Abstract
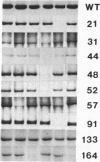
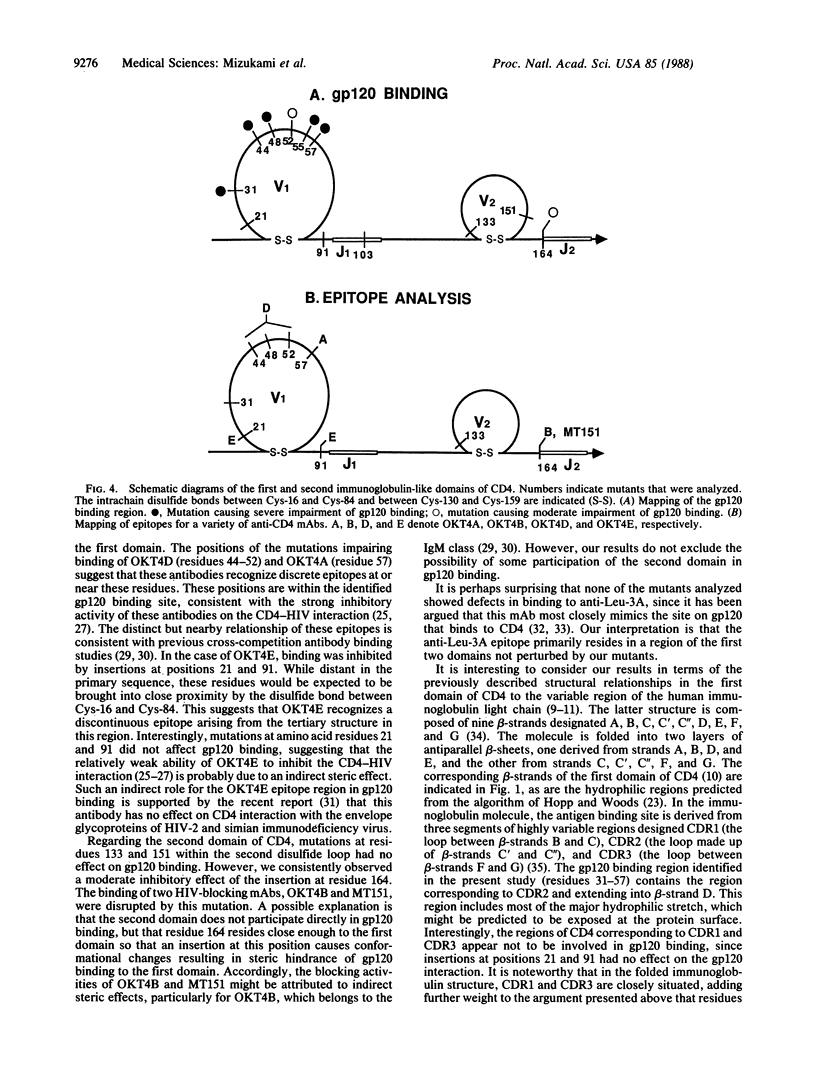
The binding region for human immunodeficiency virus (HIV) and epitopes for a panel of HIV-blocking anti-CD4 monoclonal antibodies of the CD4 molecule were defined by using in vitro site-directed mutagenesis. Codons for two amino acid residues (Ser-Arg) were inserted at selected positions within the region encoding the first and second immunoglobulin-like domains of CD4. A vaccinia virus-based expression system was used to produce soluble full-length extracellular CD4 fragments containing the insertions. The mutant proteins were tested for direct binding to soluble gp120 (the CD4-binding subunit of the viral envelope glycoprotein) and to a series of HIV-blocking anti-CD4 monoclonal antibodies. Impaired gp120 binding activity resulted from insertions after amino acid residues 31, 44, 48, 52, 55, and 57 in the first immunoglobulin-like domain. The epitopes for two HIV-blocking monoclonal antibodies, OKT4A and OKT4D, were also mapped in the gp120-binding region in the first domain. Insertions after amino acid residues 21 and 91 in the first domain had no effect on gp120 binding but impaired the binding of OKT4E, suggesting that this antibody recognizes a discontinuous epitope not directly involved in gp120 binding. Moderate impairment of gp120 binding resulted from the insertion after amino acid residues 164 in the second immunoglobulin-like domain, where the epitopes for monoclonal antibodies MT151 and OKT4B were also mapped.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzari P. M., Lascombe M. B., Poljak R. J. Three-dimensional structure of antibodies. Annu Rev Immunol. 1988;6:555–580. doi: 10.1146/annurev.iy.06.040188.003011. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Fuerst T. R., Moss B. A soluble recombinant polypeptide comprising the amino-terminal half of the extracellular region of the CD4 molecule contains an active binding site for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2357–2361. doi: 10.1073/pnas.85.7.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanh T. C., Dreesman G. R., Kennedy R. C. Monoclonal anti-idiotypic antibody mimics the CD4 receptor and binds human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3891–3895. doi: 10.1073/pnas.84.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. J., Jefferies W. A., Barclay A. N., Gagnon J., Williams A. F. Peptide and nucleotide sequences of rat CD4 (W3/25) antigen: evidence for derivation from a structure with four immunoglobulin-related domains. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1649–1653. doi: 10.1073/pnas.84.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., Hussey R. E., Steinbrich R., Ramachandran H., Husain Y., Reinherz E. L. Substitution of murine for human CD4 residues identifies amino acids critical for HIV-gp120 binding. Nature. 1988 Sep 22;335(6188):363–366. doi: 10.1038/335363a0. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Bertonis J. M., Meier W., Johnson V. A., Costopoulos D. S., Liu T., Tizard R., Walker B. D., Hirsch M. S., Schooley R. T. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988 Jan 7;331(6151):76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller T. C., Trevithick J. E., Fuller A. A., Colvin R. B., Cosimi A. B., Kung P. C. Antigenic polymorphism of the T4 differentiation antigen expressed on human T helper/inducer lymphocytes. Hum Immunol. 1984 Feb;9(2):89–102. doi: 10.1016/0198-8859(84)90031-4. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J. A., Flaherty L. E., Haggarty B. S., Rackowski J. L. Infection of T4 lymphocytes by HTLV-III does not require expression of the OKT4 epitope. J Immunol. 1986 Jan;136(2):361–363. [PubMed] [Google Scholar]
- Hussey R. E., Richardson N. E., Kowalski M., Brown N. R., Chang H. C., Siliciano R. F., Dorfman T., Walker B., Sodroski J., Reinherz E. L. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988 Jan 7;331(6151):78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- Jameson B. A., Rao P. E., Kong L. I., Hahn B. H., Shaw G. M., Hood L. E., Kent S. B. Location and chemical synthesis of a binding site for HIV-1 on the CD4 protein. Science. 1988 Jun 3;240(4857):1335–1339. doi: 10.1126/science.2453925. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landau N. R., Warton M., Littman D. R. The envelope glycoprotein of the human immunodeficiency virus binds to the immunoglobulin-like domain of CD4. Nature. 1988 Jul 14;334(6178):159–162. doi: 10.1038/334159a0. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Lundin K., Nygren A., Arthur L. O., Robey W. G., Morein B., Ramstedt U., Gidlund M., Wigzell H. A specific assay measuring binding of 125I-Gp 120 from HIV to T4+/CD4+ cells. J Immunol Methods. 1987 Feb 26;97(1):93–100. doi: 10.1016/0022-1759(87)90110-4. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Molineaux S. M., Maddon D. E., Zimmerman K. A., Godfrey M., Alt F. W., Chess L., Axel R. Structure and expression of the human and mouse T4 genes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9155–9159. doi: 10.1073/pnas.84.24.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. O., Sattentau Q. J., Beverley P. C., Hearn J. P., Fitzgerald A. K., Zuckerman A. J., Weiss R. A. HIV infection of primate lymphocytes and conservation of the CD4 receptor. Nature. 1987 Dec 3;330(6147):487–489. doi: 10.1038/330487a0. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- McDougal J. S., Nicholson J. K., Cross G. D., Cort S. P., Kennedy M. S., Mawle A. C. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J Immunol. 1986 Nov 1;137(9):2937–2944. [PubMed] [Google Scholar]
- Peterson A., Seed B. Genetic analysis of monoclonal antibody and HIV binding sites on the human lymphocyte antigen CD4. Cell. 1988 Jul 1;54(1):65–72. doi: 10.1016/0092-8674(88)90180-8. [DOI] [PubMed] [Google Scholar]
- Rao P. E., Talle M. A., Kung P. C., Goldstein G. Five epitopes of a differentiation antigen on human inducer T cells distinguished by monoclonal antibodies. Cell Immunol. 1983 Sep;80(2):310–319. doi: 10.1016/0008-8749(83)90119-3. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Clapham P. R., Weiss R. A., Beverley P. C., Montagnier L., Alhalabi M. F., Gluckmann J. C., Klatzmann D. The human and simian immunodeficiency viruses HIV-1, HIV-2 and SIV interact with similar epitopes on their cellular receptor, the CD4 molecule. AIDS. 1988 Apr;2(2):101–105. doi: 10.1097/00002030-198804000-00005. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Dalgleish A. G., Weiss R. A., Beverley P. C. Epitopes of the CD4 antigen and HIV infection. Science. 1986 Nov 28;234(4780):1120–1123. doi: 10.1126/science.2430333. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Byrn R. A., Marsters S. A., Gregory T., Groopman J. E., Capon D. J. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987 Dec 18;238(4834):1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Lüke W., Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature. 1988 Jan 7;331(6151):84–86. doi: 10.1038/331084a0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]