Abstract
Epileptogenesis is defined as the process of developing epilepsy—a disorder characterized by recurrent seizures—following an initial insult. Seizure incidence during the human lifespan is at its highest in infancy and childhood. Animal models of epilepsy and human tissue studies suggest that epileptogenesis involves a cascade of molecular, cellular and neuronal network alterations. Within minutes to days following the initial insult, there are acute early changes in neuronal networks, which include rapid alterations to ion channel kinetics as a result of membrane depolarization, post-translational modifications to existing functional proteins, and activation of immediate early genes. Subacute changes occur over hours to weeks, and include transcriptional events, neuronal death and activation of inflammatory cascades. The chronic changes that follow over weeks to months include anatomical changes, such as neurogenesis, mossy fiber sprouting, network reorganization, and gliosis. These epileptogenic processes are developmentally regulated and might contribute to differences in epileptogenesis between adult and developing brains. Here we review the factors responsible for enhanced seizure susceptibility in the developing brain, and consider age-specific mechanisms of epileptogenesis. An understanding of these factors could yield potential therapeutic targets for the prevention of epileptogenesis and also provide biomarkers for identifying patients at risk of developing epilepsy or for monitoring disease progression.
Introduction
Epilepsy, a disorder characterized by recurrent seizures, is the second most common neurological disorder, affecting more than 50 million people worldwide.1 Population studies show that seizure incidence is highest in the first month of life,2 and neonatal seizures can lead to epilepsy and other neurocognitive disorders in later life.3
Epileptogenesis is defined as the process whereby a neuronal network develops recurrent epileptic seizures de novo or following an insult, and also the process whereby seizures become more severe and frequent in chronic epilepsy. Currently available pharmacological treatments of epilepsy are actually only seizure suppressing, or `antiepileptic'. none are disease-modifying, or `antiepileptogenic'. Emerging research should yield therapeutic targets for the prevention of epileptogenesis and also provide biomarkers for identifying patients at risk of developing epilepsy or for monitoring disease progression. In this Review, we briefly discuss the evidence for epileptogenesis as a consequence of seizures in the developing brain, in addition to the factors responsible for enhanced seizure susceptibility and epileptogenesis during this period of development. Furthermore, we propose a temporal pattern of molecular, cellular and network-level changes that could contribute to the process of epileptogenesis in the immature brain, and we discuss known modulators of the altered processes that could potentially be used as antiepileptogenic agents.
Epileptogenesis in the clinic
The process of epileptogenesis has been well documented in the clinical and basic science literature relating to epilepsy. Epilepsy can develop following a variety of brain insults, but the development of epilepsy after traumatic brain injury (TBI) or stroke are the best characterized examples of epileptogenesis in adults. Acute early seizures can occur in up to 25% of cases of moderate to severe TBI,4 and chronic epilepsy develops in 10–25% of individuals following TBI.5,6 The onset of epilepsy can be delayed following the initial insult and can take 5 years or more to emerge after TBI.6 In addition, despite controlling acute seizures, the antiepileptic drugs phenytoin, phenobarbital, valproate4 and carbamazepine7 do not reduce the incidence of post-traumatic epilepsy. Poststroke seizures account for 11% of all new cases of adult-onset epilepsy; the severity of epilepsy correlates with stroke size and severity.1
Epileptogenesis also occurs in infancy and early childhood. Aside from the inherited forms of epilepsy and epileptogenic malformations of cortical development, most cases of early-life seizures are associated with external insults, such as hypoxic–ischemic encephalopathy,2 fever8 or trauma.9 These external insults can lead to the develop ment of epilepsy in later life. The highest incidence of pediatric seizures is observed during the neonatal period, and hypoxic–ischemic encephalopathy is the most common cause. Up to 27% of infants with neonatal seizures develop epilepsy and/or cognitive and behavioral deficits in later life.10 Febrile seizures are the next most common cause of seizures in the pediatric population, with a maximum prevalence during infancy and early childhood. Simple febrile seizures are relatively benign and seem to have few consequences, but prolonged complex febrile seizures have been shown to correlate with the development of cognitive deficits and epilepsy in later life.11 The ongoing Consequences of Prolonged Childhood Febrile Seizure Study (FEBSTAT) is prospectively evaluating the causal relationship between early febrile seizures and the risk of developing epilepsy in later life.8
Experimental models of epileptogenesis
Models of acquired epilepsy in the mature brain use initiating agents in adult animals to study the long-term development of epilepsy. Such initiating agents include fluid percussion injury,12 stroke,13 and chemoconvulsant-induced and electrical-stimulus-induced status epilepticus.14 The seizures initiated in these models cause early neuronal death, which is followed by the formation of new synapses from surviving neurons (mossy fiber sprouting), and, ultimately, the onset of spontaneous seizures.
The process of epileptogenesis in early life, namely infancy and early childhood, is thought to be somewhat different from that in the mature brain. Consistent with the high clinical prevalence of epilepsy in infancy and childhood, experimental evidence from a variety of animal models—with the earliest observations being made over two decades ago15,16—reveals markedly reduced seizure thresholds in the immature brain (Box 1). With respect to the developmental regulation of neurotransmitter synthesis, receptors, and transporters, as well as development of specific neuronal and glial cells populations and myelination, the first two postnatal weeks of development of the rodent brain are roughly equivalent to the neonatal period and early infancy (first year of life) in human brain development,17,18 and many rodent models employ this developmental window. In a model of neonatal seizures, hypoxia induced seizures in postnatal day (P)10 rats, resulting in increased later-life seizure susceptibility, increased neuronal injury following chemoconvulsant insults, and long-term deficits in cognitive and behavioral tasks.19–21 In addition, febrile seizures in rat pups during the second postnatal week led to the development of spontaneous seizures later in life, as well as long-term changes in hippocampal hyperexcitability. MRI shows acute increases in the T2 signal in the hippocampus following febrile seizures, which potentially reflects tissue injury and edema.22 The occurrence of spontaneous recurrent seizures has also been observed after induction of status epilepticus during the second and third postnatal weeks in rodents, by use of chemoconvulsants such as pilocarpine,23 flurothyl,24 kainate25 and tetanus toxin.26 The main difference observed between adult-brain and developing-brain models of epileptogenesis is the relative lack of acute neuronal death in younger animals.27 Epileptogenesis has been demonstrated in a number of brain regions, but most studies using experimental animal models have focused on the hippocampus, because of the region's well-characterized network architecture and afferent and efferent pathways. Hence, in this Review, much of the discussion is centered on investigations of epileptogenic alterations of hippocampal structure and function.
Seizure susceptibility factors
Seizure susceptibility seems to peak during the intense period of rapid brain growth and synaptogenesis that occurs in the immature brain. In this section, we review the major classes of factors that regulate neuronal excitability and have been implicated in conferring this enhanced susceptibility.
Enhanced excitation in the immature brain
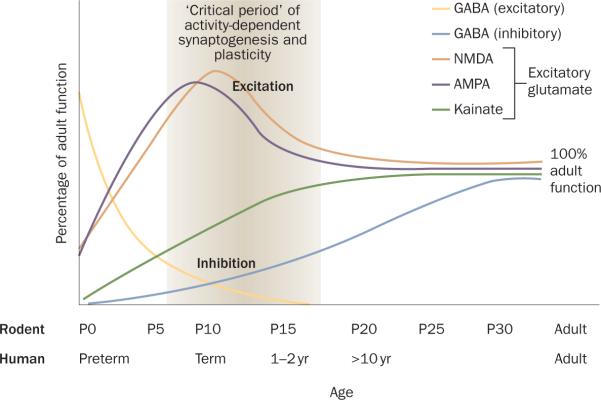
Given that neuronal activity is critical for synaptogenesis and brain development, excitation predominates over inhibition in neuronal networks of the cerebral cortex and limbic structures in the neonatal period and during the first few years of life.27 There is an overshoot of synapse and spine density in the first two weeks of life in rats and in the first year of life in humans and primates.28,29 Excitatory ion channels and transporters are expressed at levels that promote excitation, whereas inhibition is relatively underdeveloped compared with later life (Figure 1).
Figure 1. Schematic depiction of maturational changes in glutamate and GABA receptor function in the developing brain30.
Equivalent developmental periods are displayed for rats and humans on the top and bottom x-axes, respectively. Activation of GABA receptors is depolarizing in rats early in the first postnatal week and in humans up to and including the neonatal period. Functional inhibition, however, is gradually reached over development in rats and humans. Before full maturation of GABA-mediated inhibition, the NMDA and AMPA subtypes of glutamate receptors peak between the first and second postnatal weeks in rats and in the neonatal period in humans. Kainate receptor binding is initially low and gradually rises to adult levels by the fourth postnatal week. Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate; GABA, γ-aminobutyric acid; NMDA, N-methyl-D-aspartate; P, postnatal day.
Glutamate is the predominant excitatory amino acid neurotransmitter in neurons, and glutamate receptors (GluRs) are developmentally regulated in neurons and glia.30,31 Ionotropic GluRs (iGluRs) are ligand-gated ion channels that permit the flux of sodium ions, potassium ions and also calcium ions to varying degrees, depending on their subunit make-up.32 The main iGluR subtypes are N-methyl-D-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptors (AMPARs) and kainate receptors. NMDARs consist of an obligatory NMDAR1 (NR1) subunit in combination with NR2A, NR2B, NR2C, NR2D and/or NR3A subunits.32 Similarly, AMPARs are composed of combinations of GluR1, GluR2, GluR3 and/or GluR4 subunits,33 and kainate receptors are heteromeric combinations of GluR5, GluR6, GluR7, KA1, and/or KA2 subunits.34 NMDARs are always permeable to calcium, whereas the divalent cation permeability of AMPARs and kainate receptors depends on the subunit composition of the receptor. When GluR2 expression is relatively low (or absent), AMPARs are permeable to calcium,33 and kainate receptors become more permeable to calcium when the GluR5 or GluR6 subunits are absent.34 In addition to iGluRs, glutamate activates metabotropic GluRs (mGluRs), which are G-protein-coupled receptors that mediate slow synaptic responses. The mGluRs can be divided into three classes: group I (mGluR1 and mGluR5 subunits), group II (mGluR2 and mGluR3 subunits) and group III (mGluR4 and mGluR6 subunits).35
GluR subunits are differentially expressed during development (Figure 1).30 In the immature brain, neuronal NMDARs contain high levels of the NR2B, NR2A and NR3A subunits. Such elevated levels of NR2B subunits result in extended current decay times, and the high levels of NR2D and NR3A subunits reduce the sensitivity of NMDARs to magnesium ions.32 The increased expression in the immature brain of NR2B, NR2D and NR3A subunits all contribute to increased NMDAR-mediated calcium influx and lower the threshold for seizures and excitotoxic hypoxic–ischemic injury. In addition, this enhanced NMDAR-excitability has been implicated in the rapid synaptogenesis that occurs during development.
AMPARs are also maturationally regulated, and, in rats, there is a higher prevalence of GluR2 subunit-deficient receptors in the immature brain than in the adult brain, which leads to increased calcium influx.36,37 Additionally, upregulation of the flip isoform of the GluR1 subunit in early life results in reduced desensitization of AMPARs and extended current durations.27 Importantly, AMPAR expression in the developing human brain follows a similar pattern of relative GluR2 deficiency, specifically during the term and the early postnatal periods in cortical neurons.17,38 Taken together, these characteristic patterns of NMDAR and AMPAR subunit expression potentially represent age-specific therapeutic targets that could be modulated to attenuate excitotoxic neuronal injury and seizures in the neonatal brain. Indeed, the experimental NR2B subunit-specific antagonist ifenprodil suppressed seizures in immature rat pups.39 Other NMDAR antagonists, including memantine and felbamate, were neuroprotective in immature rodent stroke models,40,41 but they have not yet been tested in neonatal seizure models. AMPAR antagonists have been shown to be useful in neonatal stroke and seizure models.30 Topiramate, an FDA approved anticonvulsant and AMPAR antagonist, suppressed seizures and long-term neurobehavioral deficits in a rodent seizure model, even when administered following seizures.20 In addition, the specific AMPAR antagonist talampanel protected against neonatal seizures in one rodent model.42
Compared with the iGluRs, less information is available regarding developmental regulation of mGluRs. Results from a study in rodents, however, showed that mGluR1 expression might peak in the first postnatal week, whereas mGluR2, mGluR3 and mGluR5 levels mature by the second postnatal week.43 Agonists of group I mGluRs exert proconvulsant effects, suggesting that elevated levels of receptors from this group might have a role in early excitability. Conversely, agonists of group II and group III mGluRs have anticonvulsant properties.44
Diminished GABAergic inhibition in early life
While GluR-mediated excitation is enhanced in the immature brain, receptors and the synthetic enzyme (glutamic acid decarboxylase) for the major inhibitory neurotransmitter γ-aminobutyric acid (GABA) do not achieve maximal expression levels until the fourth postnatal week in rats.15,45 Furthermore, whereas GABA is inhibitory and hyperpolarizing in the mature brain, this neurotransmitter can be excitatory and depolarizing in the immature brain.46 GABA release can activate GABAA receptors (GABAARs), which are ligand-gated chloride channels. The paradoxical GABA-mediated excitation occurs because of maturational variations difference in the intracellular chloride ion concentration, which is maintained by the action of opposing chloride transporters. Activation of GABAARs in the mature brain hyperpolarizes neurons, by causing an influx of chloride into the cell. In immature neurons, the intracellular chloride concentration is higher than the extracellular concentration, so GABAAR activation leads to an efflux of such ions, thereby depolarizing the cell. This depolarizing effect is largely attributable to decreased expression of the chloride exporter potassium–chloride cotransporter (KCC)2 during early development.47 Furthermore, the sodium–potassium–chloride cotransporter (NKCC)2, a chloride importer, is more highly expressed in the second postnatal week than later in life.47 Similarly, NKCC1 expression is highest in the human cortex at term, whereas KCC2 is not expressed until the middle of the first year of life.47 In addition to developmental regulation, differences in chloride transporter expression and the resultant function of GABAARs have been observed between male and female rat pups.48 These expression patterns might contribute to gender differences in epileptogenesis. Furthermore, brain-derived-neurotrophic-factor (BDNF)-mediated activation of BDNF receptors reduces KCC2 messenger RNA (mRNA) and protein levels in rat hippocampal slice cultures following seizure induction, and also in adult models of kindling-induced epileptogenesis.49 Studies have demonstrated that the NKCC1 inhibitor bumetanide can block seizures in a rodent model of neonatal seizures,47,50 and this agent might have potential in the clinic as an antiepileptogenic therapy.
Ion channel expression promotes excitability
Like ligand-gated receptors, voltage-gated ion channels affect neuronal excitability, and their expression is developmentally regulated. In neonatal mouse thalamic neurons, neurotransmitter release depends on both N-type and P/Q-type voltage-gated calcium (Cav) channels.51 With maturation, this function is taken over exclusively by the P/Q-type channels, which are formed by Cav2.1 subunits.52 In humans, mutations in the gene that encodes Cav2.1 are thought to be associated with a type of absence epilepsy that has a highly age-specific presentation.52 Similarly, mutations in the genes KCNQ2 and KCNQ3, which encode voltage-gated potassium (Kv) channels Kv7.2 and Kv7.3, respectively, are associated with benign familial neonatal convulsions.53 These mutations interfere with the normal hyperpolarizing potassium ion current and promote repetitive action potential firing.54
Another potassium channel superfamily member, the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel, is also developmentally regulated. HCN channels are important for maintenance of the resting membrane potential and dendritic excitability.55 In the immature brain, the HCN1 channel isoform is expressed at a relatively low level, but expression increases with age. Rising levels of HCN1 are associated with reduced dendritic excitability.56 Selective blockers of HCN channels have been shown to disrupt synchronous epileptiform activity in the neonatal rat hippocampus,57 suggesting that these developmentally regulated channels might also be targets for therapy in neonatal seizures.
In addition to mutations in potassium channels, voltage-gated sodium (Nav) channel abnormalities also have been implicated in a number of early-life epilepsy syndromes.58 The Nav1.1, Nav1.2, Nav1.3 and Nav1.6 sodium channel subtypes, encoded by the SCN1A, SCN2A, SCN3A and SCN8A genes, respectively, are the principal sodium channels in the CNS.58,59 More than 300 mutations in SCN1A have been identified: gain-of-function mutations are a primary cause of generalized epilepsy with febrile seizures-plus (GEFS+),60–62 whereas loss-of-function mutations are associated with severe myoclonic epilepsy of infancy (SMEI).60 Studies using mouse models suggest that SMEI is caused by a selective loss of sodium ion currents and reduced excitability of GABAergic inhibitory interneurons, which leads to hyperexcitability, epilepsy and ataxia. In addition, mouse models with mutations in Scn8a and Scn2a exhibit ataxia and generalized seizures during early postnatal development.63,64
Sodium channel dysregulation seems to be critically linked to early-life seizure syndromes like GEFs+, Dravet syndrome and SMEI. Furthermore, mutations in sodium channels modify the phenotypes associated with other genetic causes of epilepsy. The inheritance of multiple genetic mutations and variants thereof could explain the variable penetrance of some genetically determined epileptic disorders. Indeed, mice with mutations in both Scn2a and Kcnq2 have more-severe forms of epilepsy than animals with only one of the mutations.65 Conversely, severe forms of epilepsy that are associated with mutations in Scn1a have been rescued by a genetic modification of Scn8a.66,67 The results from these mouse studies suggest that genetic modifiers could contribute to the clinical variability observed in epilepsy syndromes such as SMEI and GEFS+, and interactions between multiple ion channel variants might, therefore, have a disease-modifying role in epilepsy syndromes and epileptogenesis.
The inflammatory response and seizures
Microglial activation occurs after acute seizures in various animal models of epilepsy.68,69 The density of microglia reaches a maximum during brain development in the cortex, which is also when synaptogenesis is at its peak.70,71 During normal development and following injury, microglia participate in `synaptic stripping' whereby presynaptic terminals are eliminated from neurons.72 Anti-inflammatory compounds or agents that inhibit microglial activation (for example, minocycline and doxycycline) are reported to attenuate injury in models of excitotoxicity and seizure-induced neuronal death following hypoxia–ischemia.73,74
The epileptogenic `cascade'
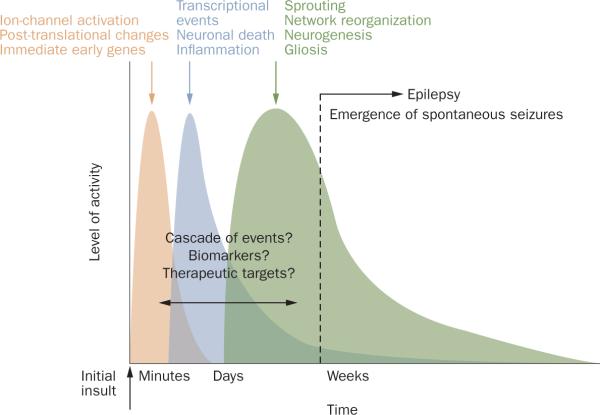
Accumulating evidence indicates that epileptogenesis is likely to be a multifactorial process. Clinical data and experimental models suggest the following temporal sequence of events. The initial insult is usually accompanied by acute seizure activity and is followed by a period (latent or silent) without overt seizure activity. Subsequently, a final stage is reached, which is characterized by the emergence of spontaneous seizures, an increase in seizure frequency, and in some cases refractoriness to antiepileptic drugs (Figure 2). In humans, this latent period can extend for months to years, while in most rodent models the latent phase lasts for 2–12 weeks.
Figure 2. Time course of epileptogenesis151.
An initial insult, such as traumatic brain injury and/or status epilepticus, is followed by a latent period lasting weeks to months or even years before the onset of spontaneous seizures. During this latent period, a cascade of molecular and cellular events occurs that alters the excitability of the neuronal network, ultimately resulting in spontaneous epileptiform activity. The alterations that occur during the latent period might provide a good opportunity for biomarker development and therapeutic intervention. The cascade of events that are presently suggested by experimental evidence can be classified temporally following the initial insult. Early changes, including induction of immediate early genes and post-translational modification of receptor and ion-channel related proteins, occur within seconds to minutes. Within hours to days, there can be neuronal death, inflammation, and altered transcriptional regulation of genes, such as those encoding growth factors. A later phase, lasting weeks to months, includes morphological alterations such as mossy fiber sprouting, gliosis, and neurogenesis.
Collectively, experimental data from animal models indicate several major stages in the epileptogenesis cascade (Figure 2). Early acute changes that occur within minutes to days following the initial insult include rapid alterations in ion channel activity, post-translational changes to existing proteins (for example, neurotransmitter receptors) and immediate early gene (IEG) activation. The subacute period of hours to weeks following the triggering insult can include processes such as activation of transcription, neuronal death and inflam mation. Chronic changes that occur over weeks to months include anatomical alterations such as neurogenesis, mossy fiber sprouting, network reorganization, and gliosis. In the sections that follow, we review the available literature regarding the temporal sequence of epileptogenesis, focusing on changes observed in the developing brain (Table 1). Furthermore, we report potential therapeutic strategies that have been suggested to target the mechanisms involved in epileptogenesis (Table 2).
Table 1.
Factors contributing to epileptogenesis in the immature brain
| Changes observed | Injury modela | Time of assessment |
|---|---|---|
| Immediate early gene induction | ||
| Increased transcription of Fos and Jun mRNA | Lithium–pilocarpine induced SE;81 hypoxia-induced seizures80,82 |
Hours |
| Post-translational changes in neurotransmitter receptors | ||
| Calcineurin activation | Hypoxia-induced seizures;87 lithium–pilocarpine-induced SE88 |
Hours to days |
| Kv2.1 dephosphorylation | Kainate-induced seizures;149 pilocarpine-induced SE91 |
Hours |
| GABAA receptor dephosphorylation | Hypoxia-induced seizures;87 low-magnesium-induced seizuresb,90 |
Hours to days |
| AMPAR phosphorylation | Hypoxia-induced seizures92 | Hours |
| NMDAR phosphorylation | Hypoxia–ischemia at P793 | Hours to days |
| Neuronal cell-death | ||
| Some models exhibit cell death | Flurothyl-induced seizures;97 febrile seizures98 |
Days |
| Neurotrophic factors | ||
| Increased levels of BDNF | Kainate-induced SEc,103 | Days |
| Inflammation | ||
| Increased levels of cytokines (for example, IL-δ, IL-1β) | Kainate-induced SE;108 febrile seizures106 |
Hours to days |
| TNF | Febrile seizures106 | Hours to days |
| Increased levels of NFκB1 and NFκB1a | Febrile seizures at P10;106 kainate-induced SE at P15108 |
Days |
| IL-1β | Kainate-induced SE at P15108 | Days |
| Neurotransmitter receptor expression | ||
| Decreased GluR2 subunit expression | Hypoxia-induced seizure;36 lithium–pilocarpine-induced SE23 |
Days |
| Increased GABAAα1 and decreased GABAAα4 subunit expression | Lithium–pilocarpine-induced SE23,112 | Days |
| Ion channel expression | ||
| HCN channels (increased or decreased levels observed in different models) | Kainate-induced SE; febrile seizures;115 hypoxia-induced seizures114 |
Days |
| Neuronal sprouting | ||
| Mossy-fiber sprouting dentate granule cells | Flurothyl-induced SE;119 kainate-induced SE150 |
Days to weeks |
| Neurogenesis | ||
| Increase in neurogenesis and neuronal stem cells | Kainate-induced SE;121 febrile seizures122 |
Weeks to months |
| Decrease in neurogenesis | Flurothyl-induced SE123 | Weeks to months |
| Gliosis | ||
| Increased glia activation | Kainate-induced SE;125 flurothyl-induced SE124 |
Weeks to months |
All experiments were conducted using in vivo preparations except where indicated.
In vitro preparation.
Experiments were conducted using in vivo and in vitro preparations.
Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor; BDNF, brain-derived neurotrophic factor; GABA, γ-aminobutyric acid; GluR, glutamate receptor; HCN, hyperpolarization-activated cyclic nucleotide-gated; IL, interleukin; Kv, voltage-gated potassium channel; mRNA, messenger RNA; NFκB, nuclear factor κB; NMDAR, N-methyl-D-aspartate receptor; P, postnatal day; SE, status epilepticus; TNF, tumor necrosis factor.
Table 2.
Potential targets for antiepileptogenic therapy for early-life seizures
| Mechanism targeted | Potential therapeutic optionsa |
|---|---|
| Acute changes | |
| Immediate early genes | Chromatin acetylation modifiers or histone deacetylation inhibitors (for example, valproate, suberoylanilide hydroxamic acid) |
| NMDAR | NMDAR inhibitors (for example, memantine, felbamate); NR2B-specific inhibitors (ifenprodil) |
| AMPAR | AMPAR antagonists (for example, topiramate, talampanel, GYKI compounds) |
| NKCC1 | NKCC1 inhibitor (bumetanide) |
| GABAAR | GABAAR agonists (for example, phenobarbital, benzodiazepines) |
| Protein phosphatases | Calcineurin inhibitors (for example, FK506) |
| Protein kinases | Kinase inhibitors (CaMKII inhibitor KN-62, PKA inhibitor KT5720, PKC inhibitor chelerythrine) |
| Subacute changes | |
| Inflammation | Anti-inflammatory compounds (ACTH); microglial inactivators (for example, minocycline, doxycycline) |
| Neuronal injury | Erythropoietin, antioxidants, nitric oxide inhibitors, NMDAR antagonists (memantine) |
| HCN channels | Ih-blocker (ZD7288) |
| CB1 | CB1 receptor antagonists (for example, SR14176A, SR141716) |
| Chronic changes | |
| Sprouting | Protein synthesis inhibitors (for example, rapamycin, cycloheximide) |
| Gliosis | Anti-inflammatory agents (for example, COX-2 inhibitors, minocycline, doxycycline) |
The compounds listed are effective in in vivo experimental models of early-life seizures and/or brain injury, and many represent classes of drugs that are already in clinical use for other indications.
Abbreviations: ACTH, adrenocorticotropic hormone; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor; CaMKII, calcium–calmodulin kinase II; CB1, cannabinoid type 1 receptor; COX-2, cyclooxygenase 2; GABAAR, γ-aminobutyric acid receptor A; GluR, glutamate receptor; GYKI, 1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine; HCN, hyperpolarization-activated cyclic nucleotide-gated; Ih, hyperpolarization-activated current; NKCC1, sodium–potassium–chloride cotransporter 1; NMDAR, N-methyl-D-aspartate receptor; NR2B, NMDAR subunit 2B; PKA, protein kinase A; PKC, protein kinase C.
Acute changes in epileptogenesis
Induction of immediate early genes
IEGs are transcriptionally activated in response to neuronal activity, and have been implicated in normal synaptic plasticity and synaptogenesis. A number of IEGs, including Fos, Jun, Egr1, Egr4, Homer1, Nurr77, and Arc, have been identified as being transcriptionally activated or upregulated in animal models of epilepsy.75 The molecular cascades associated with IEG induction are initiated by repeated intense synaptic activation, which leads to depolarization and opening of nmDAR channels. The subsequent calcium influx can lead to the activation of kinase cascades, which ultimately results in the phosphorylation of transcription factors, such as cyclic-AMP response element binding protein (CREB) and CREB binding protein (CBP).76 Following phosphorylation, the transcription factors undergo translocation to the nucleus, where they activate gene transcription. The upregulated IEGs can modulate secondary response genes and IEG `effectors', which in turn modulate synaptic function.76
An increase in the expression of IEGs has been previously described in neocortical and hippocampal tissue in patients with epilepsy.77–79 Induction of IEGs has been observed in various animal models of seizures, including chemoconvulsant-induced and electrically induced kindling models.75 The majority of the changes in IEG expression have been observed in animal models of adult epilepsy, although some components of the molecular cascades that activate IEGs have also been shown to be activated in the developing brain. For example, the induction of Fos and Jun has been observed in the developing brain in hypoxia-induced seizures and lithium-pilocarpine status epilepticus models.80–82 The roles of these effectors could potentially be enhanced in the immature brain, as a number of IEGs, including Fos and Arc, are developmentally upregulated during the early postnatal period.83
Regulation of ion channels and receptors
The calcium influx following a seizure has the potential to activate a variety of signaling cascades. Such signaling could affect mechanisms that are hypothesized to increase synaptic efficiency, including expansion of the postsynaptic density,84 enlargement of the postsynaptic dendritic spines85 and clustering of the excitatory neurotransmitter receptors at the postsynaptic density.86 In addition, calcium activates phosphatases and kinases that alter ion channel and neurotransmitter receptor function. The calcium-calmodulin-activated phosphatase calcineurin is activated following early-life hypoxia-induced seizures87 and pilocarpine-induced epileptogenesis.88 Activation of calcineurin can lead to GABAAR endocytosis, decreased inhibitory postsynaptic potential frequency and reduced network inhibition in early-life seizures in vivo.87 similarly, the in vitro induction of seizures in neuronal and whole hippocampal preparations can impair GABAAR-mediated synaptic inhibition.89 Calcineurin-induced dephosphorylation of GABAARs results in endocytosis of these receptors.87, 90 In the immature brain, seizure-induced calcineurin activation can also cause rapid dephosphorylation of Kv2.1 channels, which leads to their subsequent internalization and prolongation of neuronal depolarization.91
Seizure-sensitive kinase phosphorylation sites on GluRs have also been described in the immature brain. Protein kinase C activity and calcium-calmodulin-dependent protein kinase II activity increase within minutes after seizures in rat pups, leading to an increase in phos phorylation of ser831 on GluR1 and ser880 on GluR2. Protein kinase A activation and phos phorylation of ser845 of GluR1 are also increased.92 The phosphorylation changes at ser831 and ser845 on GluR1 have been described to increase channel conductance and open-channel probability, respectively,33 both of which lead to enhanced AMPAR-mediated potentiation following seizures.92 Increased phosphorylation of ser880 on GluR2 can lead to endocytosis of the GluR2 subunit and enhanced Ca2+ permeability.33 Importantly, AMPAR antagonists administered within the first 48 hours after a seizure can prevent kinase activation and phosphorylation of ser831 and ser845 on GluR1. In turn, the inhibition of phosphorylation attenuates altered AMPAR function and mitigates against increased seizure susceptibility in later life.92 Changes in NMDARs mediated by seizure-induced cellular sarcoma kinase activation have also been reported in adult models. Hypoxia-ischemia in the developing brain can induce phosphorylation of the NR2A and NR2B receptor subunits via activation of cellular sarcoma kinases.93
Subacute changes
Neuronal death
Status epilepticus induced by electrical or chemoconvulsant stimulation results in progressive neuronal loss in the CA3 region of the hippocampus in adult models of epilepsy.94 This neuronal loss is presumed to alter the balance between excitation and inhibition in the limbic network, and to represent an important step in epileptogenesis.95 Studies in early-life seizure models in animals have consistently failed to demonstrate neuronal death in the hippocampus or amygdala.96 Limited neuronal death in hippocampal neurons can occur in the immature brain, however, as described in the flurothyl-induced seizure model97 and in a model of febrile seizures.98
Subplate neurons have emerged as another population of cells in the immature brain that are susceptible to seizures.99 Such neurons are present in appreciable numbers in the deep cortical regions during the preterm and neonatal periods and are critical for the normal maturation of cortical networks.100 In humans and rodents, subplate neurons possess high levels of AMPARs and NMDARs.17, 38 These cells, however, lack oxidative stress defenses, so they are selectively vulnerable to hypoxic-ischemic insults.101 In young kittens, kainate-induced seizures cause a loss of sub-plate neurons, and, consequently, abnormal development of inhibitory networks.100 Importantly, administration of clinically available antioxidants, such as erythropoeitin, is neuroprotective following hypoxia-induced neonatal seizures in rats.102
Neurotrophic factors
Neurotrophic factors and their receptors are abundant and exhibit increased expression during early postnatal development compared with their levels in the adult brain, and these molecules are considered to be critical for normal synaptic development.76 Nerve growth factor and BDNF, along with their receptors—members of the tropomyosin-related kinase (Trk) family of tyrosine kinases—have been extensively studied in epilepsy. Seizures in early life can increase neurotrophic factor expression (for example, BDNF mRNA and protein levels in limbic and cortical areas).103 BDNF-mediated TrkB signaling is important in conferring epileptogenesis in adult rats;104 however the roles of TrkA and TrkC are less well understood. Mice with a conditional deletion of TrkB in neurons have been reported to be protected from epileptogenesis.105 TrkB-activated signaling pathways—including those involving the serine-threonine kinase AKT, extracellular signal-regulated kinase (ERK) and phospholipase C—have a critical role in mediating synaptic plasticity in models of long-term potentiation, and a similar process of plasticity might in part mediate epileptogenesis in kindling models.105 Neurotrophins could also promote epileptogenesis via their role in modulating the maturational onset of KCC2 expression, which enhances GABA-mediated synaptic inhibition in dentate granule cells.49
Inflammation
Subacute inflammatory processes occur after seizures, and increased levels of cytokines, such as interleukin (IL)-δ, IL-1, IL-2, IL-6, tumor necrosis factor and macrophage colony-stimulating factor, are associated with seizures.106, 107 Changes in cytokine levels (for example, the interleukins) have been observed in experimental models of adult-onset epilepsy, but few studies have explored the role of inflammation-related changes in early-life seizures. Febrile seizures at P10 increase levels of nuclear factor κB (NFκB)1 and NFκB1a, whereas activation of IL-1β is observed after kainate-induced seizures at P15.106 IL-1β binding to its receptors can trigger intracellular messenger cascades, leading to targeted activation of mitogen-activated protein kinase and NFκB1, and subsequent transcriptional activation.69, 108 Seizures also directly activate microglia, triggering an inflammatory response mediated by cytokines, complement factors and major histocompatibility class factors.69 Microglia reach their maximal density in the brain during early development,70, 71 and activation of microglia and their subsequent infiltration of the neocortex following seizures might also have a role in epileptogenesis. The involvement of microglia in synaptic modulation and elimination72 during the critical period of development in the first few postnatal weeks in rodents suggests that microglial activation following early-life seizures might also target synapses. Indeed, the microglial inactivators minocycline and doxycycline protect against neuronal death following kainate-induced status epilepticus in adult mice.74 The neuroprotective effects of microglial inactivators have not been reported in the developing brain because of the lack of neuronal death observed following kainate-induced seizures during the second postnatal week. The effects of microglial inactivators on network excitability have yet to be studied in epilepsy models in the immature brain, and might be of considerable interest.
Transcriptional changes
In addition to rapid and early post-translational regulation of receptors and ion channels, early-life seizures induce subacute and long-term changes in neurotransmitter receptor transcription and expression. For example, GluR2 subunit expression declines following lithium-pilocarpine23 or hypoxia-induced seizures in rats.36 A reduction in GluR2 protein levels could lead to an increase in the number of calcium-permeable AMPARS, and, subsequently, pro-excitatory alterations in calcium signaling and long-term synaptic potentiation. Excitotoxic insults can activate the transcriptional repressor for GluR2 (repressor element 1-silencing transcription factor),109 which could provide a mechanistic explanation for reduced expression of the subunit. In addition, changes in NMDARs following early-life seizures might downregulate network excitability: for example, a long-term decrease in NR1 and NR2B subunit expression is observed following a single early-life seizure at P7.110 With respect to mGluRs, early-life seizures seem to induce an upregulation of subunits that promote excitability, such as the mGluR1 subunit.43
Studies in the adult rat brain show that seizures cause a decrease in the expression of the GABAAR α1 subunit, which would diminish inhibition.111 By contrast, status epilepticus models that produce long-term excitability in the immature brain result in an increase in the expression of the GABAAR α1 subunit. The studies in the immature brain also show a decrease in GABAAR α4 levels, because of the activation of the transcriptional repressor inducible cyclic-AMP early repressor by calcium-calmodulin-dependent protein kinase II.23, 112 These changes could constitute a homeostatic plasticity mechanism to counterbalance hyperexcitation, but early upregulation of inhibition could also modify neuronal networks to promote long-term network epileptogenesis.
Seizures in the immature brain also exert varying effects on HCN channel expression and hyperpolarization-activated current (Ih) function. In adult rats, kainate-induced status epilepticus leads to a functional decrease in Ih in CA1 neurons and an increase in synaptic excitability.113 Similarly, hypoxia-induced seizures in P10 rats cause a decrease in the Ih.114 Hyperthermia-induced seizures in immature rat pups, however, cause an increase in Ih, as well as an increase in the number of HCN channels.57 Hence, in some situations perturbation of HCN channels and Ih during development might promote epileptogenesis.
Evidence also suggests that the induction of seizures downregulates endocannabinoid receptors in a rat model of febrile seizures. The cannabinoid type 1 receptor (CB1) is presynaptically located and modulates glutamatergic synaptic function, and levels of CB1 mRNA and protein are decreased within days following hyperthermia-induced seizures in P10 rat pups.115 Systemic administration of the CB1 antagonist sR141716A before seizures is not acutely anticonvulsant; however, it does protect against long-term increases in hippocampal network excitability, suggesting an antiepileptogenic effect for this compound.115 Notably, similar decreases in CB1 and CB1 receptor binding protein mRNA and protein levels have been observed in human mesial temporal sclerosis tissue.116
Chronic changes
Sprouting
In models of epileptogenesis in adult rodents and studies using tissue removed from chronic epileptic foci in older children and adults, dentate granule cells seem to sprout aberrant collaterals to the inner molecular layer of the dentate gyrus. These projections form monosynaptic connections between dentate granule cells, thereby creating a positive feedback loop and seizure focus.14 Attempts to block protein synthesis with agents such as cycloheximide have not had consistent effects on sprouting or the prevention of epileptogenesis.117 A studies has shown that early treatment with rapamycin, an inhibitor of the mammalian target of rapamycin pathway-mediated protein translation, blocks later-life seizure activity in a mouse model of tuberous sclerosis.118 By contrast, early-life seizures result in a different pattern of sprouting whereby the mossy fiber synapses terminate on the basal dendrites in the CA3 region and the stratum oriens.119 The difference in sprouting patterns might be partly related to the fact that—at least in the early postnatal rodent models—the seizures occur before the completion of the connectivity of the dentate granule cells, which is usually accomplished in the third to fourth postnatal week.
Neurogenesis
Human studies performed on pediatric autopsy specimens and tissue biopsies taken during epilepsy surgery show there is an increase in neurogenesis in individuals with epilepsy compared with controls.120 Rodent models in which chemoconvulsants are administered during the first four weeks of life also show increased neurogenesis during either the ictal period119 or postictal period.121 Similarly, a long-term increase in newborn neuron survival in the dentate gyrus is observed in the prolonged febrile seizure model.122 Recurrent flurothyl-induced seizures in the first postnatal week, however, result in a decrease in subsequent neurogenesis in dentate granule cells, a process that normally continues up to the third postnatal week in control rats.123 The alterations in neurogenesis observed following early-life seizures clearly varies depending on the experimental model being studied. Aberrant connections established by immature and newly born neurons following seizures might, however, have an important role in the epileptogenesis process. Such changes in network re organization during the chronic phase could be targets for pharmacological intervention. Further studies regarding the functional consequences of the aberrant neuronal connections formed during this network reorganization will elucidate the roles of these connections in epileptogenesis.
Gliosis
Astrocytic gliosis occurs in the chronic stages of epilepsy in the mature brain. The role of astrocytes and their increased abundance in epilepsy is still controversial: astrocytes can release substances that are pro-excitatory, such as glutamate, as well as inhibitory molecules, such as adenosine.124 Several studies in rats have demonstrated that astrocytic proliferation, following seizures, is more-pronounced in later life than in the first postnatal weeks.125 The robust nature of reactive astrocytosis in later life seems to correlate with the cell death observed in adult models of epilepsy.126 The astrocytic glutamate transporters (excitatory amino acid transporters 1 and 2) are developmentally regulated,127 and their effects on glutamate reuptake might modulate excitability after early-life seizures.
Clinical biomarkers
Investigations into the basic mechanisms of epileptogenesis have identified a variety of factors that are involved in epileptogenesis at the molecular, cellular and neuronal network levels. Substantial research has also been conducted to reliably quantify some of these alterations, in particular, using imaging and EEG, the findings of which could serve as clinically useful noninvasive biomarkers for epilepsy progression and epileptogenesis.
In experiments performed in young rats with lithium-pilocarpine-induced or hyperthermic-induced seizures, MRI reveals hippocampal abnormalities before the development of spontaneous seizures following the insult,128, 129 which supports the predictive value of this technique. The FEBSTAT study aims to determine the presence of a similar signature change in children who are experiencing prolonged febrile seizures.8 Structural MRI has also identified patterns of hippocampal and neocortical atrophy that might predict disease progression in temporal lobe and hippocampal epilepsy.130, 131
In addition, the noninvasive blood-oxygen-level-dependent signal of functional mRI might be useful for the study of interictal and ictal events in patients with focal epilepsy, and might help to predict the epileptogenicity of certain lesions, especially when used in combination with EEG.132 PET scanning has revealed metabolic changes that are potentially specific for epileptic lesions: the α-methyl tryptophan ligand has been shown to be increased in epileptogenic zones in patients with tuberous sclerosis, compared with controls.133
Digital EEG methods now permit recording at expanded frequency ranges, which have helped identify novel interictal epileptiform EEG abnormalities, such as very high frequency oscillations (250–600 Hz), termed `fast ripples'.134 These fast ripples might predict epileptogenic regions and the development of epilepsy, and could be noninvasively identified by means of magnetoencephalo graphy, or through the use of interictal patterns on scalp EEG in conjunction with functional mRI.135 Fast ripples have yet to be studied in human infants and children, although they might represent a promising predictive marker for these populations.
Conclusions
Epileptogenesis results from a temporal progression of cascading molecular and cellular changes that lead to network reorganization; most of which occur during the latent phase. The use of stage-specific therapies targeting the individual sequential processes, as briefly discussed in this article, might offer potential therapeutic strategies for preventing epileptogenesis following insults to the developing brain. Furthermore, the increasing comprehen sion of these epileptogenic processes might aid in the develop ment of targeted antiepileptogenic drugs to complement the currently available anticonvulsant antiepileptic agents.
Key points.
-
■
Epileptogenesis is defined as the process of developing epilepsy—a disorder characterized by recurrent seizures—following an initial insult, and the process evolves through acute, subacute and chronic phases
-
■
The currently available therapies for epilepsy are predominantly anticonvulsant and do not modify the epileptogenic process
-
■
The immature brain exhibits increased excitation and diminished inhibition, and this enhanced excitability increases the propensity for seizures and epileptogenesis in infancy and early childhood compared with later life
-
■
The epileptogenic process in the immature brain evolves via alterations in molecular, cellular and neuronal network properties; animal models provide valuable insight into this process
-
■
Targeting the different phases of epileptogenesis with appropriate therapies might help develop disease-modifying antiepileptogenic treatment paradigms
Box 1 | In vivo rodent models of epileptogenesis.
Immature brain
Adult brain
-
■
Lateral fluid percussion injury (traumatic brain injury)12,141
- ■
-
■
Lithium–pilocarpine status epilepticus144
-
■
Pilocarpine-induced status epilepticus145
-
■
intrahippocampal stimulation; perforant pathway stimulation146
-
■
Amygdala stimulation147
-
■
Cortical stroke models148
Abbreviation: P, postnatal day.
Acknowledgments
This work was supported by funding from the National Institutes of Health (grants RO1 NS31718 and DP1 OD003347 to F. E. Jensen), the Epilepsy Therapy Development Project (F. E. Jensen), and a grant from the Parents Against Childhood Epilepsy (S. N. Rakhade and F. E. Jensen). Additional support was provided from the National Institutes of Health Mental Retardation and Developmental Disabilities Center (P30 HD18655).
Footnotes
Competing interests The authors declare no competing interests
Review criteria A literature search was performed, using the PubMed and Library of Congress databases, for papers covering the main topics included in this Review. Searches were limited to articles published in English and used the following terms: “epileptogenesis”, “seizure”, “developing brain”, “epilepsy “, “neuron”, “synapse”, “pediatric”, and “neonate”. The abstracts and web content retrieved from the search were reviewed and prioritized. Full articles were obtained and additional references were reviewed when appropriate.
References
- 1.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Neurology of the Newborn. Saunders; Philadelphia: 2008. pp. 203–244. [Google Scholar]
- 3.LaFrance WC, Jr, Kanner AM, Hermann B. Psychiatric comorbidities in epilepsy. Int. Rev. Neurobiol. 2008;83:347–383. doi: 10.1016/S0074-7742(08)00020-2. [DOI] [PubMed] [Google Scholar]
- 4.Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515–524. doi: 10.1046/j.1528-1157.2001.28900.x. [DOI] [PubMed] [Google Scholar]
- 5.Garga N, Lowenstein DH. Posttraumatic epilepsy: a major problem in desperate need of major advances. Epilepsy Curr. 2006;6:1–5. doi: 10.1111/j.1535-7511.2005.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haltiner AM, Temkin NR, Dikmen SS. Risk of seizure recurrence after the first late posttraumatic seizure. Arch. Phys. Med. Rehabil. 1997;78:835–840. doi: 10.1016/s0003-9993(97)90196-9. [DOI] [PubMed] [Google Scholar]
- 7.Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(Suppl 2):10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 8.Shinnar S, et al. Phenomenology of prolonged febrile seizures: results of the FEBSTAT study. Neurology. 2008;71:170–176. doi: 10.1212/01.wnl.0000310774.01185.97. [DOI] [PubMed] [Google Scholar]
- 9.Dinner DS, Wyllie E. In: The Treatment of Epilepsy: Principles and Practice. Wyllie E, editor. Lea & Febiger; Philadelphia: 1993. pp. 654–658. [Google Scholar]
- 10.Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–1822. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- 11.VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann. Neurol. 1998;43:413–426. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 12.Kharatishvili I, Nissinen JP, Mcintosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Epsztein J, Ben-Ari Y, Represa A, Crepel V. Late-onset epileptogenesis and seizure genesis: lessons from models of cerebral ischemia. Neuroscientist. 2008;14:78–90. doi: 10.1177/1073858407301681. [DOI] [PubMed] [Google Scholar]
- 14.Williams PA, Hellier JL, White AM, Staley KJ, Dudek FE. Development of spontaneous seizures after experimental status epilepticus: implications for understanding epileptogenesis. Epilepsia. 2007;48(Suppl 5):157–163. doi: 10.1111/j.1528-1167.2007.01304.x. [DOI] [PubMed] [Google Scholar]
- 15.Swann JW, Brady RJ, Martin DL. Postnatal development of GABA-mediated synaptic inhibition in rat hippocampus. Neuroscience. 1989;28:551–561. doi: 10.1016/0306-4522(89)90004-3. [DOI] [PubMed] [Google Scholar]
- 16.Moshe SL, Sharpless NS, Kaplan J. Kindling in developing rats: variability of afterdischarge thresholds with age. Brain Res. 1981;211:190–195. doi: 10.1016/0006-8993(81)90082-2. [DOI] [PubMed] [Google Scholar]
- 17.Talos DM, et al. Developmental regulation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J. Comp. Neurol. 2006;497:42–60. doi: 10.1002/cne.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romijn HJ, Hofman MA, Gramsbergen A. At What age is the developing cerebral cortex of the rat comparable to that of the full term newborn baby? Early Hum. Dev. 1991;26:61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- 19.Jensen FE, Applegate CD, Holtzman D, Belin TR, Burchfiel JL. Epileptogenic effect of hypoxia in the immature rodent brain. Ann. Neurol. 1991;29:629–637. doi: 10.1002/ana.410290610. [DOI] [PubMed] [Google Scholar]
- 20.Koh S, Tibayan FD, Simpson JN, Jensen FE. NBQX or topiramate treatment following perinatal hypoxia-induced seizures prevents later increases in seizure-induced neuronal injury. Epilepsia. 2004;45:569–575. doi: 10.1111/j.0013-9580.2004.69103.x. [DOI] [PubMed] [Google Scholar]
- 21.Mikati MA, et al. Long-term effects of acute and of chronic hypoxia on behavior and on hippocampal histology in the developing brain. Brain Res. Dev. Brain Res. 2005;157:98–102. doi: 10.1016/j.devbrainres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Dube C, et al. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, Raol YH, Hsu FC, Coulter DA, Brooks-Kayal AR. Effects of status epilepticus on hippocampal GABAA receptors are age-dependent. Neuroscience. 2004;125:299–303. doi: 10.1016/j.neuroscience.2004.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sogawa Y, et al. Timing of cognitive deficits following neonatal seizures: relationship to histological changes in the hippocampus. Brain Res. Dev. Brain Res. 2001;131:73–83. doi: 10.1016/s0165-3806(01)00265-6. [DOI] [PubMed] [Google Scholar]
- 25.Stafstrom CE, Thompson JL, Holmes GL. Kainic acid seizures in the developing brain: status epilepticus and spontaneous recurrent seizures. Brain Res. Dev. Brain Res. 1992;65:227–236. doi: 10.1016/0165-3806(92)90184-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee CL, Hrachovy RA, Smith KL, Frost JD, Jr, Swann JW. Tetanus toxin-induced seizures in infant rats and their effects on hippocampal excitability in adulthood. Brain Res. 1995;677:97–109. doi: 10.1016/0006-8993(95)00127-c. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez RM, Jensen FE. Maturational aspects of epilepsy mechanisms and consequences for the immature brain. Epilepsia. 2001;42:577–585. doi: 10.1046/j.1528-1157.2001.12000.x. [DOI] [PubMed] [Google Scholar]
- 28.Huttenlocher PR, deCourten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci. Lett. 1982;33:247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- 29.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of primate cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 30.Silverstein FS, Jensen FE. Neonatal seizures. Ann. Neurol. 2007;62:112–120. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- 31.Jensen FE. Role of glutamate receptors in periventricular leukomalacia. J. Child Neurol. 2005;20:950–959. doi: 10.1177/08830738050200120401. [DOI] [PubMed] [Google Scholar]
- 32.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 34.Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez RM, et al. Decreased glutamate receptor 2 expression and enhanced epileptogenesis in immature rat hippocampus after perinatal hypoxia-induced seizures. J. Neurosci. 2001;21:8154–8163. doi: 10.1523/JNEUROSCI.21-20-08154.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J. Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talos DM, et al. Developmental regulation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J. Comp. Neurol. 2006;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mares P, Mikulecka A. Different effects of two N-methyl-D-aspartate receptor antagonists on seizures, spontaneous behavior, and motor performance in immature rats. Epilepsy Behav. 2009;14:32–39. doi: 10.1016/j.yebeh.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Chen HS, et al. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–1132. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- 41.Wallis RA, Panizzon KL, Fairchild MD, Wasterlain CG. Protective effects of felbamate against hypoxia in the rat hippocampal slice. Stroke. 1992;23:547–551. doi: 10.1161/01.str.23.4.547. [DOI] [PubMed] [Google Scholar]
- 42.Aujla PK, Fetell M, Jensen FE. Talampanel suppresses the acute and chronic effects of seizures in a rodent neonatal seizure model. Epilepsia. 2009;50:694–701. doi: 10.1111/j.1528-1167.2008.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avallone J, Gashi E, Magrys B, Friedman LK. Distinct regulation of metabotropic glutamate receptor (mGluR1α) in the developing limbic system following multiple early-life seizures. Exp. Neurol. 2006;202:100–111. doi: 10.1016/j.expneurol.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Ure J, Baudry M, Perassolo M. Metabotropic glutamate receptors and epilepsy. J. Neurol. Sci. 2006;247:1–9. doi: 10.1016/j.jns.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Brooks-Kayal AR, et al. γ-Aminobutyric acidA receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J. Neurochem. 2001;77:1266–1278. doi: 10.1046/j.1471-4159.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- 46.Loturco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 47.Dzhala VI, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 48.Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABAA receptors. J. Neurosci. 2008;28:1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivera C, et al. BDNF-induced TrkB activation down-regulates the K+-Cl– cotransporter KCC2 and impairs neuronal Cl– extrusion. J. Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann. Neurol. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 51.Iwasaki S, Momiyama A, Uchitel OD, Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. J. Neurosci. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noebels JL. The biology of epilepsy genes. Annu. Rev. Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- 53.Cooper EC, Jan LY. M-channels: neurological diseases, neuromodulation, and drug development. Arch. Neurol. 2003;60:496–500. doi: 10.1001/archneur.60.4.496. [DOI] [PubMed] [Google Scholar]
- 54.Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J. Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu. Rev. Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 56.Chen K, et al. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat. Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bender RA, Baram TZ. Hyperpolarization activated cyclic-nucleotide gated (HCN) channels in developing neuronal networks. Prog. Neurobiol. 2008;86:129–140. doi: 10.1016/j.pneurobio.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J. Neurosci. 2008;28:11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 60.Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. J. Clin. Invest. 2005;115:2010–2017. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kearney JA, et al. Recurrent de novo mutations of SCN1A in severe myoclonic epilepsy of infancy. Pediatr. Neurol. 2006;34:116–120. doi: 10.1016/j.pediatrneurol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Escayg A, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat. Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- 63.Burgess DL, et al. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant `motor endplate disease'. Nat. Genet. 1995;10:461–465. doi: 10.1038/ng0895-461. [DOI] [PubMed] [Google Scholar]
- 64.Kearney JA, et al. A gain-of-function mutation in the sodium channel gene Scn2a results in seizures and behavioral abnormalities. Neuroscience. 2001;102:307–317. doi: 10.1016/s0306-4522(00)00479-6. [DOI] [PubMed] [Google Scholar]
- 65.Kearney JA, et al. Severe epilepsy resulting from genetic interaction between Scn2a and Kcnq2. Hum. Mol. Genet. 2006;15:1043–1048. doi: 10.1093/hmg/ddl019. [DOI] [PubMed] [Google Scholar]
- 66.Martin MS, et al. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum. Mol. Genet. 2007;16:2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- 67.Glasscock E, Qian J, Yoo JW, Noebels JL. Masking epilepsy by combining two epilepsy genes. Nat. Neurosci. 2007;10:1554–1558. doi: 10.1038/nn1999. [DOI] [PubMed] [Google Scholar]
- 68.Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia. 2008;49(Suppl 2):33–41. doi: 10.1111/j.1528-1167.2008.01491.x. [DOI] [PubMed] [Google Scholar]
- 69.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav. Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Billiards SS, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J. Comp. Neurol. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- 71.Dalmau I, Vela JM, Gonzalez B, Finsen B, Castellano B. Dynamics of microglia in the developing rat brain. J. Comp. Neurol. 2003;458:144–157. doi: 10.1002/cne.10572. [DOI] [PubMed] [Google Scholar]
- 72.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 73.Lechpammer M, et al. Minocycline treatment following hypoxic/ischaemic injury attenuates white matter injury in a rodent model of periventricular leucomalacia. Neuropathol. Appl. Neurobiol. 2008;34:379–393. doi: 10.1111/j.1365-2990.2007.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heo K, et al. Minocycline inhibits caspase-dependent and -independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neurosci. Lett. 2006;398:195–200. doi: 10.1016/j.neulet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 75.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CreB/ATF proteins. Brain Res. Brain Res. Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 76.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 77.MacGibbon GA, et al. Expression of Fos, Jun, and Krox family proteins in Alzheimer's disease. Exp. Neurol. 1997;147:316–332. doi: 10.1006/exnr.1997.6600. [DOI] [PubMed] [Google Scholar]
- 78.Rakhade SN, et al. A common pattern of persistent gene activation in human neocortical epileptic foci. Ann. Neurol. 2005;58:736–747. doi: 10.1002/ana.20633. [DOI] [PubMed] [Google Scholar]
- 79.Rakhade SN, et al. Activity-dependent gene expression correlates with interictal spiking in human neocortical epilepsy. Epilepsia. 2007;48(Suppl 5):86–95. doi: 10.1111/j.1528-1167.2007.01294.x. [DOI] [PubMed] [Google Scholar]
- 80.Jensen FE, Firkusny IR, Mower GD. Differences in c-fos immunoreactivity due to age and mode of seizure induction. Brain Res. Mol. Brain Res. 1993;17:185–193. doi: 10.1016/0169-328x(93)90001-6. [DOI] [PubMed] [Google Scholar]
- 81.Dube C, et al. C-Fos, Jun D and HSP72 immunoreactivity, and neuronal injury following lithium-pilocarpine induced status epilepticus in immature and adult rats. Brain Res. Mol. Brain Res. 1998;63:139–154. doi: 10.1016/s0169-328x(98)00282-4. [DOI] [PubMed] [Google Scholar]
- 82.Hyde P, Stafstrom CE, Firkusny IR, Holmes GL, Jensen FE. Parallel age-dependent c-fos immunoreactivity and seizure characteristics induced by kainic acid. Epilepsia. 1992;33:44–44. [Google Scholar]
- 83.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/ arg3.1: regulation, mechanisms, and function. J. Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 87.Sanchez RM, Dai W, Levada RE, Lippman JJ, Jensen FE. AMPA/kainate receptor-mediated downregulation of GABAergic synaptic transmission by calcineurin after seizures in the developing rat brain. J. Neurosci. 2005;25:3442–3451. doi: 10.1523/JNEUROSCI.0204-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurz JE, et al. A significant increase in both basal and maximal calcineurin activity in the rat pilocarpine model of status epilepticus. J. Neurochem. 2001;78:304–315. doi: 10.1046/j.1471-4159.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- 89.Khalilov I, Holmes GL, Ben Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat. Neurosci. 2003;6:1079–1085. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- 90.Blair RE, Sombati S, Lawrence DC, McCay BD, DeLorenzo RJ. Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J. Pharmacol. Exp. Ther. 2004;310:871–880. doi: 10.1124/jpet.104.068478. [DOI] [PubMed] [Google Scholar]
- 91.Bernard C, et al. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- 92.Rakhade SN, et al. Early alterations of AMPA receptors mediate synaptic potentiation induced by neonatal seizures. J. Neurosci. 2008;28:7979–7990. doi: 10.1523/JNEUROSCI.1734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang X, et al. Activated Src kinases interact with the N-methyl-D-aspartate receptor after neonatal brain ischemia. Ann. Neurol. 2008;63:632–641. doi: 10.1002/ana.21365. [DOI] [PubMed] [Google Scholar]
- 94.Cavazos JE, Golarai G, Sutula TP. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J. Neurosci. 1991;11:2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J. Neurophysiol. 1998;79:418–429. doi: 10.1152/jn.1998.79.1.418. [DOI] [PubMed] [Google Scholar]
- 96.Holmes GL, Khazipov R, Ben Ari Y. Seizure-induced damage in the developing human: relevance of experimental models. Prog. Brain Res. 2002;135:321–334. doi: 10.1016/S0079-6123(02)35030-1. [DOI] [PubMed] [Google Scholar]
- 97.Wasterlain CG, et al. Seizure-induced neuronal death in the immature brain. Prog. Brain Res. 2002;135:335–353. doi: 10.1016/S0079-6123(02)35031-3. [DOI] [PubMed] [Google Scholar]
- 98.Toth Z, Yan XX, Haftoglu S, Ribak C, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J. Neurosci. 1998;18:4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kinney HC, Haynes RL, Folkerth RD, Golden JA, Harding B. Pathology and Genetics: Acquired and Inherited Diseases of the Developing Nervous System. Vol. 20. ISN Neuropathology Press; Basel: 2004. pp. 29–40. [Google Scholar]
- 100.Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–525. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- 101.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J. Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mikati MA, El Hokayem JA, El Sabban ME. Effects of a single dose of erythropoietin on subsequent seizure susceptibility in rats exposed to acute hypoxia at P10. Epilepsia. 2007;48:175–181. doi: 10.1111/j.1528-1167.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 103.Tandon P, et al. Neuroprotective effects of brain-derived neurotrophic factor in seizures during development. Neuroscience. 1999;91:293–303. doi: 10.1016/s0306-4522(98)00609-5. [DOI] [PubMed] [Google Scholar]
- 104.Scharfman HE, Goodman JH, Sollas AL, Croll SD. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp. Neurol. 2002;174:201–214. doi: 10.1006/exnr.2002.7869. [DOI] [PubMed] [Google Scholar]
- 105.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci. STKE. 2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 106.Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1β contributes to the generation of experimental febrile seizures. Ann. Neurol. 2005;57:152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med. J. 2008;49:1–18. doi: 10.3349/ymj.2008.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Viviani B, et al. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Calderone A, et al. Ischemic insults derepress the gene silencer REST in neurons destined to die. J. Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Benke TA. A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann. Neurol. 2007;61:411–426. doi: 10.1002/ana.21071. [DOI] [PubMed] [Google Scholar]
- 111.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat. Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 112.Hu Y, et al. Surface expression of GABAA receptors is transcriptionally controlled by the interplay of cAMP-response element-binding protein and its binding partner inducible cAMP early repressor. J. Biol. Chem. 2008;283:9328–9340. doi: 10.1074/jbc.M705110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch. Dis. Child Fetal Neonatal Ed. 2004;89:F152–F155. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang K, Peng BW, Sanchez RM. Decreased IH in hippocampal area CA1 pyramidal neurons after perinatal seizure-inducing hypoxia. Epilepsia. 2006;47:1023–1028. doi: 10.1111/j.1528-1167.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- 115.Chen K, et al. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J. Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ludanyi A, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J. Neurosci. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Toyoda I, Buckmaster PS. Prolonged infusion of cycloheximide does not block mossy fiber sprouting in a model of temporal lobe epilepsy. Epilepsia. 2005;46:1017–1020. doi: 10.1111/j.1528-1167.2005.04605.x. [DOI] [PubMed] [Google Scholar]
- 118.Meikle L, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Holmes GL, Gaiarsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann. Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 120.Takei H, Wilfong A, Yoshor D, Armstrong DL, Bhattacharjee MB. Evidence of increased cell proliferation in the hippocampus in children with Ammon's horn sclerosis. Pathol. Int. 2007;57:76–81. doi: 10.1111/j.1440-1827.2006.02060.x. [DOI] [PubMed] [Google Scholar]
- 121.Sankar R, Shin D, Liu H, Katsumori H, Wasterlain CG. Granule cell neurogenesis after status epilepticus in the immature rat brain. Epilepsia. 2000;41(Suppl 6):S53–S56. doi: 10.1111/j.1528-1157.2000.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 122.Lemmens EM, Lubbers T, Schijns OE, Beuls EA, Hoogland G. Gender differences in febrile seizure-induced proliferation and survival in the rat dentate gyrus. Epilepsia. 2005;46:1603–1612. doi: 10.1111/j.1528-1167.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 123.Schmid R, Tandon P, Stafstrom CE, Holmes GL. Effects of neonatal seizures on subsequent seizure-induced brain injury. Neurology. 1999;53:1754–1761. doi: 10.1212/wnl.53.8.1754. [DOI] [PubMed] [Google Scholar]
- 124.Jabs R, Seifert G, Steinhauser C. Astrocytic function and its alteration in the epileptic brain. Epilepsia. 2008;49(Suppl 2):3–12. doi: 10.1111/j.1528-1167.2008.01488.x. [DOI] [PubMed] [Google Scholar]
- 125.Sperber EF, Haas KZ, Romero MT, Stanton PK. Flurothyl status epilepticus in developing rats: behavioral, electrographic histological and electrophysiological studies. Brain Res. Dev. Brain Res. 1999;116:59–68. doi: 10.1016/s0165-3806(99)00075-9. [DOI] [PubMed] [Google Scholar]
- 126.Rizzi M, et al. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol. Dis. 2003;14:494–503. doi: 10.1016/j.nbd.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 127.Furuta A, Rothstein JD, Martin LJ. Glutamate transporter subtypes are expressed differentially during rat CNs development. J. Neurosci. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roch C, Leroy C, Nehlig A, Namer IJ. Predictive value of cortical injury for the development of temporal lobe epilepsy in 21-day-old rats: an MRI approach using the lithium-pilocarpine model. Epilepsia. 2002;43:1129–1136. doi: 10.1046/j.1528-1157.2002.17802.x. [DOI] [PubMed] [Google Scholar]
- 129.Dube C, Yu H, Nalcioglu O, Baram TZ. Serial MRI after experimental febrile seizures: altered T2 signal without neuronal death. Ann. Neurol. 2004;56:709–714. doi: 10.1002/ana.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kimiwada T, et al. Hippocampal and thalamic diffusion abnormalities in children with temporal lobe epilepsy. Epilepsia. 2006;47:167–175. doi: 10.1111/j.1528-1167.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 131.Lin JJ, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb. Cortex. 2007;17:2007–2018. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- 132.Tyvaert L, et al. Different structures involved during ictal and interictal epileptic activity in malformations of cortical development: an EEG-fMRI study. Brain. 2008;131:2042–2060. doi: 10.1093/brain/awn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kagawa K, et al. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with α-[11C]methyl-L-tryptophan positron emission tomography (PET) J. Child Neurol. 2005;20:429–438. doi: 10.1177/08830738050200050701. [DOI] [PubMed] [Google Scholar]
- 134.Bragin A, Wilson CL, Fields T, Fried I, Engel J., Jr. Analysis of seizure onset on the basis of wideband EEG recordings. Epilepsia. 2005;46(Suppl 5):59–63. doi: 10.1111/j.1528-1167.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- 135.Engel J., Jr. Progress in epilepsy: reducing the treatment gap and the promise of biomarkers. Curr Opin Neurol. 2008;21:150–154. doi: 10.1097/WCO.0b013e3282f4edc3. [DOI] [PubMed] [Google Scholar]
- 136.Williams PA, Dou P, Dudek FE. Epilepsy and synaptic reorganization in a perinatal rat model of hypoxia-ischemia. Epilepsia. 2004;45:1210–1218. doi: 10.1111/j.0013-9580.2004.60403.x. [DOI] [PubMed] [Google Scholar]
- 137.Rakhade SN, et al. Early life hypoxia induced seizures lead to spontaneous epilepsy in a rodent model [abstract] Epilepsia. 2008;49(S7):327. [Google Scholar]
- 138.Anderson AE, Hrachovy RA, Antalffy BA, Armstrong DL, Swann JW. A chronic focal epilepsy with mossy fiber sprouting follows recurrent seizures induced by intrahippocampal tetanus toxin injection in infant rats. Neuroscience. 1999;92:73–82. doi: 10.1016/s0306-4522(98)00746-5. [DOI] [PubMed] [Google Scholar]
- 139.Kubova H, et al. Status epilepticus in immature rats leads to behavioural and cognitive impairment and epileptogenesis. Eur. J. Neurosci. 2004;19:3255–3265. doi: 10.1111/j.0953-816X.2004.03410.x. [DOI] [PubMed] [Google Scholar]
- 140.Mateffyova A, Otahal J, Tsenov G, Mares P, Kubova H. Intrahippocampal injection of endothelin-1 in immature rats results in neuronal death, development of epilepsy and behavioral abnormalities later in life. Eur. J. Neurosci. 2006;24:351–360. doi: 10.1111/j.1460-9568.2006.04910.x. [DOI] [PubMed] [Google Scholar]
- 141.D'Ambrosio R, et al. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nadler JV, Perry BW, Cotman CW. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature. 1978;271:676–677. doi: 10.1038/271676a0. [DOI] [PubMed] [Google Scholar]
- 143.Ben-Ari Y, Lagowska J. Epileptogenic action of intra-amygdaloid injection of kainic acid [French] C. R. Acad. Sci. Hebd. Seances Acad. Sci. D. 1978;287:813–816. [PubMed] [Google Scholar]
- 144.Jope RS, Morrisett RA, Snead OC. Characterization of lithium potentiation of pilocarpine-induced status epilepticus in rats. Exp. Neurol. 1986;91:471–480. doi: 10.1016/0014-4886(86)90045-2. [DOI] [PubMed] [Google Scholar]
- 145.Turski WA, et al. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- 146.Mazarati AM, Wasterlain CG, Sankar R, Shin D. Self-sustaining status epilepticus after brief electrical stimulation of the perforant path. Brain Res. 1998;801:251–253. doi: 10.1016/s0006-8993(98)00606-4. [DOI] [PubMed] [Google Scholar]
- 147.Nissinen J, Halonen T, Koivisto E, Pitkanen A. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res. 2000;38:177–205. doi: 10.1016/s0920-1211(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 148.Karhunen H, Jolkkonen J, Sivenius J, Pitkanen A. Epileptogenesis after experimental focal cerebral ischemia. Neurochem. Res. 2005;30:1529–1542. doi: 10.1007/s11064-005-8831-y. [DOI] [PubMed] [Google Scholar]
- 149.Misonou H, et al. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat. Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- 150.Cross DJ, Cavazos JE. Synaptic reorganization in subiculum and CA3 after early-life status epilepticus in the kainic acid rat model. Epilepsy Res. 2007;73:156–165. doi: 10.1016/j.eplepsyres.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jensen FE. Post-traumatic epilepsy: treatable epileptogenesis. Epilepsia. 2009;50(Suppl 2):1–3. doi: 10.1111/j.1528-1167.2008.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]