Abstract
Neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), lack definitive diagnostic tests or biomarkers of disease progression. Most studies that investigate protein abnormalities in ALS have used biofluids such as blood or cerebrospinal fluid (CSF), while some have used post mortem tissue or CSF samples. Since ALS disease progression and post mortem effects probably induce significant alterations to protein modifications or proteolysis, we directly examined the CSF proteome from ALS subjects at various lengths of time from symptom onset and at autopsy by mass spectrometry based proteomics. CSF was also obtained from both healthy age-matched control subjects and at autopsy from healthy and Alzheimer's disease (AD) controls. We identified significant differences in the CSF proteome between living and post mortem ALS subjects, as well as living and post mortem control subjects. We also noted differences in the CSF proteome of ALS subjects that have exhibited symptoms for varying lengths of time and between ALS and AD subjects at end-stage of disease. This is the first study describing differences in the CSF proteome from post mortem and living ALS subjects using a mass spectrometric approach. These differences highlight the importance of utilizing CSF from living ALS subjects near the time of symptom onset for the identification of early protein biomarkers, although some protein alterations that occur early in the disease process are maintained throughout the course of disease and in post mortem samples.
Keywords: Cerebrospinal fluid, proteomics, mass spectrometry, biomarkers
Introduction
Although many scientific advances have been made in understanding the pathobiology of neurodegenerative diseases including Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS), few studies have yielded disease specific biomarkers or effective clinical interventions (1–3). Imaging and proteomic approaches using two-dimensional gel electrophoresis (2D-GE) or mass spectrometry have been utilized to discover surrogate or protein biomarkers for these neurologic diseases (4–7). Additional studies have revealed oxidative modifications to proteins or lipids as potential biomarkers in AD, ALS and PD (8,9). A number of individual proteins such as tau, Nogo, neurofilament (NF), vascular endothelial growth factor, S100B protein and alpha2-macroglobulin have been reported to act as biomarkers for ALS but none has achieved the level of specificity required for proper early diagnosis of the disease (3,10).
One critical component of biomarker discovery efforts is the starting material used for analysis. For diagnostic biomarkers it is critical to obtain biofluids or tissue samples as close to the time of disease onset as possible as biomarker patterns may change during disease progression or at end-stage. A recent study using two-dimensional gel electrophoresis (2D-GE) compared CSF from healthy living and post mortem subjects to uncover potential biomarkers of ischemia and neurodegeneration and identified proteins including GFAP that were previously denoted as markers of numerous neurodegenerative diseases (11). A second 2D-GE study also revealed proteomic changes between ante mortem and post mortem CSF samples in a small group of healthy subjects, including proteins previously proposed to be stroke biomarkers (12). Due to post mortem interval times and ante mortem or agonal state of the subject, protein alterations within CSF obtained at the time of autopsy may be unrelated to the disease and dramatically affect proteomic analysis and subsequent biomarker discovery efforts.
Although CSF is an appropriate biofluid for biomarker discovery, it is important to properly obtain and store these samples to maximize proteome stability and minimize blood contamination (13). We recently reported standard procedures to properly acquire and store human CSF samples to maintain proteome stability as assessed by mass spectrometry (14). We have used this CSF protocol to uncover a panel of protein biomarkers in the CSF of patients recently diagnosed with ALS (7). There are many additional factors such as differences in age, gender, and drug regimens that present as confounding factors in addition to other disease unrelated issues, including time of day that the biofluid is collected. Another confound is the use of autopsy derived tissue or biofluids that may harbor post mortem induced changes to the proteome. These factors can generate false positives when screening for disease specific protein biomarkers. Few studies have directly examined proteomic changes between samples obtained from living subjects near time of disease diagnosis compared with samples obtained at end-stage disease. No such studies have been performed for ALS. It is therefore important to evaluate proteomic alterations that occur during disease progression and protein alterations that exist between neurologic disease samples obtained from subjects early in the disease and those obtained at end-stage or post mortem.
In the current study, we utilized CSF samples obtained from living subjects diagnosed with ALS and those acquired at the time of autopsy for protein profiling using mass spectrometry based proteomics. Age-matched healthy and neurologic disease controls were included in both the living and autopsy derived subject groups. We identified 38 mass spectral peaks that exhibit statistically significant changes between these subject groups, including many proteins that were previously identified as potential ALS biomarkers. This study highlights the importance of choosing the proper starting samples for biomarker discovery efforts for ALS and other neurologic diseases and also validates prior studies regarding specific proteins as potential biomarkers for ALS.
Materials and methods
Study population
CSF from 20 recently diagnosed and living sporadic ALS subjects (L-ALS) and 17 non-ALS age-matched living control subjects (L-Ctl) were obtained by lumbar puncture upon Massachusetts General Hospital and University of Pittsburgh Institutional Review Board (IRB) approved consent. The age-matched control group included CSF obtained from 16 healthy control subjects who volunteered to participate in this study. For ALS subjects, the average time from clinical onset (date when the patient first reported symptoms) to when CSF was procured was 350 days for the living ALS patients (range 110–3325 days). All ALS patients met El Escorial criteria for clinically definite ALS, clinically probable ALS, or clinically probable ALS-laboratory supported (15). CSF was also acquired at time of autopsy from 17 ALS (PM-ALS), eight AD (PM-AD) and eight non-neurologic control (PM-Ctl) cases from the University of Pittsburgh ALS Tissue Bank and de-identified by the honest broker system at the University of Pittsburgh. The average ages (years) of the ALS and control living subjects were 49.04±2.78 and 43.4±4.23, respectively; those from the autopsy cohort were 54±2.5 and 60±3.3 for ALS and control cases, respectively. The average post mortem interval times for the autopsy ALS and control subjects were 6.7±1 and 5.9±0.45 h, respectively. The ratio of males to females in the living subject cohort was 1.8:1 and in the autopsy cohort was 1.5:1. Acquisition of CSF and processing was performed as previously described (14).
Surface enhanced laser desorption/ionization - time of flight - mass spectrometry (SELDI-TOF-MS)
Sample and protein chip preparations were performed as described previously except that 0.5–4 μg of the CSF protein was utilized (7). Ciphergen Q10 and zinc coated IMAC-30 protein chips were used for the sample analysis. 2D-Quant kit (Amersham, USA) was used to determine CSF protein concentrations (0.06 μg/μl to 0.6 μg/μl). Protein chips were read using the SELDI-TOF-MS and the total ion current profiles normalized as previously described (7). External calibration of the mass spectrometer was performed using the ‘7-in-1’ peptide mix from Ciphergen Biosystems (vasopressin (1084.2 Da), somatostatin (1637.9 Da), porcine dynorphin A (2147.5 Da), human ACTH (2933.5 Da), bovine insulin B chain (3495.9 Da), human recombinant insulin (5807.6 Da), and hirudin (7033.6 Da)). Human SOD1 (15,591.4 Da) was also added to this mix.
Data analysis
Total ion current of all profiles was used to normalize each of the spectrograms. Peak labeling was performed using second-pass peak selection with a signal to noise ratio of 1:5. All samples within each sample set were prepared and analyzed at the same time within the same experiment. Each experiment was repeated three times. Spectra were analyzed using Ciphergen ProteinChip software (Version 3.2.1). Statistical analysis of all mass peaks was performed using the non-parametric Mann-Whitney test on the maximal intensity of each peak. Peak labeling was performed using second-pass peak selection with a signal to noise ratio of 1.5. Significance threshold was set at p<0.05.
Results
We determined the protein profile of CSF from each of the 36 living subjects (20 ALS (L-ALS) and 16 controls (L-Ctl)) and 33 autopsy subjects (17 ALS (PM-ALS) and 16 controls (PM-Ctl)) by surface enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS). All ALS subjects were classified as sporadic ALS with no known mutations in genes associated with familial ALS. All samples within each experiment were performed in duplicate and analyzed for statistically significant mass peak differences between the ALS and control subjects within each sample group and differences between the living and post mortem subjects. All experiments were repeated at least twice. To demonstrate reproducibility a standard CSF sample was used on one spot of every chip and the coefficient of variation was determined to be less than 30% for each of 25 mass spectral peaks.
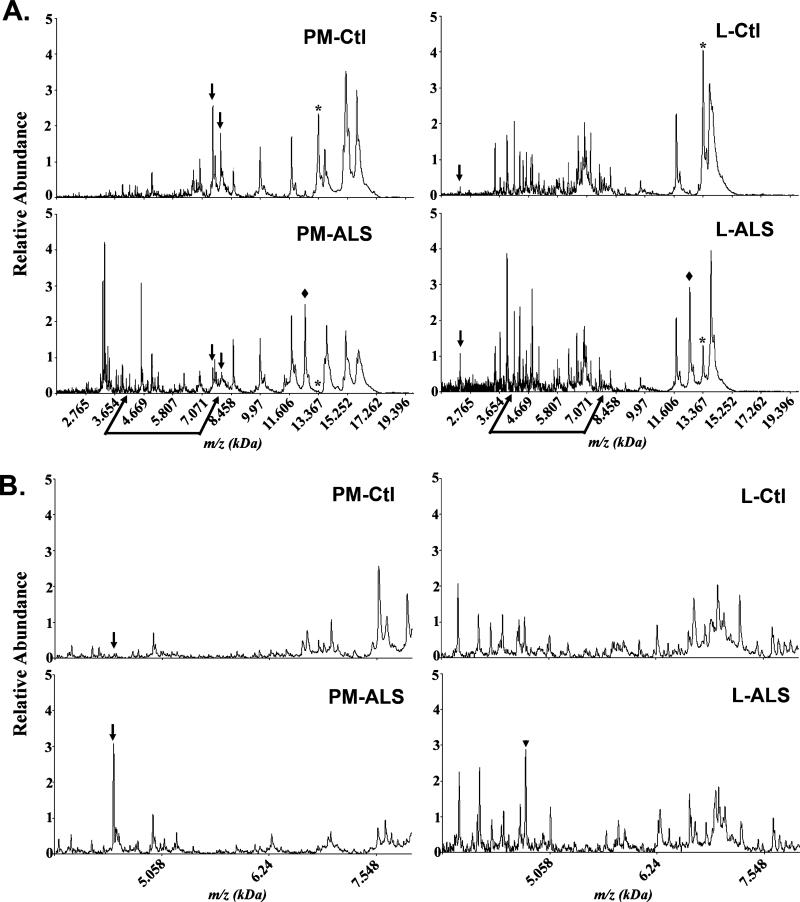
For this study we focused our efforts on comparison of CSF mass spectra in the range 2–20 kDa. A total of 317 protein peaks was detected and analyzed from the ProteinChip arrays (Ciphergen) using the Biomarker Pattern Software (BPS). Representative spectra from each of the four sample categories (PM-Ctl, PM-ALS, L-Ctl, and L-ALS) are shown in Figure 1A. While the overall proteomic profile pattern was somewhat similar between the post mortem ALS and control subjects and between the living ALS and control subjects, significant differences were evident between the overall CSF proteomic profile between post mortem and living ALS subjects (Figure 1A). One mass peak corresponding to cystatin C (13.38 kDa) exhibited increased levels in control subjects compared to ALS subjects (* in Figure 1A). This result confirms prior studies that have identified cystatin C as a putative biomarker for ALS (7,16). It is interesting to note that the relative intensity of cystatin C varied among the four groups (L-Ctl>PM-Ctl>L-ALS>PM-ALS). Cystatin C was significantly reduced in PM-ALS compared to the other three group categories (asterisk in Figure 1A). Additional mass peaks in the range 15–16 kDa are evident in the post mortem samples that were not present in CSF from the living subjects (Figure 1A). These peaks represent the α- and β-chains of hemoglobin (15.1 and 15.8 kDa, respectively) that probably have leaked into the CSF after death and/or during the CSF procurement at autopsy.
Figure 1.
Representative SELDI-TOF-MS protein profiles illustrating differences in the CSF proteome of ante mortem and post mortem control and ALS subjects in the mass range of (A) 2–20 kDa and (B) 4–8 kDa. (A) Proteomic differences are observed by comparing the CSF profile of post mortem control (PM-Ctl) and post mortem ALS (PM-ALS) subjects, as well as between living control (L-Ctl) and living ALS (L-ALS) subjects. Arrows in (A) and (B) represent differences between control and the respective ALS case. The arrowhead in (B) represents a 4.8 kDa peak with highest peak intensity value in L-ALS subjects. A protein peak at approximately 12.7 kDa is present in both ALS sample groups but absent in control subjects (◆ in panel A). The cystatin C protein peak at 13.37 kDa (*) is present at high intensity in the control subjects but reduced in L-ALS CSF and absent in the PM-ALS CSF. (B) Enhanced view of the spectrogram between the 4 and 8 kDa mass range.
Closer examination in the mass range of 4–8 kDa revealed numerous mass peaks that either change in peak intensity or presence between the post mortem and living subjects (Figure 1B). In Figure 1, arrows highlight examples of mass peak differences between ALS and control subjects within a subject group (PM or L). For example, the 7.49 kDa and 7.79 kDa m/z peaks exhibit increased levels in the PM-Ctl versus the PM-ALS case. A protein peak of approximately 12.7 kDa is present in both ALS sample groups but absent in control subjects (◆ in Figure 1A). We also note a 4.8 kDa peak present in higher levels in L-ALS compared to L-Ctl or PM-ALS subjects (arrowhead in Figure 1B). This peak has the appropriate mass for VGF, a protein previously identified in the CSF as a biomarker for ALS (16). While the overall spectral pattern between 4 and 8 kDa for the L-Ctl and L-ALS subjects appears quite similar (Figure 1B), there were specific m/z peak differences between these samples and more numerous m/z peak alterations evident between the PM-ALS and L-ALS samples (Figure 1B).
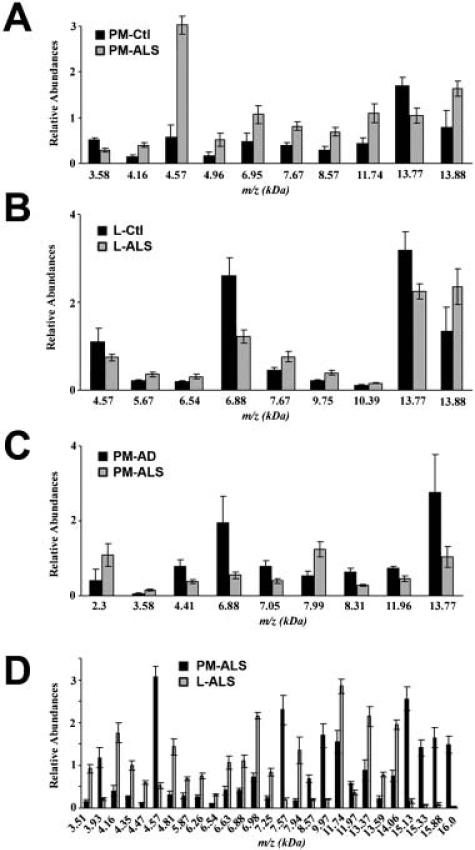
We next performed a statistical analysis of the mass peaks in the CSF proteome between post mortem and recently diagnosed ALS patients and Alzheimer's disease (AD) patients. Using the Ciphergen 3.1 biomarker wizard clustering software, peaks were analyzed using the non-parametric Mann-Whitney test. Pair-wise comparisons were made between PM-ALS/PM-Ctl; L-ALS/L-Ctl; PM-ALS/PM-AD; and PM-ALS/L-ALS from three independent experiments. The m/z peaks common to at least two different experiments and those that were statistically significant with a p-value ≤0.02 are represented by histograms (Figure 2, panels A–D). The number of m/z peaks identified by comparing PM-ALS/PM-Ctl (Figure 2A), L-ALS/L-Ctl (Figure 2B), and PM-ALS/PM-AD (Figure 2C) were 10, 9 and 9, respectively. Comparing PM-ALS to L-ALS samples yielded 27 mass peak differences (Figure 2D). Two m/z peaks (6.88 kDa and 13.77 kDa) were common to the sample pairs of PM-ALS/PM-AD and L-Ctl/L-ALS. However, the peak signal at 6.88 kDa is the double charged protonated form of the peak at 13.77 kDa. This m/z peak corresponds to transthyretin and has been previously shown to be a putative biomarker for ALS (7). Furthermore, the 13.77 kDa transthyretin peak appeared statistically different in PM-Ctl/PM-ALS samples (Figure 2A). This transthyretin peak is decreased in all ALS samples compared to healthy controls and AD subjects. The four m/z peaks in Figure 2B of greatest relative intensity values (4.57, 6.88, 13.77 and 13.88 kDa) were identified as potential ALS biomarkers in our previous proteomic study comparing ALS and control subjects (7). While this study confirms our previous results, it should be noted that there is overlap between the living control and ALS subjects used in these studies. Panel 2C compares post mortem ALS to post mortem AD CSF and identifies a group of protein peaks that differentiate these two neurologic diseases. Included in these peaks are a 3.58 kDa peak that also differentiates PM-ALS from healthy control CSF (Figure 2A), and the 13.77 transthyretin peak. Prior 2D-GE and mass spectrometry based proteomic studies have found that levels of the 13.77 kDa transthyretin peak in the CSF of AD patients are decreased compared to control (5), and the present study further determined that ALS patients exhibit decreased levels of the 13.77 kDa transthyretin peak compared to AD.
Figure 2.
Average intensities of significant (p<0.02) protein peaks differentially expressed between the subject groups. (A) Ten m/z peaks exhibit statistically significant differences when comparing CSF with post mortem ALS (PM-ALS, grey bars, n=17) and post mortem control (PM-Ctl, black bars, n=8) cases. (B) CSF protein peaks that exhibit significant differences between recently diagnosed ALS patients (grey bars, n=20) and age-matched controls (black bars, n=16). (C) CSF protein peaks with statistically significant differences in peak intensity values between PM-ALS (grey bars, n=17) and post mortem AD (PM-AD black bars, n=8) cases. (D) CSF protein peaks with significantly different peak intensity values when comparing recently diagnosed ALS subjects (L-ALS, grey bars, n=20) and PM-ALS cases (black bars, n=17).
The greatest number of statistically significant m/z peak alterations was observed in comparisons of the PM-ALS and L-ALS samples (Figure 2D). Of these peaks, the 3.5 kDa peak is a fragment of the neuroendocrine protein 7B2, the 4.8 kDa peak is VGF, the 6.88 kDa peak represents a double-charged species of transthyretin, the 11.7 kDa peak corresponds in size to β2-microglobulin, the 13.3 kDa peak is cystatin C, and the 15.1 kDa and 15.88 kDa peaks correspond to the molecular mass of α- and β-chains, respectively, of hemoglobin.
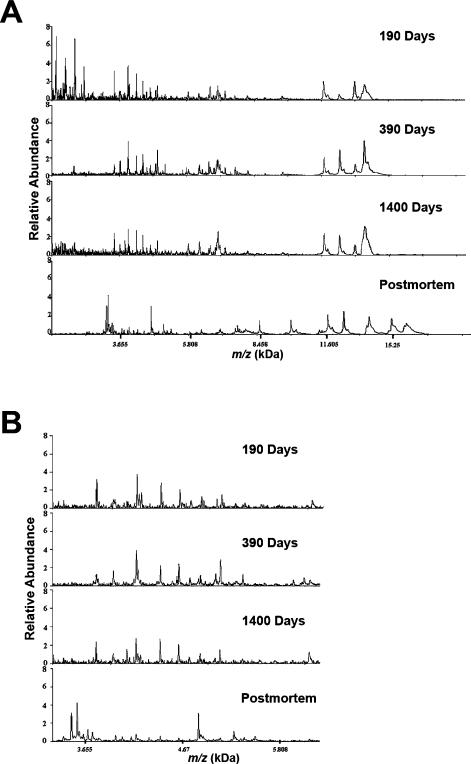
We also examined CSF from individual ALS patients with varying lengths of time between symptom onset and lumbar draw (Figure 3). While the overall protein peak pattern of CSF was similar in ALS patients early after disease onset (190 days) to patients at 390 and 1400 days after symptom onset, the protein peak pattern was strikingly different to CSF obtained at autopsy (Figure 3A). These differences are most probably due to biological alterations, since the sample handling and storage conditions for all of these samples were equivalent. Figure 3B represents a higher resolution of the spectrograms between 3.2 and 6.2 kDa. The CSF spectra for ALS patients at 190, 390 and 1400 days are quite similar with no significant mass peak alterations. However, the representative spectra for the post mortem ALS subjects (bottom panel) exhibit significant mass peak variations compared to the living patients.
Figure 3.
Differences in protein profiles of CSF from living ALS patients compared to post mortem ALS subjects. (A) Protein profiles between 2 and 20 kDa mass ranges of CSF obtained from individual living ALS patients 190, 390 or 1400 days from time of symptom onset, and a post mortem ALS patient. (B) Expanded image of the protein profiles between 3.2 and 6.2 kDa mass ranges.
Discussion
The goal of this study was to evaluate the CSF proteome of control and ALS subjects by mass spectrometry. We utilized living and autopsy derived CSF samples from control and ALS subject groups. We identified significant changes in the CSF proteome between ALS patients near the time of disease diagnosis and CSF obtained at autopsy with a short post mortem interval. These results are illustrated by the presence of 27 protein peaks with statistically significant differences between the PMALS and L-ALS CSF samples (Figure 2). In addition, we also found protein mass peaks that exhibit alterations early after clinical onset of ALS and are retained at similar levels in autopsy derived samples. The post mortem samples provided us with proteomic profiles typical of end-stage disease and identified protein mass peaks that distinguish ALS from AD. It is intuitive that the protein alterations post mortem will be very different from samples obtained from living subjects. However, this is the first study that defines these changes using a proteomic approach. In addition, the use of post mortem samples suggested that there are some proteomic alterations that could be used as markers of disease onset but not disease progression. These studies underscore the importance of carefully defining the samples used for biomarker discovery efforts and potential to uncover proteins within post mortem samples that may reflect post mortem alterations instead of disease related mechanisms. However, the use of post mortem CSF permits collection of a larger volume of CSF than can be obtained from living subjects and a direct correlation of candidate biomarkers to specific histopathology within the patient.
While a large number of protein mass peaks were identified that exhibit statistically significant differences between living and post mortem ALS subjects (Figure 2), we also observed mass peaks that exhibit differences between ALS and control subjects that occur near the time of disease diagnosis, and which remain evident in the post mortem CSF samples. These include mass peaks at 4.57, 7.67, 13.77, and 13.88 kDa (Figure 2A, B). These probably represent proteomic changes that occur near the time of symptom onset that remain throughout disease progression and in post mortem samples. Therefore, these protein peaks may be relevant as biomarkers of ALS onset but not for disease progression and highlight biochemical pathways altered early in ALS that remain altered throughout the course of disease. It is interesting to note that the 4.57 kDa peak exhibits increased levels in post mortem ALS patients compared to controls, but decreased levels in living ALS patients compared to living control subjects. A carefully controlled longitudinal study is required to evaluate the role of any protein as a potential surrogate marker of disease progression.
In this study we utilized CSF samples obtained from ALS patients at various times from symptom onset. We note that while protein peaks of low mass (>3 kDa) and between 13 and 15 kDa exhibit alterations in different ALS subjects over time, the overall proteomic profiling pattern remains quite consistent between 190 and 1400 days after symptom onset (Figure 3). However, numerous changes in the proteomic profile are apparent in post mortem CSF samples compared to living ALS patients. Protein alterations observed in post mortem CSF may represent proteolytic fragments of proteins, altered post-translational modifications, or disease-related alterations that reflect end-stage disease. CSF samples from living and post mortem subjects were processed and stored in identical conditions and storage tubes/temperature.
We also included a number of CSF samples obtained from AD patients at autopsy. Comparison of post mortem CSF from ALS and AD patients uncovered nine mass peak alterations between these neurologic diseases that may represent biomarkers that distinguish ALS from AD subjects at end-stage disease (Figure 2C). The 3.58 and 13.77 kDa peaks are common between post mortem control versus post mortem ALS and post mortem AD versus post mortem ALS. The 13.77 kDa peak represents a mass peak for a specific post-translation modified form of transthyretin, a protein that has been shown to be altered in both ALS and AD (7,17,18). Interestingly, the relative abundance of this transthyretin peak is reduced in ALS compared to AD subjects and also reduced in ALS compared to healthy controls. The 7.99 kDa mass peak is increased in the CSF of ALS compared to either AD or healthy controls, suggesting that this protein is elevated in ALS patients relative to two control groups at end-stage disease.
The majority of mass peaks that exhibit statistically significant differences between living and post mortem ALS patients have not yet been sequenced to determine the protein identity (Figure 2D). However, the 3.5 kDa peak is consistent with the protein peak we previously identified as the carboxy-terminal fragment of the neuroendocrine protein 7B2 and the 13.37 kDa peak is cystatin C (7). In addition, the 4.8 kDa peak is probably the VGF protein previously noted as a putative ALS biomarker (16). The 6.88 kDa peak is a double-charged species of transthyretin and the 11.74 kDa peak is consistent with β2-microglobulin, a protein that has previously been reported to exhibit increased protein levels in the CSF of AD patients by SELDI-TOF-MS (6). The 15.13 and 15.88 kDa peaks probably represent α- and β-chains, respectively, of hemoglobin and are present in the post mortem samples due to breach of the blood-brain barrier after death or during collection of the sample.
By performing a mass spectrometry-based proteomic analysis of CSF obtained from living and post mortem control and ALS subjects, we identified several protein peaks that demonstrate altered levels between control and ALS subjects and between post mortem ALS and AD subjects. We also observed a subset of protein peaks that exhibit alterations at the time of disease diagnosis that remain apparent after death. Future studies will determine the protein identity for all mass peaks specifically altered in ALS subjects, as well as further develop quantitative measures for these proteins using either quantitative mass spectrometry or ELISA. Such quantitative measurements could then be applied to additional ALS and control subjects to evaluate the ability of these potential biomarkers to accurately classify ALS subjects at time of symptom onset. These results further indicate that biomarker discovery efforts should be focused on biologic samples obtained as close as possible to disease onset and that long-itudinal studies within a single patient cohort are needed to further evaluate proteins for use as surrogate markers of disease progression.
Acknowledgements
The authors thank Merit Cudkowicz from Massachusetts General Hospital for providing some of the CSF samples from recently diagnosed ALS patients. This work was supported by funding from the NIH (ES013469) and The ALS Association to RB.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- 1.Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx. 2004;1:189–95. doi: 10.1602/neurorx.1.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coon KD, Dunckley T, Stephan DA. Biomarker identification in neurologic diseases: improving diagnostics and therapeutics. Expert Rev Mol Diagn. 2004;4:361–75. doi: 10.1586/14737159.4.3.361. [DOI] [PubMed] [Google Scholar]
- 3.Bowser R, Cudkowicz M, Kaddurah-Daouk R. Biomarkers for amyotrophic lateral sclerosis. Expert Rev Mol Diagn. 2006;6:387–98. doi: 10.1586/14737159.6.3.387. [DOI] [PubMed] [Google Scholar]
- 4.Miller DH. Biomarkers and surrogate outcomes in neurodegenerative disease: lessons from multiple sclerosis. NeuroRx. 2004;1:284–94. doi: 10.1602/neurorx.1.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puchades M, Hansson SF, Nilsson CL, Andreasen N, Blennow K, Davidsson P. Proteomic studies of potential cerebrospinal fluid protein markers for Alzheimer's disease. Mol Brain Res. 2003;118:140–6. doi: 10.1016/j.molbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Carrette O, Demalte I, Scherl A, Yalkinoglu O, Corthals G, Burkhard P, et al. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer's disease. Proteomics. 2003;3:1486–94. doi: 10.1002/pmic.200300470. [DOI] [PubMed] [Google Scholar]
- 7.Ranganathan S, Williams E, Ganchev P, Gopalakrishnan V, Lacomis D, Urbinelli L, et al. Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J Neurochem. 2005;95:1461–71. doi: 10.1111/j.1471-4159.2005.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–74. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 9.Quinn JF, Montine KS, Moore M, Morrow JD, Kaye JA, Montine TJ. Suppression of longitudinal increase in CSF F2-isoprostanes in Alzheimer's disease. J Alzheimer's Dis. 2004;6:93–7. doi: 10.3233/jad-2004-6110. [DOI] [PubMed] [Google Scholar]
- 10.Brettschneider J, Petzold A, Submuth SD, Ludolph AC, Tumani H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology. 2006;66:852–6. doi: 10.1212/01.wnl.0000203120.85850.54. [DOI] [PubMed] [Google Scholar]
- 11.Lescuyer P, Allard L, Zimmerman-Ivol CG, Burgess JA, Hughes-Frutiger S, Burkhard PR, et al. Identification of post mortem cerebrospinal fluid proteins as potential biomarkers of ischemia and neurodegeneration. Proteomics. 2004;4:2234–41. doi: 10.1002/pmic.200300822. [DOI] [PubMed] [Google Scholar]
- 12.Finehout EJ, Franck Z, Relkin N, Lee KH. Proteomic analysis of cerebrospinal fluid changes related to post mortem interval. Clin Chem. 2006;52:1906–13. doi: 10.1373/clinchem.2006.070508. [DOI] [PubMed] [Google Scholar]
- 13.You J-S, Gelfanova V, Knierman MD, Witzmann FA, Wang M, Hale JE. The impact of blood contamination on the proteome of cerebrospinal fluid. Proteomics. 2005;5:290–6. doi: 10.1002/pmic.200400889. [DOI] [PubMed] [Google Scholar]
- 14.Ranganathan S, Polshyna A, Nicholl G, Lyons-Weiler J, Bowser R. Assessment of protein stability in cerebrospinal fluid using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry protein profiling. Clin Proteomics. 2006;2:91–102. doi: 10.1385/CP:2:1:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lat Scler. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 16.Pasinetti GM, Ungar LH, Lange DJ, Yemul S, Deng H, Yuan X, et al. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66:1218–22. doi: 10.1212/01.wnl.0000203129.82104.07. [DOI] [PubMed] [Google Scholar]
- 17.Serot JM, Christmann D, Dubost T, Couturier M. Cerebrospinal fluid transthyretin: aging and late onset Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1997;63:506–8. doi: 10.1136/jnnp.63.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biroccio A, del Boccio P, Panella M, Bernardini S, Dillio C, Gambi D, et al. Differential post-translational modifications of transthyretin in Alzheimer's disease: a study of the cerebral spinal fluid. Proteomics. 2006;6:2305–13. doi: 10.1002/pmic.200500285. [DOI] [PubMed] [Google Scholar]