The human intestine harbors and is in constant contact with 1000 trillion microbes, composed of an estimated 15,000 strains1. Recent studies have changed our perspective of commensal microbes from benign but inert passengers, to active participants in the processing of food into useful metabolic components, the post-natal development of mucosal and systemic immunity, and in its long-term steady-state function. Although mucosal surfaces have to constitutively integrate a multitude of microbial derived signals, new evidence suggests that defined bacteria or microbial products can play a dominant role in the induction of distinct class of immune responses. In this review we will focus on recent findings associating microbes that colonize or invade the gut, specialized mucosal DCs, and induction of effector or regulatory response in the GI tract.
The homeostasis of the GI tract: balance of inflammatory and regulatory signals
As the intestine is constantly exposed to antigens derived from food and commensal bacteria, a commonly held preconception is that tolerance and the induction of regulatory responses constitute the default response to antigens presented in this environment. Nevertheless, this view has to be adjusted as it is becoming clear that a certain degree of constitutive effector response and inflammation is beneficial for the host, not only to maintain integrity of the tissue but also to allow the host to develop protective responses when required. This implies that the steady state regulation of this environment relies on the maintenance of a balance of antagonistic signals allowing the induction and maintenance of various classes of effector lymphocytes. Indeed, at steady state, the gut is home to a large number of lymphocytes that have the capacity to produce regulatory (e.g. IL-10 or TGF-β) and/ or effector cytokines (e.g. IL-17 and IFN-γ2, 3). This constitutive production of cytokines is tightly controlled by the flora as germ-free mice show extensive deficiencies in intestinal immune system development and basal cytokine production2, 4. In the absence of flora, the CD4+ T cell population is diminished, disproportionately affecting Th1 and Th17 cells, while Treg frequencies are maintained or increased2. Colonization of germ free mice with complex microbiota orchestrates a broad spectrum of T helper (Th1, Th17) and regulatory T cell responses 5. Some of the factors that govern the induction of constitutive effector responses in the GI tract and how such conditioning affect subsequent effector responses against pathogens will be discussed in this review
Steady state and induction of regulatory responses
The intestinal mucosa contains a large number of antigen presenting cells whose complexity has only begun to be addressed6. Recent studies demonstrated that in the lamina propria two main subsets of DCs can be distinguished on the basis of the expression of CD103 or CX3CR1 7–9. CD103+DCs are shorter-lived and capable of efficiently presenting antigen to naive T cells, in part due to their increased ability to traffic to draining lymph nodes, while CX3CR1+ DCs are longer-lived, tissue resident and poor antigen presenting cells9. This apparent functional dichotomy can be also traced to their distinct origin. Indeed, DCs expressing CD103 arise from macrophage–DC precursors through a Flt-3 ligand growth factor mediated pathway, while CX3CR1+ Lp DCs derived from grafted Ly6Chi monocytes under the control of GMCSF 7, 8. Using infectious agents and colitis models, several groups reported that in the gut, inflammation and IL-17 induction is usually associated with DCs that do not express CD1037, 8, 10. For instance, colitis can be induced by TNF-α secreting CX3CR1+ Lp DCs and an increased frequency of these cells can lead to intestinal inflammation7. On the other hand, under steady state conditions, the gut-associated lymphoid tissue is a preferential site for the peripheral induction of regulatory T cells 11–14 suggesting that the pro-inflammatory nature of CX3CR1+DCs is counterbalanced by other subsets of DCs. Recent evidence suggests that CD103+DCs are playing a major role in the maintenance of this equilibrium. One of the specific features of CD103+DCs is their capacity to metabolize the vitamin A metabolite RA, previously shown to imprint gut homing receptors on lymphocytes 15–18 and promote IgA secretion by gut activated B cells 17. Another effect of RA on the immune regulation of the GI tract is associated with its capacity to enhance the TGF-β mediated generation of Foxp3+ Treg cells 11–13, 19–23. Of note, other cells, including epithelial cells and MLN stromal cells can express enzymes associated with vitamin A metabolism 24 25 suggesting that CD103- APCs may acquire RA from alternative sources and store it23. A recent report demonstrated that stimulation of DCs via TLR2 induced vitamin A metabolizing enzymes, suggesting that these enzymes may be tightly controlled by interaction with microbes 26. In addition to inducing Foxp3 expression by T cells, RA can conversely inhibit the generation of Th-17 13, 19, 21, 22 suggesting that RA may play an important role in maintaining the balance between effector and regulatory populations in the GI tract. The actions of RA are mediated by nuclear receptors which are ligand-inducible transcriptional regulators belonging to two distinct families: RAR and RXR. Each family consists of the three genetic isotypes (RAR- or RXRα, β and γ). Foxp3 induction was abolished in RARα deficient T cells 27 and conversely, these cells produced elevated levels of both IL-17 and IFN-γ. At least two mechanisms have been identified by which RA can enhance the capacity of TGF-β to induce Treg cells. One of these mechanisms is the capacity of RA to suppress the production of effector cytokines that interfere with Foxp3 expression 27–29. Additionally, RA can also act directly on naïve T cells to favor TGF-β mediated Foxp3+ Treg cell induction 30. Despite the recent identification of these LpDC subsets, the current characterization of mucosal APC-T cell interaction remains an oversimplification. That there is no clear consensus on the nomenclature or gating strategies used by individual groups precludes major synthesis. In addition, the composition and function of gut APCs is likely to be affected by inflammation as well as their location along the length of the intestine, as will be discussed in more detail below.
Negative control of Treg induction by microbes
It is becoming clear that activation status as well as inflammatory mediators modulate the capacity of DCs to induce Treg cells de novo 85–90. For instance IL-6 and TGF-β in tandem can direct the production of IL-17 secreting T cells over Treg cells 85, 86 and Th1 and Th2 effector cytokines have an antagonistic effect on Treg cell conversion 29. In addition, activated DCs or strong costimulation limit the induction of Foxp3+ Treg cell in favor of the induction of effector responses 20, 91, 92.When activated with commensal derived DNA, LpDCs produce large amount of IL-6 and consequently are impaired in their capacity to induce Treg 3. Notably, other representative bacterial TLR ligands including 2 and 4 did not markedly affect LpDC induced Treg cell conversion in vitro consistent with the regulatory properties of TLR4 and 5 in this environment65, 67, 70. In absence of commensal DNA-derived signal such as in mice deficient in TLR9, Treg cell frequencies are dramaticaly increased within intestinal effector sites77. This observation suggests that via TLR9, commensals can constrain the constitutive generation of Treg cells in the GI tract and contribute to the maintenance of the tonicity of this environment
Because inflammatory signals can interfere with Treg cell induction, acute infections in the GI tract are expected to prevent expression of Foxp3. Indeed, the constitutive induction of Treg following exposure to oral antigens is inhibited after oral infection with the protozoan T.gondii93. Upon infectious challenge, the gut-resident APCs are replaced by inflammatory cells that have not been conditioned by the gut environment 94. Indeed, LpDCs from infected mice are less efficient at inducing Treg cells in vitro via their capacity to induce IFN-γ producing Teff 93. Similarly, gut DCs exposed to Toxoplasma antigen in vitro are impaired in their capacity to induce Treg cells demonstrating that tolerogenic DCs can lose their capacity to induce Treg cells when activated by defined commensal products (Bacterial DNA) or pathogens, in favor of the induction of effector cells93. Previous reports examining both gut and lung inflammation support the idea that restricted or defective Treg cell conversion can enhance immunopathology95, 96 supporting the idea that interference with constitutive Treg induction during infection may directly contribute to the pathological process.
Contextual roles of commensals on host homeostasis
There is clear evidence that some dietary microorganisms, termed probiotics, can play a beneficial role in the health of their host 31. Promising results have been obtained with probiotics in the treatment of human inflammatory diseases of the intestine and in the prevention and treatment of atopic eczema in neonates and infants32. There can be exquisite specificity in the effects of individual commensal species; for example, within the genus Lactobacillus different species are associated with differing in vivo allergic status in infants33 and contrasting capacity to induce regulatory cytokines in vitro 34. Moreover, different strains or mutants of particular Lactobacillus species stimulated very different immunological outcomes in mice 35, 36.
Some of the effects of probiotic treatment appear to be associated with an increase in the frequency of Foxp3+Treg cells as well as induction of lymphocytes (expressing Foxp3 or not) producing regulatory cytokines. For instance, in mice, probiotic Lactobacillus and/or Bifidobacterium treatment suppressed TNBS-induced colitis 37, 38, as well as allergic responses via induction of TGF-β 39 and activation of Treg cells40. Similarly, treatment of colitic mice with the probiotic cocktail VSL3 increased TGF and IL-10 producing T cells 41. In a model of pathogen-induced inflammation, treatment of mice with Bifidobacteria infantis activated Treg cells that could inhibit inflammation and induced activation of NF-kB in recipient mice42. Some of the regulatory effects of probiotics are likely to occur via modulation of DCs 34, 43 but could also arise as a consequence of their interaction with epithelial cells. Indeed, some commensal-derived products can decrease tight junction permeability44 and control the induction of antimicrobial peptides45, 46.
On the other hand, the flora itself can become a liability as the breakdown of tolerance to commensal bacteria can contribute to the progression of IBD. Inflammatory responses in human and experimental IBD are directed toward certain subsets of commensal organisms that have pathogenic potential, such as Helicobacter, Clostridium and Enteroccoccus species, with some microorganisms, such as E. coli, reported to be strongly associated with Crohn’s disease47. Furthermore, lines of evidence suggest that perturbations in gut flora alone, can lead to gastrointestinal dysfunction50. However these bacteria often can peacefully coexist in their host as part of the constitutive flora and their capacity to trigger or contribute to these disorders is partly controlled by host genetic factors. Indeed, genome wide association studies identified disease-associated mutation in genes that are involved in sensing of bacteria (NOD2) 48 and control of innate responses (IL23R) 49.
That gut inflammation is triggered by a combination of dysregulated microbiota and host genetic factors is best illustrated by a recent work looking at the role of the transcription factor Tbet in innate immune responses. In the absence of T-bet, RAG2−/− mice (TRUC) spontaneously develop a juvenile ulcerative colitis resulting from pro-inflammatory response to commensal microbiota51. The mechanism underlying this pathological process appears to be two fold. First, TRUC mice display enhanced TNF-α production. Secondly, these mice harbor a colitogenic flora that can be transmitted to T-bet competent mice51. T-bet deficiency by DCs can also lead to the spontaneous development of colorectal cancer dependent on inflammation responses to commensal bacteria in a MyD88 independent fashion52. The association between chronic Helicobacter pylori and gastric cancer constitute the best documented example of microbe-associated inflammation as trigger of carcinogenesis53. More recently, the human commensal Enterotoxigenic Bacteroides fragillis (ETBF) has also been shown to trigger colonic tumors in the multiple intestinal neoplasia (Min) strain of mice via induction of IL-17 54.
Furthermore, there can be significant interactions between commensals and pathogens. For example, a shift in bacterial composition can aggravate the immunopathology caused by toxoplasmosis 55. During infection with T. gondii, there is reduced floral complexity, either because of relative loss of more “regulatory” strains, or simply as a broad reflection of an altered homeostasis accompanying pathogenesis. Disruption of the microbiota composition has been also documented in the context of other enteric infections such as Citrobacter rodentium or Salmonella typhimurium56, 57. Understanding how shifts in the microbiota induced by pathogens contributes to both pathology and protective responses in the GI tract need to be further explored.
Role of Bacteroides fragillis and Polysaccharide A in gut homeostasis
Whereas most individual bacteria failed to efficiently stimulate intestinal T cell responses, a restricted number of individual bacteria can control the tonicity of the gut immune system5. The first demonstration that a single commensal molecule could promote beneficial immune responses was provided by the identification of the polysaccharide A (PSA). PSA is produced in large quantities by a prominent human symbiont Bacteroides fragillis 58. Colonization of germ free mice with B. fragillis or treatment with purified PSA is sufficient to restore most of the defects observed in the development the immune system 58. Furthermore, via PSA expression, Bacteroides fragillis can protect mice from experimental colitis induced by Helicobacter hepaticus, a commensal bacterium with pathogenic potential 59. This protective activity is associated with the capacity of PSA to induce or expand IL-10 producing CD4+T cells 59. Hence, immunoregulation may revolve around highly specific host-microbial molecular interactions, presumably reflecting a long and intimate co-evolution of their symbiotic relationship.
Control of IL-17 production by Segmented Filamentous Bacteria
A further demonstration of the impact of specific commensal organisms on immune system development and skewing was illustrated by the observation that mice lacking a key group of microbes (since identified as Segmented Filamentous Bacteria (SFB)) were unable to mount a Th17 response in the small intestine. In addition, the Treg cell compartment of these mice was expanded 2 5, 60. When administered to mice in a defined bacterial cocktail, SFB was sufficient to induce intestinal inflammation in SCID mice reconstituted with CD45RBhighCD4+ T cells 61. As shown by scanning electron microscopy, SFB adhere tightly to Peyer’s Patches of the small intestine and concomitantly induce local expression of IFN-γ, IL-10 and IL-17. Compared to germ free mice, SFB colonization increased IFN-γ producing CD4 cells, IL-17 producing cells and Foxp3+ Tregs in the small intestine and colon. However, absolute numbers of these cells were not restored to the level those of mice with a complete microbiota suggesting that as expected, SFB may be sufficient for such changes but need to synergize with other microorganisms to coordinate the full maturation of the intestinal immune system.
Role of TLRs in the control of GALT Treg/Teff responses
Paradoxically, commensal and pathogenic microbes interact with the host immune system through conserved ligands that are cardinal features of microorganisms 62. Many of these ligands signal through the Toll-like family of receptors (TLR) 62. TLRs are widely expressed by cells of hematopoietic origin, as well as non-hematopoietic cells, including the epithelial cells lining the intestinal tract 63. The initial identification of TLRs in the splenic and peripheral blood leukocyte compartments paved our understanding of how engagement of these molecules by invasive pathogens can activate inflammatory cascades that initiate adaptive immunity 64. However, sites colonized by commensals differ in that they are in constant contact with TLR ligands. Yet, the response to TLR signaling by the commensal flora has only recently begun to be elucidated. For example, it is now clear that TLR signaling in the intestinal epithelial compartment is crucially involved in the maintenance of intestinal homeostasis and tissue repair 65. Commensal floral interactions with TLRs have also been shown to mediate tolerance to food antigens 67. Furthermore, these signals also positively regulate the sampling of luminal contents by DCs from the underlying lamina propria compartment 66.
Role of Flagellin in the control of gut immunity
Bacterial flagellin is a structural protein that forms the main portion of flagella and promotes bacterial chemotaxis, adhesion and invasion of host tissue. TLR5 recognizes the conserved domain of flagellin monomers. Unlike other TLR family members, TLR5 is not expressed on macrophages or conventional DCs in mice and poorly expressed by intestinal epithelial cells. Instead TLR5 is highly expressed in lamina propria DCs from the small intestine and in particular on CD11chiCD11bhi dendritic cells (containing both CX3CR1 and CD103+DCs)68, 69. Flagellin stimulated LpDCs do not produce IL-10 and TNFα but instead express chemokines, prostaglandin and antimicrobial peptides 68. In addition, LpDCs stimulated with flagellin produce both IL-6 and IL-12 68. Whereas DCs from non-GALT tissues induce Th1 cells in response to TLR ligands, CD11chiCD11bhi LpDCs induce RORγT functional Th17 cells as well as Th1 cells from naïve CD4+T cells in response to flagellin in vitro and in vivo 69. Recent studies have shown that RA negatively regulates Th17 cell differentiation induced by IL-6 plus TGF-β in a dose dependent manner 13. However, LpDCs and in particular the CD11chiCD11bhi subset of LpDCs, can specifically induce Th17 in the context of TLR5 stimulation, despite their expression of enzymes associated with the generation of RA, 69. Interestingly the effect of RA on DC mediated Th17 differs according to its concentration. At high concentration, RA inhibits both Th1 and Th17 while at low concentration RA clearly favor Th17 induction while limiting Th1 responses 69. Such a role for RA in promoting Th17 is highly compatible with the observation that the GALT that is naturally exposed to RA, contains a high number of resident IL-17 producing cells. Thus, such interaction may be critical to trigger effector responses to pathogenic flagellated bacteria. Intriguingly, some commensal bacteria also have flagella, suggesting that they may have evolved mechanisms to escape TLR5-mediated host detection in the intestine. On the other hand, even in absence of pathogens, TLR5 ligands are clearly sensed as evidenced by the observation that mice lacking TLR5 spontaneously develop colitis 70. Furthermore, mice lacking TLR5 over-express genes associated with innate and adaptive immunity contributing not only to colitis development but also to their enhanced protection against infection to enteric pathogens such as Salmonella 71. Recent studies identified that in the context of Crohn’s Disease, flagellins are immunodominant antigens of the microbiota 72. An explanation of how the mucosal immune system prevents harmful responses against flagellin was provided by a study demonstrating that intestinal IgA can regulate the activation of peripheral flagellin-specific CD4+T cells73. Importantly, Treg cells control such antigen IgA B cell responses in an antigen specific manner via production of TGF-β This study uncovered a new role for Treg as a major helper T cell for the induction and maintenance of intestinal IgA B cell responses and prevention of responses against major flora antigen under steady state condition 73.
Role of commensal derived DNA
TLR9 recognizes unmethylated cytosine phosphate guanosine (CpG) dinucleotides, which are abundant in prokaryotic DNA found in intestinal flora. Using synthesized sequences containing CpG, it has been shown that engagement of TLR9 expressed on DCs, Treg and conventional T cells can limit Treg cell suppressive function 74, 75. Previous work identified an association between Crohn’s disease and a promoter polymorphism in the TLR9 gene in humans 76. Such an association supports the idea of a role for gut floral DNA (gfDNA) sensing in the pathophysiology of inflammatory bowel diseases (IBD). In mice, gut flora derived DNA (gfDNA) plays a major role in intestinal homeostasis through toll like receptor 9 (TLR9) engagement 77 and constitutive interaction between gfDNA and TLR9 in the gut can act as an immunological adjuvant and critically controls the balance between Treg and Teff 77. Naïve TLR9 deficient mice displayed a striking increase in the frequency of Foxp3+ Treg within intestinal effector sites, accompanied by a significant reduction in constitutive IL-17 and IFN-γ production by Teff cells. Complementing this, gfDNA, strongly constrained the capacity of lamina propria DCs to induce Treg conversion in vitro. Further, Treg/Teff disequilibrium in TLR9 deficient mice led to impaired immune responses to oral infection with microsporidia and to oral vaccination, which could be rescued through neutralization of Treg 77. Other studies have shown that in vitro, gut flora bacteria are not all equal in their capacity to stimulate TLR9 and do so with varying levels of efficiency that correlate with the frequency of [CG] dinucleotides 77, 78. Thus, it is tempting to speculate that alteration of Treg cell homeostasis mediated by TLR9 signaling may be differentially regulated by specific gut flora species.
Role of commensal derived ATP
Adenosine 5'-triphosphate (ATP), can modulate immune cell function by means of activation of the ATP sensor PX and P2Y receptors. Commensal bacteria have been shown to generate large amounts of ATP 79. Consistent with this observation, germ free animals have reduced ATP in their feces compared to conventional mice. ATP derived from commensal bacteria can activate a subset of lamina propria cells, CD70highCD11clow cells, leading to the differentiation of Th17 cells79. Systemic or rectal administration of ATP into these germ-free mice results in a marked increase in the number of lamina propria Th-17 cells. A CD70highCD11clow subset of the lamina propria cells expresses IL-6, IL-23p19 and TGF-β-activating integrin-αV and -β8, in response to ATP stimulation, and preferentially induces Th17 differentiation. The critical role of ATP is further underscored by the observation that administration of ATP exacerbates a T-cell-mediated colitis model with enhanced Th17 differentiation79. This data provide the first evidence that a commensal-derived metabolite could direct a specific immune response.
Role of short-chain fatty acid (SCFAs)
Short chain fatty acids (SCFA) are produced by colonic bacteria after fermentation of dietary fibers. The type of SCFA produced depends on the metabolizing bacteria, with Bacteroides producing high level of acetate and propionate while firmicutes produce high amount of butyrate80. During the onset of colitis the amount of SCFA is significantly reduced 81 and increased intake of either dietary fibers or SCFA is clinically beneficial in the treatment of various forms of colitis82, 83. A recent study demonstrated that interaction between SCFA and its receptor the G-protein coupled receptor 43 (GPR43) was required to control inflammatory responses during experimental colitis or asthma84. Evidence also suggests that expression of GPR43 is associated with the expression of other innate receptors such as TLRs or C5aR in the GI tract84. Thus SCFA provides another example of a link between dietary elements and control of gut homeostasis.
Systemic effect of mucosal microbial populations
One consequence of the immune system’s reliance on microflora for optimal immunoregulation, is that alteration of microbial communities may result in unintended activation of immune effector mechanisms. Such alteration in microbial communities can arise as a consequence of exogeneous factors including urbanization, global travel, dietary changes 97 and perhaps most importantly, in response to antibiotic treatment. Recent evidence suggests that acute antibiotic treatment has dramatic consequences on the taxonomic richness and diversity of the commensal microbial community that can last for an extended period of time98, 99. In experimental models, antibiotic associated disturbance of the microbiota disrupts not only local responses to food antigen or infection 67, 100 77 but has also consequences on systemic responses. Indeed, treatment of mice with antibiotics led to a reduction in the incidence of experiment autoimmune encephalitis (EAE)101. As well, diabetes in the Non-Obese Diabetes (NOD) mouse model has been related to their housing conditions and presumably their commensal flora. When NOD mice were made deficient for MyD88, induction of disease was delayed and connected with a divergent commensal flora compared to MyD88 intact controls102. The long-term consequences of such perturbations for the human–microbial symbiosis are more difficult to discern, but chronic conditions such as asthma and atopic disease have been associated with childhood antibiotic use and an altered intestinal microbiota 104–106. Therefore the role of microbes or microbe metabolites in shaping regulation of innate and adaptive response extends beyond mucosal sites to provide systemic control of host homeostasis.
If changes in the commensal population impact upon systemic immune responses then it is not surprising to find that infection occurring in the same milieu, directly or via shift in microbial community, can also exert substantial systemic effects. The influence of infection on “bystander” or unrelated immune responses, provides a mechanistic explanation of the more general “hygiene hypothesis”. This hypotheis posits that increasing rates of allergy and asthma in Western countries could be the consequence of reduced infectious stresses during early childhood 107. Experimental work has lent strong support for this hypothesis. For example, during gastrointestinal infection, helminth-driven Treg cell suppression of effector functions protects against subsequent airway inflammation 108. Similar infections change responses to blood-stage malaria 109 and interfere with vaccinations 110, 111. Evidence for bystander suppression in human GI helminth infection is also accumulating, with lower allergy rates in infected children 112, 113, and lower inflammatory responses to autoantigen during MS 114. Indeed, helminth therapy is being tested as a potential strategy to ameliorate intestinal inflammation in Crohn’s Disease and Ulcerative Colitis 115. Notably, various suppressive cell types are observed in these infections, including “regulatory B cells” and alternatively activated macrophages, although the interdependence and sequence of activation of these regulatory components have yet to be discerned 116. The presence of symbiotic and chronic infections in the GI tract could lead to the maintenance of a pool of cells with regulatory activities that would maintain host immune homeostasis and enhance the threshold required for immune activation. Over time, established GI infections may create a new homeostatic set point, in which reactivity to the chronic pathogen is minimized, with wider implications for responsiveness to self-antigens and allergens. At this point, it remains unclear to what extent any recalibration of host immunity is purely induced by the pathogen, or by perturbation of the commensal population, or is a result of endogenous controls within the immune system itself. On the basis of both human and experimental studies discussed above, it seems likely that all three components play an essential role in reaching a stable and nonpathogenic steady state for the longer term.
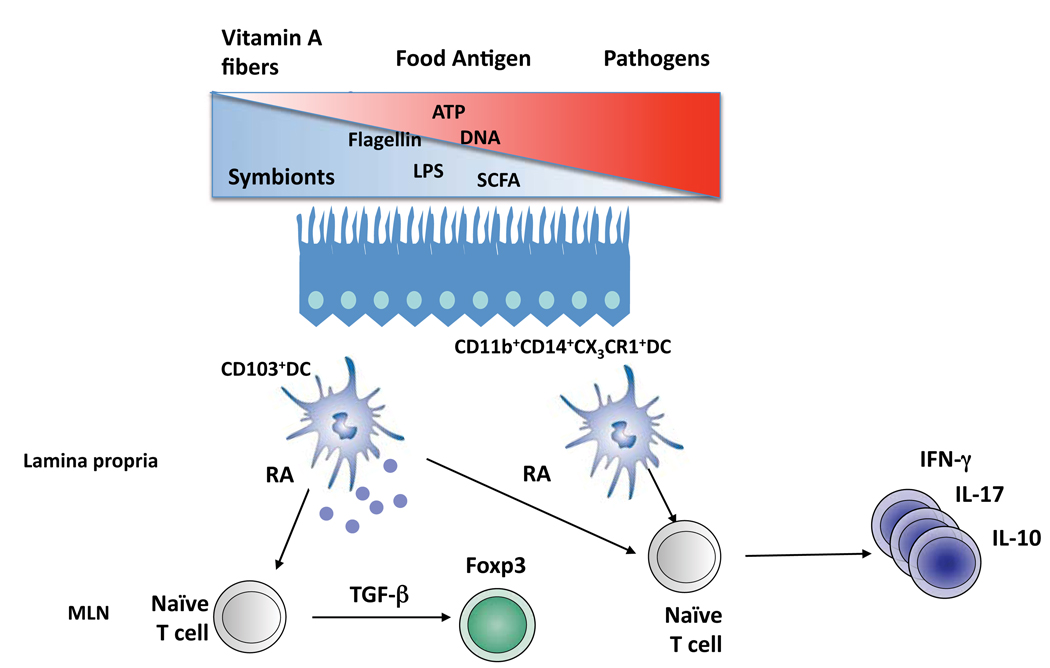
Figure 1. Conditioning of T cell by commensals.
The gut is colonized by a complex microbiota composed of microbes with potential beneficial (symbionts) or pathogenic effects for their host. This site is constantly conditioned by dietary elements such as vitamin A or fiber that can regulate the homeostasis of this site via their metabolites (respectively retinoic acid (RA) or short chain fatty acid (SCFA)). In the lamina propria or Peyer’s patches, various subsets of DCs (e.g. for the lamina propria CD11c+CD103+ or CD11b+CD14+CXCR1+DC) can be found. These DCs can induce Foxp3+Treg via their capacity to metabolizing and release RA (CD103+DCs) or induce various classes of effector responses following exposure to microbial derived products (e.g. Flagellin, Bacterial DNA) or metabolites (ATP).
Acknowledgements
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Ivanov II, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. •• This report first showed that the presence of Th17 cells in the lamina propria of the small intestine was dependent upon a diverse commensal flora.
- 3.Hall JA, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008 doi: 10.1016/j.immuni.2008.08.009. •• CpG DNA from commensal organisms was shown to tbe critical for the balance between Treg and effector T cell fates as TLR9 deficient animals had significantly increased Tregs. As well, DNA from commensal organisms was shown to act as an adjuvant in a model of infection
- 4.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 5.Gaboriau-Routhiau V, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. • Along with reference 45 this paper outlines the specific role of a single commensal species: Segmented Filamentous Bacteria in sustaining Th17 cells in the lamina propria and suppports the idea that specific bacterial organisms can have far-reaching immunological effects.
- 6.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varol C, et al. Intestinal Lamina Propria Dendritic Cell Subsets Have Different Origin and Functions. Immunity. 2009 doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Bogunovic M, et al. Origin of the Lamina Propria Dendritic Cell Network. Immunity. 2009 doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz O, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortin G, et al. A role for CD47 in the development of experimental colitis mediated by SIRPalpha+CD103- dendritic cells. J Exp Med. 2009;206:1995–2011. doi: 10.1084/jem.20082805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007 doi: 10.1084/jem.20070602. •• This works identifies retinoic acid produced from lamina propria-derived dendritic cells as a necessary component in the induction of Tregs in the periphery, particularly to antigens introduced into the GI tract as food.
- 12.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta}- and retinoic acid-dependent mechanism. J Exp Med. 2007 doi: 10.1084/jem.20070590. •• This works identifies retinoic acid produced from lamina propria-derived dendritic cells as a necessary component in the induction of Tregs in the periphery, particularly to antigens introduced into the GI tract as food.
- 13.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. •• This works identifies retinoic acid produced from lamina propria-derived dendritic cells as a necessary component in the induction of Tregs in the periphery, particularly to antigens introduced into the GI tract as food.
- 14.Mucida D, et al. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler TR, Evans ME, Fernandez-Estivariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229–261. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 16.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. •• Retinoic acid is shown to induce homing to the intestinal mucosa via up-regulation of α4β7 and CCR9.
- 17.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 18.Siewert C, et al. Induction of organ-selective CD4(+) regulatory T cell homing. Eur J Immunol. 2007;37:978–989. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- 19.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 20.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 22.Elias KM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 24.Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J Immunol. 2007;179:3504–3514. doi: 10.4049/jimmunol.179.6.3504. [DOI] [PubMed] [Google Scholar]
- 25.Hammerschmidt SI, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manicassamy S, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill JA, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantorna MT, Nashold FE, Chun TY, Hayes CE. Vitamin A down-regulation of IFN-gamma synthesis in cloned mouse Th1 lymphocytes depends on the CD28 costimulatory pathway. J Immunol. 1996;156:2674–2679. [PubMed] [Google Scholar]
- 29.Wei J, et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mucida D, et al. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–472. doi: 10.1016/j.immuni.2009.03.008. author reply 472-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. Journal of gastroenterology. 2009;44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 33.Kalliomaki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol. 2003;3:15–20. doi: 10.1097/00130832-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Smits HH, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 35.Fujiwara D, Inoue S, Wakabayashi H, Fujii T. The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance. Int Arch Allergy Immunol. 2004;135:205–215. doi: 10.1159/000081305. [DOI] [PubMed] [Google Scholar]
- 36.Grangette C, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A. 2005;102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-b-bearing regulatory cells. J Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 38.Zoumpopoulou G, et al. Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. International journal of food microbiology. 2008;121:18–26. doi: 10.1016/j.ijfoodmicro.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Feleszko W, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 40.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 41.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 42.O'Mahony C, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foligne B, et al. A key role of dendritic cells in probiotic functionality. PLoS ONE. 2007;2:e313. doi: 10.1371/journal.pone.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnich N, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hampe J, et al. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet. 2001;357:1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 49.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conte MP, et al. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760–1767. doi: 10.1136/gut.2005.078824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrett WS, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. •• This paper outlines a role for T-bet in controlling innate immune responses to commensals via TNFα. T-bet/Rag2 double knockout mice over-produced this cytokine leading to ulcerative colitis. Surprisingly, transfer of the commensal microbiota of these mice to wild-type mice was sufficient for colitis induction, highlighting that both microbial and host factors contribute to colitis
- 52.Garrett WS, et al. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell. 2009;16:208–219. doi: 10.1016/j.ccr.2009.07.015. • Uncontrolled DC-driven inflammation is shown to drive colorectal cancer in a MyD88-dependent manner.
- 53.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2000;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 54.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heimesaat MM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 56.Lupp C, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:204. doi: 10.1016/j.chom.2007.08.002. • Using a number of models, inflammatory responses are shown to profoundly affect the intestinal microbiota and promote the outgrowth of gram-negative Enterobacteriaceae family bacteria.
- 57.Stecher B, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. •• A single polysaccharide component, PSA, from a common commensal bacterium B. fragilis is sufficient for the complementation of the immune defects associated with germ-free mice.
- 59.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. • PSA is shown to significantly reduce pathology in a number of models of inflammatory bowel disease via the induction of IL-10 production.
- 60.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stepankova R, et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis. 2007;13:1202–1211. doi: 10.1002/ibd.20221. [DOI] [PubMed] [Google Scholar]
- 62.Sansonetti PJ, Di Santo JP. Debugging how bacteria manipulate the immune response. Immunity. 2007;26:149–161. doi: 10.1016/j.immuni.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 64.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 65.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. •• Toll-like receptor signaling from commensal bacteria is shown to be important for the amelioration of intestinal injury and associated inflammation.
- 66.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 68.Uematsu S, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. •• This work shows the importance of TLR5 activation by flagellin of lamina propria dendritic cells in the induction of immune responses at the intestinal mucosa. TLR5 activation is shown to induce retinoic acid production, which at lower levels supports the differentiation of Th17 cells and IgA producing B cells.
- 69.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 70.Vijay-Kumar M, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vijay-Kumar M, et al. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect Immun. 2008;76:1276–1281. doi: 10.1128/IAI.01491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lodes MJ, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [comment] [DOI] [PubMed] [Google Scholar]
- 75.Larosa DF, et al. CpG DNA inhibits CD4+CD25+ Treg suppression through direct MyD88-dependent costimulation of effector CD4+ T cells. Immunol Lett. 2007;108:183–188. doi: 10.1016/j.imlet.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torok HP, et al. Crohn's disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology. 2004;127:365–366. doi: 10.1053/j.gastro.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 77.Hall JA, et al. Commensal DNA Limits Regulatory T Cell Conversion and Is a Natural Adjuvant of Intestinal Immune Responses. Immunity. 2008 doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dalpke A, Frank J, Peter M, Heeg K. Activation of toll-like receptor 9 by DNA from different bacterial species. Infect Immun. 2006;74:940–946. doi: 10.1128/IAI.74.2.940-946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. •• ATP derived from commensal organisms is shown to induce the production of effector Th17 T cells by a specific subset of lamina propria dendritic cells. Introduction of ATP into the lumen is shownto exacerbate colitis.
- 80.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 81.Treem WR, Ahsan N, Shoup M, Hyams JS. Fecal short-chain fatty acids in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1994;18:159–164. doi: 10.1097/00005176-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 83.Kanauchi O, et al. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J Gastroenterol. 2002;37 Suppl 14:67–72. doi: 10.1007/BF03326417. [DOI] [PubMed] [Google Scholar]
- 84.Maslowski KM, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. •• Neutrophil sensing of single-chain fatty acids, a metabolite produced by the intestinal flora is required for the resolution of inflammatory responses. Shows that the immune system interacts with the metabolic products of commensals.
- 85.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 87.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 89.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nature immunology. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 90.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature immunology. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 91.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 92.Wang L, et al. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oldenhove G, et al. Decrease of Foxp3(+) Treg Cell Number and Acquisition of Effector Cell Phenotype during Lethal Infection. Immunity. 2009 doi: 10.1016/j.immuni.2009.10.001. •• Infection with T. gondii is shown to prevent the conversion of Treg in the Lp as well as inhibit regulation of pre-existing Treg via apoptosis and the induction of a Teff phenotype.
- 94.Dunay IR, et al. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Izcue A, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Curotto de Lafaille MA, et al. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. •• these works demonstrate that antibiotic treatment led to a profund and long lasting modification of floral composition. Most species of bacteria reconstitute 4 weeks after cessation of treatment, whereas others still remain absent six months hence.
- 98.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hill DA, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2009 doi: 10.1038/mi.2009.132. •• these works demonstrate that antibiotic treatment led to a profund and long lasting modification of floral composition. Most species of bacteria reconstitute 4 weeks after cessation of treatment, whereas others still remain absent six months hence.
- 100.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun. 2005;73:30–38. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ochoa-Reparaz J, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 102.Wen L, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. •• Deficiency the TLR-signaling adaptor protein MyD88 is shown to prevent the development of Type 1 diabetes in a mouse model of the disease. This prevention is dependent upon the possession of a unique commensal population and protection from diabetes can be conferred via the transfer of commensals from MyD88 knockout mice to wild-type controls.
- 103.Wolowczuk I, et al. Feeding our immune system: impact on metabolism. Clin Dev Immunol. 2008;2008:639803. doi: 10.1155/2008/639803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noverr MC, Huffnagle GB. The 'microflora hypothesis' of allergic diseases. Clin Exp Allergy. 2005;35:1511–1520. doi: 10.1111/j.1365-2222.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- 105.Prioult G, Nagler-Anderson C. Mucosal immunity and allergic responses: lack of regulation and/or lack of microbial stimulation? Immunol Rev. 2005;206:204–218. doi: 10.1111/j.0105-2896.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 106.Marra F, et al. Does antibiotic exposure during infancy lead to development of asthma?: a systematic review and metaanalysis. Chest. 2006;129:610–618. doi: 10.1378/chest.129.3.610. [DOI] [PubMed] [Google Scholar]
- 107.Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:969–977. doi: 10.1016/j.jaci.2006.03.003. quiz 978. [DOI] [PubMed] [Google Scholar]
- 108.Wilson MS, et al. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su Z, Segura M, Morgan K, Loredo-Osti JC, Stevenson MM. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect Immun. 2005;73:3531–3539. doi: 10.1128/IAI.73.6.3531-3539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Su Z, Segura M, Stevenson MM. Reduced protective efficacy of a blood-stage malaria vaccine by concurrent nematode infection. Infect Immun. 2006;74:2138–2144. doi: 10.1128/IAI.74.4.2138-2144.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Urban JF, Jr, et al. Infection with parasitic nematodes confounds vaccination efficacy. Vet Parasitol. 2007;148:14–20. doi: 10.1016/j.vetpar.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cooper PJ, et al. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003;111:995–1000. doi: 10.1067/mai.2003.1348. [DOI] [PubMed] [Google Scholar]
- 113.Dagoye D, et al. Wheezing, allergy, and parasite infection in children in urban and rural Ethiopia. Am J Respir Crit Care Med. 2003;167:1369–1373. doi: 10.1164/rccm.200210-1204OC. [DOI] [PubMed] [Google Scholar]
- 114.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 115.Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 116.Fallon PG, Mangan NE. Suppression of Th2-type allergic reactions by helminth infection. Nat. Rev. Immunol. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- 117.Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12:563–568. doi: 10.1016/s0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]