regulated in CTLs specific for cytomegalovirus, adenovirus, and cancer testis antigens. Multivirus-specific CTLs were generated as previously described(25). Briefly PBMC obtained from healthy seropositive donors were stimulated with autologous monocytes and EBV-LCLs transduced with an adenovirus vectors encoding the CMV pp65 protein (AdF35pp65-I-GFP)(29) as a source of CMV and Adenovirus antigens. IL-2 (50 U/mL) was added to the culture at day 14. The expression of IL-7Rα was monitored by FACS analysis during culture. Panel a shows that IL-7Rα expression was down regulated in T lymphocytes during the generation of virus-specific CTLs. IL-7Rα was undetectable in CMV- and Ad-specific CTLs quantified using NLV- and TDL-specific tetramer staining, respectively (Panel b). Panels c-f illustrate the functional characterization of multivirus-specific CTL lines using IFNγ Elispot assay (panels c and e) and cytotoxicity assay (panels d and f).
Growth in IL-7 does not impair the antigen specificity of IL-7Rα+EBV-CTLs. CTL clones specific for tumor-associated antigens such as PRAME were generated as previously described(24). Briefly, polyclonal T-cell lines obtained after 3 stimulations with artificial antigen presenting cells loaded with peptides derived from PRAME antigen were cloned by limiting dilution assay in 96 well plates, in the presence of IL-2 (100 U/mL), CD3 antibody (50 ng/mL OKT3; Ortho Biotech, Bridgewater, NJ) and irradiated (4,000 rad) allogeneic PBMC as feeder cells. Panel a show that these clones lack the expression of IL-7Rα by FACS analysis. Panels b and c illustrate that these clones are specific for the ALY-PRAME peptides using IFNγ Elispot assay (panel b) and cytotoxicity assay (panels c) against PHA-blasts loaded with ALY-peptide or an irrelevant peptide as target cells.
Expression of IL-7Rα in MART-1 specific CTLs restores responsiveness to IL-7. Panel a. MART-1 specific CTLs were generated as previously described(24). Control MART-1 CTLs lacking the expression of IL-7Rα were transduced either with the SFG.IL-7Rα gamma retroviral vector or with the control vector ΔCD34. After transduction, IL-7Rα was re-expressed by CTLs transduced with SFG.IL7Rα but not by CTLs transduced with the control vector. Panel b illustrates that, after transduction, IL-7Rα was expressed by MART-1 tetramer+ CTLs. Panel c illustrates that transgenic IL-7Rα in MART-1 CTLs is functional as Stat5 is phosphorylated after incubation with IL-7. Panel d illustrates that after transduction the percentage of IL-7Rα+ MART-1 CTLs increased when the cells were stimulated once a week with antigen-presenting cells loaded with the MART-1 peptide and in presence of IL-7. Panel e shows that IL-7Rα+ MART-1 CTLs proliferate in response to IL-2 (50 U/ml) and IL-7 (5 ng/mL), while control MART-1 CTLs proliferate only in response to IL-2. Data represent two different MART-1 CTL lines. Cells were stimulated with antigen-presenting cells loaded with MART-1 peptide. CTL proliferation was quantified using the H3Thymidine incorporation assay.
Phenotypic analysis for memory markers of EBV-CTL lines.
Tetramer analysis of EBV-CTL lines.
Repertoire analysis of EBV-CTL lines generated from 4 representative donors.
Acknowledgments
This work was supported in part from Leukemia and Lymphoma Society Specialized Center of Research (SCOR; grant no. 7018) (H.E.H.), NIH P50CA126752 (H.E.H.) and U54 HL081007 Specialized Centers for Cell-based Therapy and Data & Coordinating Center (M.K.B.). B.S. is supported by NIH RO1CA131027 and Leukemia and Lymphoma Society Translational Research grant. G.D. is supported by the Doris Duke Charitable Foundation/Clinical Scientist development award and by a Leukemia and Lymphoma Society Translational Research grant. The authors declare no competing financial interests. J.F.V. is supported by When Everyone Survives (WES) foundation.
References
- Dudley ME., and , Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus--specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- Comoli P, Pedrazzoli P, Maccario R, Basso S, Carminati O, Labirio M, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–8949. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Koka R., and , Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA., and , Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadzadeh M., and , Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC., and , Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD., and , Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Nanjappa SG, Walent JH, Morre M., and , Suresh M. Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J Clin Invest. 2008;118:1027–1039. doi: 10.1172/JCI32020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpdogan O, Muriglan SJ, Eng JM, Willis LM, Greenberg AS, Kappel BJ, et al. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2003;112:1095–1107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sili U, Huls MH, Davis AR, Gottschalk S, Brenner MK, Heslop HE, et al. Large-scale expansion of dendritic cell-primed polyclonal human cytotoxic, T-lymphocyte lines using lymphoblastoid cell lines for adoptive immunotherapy. J Immunother. 2003;26:241–256. doi: 10.1097/00002371-200305000-00008. [DOI] [PubMed] [Google Scholar]
- Leen AM, Sili U, Savoldo B, Jewell AM, Piedra PA, Brenner MK, et al. Fiber modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004;103:1011–1019. doi: 10.1182/blood-2003-07-2449. [DOI] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- Quintarelli C, Dotti G, De, Angelis B, Hoyos V, Mims M, Luciano L, et al. Cytotoxic T lymphocytes directed to the Preferentially Expressed Antigen of Melanoma (PRAME) target chronic myeloid leukemia. Blood. 2008;112:1876–1885. doi: 10.1182/blood-2008-04-150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DJ, Jr., Dudley ME, Robbins PF., and , Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B, Huls MH, Liu Z, Okamura T, Volk HD, Reinke P, et al. Autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for the treatment of persistent active EBV infection. Blood. 2002;100:4059–4066. doi: 10.1182/blood-2002-01-0039. [DOI] [PubMed] [Google Scholar]
- Savoldo B, Goss JA, Hammer MM, Zhang L, Lopez T, Gee AP, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs) Blood. 2006;108:2942–2949. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- Quintarelli C, Vera JF, Savoldo B, Giordano, Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B, Rooney CM, Di, Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30ζ artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive Cell Therapy for Patients With Metastatic Melanoma: Evaluation of Intensive Myeloablative Chemoradiation Preparative Regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erard F, Wild MT, Garcia-Sanz JA., and , Le GG. Switch of CD8 T cells to noncytolytic CD8-CD4- cells that make TH2 cytokines and help B cells. Science. 1993;260:1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- Kienzle N, Olver S, Buttigieg K, Groves P, Janas ML, Baz A, et al. Progressive differentiation and commitment of CD8+ T cells to a poorly cytolytic CD8low phenotype in the presence of IL-4. J Immunol. 2005;174:2021–2029. doi: 10.4049/jimmunol.174.4.2021. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PF, Carrington J, Nathwani A., and , Vanin EF. RD114-pseudotyped oncoretroviral vectors. Biological and physical properties. Ann N Y Acad Sci. 2001;938:262–276. [PubMed] [Google Scholar]
- Kim YJ, Dubey P, Ray P, Gambhir SS., and , Witte ON. Multimodality imaging of lymphocytic migration using lentiviral-based transduction of a tri-fusion reporter gene. Mol Imaging Biol. 2004;6:331–340. doi: 10.1016/j.mibio.2004.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IL-7Rα is down regulated in CTLs specific for cytomegalovirus, adenovirus, and cancer testis antigens. Multivirus-specific CTLs were generated as previously described(25). Briefly PBMC obtained from healthy seropositive donors were stimulated with autologous monocytes and EBV-LCLs transduced with an adenovirus vectors encoding the CMV pp65 protein (AdF35pp65-I-GFP)(29) as a source of CMV and Adenovirus antigens. IL-2 (50 U/mL) was added to the culture at day 14. The expression of IL-7Rα was monitored by FACS analysis during culture. Panel a shows that IL-7Rα expression was down regulated in T lymphocytes during the generation of virus-specific CTLs. IL-7Rα was undetectable in CMV- and Ad-specific CTLs quantified using NLV- and TDL-specific tetramer staining, respectively (Panel b). Panels c-f illustrate the functional characterization of multivirus-specific CTL lines using IFNγ Elispot assay (panels c and e) and cytotoxicity assay (panels d and f).
Growth in IL-7 does not impair the antigen specificity of IL-7Rα+EBV-CTLs. CTL clones specific for tumor-associated antigens such as PRAME were generated as previously described(24). Briefly, polyclonal T-cell lines obtained after 3 stimulations with artificial antigen presenting cells loaded with peptides derived from PRAME antigen were cloned by limiting dilution assay in 96 well plates, in the presence of IL-2 (100 U/mL), CD3 antibody (50 ng/mL OKT3; Ortho Biotech, Bridgewater, NJ) and irradiated (4,000 rad) allogeneic PBMC as feeder cells. Panel a show that these clones lack the expression of IL-7Rα by FACS analysis. Panels b and c illustrate that these clones are specific for the ALY-PRAME peptides using IFNγ Elispot assay (panel b) and cytotoxicity assay (panels c) against PHA-blasts loaded with ALY-peptide or an irrelevant peptide as target cells.
Expression of IL-7Rα in MART-1 specific CTLs restores responsiveness to IL-7. Panel a. MART-1 specific CTLs were generated as previously described(24). Control MART-1 CTLs lacking the expression of IL-7Rα were transduced either with the SFG.IL-7Rα gamma retroviral vector or with the control vector ΔCD34. After transduction, IL-7Rα was re-expressed by CTLs transduced with SFG.IL7Rα but not by CTLs transduced with the control vector. Panel b illustrates that, after transduction, IL-7Rα was expressed by MART-1 tetramer+ CTLs. Panel c illustrates that transgenic IL-7Rα in MART-1 CTLs is functional as Stat5 is phosphorylated after incubation with IL-7. Panel d illustrates that after transduction the percentage of IL-7Rα+ MART-1 CTLs increased when the cells were stimulated once a week with antigen-presenting cells loaded with the MART-1 peptide and in presence of IL-7. Panel e shows that IL-7Rα+ MART-1 CTLs proliferate in response to IL-2 (50 U/ml) and IL-7 (5 ng/mL), while control MART-1 CTLs proliferate only in response to IL-2. Data represent two different MART-1 CTL lines. Cells were stimulated with antigen-presenting cells loaded with MART-1 peptide. CTL proliferation was quantified using the H3Thymidine incorporation assay.
Phenotypic analysis for memory markers of EBV-CTL lines.
Tetramer analysis of EBV-CTL lines.
Repertoire analysis of EBV-CTL lines generated from 4 representative donors.