Summary
The plasminogen activator/plasmin system is believed to play an important role in diverse pathophysiological processes, including wound healing, vascular remodeling and pulmonary fibrosis. Our recent studies show that plasmin upregulates the expression of Cyr61, a growth factor-like gene that has been implicated in cell proliferation and migration. In the present study, we investigated whether plasmin promotes fibroblast proliferation and, if so, determine the role of Cyr61 in the plasmin-induced response. Human lung fibroblasts were exposed to varying concentrations of plasmin and DNA synthesis was monitored by measuring the incorporation of 3H-thymidine into DNA. Plasmin increased DNA synthesis of fibroblasts in a dose-dependent manner. Protease-activated receptor-1 (PAR-1)-specific antibodies, but not PAR-2-specific antibodies, reduced the plasmin-induced DNA synthesis. Consistent with this, plasmin had no substantial effect on the DNA synthesis in PAR-1-deficient mouse fibroblasts. Plasmin activated both p38 and p44/42 MAPKs and specific inhibitors of these pathways inhibited the plasmin-induced DNA synthesis. Plasmin-induced increase in the DNA synthesis was completely abrogated by anti-Cyr61 antibodies. Interestingly, thrombin, which is a potent inducer of Cyr61, had only a minimal effect on fibroblast proliferation. Additional experiments suggested that plasmin cleaved cell/extracellular matrix-associated Cyr61 and the conditioned media from plasmin-treated cells could support the cell proliferation. Overall, these data suggest that plasmin promotes fibroblast proliferation by a novel pathway, involving two independent steps. In the first step, plasmin induces Cyr61 expression via activation of PAR-1, and in the second step, plasmin releases Cyr61 deposited in the extracellular matrix, thus making it accessible to act on cells.
Keywords: cell proliferation, Cyr61, plasmin, protease-activated receptors
Introduction
The plasminogen activator/plasmin system plays an important role, in addition to fibrinolysis, in many pathophysiological processes, including cancer, atherosclerosis, vascular remodeling and wound healing [1-4]. Plasmin is shown to affect various cellular processes primarily through proteolysis of extracellular matrix (ECM) and activation of matrix metalloproteinases [2,3]. Plasmin also activates and liberates growth factors, such as transforming growth factor-β1 [5-7] and basic fibroblast growth factor (bFGF) [8,9], from the ECM, which can mediate various effects of plasmin on tissue remodeling and angiogenesis.
Cellular expression of plasminogen activators, particularly urokinase-type plasminogen activator (uPA), which ensures the generation of plasmin at cell surfaces, was shown to increase DNA synthesis in various cell and other model systems [9-16]. However, u-PA was shown primarily to stimulate cell proliferation independent of plasminogen [10-13], probably by binding to its cellular receptor urokinase-type plasminogen activator receptor (uPAR) and eliciting signals via the receptor-mediated signaling. Earlier studies that directly examined the ability of plasmin to promote cell proliferation gave conflicting results. Herbert et al. [14] reported that plasmin had no effect on smooth muscle cell (SMC) proliferation whereas tissue-type plasminogen activator induced a 6–7-fold increase in the cell number. Similarly, plasmin had no significant effect on proliferation of prostatic cancer cells [12,15]. Grainger et al. [17] reported that plasmin inhibits the proliferation of SMC. Data from more recent studies suggest that plasmin may directly potentiate the proliferation of hepatocytes in primary cultures [16] and SMC of human saphenous vein [9] since plasmin inhibitors suppressed the proliferation of these cells. Thus, at present, the mechanistic role of plasmin in cell proliferation is unclear.
Our recent studies [18] show that plasmin upregulates the expression of Cyr61, a growth factor like immediate early response gene, in fibroblasts via PAR-1-coupled signaling mechanism. Cyr61 belongs to a novel class of cysteine-rich secreted proteins and acts as an ECM-associated signaling molecule [19]. Recent studies suggest that Cyr61 influences a wide array of important biological functions [19] and activates a genetic program for wound repair in skin fibroblasts [20]. Unlike classical growth factors, Cyr61 does not act as a mitogen by itself, but augments growth factor-induced DNA synthesis in fibroblasts and endothelial cells [21,22]. Our current knowledge of potential functions of Cyr61 is based primarily on experiments in which cells were treated with relatively high concentrations (25–225 nm) of purified recombinant Cyr61 [20-23]. Although Cyr61 is strongly induced by growth factors [24] and proteases [18,25], it seems unlikely that actual local concentration of Cyr61 protein in tissues could reach a high nanomolar concentration range. Further, at present, there is no convincing evidence that Cyr61 induced in response to pathophysiological stimuli altered cellular functions.
In the present study, we investigated whether plasmin induces DNA synthesis in fibroblasts and the role of PAR-1 and Cyr61 in plasmin-induced mitogenesis. The data presented here show that plasmin promotes fibroblast proliferation by a novel mechanism. In this mechanism, plasmin not only induces Cyr61 expression but also releases it from ECM, thus making it readily accessible to act on cells.
Materials and methods
Cell culture
A human fibroblast cell line (WI-38) was obtained from ATCC (Rockville, MD, USA). Immortalized PAR-1-deficient (PAR-1−/−) and wild-type murine embryonic myofibroblasts were kindly provided by P. Andrade-Gordon (Drug Discovery, The R.W. Johnson Pharmaceuticals Research Institute, Spring House, PA, USA). Fibroblast cells lines were grown as described earlier [18].
Enzymes and other reagents
Human plasmin, thrombin and other coagulant and fibrinolytic proteases were obtained from either Enzyme Research Laboratories (South Bend, IN, USA) or Hematologic Technologies Inc. (Essex Junction, VT, USA). Recombinant tissue factor pathway inhibitor-2 (TFPI-2) was a gift from W. Kisiel (University of New Mexico, New Mexico, USA). Active siteinhibited plasmin was prepared by incubating plasmin with a 100-fold molar excess of D-Phe-L-Phe-L-Arg chloromethyl ketone (PPACK) (Calbiochem, San Diego, CA, USA) for 1 h at 37 °C and then removing the free inhibitor by exhaustive dialysis at 4 °C against 10 mm HEPES, 0.15 m NaCl, pH 7.5. PAR-1-specific monoclonal antibodies, WEDE-15 and ATAP-2, were obtained from Beckman Coulter (Fullerton, CA, USA) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), respectively. W. Ruf (Scripps Research Institute, La Jolla, CA, USA) provided PAR-2-specific antibodies. Basic FGF and antibodies against bFGF were obtained from R&D Systems (Minneapolis, MN, USA). Antibody kits for the analysis of activation of p44/42 MAPK, p38 MAPK and JNK were obtained from Cell Signaling Technologies (Beverly, MA, USA).
Cyr61 antibodies
To obtain antibodies against human Cyr61, we first expressed recombinant human Cyr61 in insect cells. Recombinant Cyr61 from the conditioned media was purified essentially as described earlier using SP-Sepharose chromatography [22]. The recombinant Cyr61 was further purified by affinity chromatography on a nickel column to obtain a near homogeneous protein. Such purified recombinant Cyr61 protein was used to raise antibodies against Cyr61 in rabbit using a standard protocol. IgG was purified by DEAE Affi-Gel-Blue (Bio-Rad, Hercules, CA, USA) chromatography. Cyr61 polyclonal antibodies (H78) for epitope corresponding to the internal region of Cyr61 (aa 163–240) were obtained from Santa Cruz Biotechnology, Inc. for use in immunoblot analysis.
Immunoblot analysis
Following specific treatments, overlying media were removed and the cells were lyzed in 100 μL (six-well plate) of lysis buffer [20 mm Tris–HCl, pH 6.8, 2% SDS, 10% glycerol, 0.1% Triton X-100, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 5 mm sodium pyrophosphate and 1 × proteinase inhibitor cocktail (Sigma, St Louis, MO, USA)]. Fifteen microliters of cell lysates were subjected to SDS–PAGE on 10% polyacrylamide gel, transferred onto PVDF membrane by electroblotting and probed with specific antibodies using standard procedures. Where conditioned media were used for immunoblotting, they were concentrated (~ 10-fold) by ultrafiltration (10 000 Mr cut-off filter) before being subjected to SDS–PAGE.
DNA mitogenesis
The quiescent cells (50–60% confluent and serum starved for overnight) were treated with plasmin or other stimulants in serum-free medium in the presence of 1 μCi mL−1 of 3H-methyl thymidine. After 48 h of incubation, the cells were washed three times with PBS (pH 7.2) and fixed with ice-cold 10% (v/v) trichloroacetic acid (TCA). The labeled DNA was dissolved in 250 μL of 0.2 n NaOH, mixed with 3.25 mL of scintillation cocktail (Ecolite +, ICN Biomedicals Inc., Costa Mesa, CA, USA) and the radioactivity associated with DNA was counted using a LS6500 Multi Purpose Scintillation Counter (Beckman Coulter). All experiments were done in triplicate wells and repeated at least three times. Data within an experiment were normalized to 3H-thymidine incorporated into control (non-treated) cells, which was taken as 100%. Data shown in figures represent mean values ± SE of three to seven independent experiments.
Results
Effect of plasmin and other proteases on fibroblast mitogenesis
Our recent studies show that plasmin [18], thrombin and factor (FVIIa)/tissue factor (TF) [25] induce Cyr61, a growth factor-like gene, expression in fibroblasts through a protease activity-dependent signaling mechanism. Since Cyr61 is shown to be involved in the regulation of cell proliferation in fibroblasts [21], we investigated whether the above proteases induce cell proliferation in fibroblasts. Human fibroblasts (WI-38) were treated with plasmin, FVIIa and thrombin (in concentrations that were shown to induce Cyr61 expression maximally) for 48 h, and 3H-thymidine incorporation into DNA was determined as an index for mitogenesis. As shown in Fig. 1A, thrombin and FVIIa increased the DNA synthesis in fibroblasts by about 20–30% over the unstimulated fibroblasts. Plasmin treatment, in contrast to FVIIa/TF and thrombin, increased 3H-thymidine incorporation markedly over the control (unstimulated cells) (P < 0.001). Plasmin-induced increase in the DNA synthesis was significantly higher than that observed with thrombin or FVIIa (P = 0.004). Similar results were obtained with primary human lung fibroblasts (data not shown).
Fig. 1.
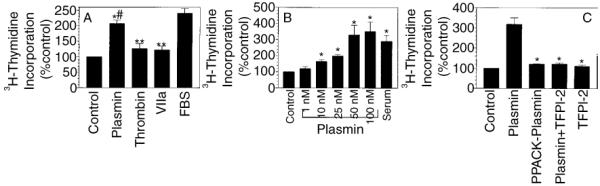
Effect of plasmin, thrombin and factor FVIIa on DNA synthesis of fibroblasts. (A) Quiescent monolayers of WI-38 cells were treated with plasmin (50 nm), thrombin (10 nm), FVIIa (100 nm) or fetal bovine serum (FBS) (10% v/v) for 48 h. 3H-methyl thymidine (1 μCi mL−1) was included in the medium. At the end of 48 h, 3H-thymidine incorporated into DNA was determined as described in Materials and methods (n = 6, mean ± SE). *P < 0.001; **P < 0.05, compared with the control value #(P = 0.004), compared with the values obtained with thrombin or FVIIa treatment. (B) Quiescent monolayers of WI-38 cells were treated with various concentrations of plasmin (1–100 nm) or FBS (10% v/v) and 3H-thymidine incorporated into DNA was determined as in (A) (mean ± SE, n = 6). *Values differ significantly (P < 0.01) from the control value. (C) Monolayers were treated with plasmin (50 nm), active site-inhibited plasmin (PPACK-plasmin, 50 nm) or plasmin (50 nm) pretreated with tissue factor pathway inhibitor (TFPI)-2 (250 nm) and 3H-thymidine incorporated into DNA was determined at the end of 48 h (mean ± SE from three experiments). *Values differ significantly (P < 0.001) from the value obtained with plasmin treatment.
Plasmin-induced DNA synthesis
Next, we investigated dose-dependency of plasmin-induced DNA synthesis by treating WI-38 cells with varying concentrations of plasmin, 1–100 nm, for 48 h. As shown in Fig. 1B, plasmin-induced increase in 3H-thymidine incorporation was evident and statistically significant (P < 0.01) with as low as 10 nm of plasmin, and reached the maximum level at 50 nm of plasmin. With 50 nm of plasmin, we observed a 2–5-fold increase in 3H-thymidine incorporation over unstimulated fibroblasts. The fold-increase in the DNA synthesis observed in fibroblasts with plasmin treatment was similar to the fold-increase attained with serum treatment, suggesting that plasmin enhanced DNA synthesis maximally in fibroblasts. In contrast to plasmin, active site-inhibited plasmin (PPACK-plasmin) failed to increase the DNA synthesis (Fig. 1C), suggesting that the protease activity of plasmin is essential for plasmin-induced cell proliferation. These data were further confirmed in experiments where preincubation of plasmin with TFPI-2, an effective inhibitor of plasmin [26], completely abrogated the plasmin-induced DNA synthesis (Fig. 1C). To test whether plasmin-induced cell proliferation is mediated through its activation of matrix metalloproteases, we examined the effect of GM 6001 and GM 1489, potent and broad range inhibitors of matrix metalloproteases (MMP1, MMP2, MMP3, MMP8 and MMP9), on plasmin-induced cell proliferation. Both the inhibitors, even at a high concentration of 40 μm, failed to inhibit plasmin-induced cell proliferation (data not shown), ruling out the involvement of MMPs in plasmin-induced cell proliferation.
Involvement of PAR-1 in plasmin-induced cell proliferation
To investigate whether plasmin-induced fibroblast proliferation is mediated via PAR-1, as observed with plasmin-induced Cyr61 gene expression, wild-type and PAR-1-deficient mouse fibroblasts were treated with plasmin. As shown in Fig. 2A, plasmin increased 3H-thymidine incorporation into DNA in the wild-type mouse fibroblasts in a dose-dependent manner. At 25 nm of plasmin concentration, 3H-thymidine incorporation was 110% higher than that observed with unstimulated cells. In contrast, plasmin treatment had only a minimal effect (~ 30% increase over the control) in PAR-1-deficient cells. Plasmin-induced DNA synthesis in wild-type mouse fibroblasts was significantly higher (P < 0.05) than that observed in PAR-1-deficient cells. Thrombin (10 nm) treatment enhanced 3H-thymidine incorporation by 36% in wild-type mouse fibroblasts and 24% in PAR-1-deficient cells (data not shown). As expected, both cell types responded well to serum stimulation. To confirm further the involvement of PAR-1 in plasmin-induced fibroblast proliferation, we have investigated the effect of PAR-1-specific antibodies on plasmin-induced DNA synthesis in human fibroblasts. WI-38 cells were pretreated for 1 h with PAR-1-specific monoclonal antibody (ATAP-2), followed by plasmin for 48 h. As shown in Fig. 2B, PAR-1-specific antibody significantly inhibited plasmin-induced DNA synthesis. In contrast, PAR-2-specific antibodies, which effectively neutralize factor Xa and FVIIa/TF-induced PAR-2-mediated signaling [27], had no significant effect on plasmin-induced DNA synthesis.
Fig. 2.
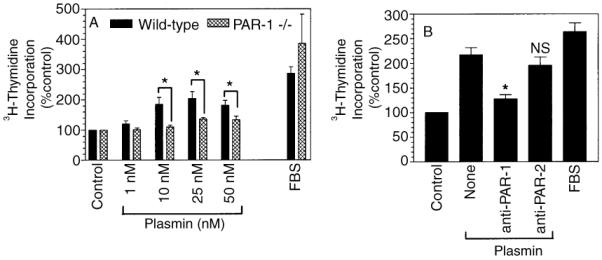
Involvement of protease-activated receptor (PAR)-1 in plasmin-induced DNA synthesis. (A) Quiescent monolayers of wild-type mouse fibroblasts (filled bars) or PAR-1-deficient mouse fibroblasts (hatched bars) were treated with varying concentrations of plasmin or serum (10% v/v) in the presence of 3H-methyl thymidine (1 μCi mL−1). At the end of 48 h stimulation, 3H-thymidine incorporated into DNA was determined (mean ± SE, n = 3–6). *P < 0.05. (B) WI-38 cells were pretreated with PAR-1-specific monoclonal antibody, ATAP-2 (25 μg mL−1) or PAR-2-specific polyclonal antibodies (500 μg mL−1) for 1 h, followed by plasmin (50 nm) stimulation. At the end of 48 h stimulation, 3H-thymidine incorporated into DNA was determined (mean ± SE, n = 3–7). *P < 0.001; ns, no significant difference from the value obtained with plasmin treatment alone.
Involvement of MAPK signaling pathway in plasmin-induced DNA synthesis
To investigate the involvement of MAPKs in plasmin-induced mitogenesis, we investigated the effect of plasmin on the activation of p44/42 MAPK, p38 MAPK and JNK, and analyzed the effect of MAPK pathway inhibitors on plasmin-induced DNA synthesis. As shown in Fig. 3A,B, plasmin markedly induced the activation of p44/42 MAPK. PD98059 and U0126, the selective inhibitors of MEK1 and MEK2, fully inhibited plasmin-induced activation of p44/42 MAPK. Plasmin also induced the activation of p38 MAPK, but the activation was modest (Fig. 3C). Plasmin appeared to have no effect on JNK activation, as we were unable to detect the phosphorylated JNK protein on the Western blot analysis using phosphospecific JNK antibodies (data not shown). Treatment of fibroblasts with PD98059 and U0126 inhibited plasmin-induced DNA synthesis by about 60–80% (Fig. 3D), indicating the involvement of p44/42 MAPK pathway in plasmin-induced DNA synthesis. p38 MAPK specific inhibitor, SB203580, also inhibited plasmin-induced DNA synthesis by 50% (Fig. 3D). It should be noted that the above inhibitors, in concentration used herein, did not affect the cell viability as measured in MTT assay or trypan blue dye exclusion method.
Fig. 3.
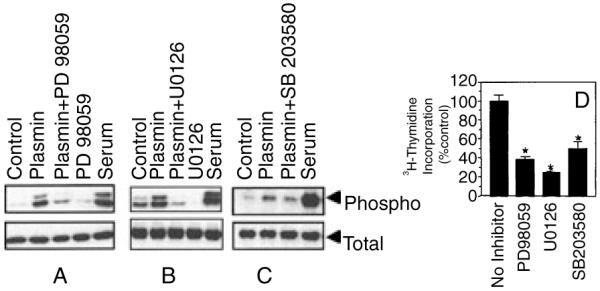
Involvement of p44/42 MAPK and p38 MAPK activation in plasmin-induced DNA synthesis. Quiescent monolayers of WI-38 cells were preincubated with or without specific inhibitors for 30 min and then stimulated with plasmin (50 nm) for 5 min (C) or 20 min (A,B). Cell lysates were subjected to Western blot analysis to detect phosphospecific and total p38 and p44/42 MAPK. (D) The effect of inhibitors on plasmin-induced DNA synthesis (mean ± SE, n = 4). Inhibitor concentrations as follows: PD98059, 50 μm; U0126, 10 μm; SB203580, 25 μm.Values obtained with the inhibitors significantly differ (P < 0.01) from the value obtained with plasmin alone.
Anti-Cyr61 antibodies inhibit plasmin-induced DNA synthesis
To test whether plasmin-induced Cyr61 plays a role in fibroblast proliferation observed with the plasmin treatment, WI-38 cells were treated with anti human Cyr61 IgG for 1 h before the addition of plasmin. As shown in Fig. 4, anti-Cyr61 antibodies fully attenuated plasmin-induced cell proliferation. No differences were observed in 3H-thymidine incorporation between the unstimulated cells and the cells that were treated with plasmin in the presence of anti-Cyr61 antibodies. An irrelevant antibody raised using the same protocol had no effect on plasmin-induced mitogenesis. Since earlier studies suggest that Cyr61 has no detectable mitogenic activity by itself, but enhances the mitogenic effect of growth factors, such as bFGF [21], we investigated the potential contribution of bFGF in plasmin-induced mitogenesis. Fibroblasts were treated with bFGF neutralizing antibodies and then stimulated with plasmin. In contrast to Cyr61 antibodies, anti-bFGF had no significant effect on plasmin-induced DNA synthesis (data not shown). Further, addition of bFGF to fibroblasts did not enhance the plasmin-induced DNA synthesis.
Fig. 4.
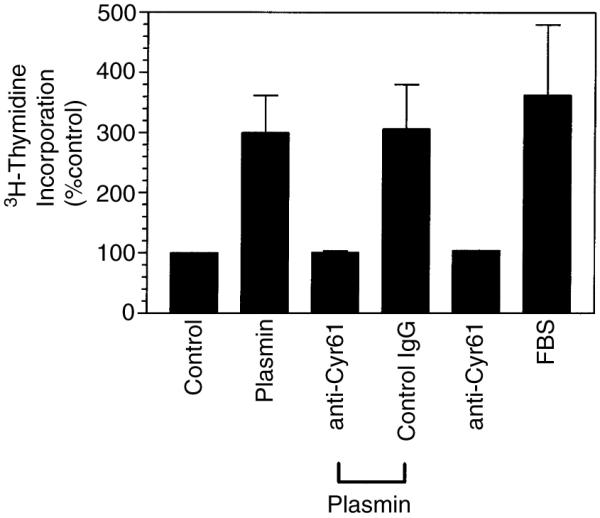
Anti-Cyr61 antibodies abolish plasmin-induced DNA synthesis. Quiescent monolayers of WI-38 cells were pretreated with antihuman Cyr61 IgG (100 μg mL−1) or an irrelevant IgG as a control (100 μg mL−1) for 1 h. The cells were stimulated with plasmin (50 nm) for 48 h and 3H-thymidine incorporated into DNA was determined (n = 3).
Plasmin mediates the release of cell/ECM-associated Cyr61
Our earlier studies show that thrombin is a potent inducer of Cyr61 gene expression in fibroblasts [18,25]. Therefore, it is intriguing to observe in the present study that plasmin, but not thrombin, effectively promotes fibroblast proliferation. To obtain potential clues to explain this anomaly, we examined the effect of plasmin and thrombin on Cyr61 protein expression by Western blot analysis. When cell lysates were analyzed for the expression of Cyr61, we found only trace levels of Cyr61 in unstimulated fibroblasts. Thrombin treatment markedly upregulated the expression of Cyr61 protein (Fig. 5). Surprisingly, we found no detectable increase in Cyr61 protein in cell lysates of fibroblasts that were treated with plasmin (Fig. 5). In fact, plasmin treatment appeared to reduce basal levels of Cyr61 observed in unstimulated cells. These data suggest that plasmin might be cleaving Cyr61, which associates with ECM and cell surfaces [28]. However, we were unable to detect Cyr61 protein or its fragments in the conditioned media of plasmin-treated cells. This could be due to the inability of Cyr61 antibodies to bind effectively to Cyr61 peptide fragments on immunoblot analysis. The data obtained with recombinant Cyr61 and plasmin support such a scenario. In these experiments recombinant Cyr61 (~ 10 μg mL−1) was incubated with plasmin or thrombin (10 nm each) for 1 h and the samples were subjected to immunoblot analysis. Upon plasmin treatment, either intact Cyr61 protein or Cyr61 peptide fragment (28 kDa) was barely detectable, suggesting complete proteolysis of Cyr61 by plasmin. In contrast, thrombin had no effect on Cyr61 proteolysis.
Fig. 5.
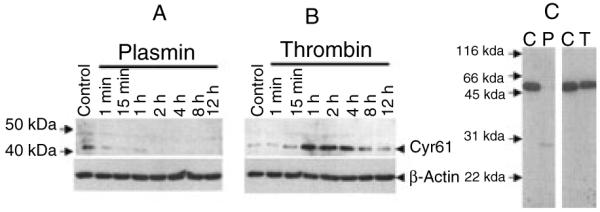
Effect of plasmin and thrombin on Cyr61 protein expression in fibroblasts and recombinant Cyr61. Quiescent monolayers of WI-38 cells were treated with plasmin (50 nm) (A) or thrombin (10 nm) (B). At varying times, the overlying medium was removed, the cells were solubilized in cell lysis buffer and cell lysates were subjected to immunoblot analysis and probed with anti-Cyr61 antibodies. (C) Recombinant human Cyr61 (10 μg mL−1) was incubated with a control buffer (C), plasmin (10 nm) or thrombin (10 nm) for 1 h and the samples were subjected to immunoblot analysis with Cyr61 antibodies. Recombinant Cyr61 migrated at a higher Mr because it contained V5-His tag at the C-terminus.
To test our hypothesis that plasmin release of Cyr61 from ECM plays a role in promoting DNA synthesis, we investigated the effect of conditioned media from control and plasmin-treated fibroblasts in promoting DNA synthesis. For these experiments fibroblasts were treated with plasmin or thrombin for 2 h, then the conditioned medium was collected and the plasmin protease activity in the medium was inactivated with TFPI-2. The conditioned medium was added to fresh cells to evaluate its effect on DNA synthesis. The data revealed that the conditioned media of plasmin-treated cells and not others significantly increased 3H-thymidine incorporation in fibroblasts. Addition of Cyr61 antibodies to the conditioned media of plasmin-treated cells attenuated the enhancing effect of the conditioned media (Fig. 6). These data suggest that plasmin-released Cyr61, following the induction of Cyr61, is responsible for increased DNA synthesis in fibroblasts exposed to plasmin.
Fig. 6.
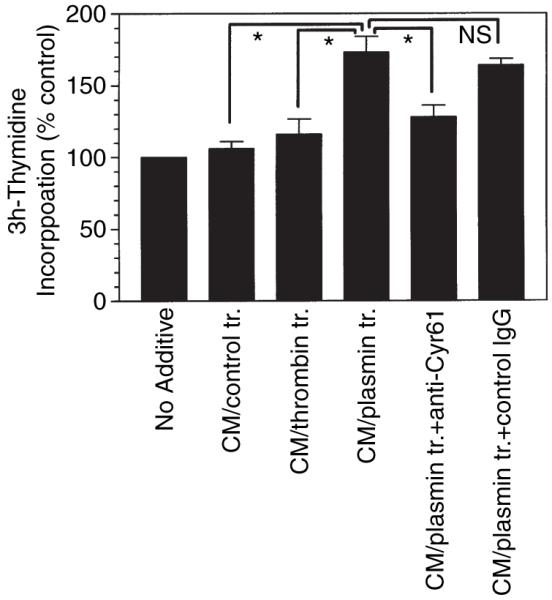
Evidence for plasmin release of Cyr61 contributes to increased DNA synthesis in fibroblasts. Quiescent monolayers of WI-38 cells were exposed to control medium or medium containing plasmin (50 nm) or thrombin (10 nm). At the end of 2 h, the supernatant media were collected and the protease activity of plasmin in the conditioned media was inactivated with tissue factor pathway inhibitor (TFPI)-2 (250 nm, for 30 min). The conditioned media of plasmin-treated cells were incubated for 30 min with control IgG or anti-Cyr61 IgG (100 μg mL−1) before being added to fresh fibroblasts. 3H-thymidine incorporated into DNA was determined at the end of 48 h (mean ± SE, n = 4). *Paired values differ significantly from each other (P < 0.005). ns, Not significant.
To strengthen further the above observation, we investigated the effect of full-length recombinant Cyr61 and plasmin-cleaved recombinant Cyr61 on DNA synthesis in fibroblasts. Recombinant Cyr61 was digested with plasmin (50 : 1 w/w ratio) for 30 min and the protease activity of plasmin was neutralized with TFPI-2. Fibroblasts were treated with either the uncleaved or plasmin-cleaved Cyr61 (10 nm) for 48 h and 3H-thymidine incorporation into DNA was measured. The data revealed that the uncleaved Cyr61 had no significant effect (109 ± 4.9% of the control) whereas plasmin-cleaved Cyr61 substantially increased (185 ± 19.9%, n = 3, P < 0.05 vs. uncleaved Cyr61) the DNA synthesis.
Discussion
The plasminogen activator/plasmin system is shown to play an important role, in addition to its established role in fibrinolysis, in many pathophysiological processes, including development, malignancy, vascular remodeling and wound healing. At present, it is believed that plasmin contributes to these processes by clearing fibrin, degrading ECM and liberating/activating sequestered growth factors from ECM. Our recent studies [18] show that plasmin induces an angiogenic and wound-healing mediator, Cyr61, in fibroblasts through the activation of PAR-1-mediated cellular signaling, a mechanism that was not fully appreciated in earlier studies. These data raise a possibility that plasmin may directly affect various cellular processes, independent of its action on fibrin and ECM degradation. The data presented here provide strong support to this hypothesis. The data show that plasmin promotes fibroblast proliferation in a novel mechanism involving two steps: first, inducing Cyr61 expression via activation of PAR-1-mediated signaling, and then releasing Cyr61 from ECM/cell surfaces.
Although a number of earlier studies showed that the plasminogen activator/plasmin system promotes proliferation of various cell types [9-16], in most of these studies the cell proliferation appeared to be independent of plasmin. Only in limited studies was the increased DNA synthesis shown to be dependent on plasmin generation [9,16]. In the human saphenous vein model system, where plasmin was shown to be responsible for the mitogenesis of SMC [9], it was suggested that plasmin-mediated mobilization of FGF-2 from the ECM was responsible for the cell proliferation since FGF-2 antibodies completely blocked the cell proliferation. It is unlikely that such a mechanism plays a role in plasmin-mediated fibroblast proliferation since FGF-2 antibodies had no significant effect on plasmin-induced DNA synthesis in fibroblasts. It is also unlikely that plasmin activation of promatrix metalloproteinases is involved in plasmin-induced DNA synthesis since the inhibitors of matrix metalloproteinases had no effect on plasmin-induced DNA synthesis.
Since in earlier studies very high concentrations of plasmin were required to activate PAR-1 and plasmin was shown to cleave PAR-1 rapidly at multiple sites downstream of the activation site, it was believed that plasmin was unlikely to transmit signaling through the activation of PAR-1; rather, it was predicted to inactivate PAR-1 [29-33]. However, our recent studies show that plasmin, at a physiologically relevant concentration range, can induce the expression of a growth factor-like gene, Cyr61, through a PAR-1-dependent mechanism. The data presented in this manuscript provide further support for the ability of plasmin to transmit cellular signaling through PAR-1. It is important to note that as low as 10 nm plasmin is sufficient to induce Cyr61 gene expression [18] and promote DNA synthesis in fibroblasts (the present study). This concentration of plasmin in vivo could be achieved by conversion of less than 1% of the zymogen to the active form. Measurement of plasmin–antiplasmin complexes, as a marker for plasmin generation in vivo, showed that a substantial amount of plasmin (5–10 nm) was generated under basal conditions and various pathophysiological conditions increased plasmin generation markedly (15–250 nm range) [34-36].
The observation that Cyr61 antibodies fully attenuated plasmin-induced DNA synthesis indicates that plasmin-induced Cyr61 is responsible for the enhanced DNA synthesis. It is interesting to note that thrombin, a potent inducer of Cyr61, had a lesser effect than plasmin in promoting the DNA synthesis of fibroblasts. At present, the reason for this discrepancy is unknown. One likely explanation for the ineffectiveness of thrombin is that thrombin-induced Cyr61 is sequestered at the cell surface or ECM, and thus the newly synthesized Cyr61 was unavailable to exert its effect on fibroblasts. Consistent with this explanation, our data show that Cyr61 induced by thrombin is associated entirely with cells/ECM, whereas no Cyr61 protein was detected in cell lysates of plasmin-treated cells. This could be explained if plasmin cleavage of cell-associated Cyr61 may proceed at a much faster rate than plasmin-induced de novo synthesis of Cyr61. Although one expects to detect Cyr61 in the conditioned media of plasmin treated cells, at present we are unable to demonstrate Cyr61 or its cleaved products in the conditioned media of plasmin-treated fibroblasts. A potential reason for this could be inability of anti-Cyr61 antibodies to effectively recognize cleaved Cyr61 fragment(s). Further, prolonged incubation of cells with plasmin may cleave the protein at multiple sites. Preliminary studies conducted with recombinant Cyr61 suggest that plasmin cleaves Cyr61 and the Cyr61 peptide fragment was poorly detected by the antibodies in immunblot analysis. However, we were able to detect the release of a Cyr61 peptide fragment into the supernatant conditioned media when tumor cells, which constitutively express abundant Cyr61, were exposed to plasmin (data not shown).
In earlier studies where recombinant Cyr61 was added to cells, it was found that Cyr61 does not act as a mitogen by itself but enhances the mitogenic effect of other growth factors, such as bFGF [21]. Since plasmin was shown to release biologically active bFGF from ECM [8,9], it is possible that plasmin-induced mitogenesis could have stemmed from a combined action of Cyr61 and bFGF. However, in contrast to Cyr61 antibodies, bFGF antibodies failed to attenuate plasmin-induced response. Moreover, exogenously added bFGF did not further enhance plasmin-induced DNA synthesis in fibroblasts (data not shown). These data raise the possibility that exogenously added recombinant Cyr61 may not mimic the endogenously synthesized Cyr61. An alternative explanation could be that plasmin-cleaved Cyr61 fragment(s) and not native Cyr61 acts as a potent mitogen or plasmin may be inducing other cellular factors that support Cyr61 action. Preliminary studies performed in the present study with a full-length and plasmin-cleaved recombinant Cyr61 support the possibility that plasmin cleavage of Cyr61 enhances the mitogenic effect of Cyr61. However, further experiments are needed to confirm and extend this novel finding.
In summary, the data presented herein provide a novel mechanism by which plasmin promotes cell proliferation. In this mechanism, plasmin first induces the expression of Cyr61, a growth factor-like molecule that associates with ECM [20], via activation of PAR-1-mediated signaling. Second, plasmin releases/cleaves newly synthesized Cyr61 from the ECM, making it accessible to cells. The ability of plasmin to induce mitogenesis, in addition to its pericellular proteolysis, may provide a coordinated spatiotemporal effect that is required for wound healing and tissue remodeling.
Acknowledgements
This study was supported in part by National Institute of Health Grant HL65500 (to U.R.P.).
References
- 1.Carmeliet P, Collen D. Development and disease in proteinase-deficient mice: role of the plasminogen, matrix metalloproteinase and coagulation system. Thromb Res. 1998;91:255–85. doi: 10.1016/s0049-3848(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 2.Pepper MS. Role of the matrix metalloproteinases and plasminogen activator–plasmin system in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–17. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 3.Lijnen HR. Plasmin and matrix metalloproteinases. Thromb Haemost. 2001;86:324–33. [PubMed] [Google Scholar]
- 4.Ploplis VA, Castellino FJ. Gene targeting of components of the fibrinolytic system. Thromb Haemost. 2002;87:22–31. [PubMed] [Google Scholar]
- 5.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990;110:1361–7. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yee JA, Yan L, Dominguez JC, Allan EH, Martin TJ. Plasminogen-dependent activation of latent transforming growth factor beta by growing cultures of osteoblast-like cells. J Cell Physiol. 1993;157:528–34. doi: 10.1002/jcp.1041570312. [DOI] [PubMed] [Google Scholar]
- 7.Grainger DJ, Wakefield L, Bethell HW, Farndale RW, Metcalfe JC. Release and activation of platelet latent TGF-B in blood clots during dissolution with plasmin. Nat Med. 1995;1:932–7. doi: 10.1038/nm0995-932. [DOI] [PubMed] [Google Scholar]
- 8.Saksela O, Rifkin DB. Release of basic fibroblast growth factor–heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990;110:767–75. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George SJ, Johnson JL, Smith MA, Jackson CL. Plasmin-mediated fibroblast growth factor-2 mobilisation supports smooth muscle cell proliferation in human saphenvous vein. Vascular Res. 2001;38:492–51. doi: 10.1159/000051082. [DOI] [PubMed] [Google Scholar]
- 10.Kirchheimer JC, Wojta J, Christ G, Binder BR. Proliferation of human epidermal tumor cell line stimulated by urokinase. FASEB J. 1987;1:125–8. doi: 10.1096/fasebj.1.2.3038646. [DOI] [PubMed] [Google Scholar]
- 11.Baron-Van Evercooren A, Leprince P, Rogister B, Lefebvre PP, Delree P, Selak I, Moonen G. Plasminogen activators in developing peripheral nervous system, cellular origin and mitogenic effect. Brain Res. 1987;433:101–8. doi: 10.1016/0165-3806(87)90068-x. [DOI] [PubMed] [Google Scholar]
- 12.Kirchheimer JC, Wojta J, Hienert G, Christ G, Heger ME, Pfluger H, Binder BR. Effect of urokinase on the proliferation of primary cultures of human prostatic cells. Thromb Res. 1987;48:291–8. doi: 10.1016/0049-3848(87)90441-5. [DOI] [PubMed] [Google Scholar]
- 13.Binder BR. Influence of urokinase on cell proliferation and invasion. Blood Coag Fibrinol. 1990;1:717–20. [PubMed] [Google Scholar]
- 14.Herbert JM, Lamarche I, Prabonnaud V, Dol F, Gauthier T. Tissue-type plasminogen activator is a potent mitogen for human aortic smooth muscle cells. J Biol Chem. 1994;269:3076–80. [PubMed] [Google Scholar]
- 15.Festuccia C, Dolo V, Guerra F, Violini S, Muzi P, Pavan A, Bologna M. Plasminogen activator system modulates invasive capacity and proliferation in prostatic tumor cells. Clin Exp Metastasis. 1998;16:513–28. doi: 10.1023/a:1006590217724. [DOI] [PubMed] [Google Scholar]
- 16.Akao M, Hasebe Y, Okumura N, Hagiwara H, Seki T, Ariga T. Plasminogen activator–plasmin system potentiates the proliferation of hepatocytes in primary culture. Thromb Res. 2002;107:169–74. doi: 10.1016/s0049-3848(02)00258-x. [DOI] [PubMed] [Google Scholar]
- 17.Grainger DJ, Kirschenlohr HL, Metcalfe JC, Weissberg PL, Wade DP, Lawn RM. Proliferation of human smooth muscle cells promoted by lipoprotein(a) Science. 1993;260:1655–8. doi: 10.1126/science.8503012. [DOI] [PubMed] [Google Scholar]
- 18.Pendurthi UR, Ngyuen M, Andrade-Gordon P, Petersen LC, Rao LVM. Plasmin induces Cyr61 gene expression in fibroblasts via protease activated receptor-1 and p44/42 mitogen-activated protein kinase-dependent signaling pathway. Arterioscler Thromb Vasc Biol. 2002;22:1421–6. doi: 10.1161/01.atv.0000030200.59331.3f. [DOI] [PubMed] [Google Scholar]
- 19.Lau LF, Lam S. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 20.Chen C-C, Mo F-E, Lau LF. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001;276:47329–34. doi: 10.1074/jbc.M107666200. [DOI] [PubMed] [Google Scholar]
- 21.Kireeva ML, Mo F-E, Yang GP, Lau LF. Cyr61, a product of growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16:1326–34. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolesnikova T, Lau LF. Human CYR61-mediated enhancement of bFGF-induced DNA synthesis in human umbilical vein endothelial cells. Oncogene. 1998;16:747–54. doi: 10.1038/sj.onc.1201572. [DOI] [PubMed] [Google Scholar]
- 23.Grzeszkiewicz TM, Kirschling DJ, Chen N, Lau LF. CYR61 stimulates skin fibroblast migration through integrin αvβ5 and enhances mitogenesis through integrin αvβ3, independent of its carboxyl-terminal domain. J Biol Chem. 2001;276:21943–50. doi: 10.1074/jbc.M100978200. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990;10:3569–77. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendurthi UR, Allen KE, Ezban M, Rao LVM. Factor VIIa and thrombin induce the expression of Cyr61 and connective tissue growth factor, extracellular matrix signaling proteins that could act as possible downstream mediators in factor VII: tissue factor-induced signal transduction. J Biol Chem. 2000;275:14632–41. doi: 10.1074/jbc.275.19.14632. [DOI] [PubMed] [Google Scholar]
- 26.Petersen LC, Sprecher CA, Foster DC, Blumberg H, Hamamoto T, Kisiel W. Inhibitory properties of a novel human Kunitz-type protease inhibitor homologous. Biochem. 1996;35:266–72. doi: 10.1021/bi951501d. [DOI] [PubMed] [Google Scholar]
- 27.Ruf W, Dorfleutner A, Riewald M. Specificity of coagulation factor signaling. J Thromb Haemost. 2003;1:1495–503. doi: 10.1046/j.1538-7836.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 28.Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF. Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res. 1997;233:63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- 29.Kimura M, Andersen TT, Fenton IIJW, Bahou W, Aviv A. Plasmin–platelet interaction involves cleavage of functional thrombin receptor. Am J Physiol. 1996;271:C54–C60. doi: 10.1152/ajpcell.1996.271.1.C54. [DOI] [PubMed] [Google Scholar]
- 30.Kuliopulos A, Covic L, Seeley SK, Sheridan PJ, Helin J, Costello CE. Plasmin desensitization of the PAR1 thrombin receptor: kinetics, sites of truncation, and implications for thrombolytic therapy. Biochem. 1999;38:4572–85. doi: 10.1021/bi9824792. [DOI] [PubMed] [Google Scholar]
- 31.Loew D, Perrault C, Morales M, Moog S, Ravanat C, Schuhler S, Arcone R, Pietropaolo C, Cazenave JP, van Dorsselaer A, Lanza F. Proteolysis of the exodomain of recombinant protease-activated receptors: prediction of receptor activation or inactivation by MALDI mass spectrometry. Biochem. 2000;39:10812–22. doi: 10.1021/bi0003341. [DOI] [PubMed] [Google Scholar]
- 32.Parry MA, Myles T, Stone SR, Tschopp J. Cleavage of the thrombin receptor: identification of potential activators and inactivators. Biochem J. 1996;320:335–41. doi: 10.1042/bj3200335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafer AI, Mass AK, Ware JA, Jhonson PC, Rittenhouse SE, Salzman EW. Platelet protein phosphorylation, elevation of cytosolic calcium, and inositol phospholipid breakdown in platelet activation induced by plasmin. J Clin Invest. 1986;78:73–9. doi: 10.1172/JCI112576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suffredini AF, Harpel PC, Parrillo JE. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. N Engl J Med. 1989;320:1165–72. doi: 10.1056/NEJM198905043201802. [DOI] [PubMed] [Google Scholar]
- 35.Renckens R, Weijer S, de Vos AF, Pater JM, Meijers JC, Hack CE, Levi M, van der Poll T. Inhibition of plasmin activity by tranexamic acid does not influence inflammatory pathways during human endotoxemia. Arterioscler Thromb Vasc Biol. 2004;24:483–8. doi: 10.1161/01.ATV.0000118280.95422.48. [DOI] [PubMed] [Google Scholar]
- 36.Taguchi O, Gabazza EC, Yoshida M, Yamakami T, Kobayashi H, Shima T. High plasma level of plasmin–alpha 2-plasmin inhibitor complex is predictor of poor prognosis in patients with lung cancer. Clinica Chimica Acta. 1996;244:69–81. doi: 10.1016/0009-8981(95)06196-7. [DOI] [PubMed] [Google Scholar]


