Abstract
The CD40–CD40 ligand (CD40L) signaling axis plays an important role in immunological pathways. Consequently, this dyad is involved in chronic inflammatory diseases, including atherosclerosis. Inhibition of CD40L in apolipoprotein E (Apoe)–deficient (Apoe−/−) mice not only reduced atherosclerosis but also conferred a clinically favorable plaque phenotype that was low in inflammation and high in fibrosis. Blockade of CD40L may not be therapeutically feasible, as long-term inhibition will compromise systemic immune responses. Conceivably, more targeted intervention strategies in CD40 signaling will have less deleterious side effects. We report that deficiency in hematopoietic CD40 reduces atherosclerosis and induces features of plaque stability. To elucidate the role of CD40–tumor necrosis factor receptor-associated factor (TRAF) signaling in atherosclerosis, we examined disease progression in mice deficient in CD40 and its associated signaling intermediates. Absence of CD40-TRAF6 but not CD40-TRAF2/3/5 signaling abolishes atherosclerosis and confers plaque fibrosis in Apoe−/− mice. Mice with defective CD40-TRAF6 signaling display a reduced blood count of Ly6Chigh monocytes, an impaired recruitment of Ly6C+ monocytes to the arterial wall, and polarization of macrophages toward an antiinflammatory regulatory M2 signature. These data unveil a role for CD40–TRAF6, but not CD40–TRAF2/3/5, interactions in atherosclerosis and establish that targeting specific components of the CD40–CD40L pathway harbors the potential to achieve therapeutic effects in atherosclerosis.
Atherosclerosis is a chronic inflammatory disease of the large arteries that involves multiple immunological processes (Hansson, 2005; Weber et al., 2008). During the progression of atherosclerosis, ongoing activation of the immune system causes continuous recruitment of inflammatory cells into the plaque and degradation of its extracellular matrix. This creates a so-called vulnerable plaque, which is prone to rupture and therefore likely to cause acute complications such as myocardial infarction or stroke (Virmani et al., 2000).
The CD40–CD40 ligand (CD40L) dyad, a costimulatory receptor-ligand pair, plays a crucial role in enhancing immune responses and inflammation and contributes to a plethora of chronic inflammatory diseases, for example, colitis, arthritis, allergic encephalitis, and multiple sclerosis (Durie et al., 1993; Gerritse et al., 1996; Lutgens et al., 2007; Vowinkel et al., 2007). Disruption of the CD40L gene in apolipoprotein E (Apoe)−/− mice abrogated atherosclerosis and caused a plaque phenotype with only few inflammatory cells and a high percentage of extracellular matrix (Lutgens et al., 1999), which is reminiscent of a clinically favorable stable atherosclerotic plaque in humans (Virmani et al., 2000). This phenotype was copied when Apoe−/− mice with initial plaques or established atheromata were treated with a blocking anti-CD40L antibody (Mach et al., 1998; Lutgens et al., 2000; Schönbeck et al., 2000).
In principle, these findings render targeting of CD40L a promising strategy to reduce atherosclerosis and to stabilize atherosclerotic plaques. Unfortunately, clinical trials using an anti-CD40L antibody have been omitted as a result of thromboembolic complications, particularly because CD40L is present on platelets and can interact with the integrin αIIbβ3 in platelets (Kawai et al., 2000; André et al., 2002).
Antagonizing CD40, the receptor for CD40L, or its signaling intermediates would be an alternative approach for treating human atherosclerosis. However, information on the effects of CD40 and CD40-associated signal transduction pathways in atherosclerosis is scarce. It has been shown that low-density lipoprotein receptor (Ldlr)–deficient (Ldlr−/−) mice lacking the CD40 gene do not develop smaller atherosclerotic lesions than control Ldlr−/− mice (Zirlik et al., 2007b), although a proatherogenic role for endothelial CD40–TNF receptor-associated factor (TRAF) 1, 2, 3, 5, and 6 signaling in atherosclerosis has been claimed (Zirlik et al., 2007a). Using mice carrying a CD40 transgene with targeted mutations at the CD40-TRAF6 recognition site, we found that CD40-TRAF6 signaling is specifically required for arterial neointima formation (Donners et al., 2008).
In the present study, we tried to elucidate the role of CD40 and its associated signaling intermediates, the TRAFs, in primary atherosclerosis in vivo in detail. We found that CD40−/−Apoe−/− mice had a reduction in atherosclerosis and developed a stable atherosclerotic plaque phenotype. BM transplantation of CD40−/− BM into Ldlr−/− mice revealed that hematopoietic CD40 was responsible for the observed phenotype. Surprisingly, when CD40–TRAF6, but not CD40–TRAF2/3/5, interactions were defective, atherosclerosis was completely abrogated.
RESULTS
CD40 deficiency reduces atherosclerosis
We generated CD40−/−Apoe−/− mice and analyzed the extent and phenotype of atherosclerosis at 26 wk of age on a normal chow diet. Body weight, as well as cholesterol content or plasma sCD40L levels did not differ between the groups (Table I).
Table I.
Body weights, plasma cholesterol levels, and sCD40L serum levels did not differ between the genotypes (P > 0.05)
| Genotype | Body weight | Cholesterol | sCD40L |
| g | mg/dl | ng/ml | |
| Apoe−/− | 28.7 ± 0.8 | 275 ± 16 | 4.0 ± 0.8 |
| CD40−/−Apoe−/− | 29.2 ± 0.8 | 299 ± 24 | 5.6 ± 1.6 |
| CD40-Twt | 26.1 ± 0.8 | 328 ± 26 | 3.3 ± 1.0 |
| CD40-T2/3/5−/− | 24.9 ± 0.7 | 297 ± 33 | 5.0 ± 2.6 |
| CD40-T6−/− | 24.8 ± 0.8 | 283 ± 12 | 2.9 ± 0.5 |
| CD40-T2/3/5/6−/− | 28.9 ± 0.7 | 330 ± 36 | 5.3 ± 1.9 |
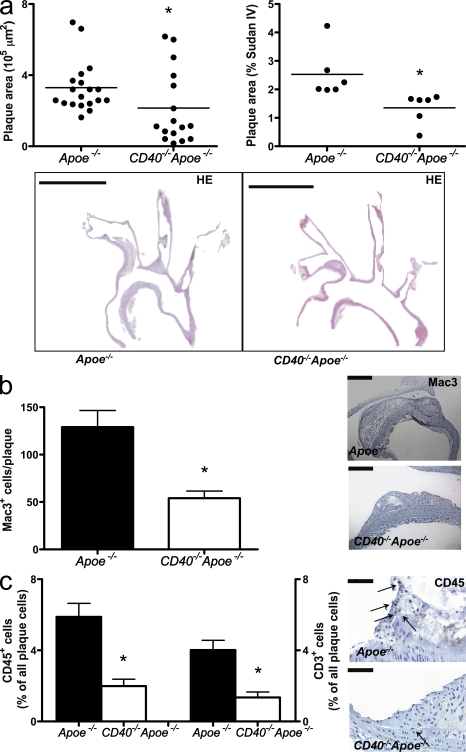
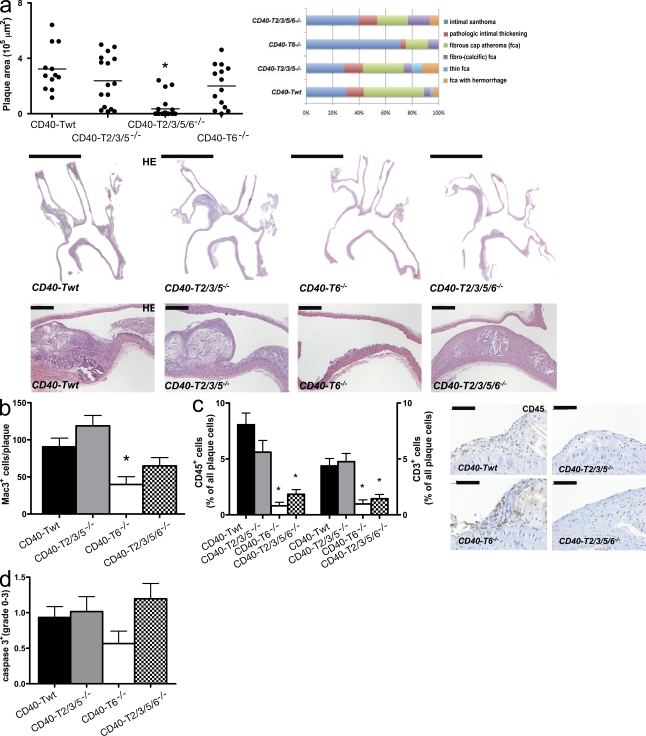
As in CD40L−/−Apoe−/− mice, deficincy of CD40 reduced the atherosclerotic plaque area in the aortic arch and its branch points, as well as in the thoraco-abdominal aorta (Fig. 1 a). This was associated with a less inflammatory and more fibrotic plaque quality, as reflected by the low amount of thin fibrous cap atheromata (Virmani et al., 2000) that developed in absence of CD40 (Fig. S1 a). The absence of CD40 reduced the lipid core size (ApoE−/−, 35.4% vs. CD40−/−ApoE−/−, 23.3%; P < 0.05), the number of plaque macrophages, and the content of CD45+ cells and CD3+ T lymphocytes (Figs. 1, b and c). The number of Foxp3+ regulatory T cells in plaque or adventitia was unaffected (Fig. S1, b and c). The numbers of cleaved caspase 3+ (apoptotic) cells (Fig. S1 d), as well as the degree of iron or fibrin deposition in the plaque (not depicted), were unaffected.
Figure 1.
Role of CD40 in atherosclerosis. (a–c) Apoe−/− mice (n = 19) were fed a normal chow diet for 26 wk and were compared with CD40−/−Apoe−/− (n = 16). Sections of the aortic arch and its main branch points (brachiocephalic artery, right subclavian artery, right carotid artery, left carotid artery, and left subclavian artery) were stained with hematoxylin and eosin (HE; representative sections in a, bottom) to analyze the extent of atherosclerosis (plaque area; a, top left: plaque area aortic arch and branch points; a, top right: en face staining thoraco-abdominal aorta; bars, 2 mm; horizontal bars represent mean) and plaque phenotype (b and c). (b) Macrophage infiltration was expressed as the absolute number of Mac3+ cells per plaque (bars, 100 µm). (c) CD45 and CD3 content were expressed as the percentage of CD3+ or CD45+ cells of all plaque cells (arrows indicate CD45+ cells; bars, 50 µm). Error bars represent mean ± SEM. *, P < 0.05.
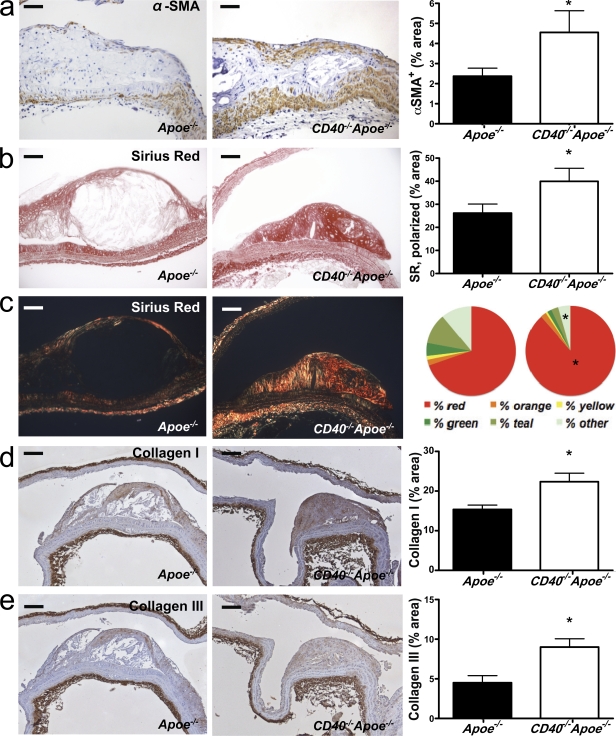
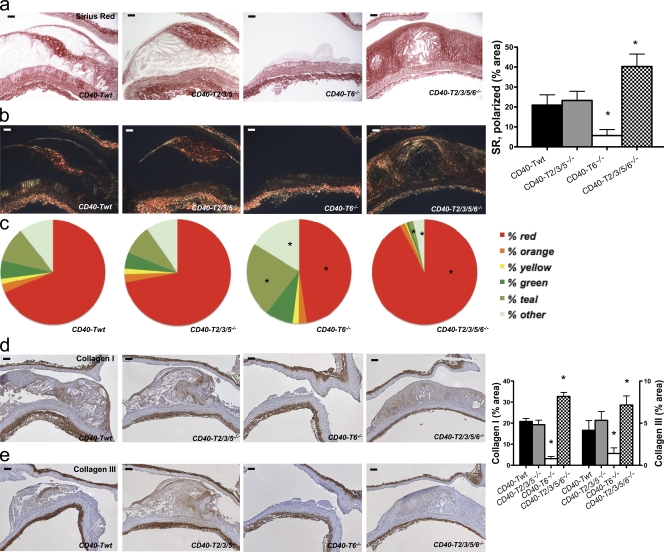
Besides the decrease in inflammatory cell content, plaques of CD40−/−Apoe−/− mice had a more fibrotic appearance. The content of α–smooth muscle actin (SMA; α-SMA+ cells; Fig. 2 a) and collagen (Fig. 2 b) was significantly increased. Bright-field polarization microscopy revealed that in the absence of CD40, the color distribution of the collagen deviated toward the red spectrum, indicating larger collagen fibrils (MacKenna and Omens, 1996). Concordant with these findings, we found an increase in the amount of collagen I and III in the plaque (Fig. 2, d and e).
Figure 2.
Deficiency of CD40 induces plaque fibrosis. Sections of the aortic arch and its main branch points (brachiocephalic artery, right and left subclavian artery, and right and left carotid artery) of Apoe−/− mice (n = 19) and CD40−/−Apoe−/− (n = 16) mice fed a normal chow diet for 26 wk were stained for α-SMA to detect the content of SMCs (a, representative sections on the left; bars, 50 µm), which was quantified as the percentage of α-SMA–positive plaque area (a, right). (b and c) Consecutive sections were stained with Sirius red to assess the content of collagen in the plaque by light (b) and polarized light (c) microscopy (representative sections on the left; bars, 50 µm). In addition, the content of collagen was expressed as the percentage of α-SMA–positive plaque area (b, right) and the color spectrum obtained using polarization microscopy was analyzed (c, right). (d and e) The content of collagen I (d) and collagen III (e) was assessed by immunohistochemistry (representative sections on the left; bars, 100 µm) and expressed as the percentage of positively stained plaque area (right). Error bars represent mean ± SEM. *, P < 0.05.
Recent studies have identified the involvement of various matrix metalloproteinases in atherosclerotic lesion formation and their contribution to fibrous cap thinning by degrading extracellular matrix (Hansson and Libby, 2006). Interestingly, MMP-2 and MMP-9 expression was decreased in the plaques of CD40−/−Apoe−/− mice (Fig. S2, a and b). To confirm an impairment of proteolytic activity and effects of tissue inhibitor of metalloproteinase (TIMP) 1, we measured gelatinase activity and TIMP-1 messenger RNA (mRNA) levels in macrophages stimulated with the CD40-clustering antibody FGK45. We found that gelatinase activity was decreased (Fig. S2 c), whereas TIMP-1 levels were increased in CD40-deficient macrophages (Fig. S2 d).
Leukocyte CD40 deficiency reduces atherosclerosis and impairs macrophage migration
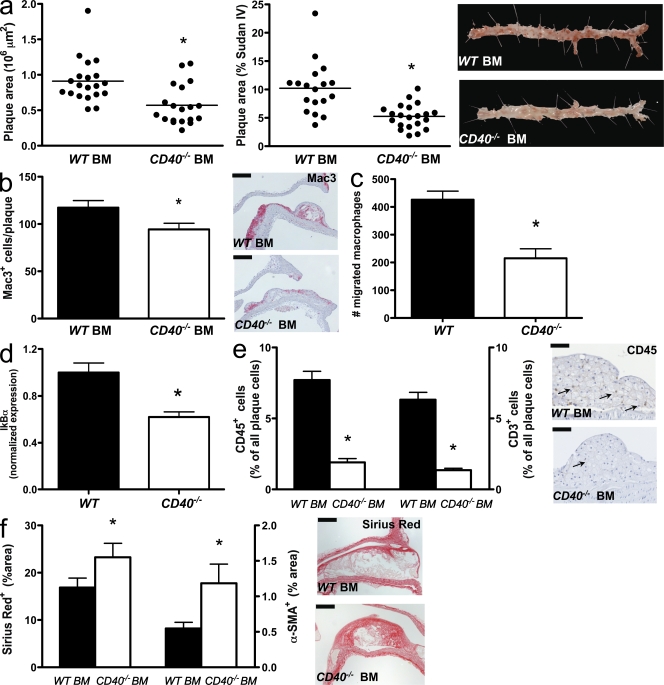
To unequivocally establish the involvement of leukocyte versus nonleukocyte CD40 in atherosclerosis, we reconstituted lethally irradiated Ldlr−/− mice with CD40−/− or WT BM and fed the mice with a high-fat diet for 24 wk. Body weights and plasma cholesterol levels did not differ between the groups (body weight, 20.1 vs. 19.7 g in Ldlr−/−; P > 0.05; cholesterol, 368 vs. 357 mg/dL Ldlr−/−). Chimeric Ldlr−/− mice with CD40−/− BM exhibited markedly reduced atherosclerosis in the aortic arch including its branch points and in the thoraco-abdominal aorta (Fig. 3 a). Similar to CD40−/−Apoe−/− mice, atherosclerotic plaques of chimeric CD40−/−Ldlr−/− mice contained less Mac3+ macrophages (Fig. 3 b). In culture, CD40−/− macrophages showed an impaired migration toward CCL2 (Fig. 3 c). Moreover, the percentage of CD45+ cells and CD3+ T lymphocytes in the plaque was decreased (Fig. 3 e). In accordance with this antiinflammatory profile in the plaque, absence of CD40 strongly decreased the expression of IκBα in FGK45-stimulated macrophages (Fig. 3 d), suggesting that the phenotypic effects are mediated by NF-κB signaling. Total collagen content (Fig. 3 f) and smooth muscle cell (SMC) content (Fig. 3 f) were increased in the plaque in absence of CD40. These data reveal a crucial role for leukocyte-dependent CD40 signaling in atherosclerosis.
Figure 3.
Role of hematopoietic cell–derived CD40 in diet-induced atherosclerosis. (a–f) WT→Ldlr−/− chimeras (n = 19) were fed a normal chow diet for 26 wk and were compared with CD40−/−→Ldlr−/− BM chimeras (n = 19). Sections of the aortic arch and its main branch points (brachiocephalic artery, right and left subclavian artery, and right and left carotid artery) were stained with hematoxylin and eosin and the thoraco-abdominal aorta was stained with Sudan IV (representative examples in a, right) to analyze the extent of atherosclerosis (a, left: plaque area in the arch; a, middle: plaque area in the thoraco-abdominal aorta; horizontal bars represent mean) and phenotype (b–f). (b) Macrophage infiltration was expressed as the absolute number of Mac3+ cells per plaque (bars, 100 µm). (c) Migration of BM-derived macrophages toward CCL2 for 4 h was analyzed in a Transwell migration assay (n = 6 per group, three independent experiments). (d) Levels of IκBα in FGK45-stimulated macrophages were determined by real-time PCR (n = 6). (e) CD45 and CD3 content were expressed as the percentage of CD3+ or CD45+ cells of all plaque cells (arrows indicate CD45+ cells; bars, 50 µm). (f) The content of collagen (representative sections on the right; bars, 100 µm) and α-SMA was expressed as the percentage of positively stained plaque area. Error bars represent mean ± SEM. *, P < 0.05.
Deficiency of CD40 polarizes macrophages toward an M2 phenotype
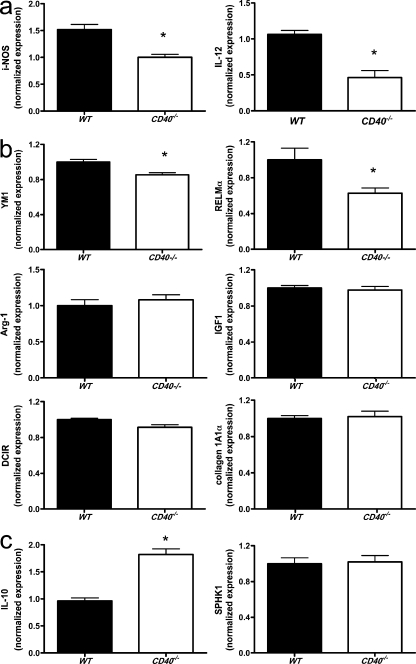
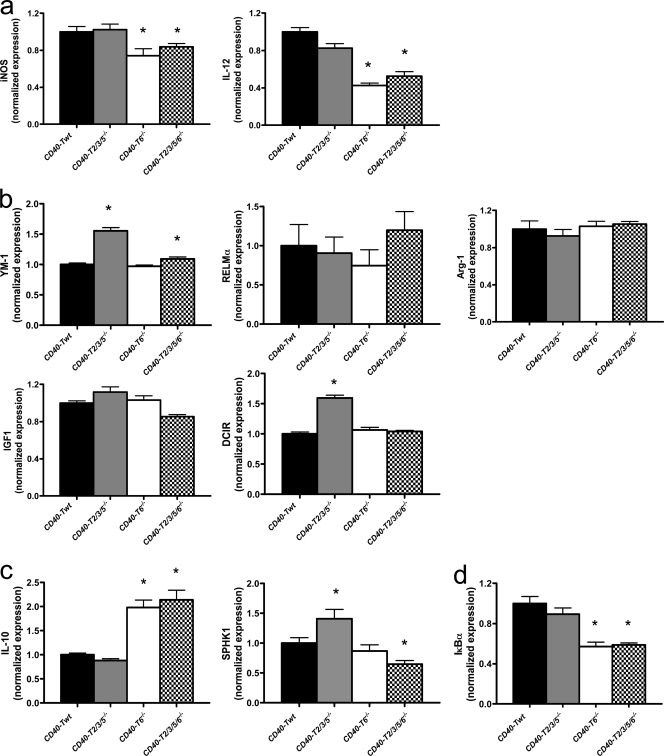
Stimulation of CD40 with the clustering antibody FGK45 in BM-derived macrophages resulted in a significant induction of the proinflammatory cytokines IL-12, iNOS (Fig. 4 a), and CCL-2 (not depicted) and a significant reduction of IL-10 (Fig. 4 c) in WT but not in CD40−/− macrophages, indicating that absence of CD40 polarizes macrophages toward an M2 phenotype (Martinez et al., 2009). To further differentiate the M2 subset into wound-healing and regulatory macrophages (Mosser and Edwards, 2008), real-time PCR analysis for respective markers was performed in FGK45-stimulated BM-derived macrophages. We observed that CD40 deficiency significantly reduced the inflammatory M1 markers iNOS and IL-12 (Fig. 4 a), only slightly reduced the expression of the wound-healing macrophage markers YM-1 and RELM-α, and did not affect the expression of arginase-I, IGF-1, or DCIR (Fig. 4 b), suggesting that CD40 signaling is essential for polarization of macrophages toward the M1 subtype but not toward wound-healing macrophages. Moreover, absence of CD40 did not result in an increase in collagen 1A1α production in these macrophages (Fig. 4 b). In contrast, deficiency of CD40 strongly induced the regulatory macrophage marker IL-10, whereas SPKH1 expression was not affected (Fig. 4 c), indicating that CD40 deletion shifts the macrophage phenotype to an antiinflammatory regulatory signature.
Figure 4.
Deficiency in CD40 induces polarization of macrophages toward a regulatory M2 macrophage phenotype. BM-derived macrophages of WT and CD40−/− mice were cultured, stimulated with 1 µg/ml of the CD40-clustering antibody FGK45, and analyzed for expression of different M1 (a) and M2 (b, wound-healing macrophages; c, regulatory macrophages) subset markers by real-time PCR. n = 6 per group of two independent experiments. Error bars represent mean ± SEM. *, P < 0.05.
CD40 deficiency affects T cell and DC phenotype
To elaborate on the immune phenotyping, we performed flow cytometry analysis of blood, spleen, and lymph node cells (Fig. S3). Consistent with the atherosclerotic phenotype, analysis of the different T lymphocyte and DC subsets revealed an antiinflammatory profile in CD40−/−Apoe−/− versus Apoe−/− mice. Whereas effector memory (CD44highCD62Llow) CD4+ and CD8+ T lymphocytes were decreased, naive (CD44lowCD62Lhigh) CD4+ and CD8+ T lymphocytes were increased (Fig. S3 a). Moreover, CD11c+CD8−CD4+ DCs, a population associated with antiinflammatory responses, was more prominent, whereas CD11c+CD4−CD8− and plasmacytoid DCs, populations associated with proinflammatory responses, were reduced (Fig. S3 b) (Shortman and Liu, 2002).
CD40-TRAF signaling in atherosclerosis
We next examined which of the CD40-associated signal transduction pathways was responsible for the phenotype observed in CD40−/−Apoe−/− mice. CD40 has no intrinsic signaling ability but requires adaptor molecules, TRAFs, to confer signaling. The cytoplasmic tail of CD40 contains three binding domains: a proximal domain that binds TRAF6 and two distal domains that bind TRAF1, TRAF2, TRAF3, and, indirectly, TRAF5 (Zapata, 2003; Lu et al., 2007; Lutgens et al., 2007). The separate TRAF binding sites are coupled to divergent signaling pathways involving different CD40 downstream modulators and effectors (Lutgens et al., 2007; Engel et al., 2009; Lievens et al., 2009), depending on the target cell types involved. To unravel the contribution of CD40-TRAF2/3/5 versus CD40-TRAF6 signaling in MHCII+ cells to atherosclerosis, we used CD40−/− mice expressing a chimeric CD40 transgene with mutations at the TRAF6 and/or TRAF2/3/5 binding site under control of the MHCII promotor (CD40-T2/3/5−/−, CD40-T6−/−, and CD40-T2/3/5/6−/− mice, respectively) or CD40−/− mice carrying the transgene without mutations (CD40-Twt mice; Ahonen et al., 2002; Donners et al., 2008) and backcrossed them with Apoe−/− mice. At 26 wk of age on a normal chow, atherosclerosis was analyzed in the aortic arch, including its main branch points, and the thoraco-abdominal aorta, including the iliac artery bifurcation (unpublished data). Body weight, plasma cholesterol level, and serum sCD40L level did not differ between the genotypes (Table I).
CD40-TRAF2/3/5 signaling does not affect atherosclerosis
The atherosclerotic plaque area, the plaque phenotype (Virmani et al., 2000), the plaque quality, as evident by the content of the lipid core, Mac-3+ cells, CD45+ cells, CD3+ T cells, collagen (I and III), SMCs, cleaved caspase 3, and macrophage polarization did not differ between CD40-T2/3/5−/− and CD40-Twt mice, indicating that deficiency in CD40-TRAF2/3/5 signaling does not affect atherosclerosis (Figs. 5 and 6). In fact, CD40-T2/3/5−/− mice displayed even higher numbers of CD4+ cells (Fig. S4 a) and CD8+CD44highCD62Llow effector memory T lymphocytes in blood, spleen, and/or lymph nodes than CD40-Twt mice and elevated CD8+ resident DCs (Fig. S3, c and d), a subtype which is characterized by increased IL-12 production (Shortman and Liu, 2002). This apparently proinflammatory profile was counterbalanced by elevated numbers of CD4+CD25+FoxP3+ regulatory T lymphocytes in blood, spleen, and lymph nodes of CD40-T2/3/5−/− mice (Fig. S4 b). Regulatory T cell function had not changed (Fig. S4 c), and because of the balanced effector T cell and regulatory T cell ratio systemic levels of IFN-ɣ, IL-6, TNF-α, IL-12, or IL-10 were unaltered (not depicted). Notably, in CD40-T2/3/5−/− mice the content of CD4+CD25+FoxP3+ regulatory T lymphocytes was also increased in the adventitia underlying the plaques but not in the plaque itself (Fig. S4 d).
Figure 5.
Role of CD40-TRAF signaling in atherosclerosis. CD40-Twt (n = 12), CD40-T2/3/5−/− (n = 16), CD40-T6−/− (n = 19), and CD40-T2/3/5/6−/− (n = 14) were backcrossed to Apoe−/− mice and fed a normal chow diet for 26 wk. (a–d) Sections of the aortic arch and its main branch points (a; brachiocephalic artery, right subclavian artery, right carotid artery, left carotid artery, and left subclavian artery) were stained with hematoxylin and eosin (HE) to analyze the extent of atherosclerosis (plaque area) and plaque phenotype. (a) Plaque area and distribution of morphological plaque phenotypes according to the classification by Virmani et al. (2000; top; horizontal bars represent mean). Representative hematoxylin and eosin–stained sections in the middle and on the bottom reveal only limited atherosclerosis in absence of CD40-T6 signaling (bars: [middle] 2 mm; [bottom] 100 µm). (b–d) CD40-T6−/− mice have small atherosclerotic lesions that contain few macrophages (b), CD45+ cells, and CD3+ T cells (c). The level of cleaved caspase 3 was determined by grading plaque from 0 (no expression) to 3 (high expression; d). Error bars represent mean ± SEM. *, P < 0.05.
Figure 6.
Role of CD40-TRAF signaling in plaque fibrosis. (a–c) Sirius red–stained sections showed low levels of collagen in the initial plaques of CD40-T6−/− mice and increased levels in plaques of CD40-T2/3/5/6−/− mice (bars, 50 µm). Collagen content was assessed by light (a) and polarized light (b) microscopy and expressed as the percentage of Sirius red–stained plaque area (right). In addition, the color spectrum obtained under polarization microscopy was analyzed (c). (d and e) The content of collagen I (d) and collagen III (e) was assessed by immunohistochemistry (representative sections on the left; bars, 100 µm) and expressed as the percentage of positively stained plaque area (right). Error bars represent mean ± SEM. *, P < 0.05.
Inhibition of CD40-TRAF6 signaling abolishes atherosclerosis
In a remarkable contrast, deficiency of CD40-TRAF6 signaling almost completely abolished the development of atherosclerosis, as only few intimal xanthomas or fatty-streak lesions (classified according to Virmani et al. [2000]) were detectable in the aortic arch and its branch points (Fig. 5 a), and no plaques were present in the thoraco-abdominal aorta of CD40-T6−/− mice (Fig. 5 a). This reduction of atherosclerosis was even more pronounced than in CD40−/−Apoe−/− mice, likely because of the effects of residual CD40-TRAF2/3/5 signaling on regulatory T lymphocytes. Concomitantly, macrophage, CD45+ cell, and CD3+ T lymphocyte infiltration, but also collagen (I and III), α-SMA, and MMP-2 and MMP-9 content, were substantially reduced in the hardly evolved lesions of CD40-T6−/− mice (Fig. 5, b and c; Fig. 6; and Fig. S5).
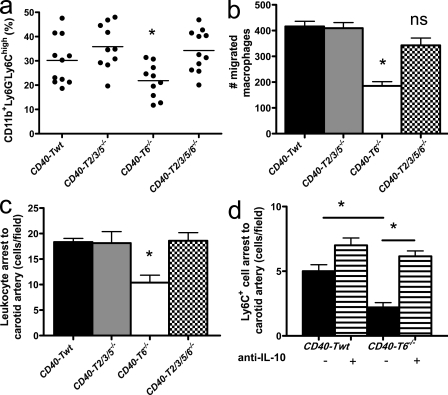
The population of peripheral blood Ly6Chigh monocytes was significantly declined (Fig. 7 a). Moreover, CD40-T6−/− macrophages had an impaired capacity to migrate toward CCL2 (Fig. 7 b). Intravital microscopy showed that the adhesion of circulating leukocytes and, in particular, that of Ly6C+ monocytes to carotid arteries was significantly impaired in CD40-T6−/− mice (Fig. 7, c and d).
Figure 7.
CD40-TRAF signaling affects monocyte homeostasis and recruitment. (a) The percentage of Ly6Chigh monocytes is reduced in the peripheral blood of CD40-T6−/− mice. Horizontal bars represent the mean. (b) Macrophages deficient in CD40-TRAF6 signaling have an impaired capacity to migrate toward CCL2 in a Transwell migration assay. (c and d) Accordingly, rhodamine-labeled leukocytes (c) and Ly6C+ monocytes (d) display an impaired luminal adhesion to the wall of carotid arteries in absence of CD40–T6 interactions in vivo, which is reversed by pretreatment with a blocking antibody to IL-10 (d). Error bars represent mean ± SEM. *, P < 0.05.
CD40-TRAF6 deficiency polarizes macrophages toward a regulatory M2 phenotype
BM-derived macrophages of CD40-T6−/− mice stimulated with FGK45 produced less IL-12 and iNOS but increased amounts of IL-10 (Fig. 8), indicating that CD40-TRAF6 deficiency skews macrophage polarization toward an antiinflammatory M2 phenotype. Further subtyping of the M2 macrophages into wound-healing and regulatory macrophages (Mosser and Edwards, 2008) showed that CD40–TRAF6 interactions did not affect the wound-healing macrophage markers YM-1, RELM-α, arginase I, IGF1, or DCIR but markedly dampened the M1 polarization response and, rather, induced a regulatory macrophage subtype that produces high amounts of IL-10. Importantly, the process of monocyte recruitment driven by CD40-TRAF6 was mediated by IL-10, as treatment of CD40-T6−/− mice with a blocking antibody to IL-10 reversed the protective effect on Ly6C+ cell numbers and adhesion (Fig. 7 d). The phenotype that occurs in CD40-T6−/− mice is likely associated with an impaired NF-κB signaling because IκBα levels in macrophages were markedly decreased (Fig. 8 d). Consistent with findings in CD40-deficient macrophages, CD40-T6−/− macrophages displayed a reduced gelatinolytic activity and increased expression of TIMP-1 (Fig. S5, c and d). Flow cytometry analysis further revealed reduced numbers of CD4+CD44highCD62Llow promigratory effector memory T lymphocytes and plasmacytoid DCs (Figs. S3, c and d).
Figure 8.
Deficiency in CD40-TRAF6 interactions induces polarization of macrophages toward a regulatory M2 macrophage phenotype. (a–c) BM-derived macrophages of CD40-Twt, CD40-T2/3/5−/−, CD40-T6−/−, and CD40-T2/3/5/6−/− mice were cultured, stimulated with 1 µg/ml of the CD40-clustering antibody FGK45, and analyzed for expression of different M1 (a) and M2 (b, wound-healing macrophages; c, regulatory macrophages) subset markers by real-time PCR. n = 6 per group of two independent experiments. (d) The levels of IκBα in FGK45-stimulated macrophages were decreased in BM-derived macrophages of CD40-T6−/− and CD40-T2/3/5/6−/− mice (n = 6). Error bars represent mean ± SEM. *, P < 0.05.
Deficiency of CD40–TRAF2/3/5/6 interactions induces plaque fibrosis
It is of note that mice with a combined deficiency in CD40-TRAF2/3/5/6 binding were characterized by an intermediate phenotype, further supporting counter-curing effects of CD40-TRAF2/3/5 and CD40-TRAF6 signaling in atherosclerosis. CD40-T2/3/5/6−/− mice showed only a slight reduction in atherosclerosis (Fig. 5 a) but displayed features of atherosclerotic plaque stability (Fig. 5 a), with an increased number of fibrocalcific plaques containing smaller lipid cores and reduced CD45+ cell and CD3+ T lymphocyte infiltrations.
Plaques of CD40-T2/3/5/6−/− mice exhibited high levels of collagen (Fig. 6, a–c) and α-SMA–positive SMCs (3.1 ± 0.7 vs. 5.1 ± 0.9; P < 0.05). Polarized light microscopy on Sirius red–stained sections further showed that the majority of this collagen consisted of large fibrils, as evident by the high percentage of the red fraction (Fig. 8, c and d). In concordance with this finding, plaque levels of collagen I were significantly increased, whereas collagen III levels were unaffected (Fig. 6, e and f). Moreover, plaque MMP-2 and MMP-9 levels were significantly reduced in CD40-T2/3/5/6−/− mice (Fig. S5, a and b), indicating a decreased proteolytic activity in these plaques. Accordingly, BM-derived macrophages of CD40-T2/3/5/6−/− mice treated with CD40-clustering antibody FGK45 displayed reduced gelatinolytic activity and elevated levels of TIMP-1 mRNA (Fig. S5, c–e).
As in CD40-T6−/− mice, macrophages of CD40-T2/3/5/6−/− mice stimulated with FGK45 exhibited an M2 macrophage profile with decreased IL-12 production and increased production of IL-10, whereas ly6Chigh monocyte numbers and adhesive function were unaffected (Figs. 7 and 8). CD40-T2/3/5/6−/− mice shared increased CD8+ effector memory T lymphocytes with CD40-T2/3/5−/− mice and decreased plasmacytoid DC numbers with CD40-T6−/− mice (Fig. S3, c and d). This indicates that some features, namely reduced inflammatory monocyte arrest and macrophage polarization, are exclusive to CD40-T6−/− mice and are not compensated for by deficiency of both TRAF binding sites.
DISCUSSION
In this study, we found a clearly divergent action of the two TRAF binding sites of CD40 in atherosclerosis. Deficiency of CD40–TRAF6 interactions in MHCII+ cells nearly abrogated atherosclerosis, an effect more marked than CD40 deletion itself, whereas deficiency of CD40–TRAF2/3/5 interactions did not affect atherosclerosis but, rather, showed a tendency to aggravate atherosclerosis. The CD40–TRAF6 interactions appeared to be specifically required for the proatherogenic activity exerted by cells of the myeloid lineage. Deficiency of CD40–TRAF6 interactions limits the subset of Ly6Chigh inflammatory monocytes and attenuates their adhesion to and infiltration into the arterial wall, thereby abolishing atherosclerotic plaque formation. The few monocytes that succeed in infiltrating the arterial wall, rather, differentiate into macrophages with an antiinflammatory regulatory M2 signature, thereby preventing plaque inflammation and progression.
In this paper, we provide conclusive evidence for an important role of CD40–TRAF6 interactions in the recruitment and fate of monocytes/macrophages. Diversity and plasticity are a hallmark of the monocyte/macrophage lineage, and their phenotype strongly depends on their microenvironment and the inflammatory signals they perceive (Gordon, 2003; Mosser and Edwards, 2008; Auffray et al., 2009). We found that CD40-TRAF6 signaling affects the homeostasis of both monocyte and macrophage polarization. CD40-T6−/− mice showed reduced numbers and reduced arterial recruitment of Ly6Chigh monocytes, the inflammatory monocyte subset which dominates in hyperlipidemia (Swirski et al., 2007; Tacke et al., 2007), can easily enter the arterial wall, and can differentiate into intimal macrophages. In parallel, CD40-T6−/− mice showed a reduction in the classically activated M1 macrophage subset producing iNOS and IL-12, which is involved in clearing intracellular parasites or tumors and eliciting tissue disruption (Gordon, 2003; Mosser and Edwards, 2008). Macrophages of CD40-T6−/− mice were skewed toward the M2 phenotype and particularly toward the IL-10–producing regulatory subtype (Mosser and Edwards, 2008). Interestingly, the reduced recruitment of Ly6Chigh monocytes in the carotid artery of CD40-T6−/− mice could be reversed by blocking IL-10, indicating that there could be a link between the IL-10–producing macrophage phenotype and effects on subsequent monocyte recruitment. Surprisingly, markers of the eponymously termed wound-healing macrophages, which contribute to the healing response after tissue destruction, were unaltered in the absence of CD40–TRAF6 interactions, although high levels of plaque fibrosis were observed in CD40-T2/3/5/6−/− mice. However, the relevance of these data has to be further substantiated and validated because the functional role of different monocyte and macrophage subsets in atherosclerosis remains to be fully elucidated (Mantovani et al., 2009; Swirski et al., 2009). For now, our data clearly reveal that a deficiency of CD40–TRAF6 interactions confers an immune-regulatory phenotype in the monocyte/macrophage lineage, which reduces atherosclerosis and is capable of inducing plaque stability.
In vascular pathobiology, only few data are available on the role CD40–TRAF6 interactions. Recently, CD40–TRAF6 interactions in MHCII+ cells have also been implicated in neointima formation in a mouse ligation model (Donners et al., 2008). Moreover, it has been observed that stimulation with CD40L induces CD40–TRAF6 interactions in endothelial cells but that their disruption surprisingly results in increased expression of CCL2 (Zirlik et al., 2007a). More evidence has been gathered regarding the role of CD40–TRAF6 interactions in hematopoietic cell types including cells of the myeloid lineage. In B cells, effects of CD40-TRAF6 signaling comprise germinal center formation, NF-κB activation, IL-6 production, and up-regulation of costimulatory molecules (Tsukamoto et al., 1999; Ahonen et al., 2002; Hostager, 2007). In monocytes/macrophages, CD40–TRAF6 interactions induce NF-κB and ERK activation, as well as the production of TNF-α and IL-6 (Mukundan et al., 2005). In DCs, CD40-TRAF6 signaling is required for MHCII surface expression and IL-6 and IL-12 production (Kobayashi et al., 2003). Differences in the effector functions of CD40-TRAF6 signaling between the various cell types may be a result of context-specific embedding of downstream pathways.
Our data further show that CD40–TRAF2/3/5 interactions on MHCII+ cells appear to be required for maintaining T lymphocyte homeostasis in atherosclerosis. Absence of CD40–TRAF2/3/5 interactions increased the numbers of CD4+ T cells and promigratory CD8+CD44highCD62Llow effector memory T lymphocytes, which by itself would likely be sufficient to aggravate atherosclerosis. However, the increase in T cells was compensated by an increase in CD4+CD25+FoxP3+ regulatory T lymphocytes, both systemically and in the vasculature. This expansion of regulatory T cells as an atheroprotective T cell subtype (Ait-Oufella et al., 2006) may protect CD40-T2/3/5−/− mice against the T cell–induced exacerbation of atherosclerosis.
Data on the role of CD40-T2/3/5 signaling in vascular biology are scarce and not uniform, as the induction of CD40-TRAF2/3/5 signaling yields different outcomes depending on the cell types and pathologies involved. Consistent with the present study, we did not find an effect on neointima formation after carotid artery ligation in CD40-T2/3/5−/− mice (Donners et al., 2008). However, TRAF2−/− and TRAF5−/− endothelial cells failed to produce IL-6 upon stimulation with CD40L, whereas CCL2 induction was unaffected (Zirlik et al., 2007a). In areas with high shear stress, TRAF3 expression in endothelial cells covering human atherosclerotic plaques is up-regulated and may act as a feedback mechanism limiting endothelial activation, as its overexpression prevented CD40-induced proinflammatory cytokine expression (Urbich et al., 2001).
A clearer picture of the function of CD40–TRAF2/3/5 interactions has emerged in B cell biology. CD40-TRAF2 signaling is crucial for germinal center formation, induces JNK activation, and, in particular, NF-κB2 activation (Tsukamoto et al., 1999; Au and Yeh, 2007; Vallabhapurapu et al., 2008). This is in accordance with our findings that IκBα mRNA expression was not significantly decreased in CD40-T2/3/5−/− macrophages. Together with CD40–TRAF5 interactions, which are also required for Ig production, CD40-TRAF2 signaling up-regulates costimulatory molecules (Au and Yeh, 2007). In contrast, TRAF3 acts as an inhibitor reducing costimulatory molecule expression and IgM production (Bishop and Xie, 2007). In cell types and organs other than B cells or lymph nodes, however, most of these functions are irrelevant. Consistent with our data, the overall effect of CD40-TRAF2/3/5 signaling with regards to atherogenesis appears to be balanced.
Our findings may harbor new and interesting therapeutic possibilities. The complete inhibition of CD40–CD40L signaling in atherosclerosis is not therapeutically feasible because long-term treatment will compromise systemic immune responses and also entails thromboembolic complications. Therefore, inhibition of the TRAF6 binding site on CD40, using small molecules or an antagonizing CD40 antibody that changes the conformation of the CD40-TRAF binding sites, may be a suitable alternative. It is conceivable that the protection by CD40-TRAF6 blockade is conferred by skewing the immune response to a more antiinflammatory profile, namely through increased production of IL-10 and its effects on mitigating the relative propensity, recruitment, and differentiation of proinflammatory monocytes. Not only could inhibition of the CD40-TRAF6 binding site reduce atherosclerosis more effectively than a complete inhibition of CD40 or CD40L by avoiding an interference with potentially protective effects by remaining CD40-signaling pathways, it may also leave the normal functions of these pathways unaffected, and would therefore be expected to only cause limited side effects. However, although our results in the mouse model are promising, caution should be applied when extrapolating these experimental data to the human situation. The effects of such selective targeting strategies will have to be meticulously scrutinized before being translated into a clinical setting.
MATERIALS AND METHODS
Animals.
CD40−/−, CD40-Twt, CD40-T2/3/5−/−, CD40-T6−/−, and CD40-T2/3/5/6−/− mice (all on a C57Bl6 background; Ahonen et al., 2002) were backcrossed for seven generations to Apoe−/− mice. Ldlr−/− mice were purchased from The Jackson Laboratory. All study protocols involving animal experiments were approved by the Animal Care and Use committee of the University of Maastricht and were performed according to official rules formulated in the Dutch law on care and use of experimental animals, which are highly similar to those of the National Institutes of Health.
Primary atherosclerosis.
CD40−/−Apoe−/− (n = 16), CD40+/+Apoe−/− (n = 19), CD40-Twt (n = 14), CD40-T2/3/5−/− (n = 17), CD40-T6−/− (n = 26), and CD40-T2/3/5/6−/− (n = 14) mice were fed a normal chow diet throughout the experiment.
BM transplantation.
Ldlr−/− mice (n = 44) were maintained in filter-top cages and given water containing 60,000 U/liter polymyxin B sulfate (Invitrogen) and 100 mg/liter neomycin (Invitrogen) from 1 wk before BM transplantation until 4 wk thereafter. Mice were lethally irradiated (10 Gy, 0.5 Gy/min; MU 15F/225 kV; Philips) and i.v. injected with 107 BM cells from CD40−/− mice or CD40+/+ mice. 4 wk after transplantation, mice were fed a 1.25% cholesterol diet for 24 wk.
Atherosclerosis experiments.
Mice were sacrificed and the arterial tree was perfused. The aortic arch and its main branch points were excised, fixed overnight, and embedded in paraffin. The rest of the arterial tree was fixed, opened, pinned, and stained with Oil-red-O for en face analysis. Longitudinal sections of the aortic arch were analyzed for plaque extent and phenotype as previously described (Virmani et al., 2000; Lutgens et al., 2006). For phenotypic parameters, immunohistochemistry was performed for CD3 (Dako), CD45 (BD), mac-3 (BD), FVIII (Dako), α-SMA (Sigma-Aldrich), caspase 3 (Cell Signaling Technology), FoxP3 (eBioscience), collagen I (Abcam), collagen III (Abcam), MMP-2 (Cell Signaling Technology), MMP-9 (Santa Cruz Biotechnology, Inc.). In addition, Perl's iron staining and Sirius red staining were as previously described (Lutgens et al., 2006). Morphometric analyses were performed using a Quantimet (Leica) with Qwin3 software (Leica). Plasma cholesterol levels were measured enzymatically (Roche), organs were analyzed by hematoxylin and eosin staining, and no abnormalities were observed. Serum sCD40L levels were measured by ELISA (eBioscience).
In vitro macrophage culture.
BM cells were isolated from n = 6 mice/group and cultured in RPMI supplemented with L929 conditioned medium to generate BM-derived macrophages as previously described (Kanters et al., 2003). Cells were pretreated with 100 U IFN-ɣ for 24 h to induce MHCII expression and then treated for 3 h with 100 ng/ml FGK45, a CD40-stimulating antibody. Cytokine levels in the medium were measured by FACS using a cytometric bead assay (BD), and quantitative gene expression of cytokines, M1, and M2 macrophage markers were analyzed as previously described (Beckers et al., 2007).
Macrophage migration assay.
Macrophage migration was assessed using 24-well Transwell migration chambers (Costar; Corning) with a pore size of 6 µm. BM-derived macrophages (0.5 × 106) of the respective genotypes, suspended in serum-free medium, were added to each chamber. Complete medium, including 100 ng/ml CCL2 (R&D Systems), was added to the lower chambers and migration was performed at 37°C for 4 h. Nonmigrated cells were removed from the membranes, and migrated cells within the membrane were fixed with methanol and stained with toluidine blue. Membranes were cut out of inserts and mounted onto slides in immersion oil. The number of migrated cells was counted on five randomly chosen fields of each membrane.
Gelatinase assay.
Gelatinase activity in the supernatant of FGK45-treated macrophages was determined using the EnzChek gelatinase/collagenase assay kit (Invitrogen). Substrate hydrolysis was analyzed after 24 h at room temperature and fluorescence was detected using a fluorescence microplate reader.
Intravital microscopy.
Leukocyte endothelial interactions were analyzed by intravital microscopy of the left carotid artery (n = 8 mice/group; Koenen et al., 2009). 6 h after i.p. injection of 1.0 µg of mouse TNF (PeproTech), mice were anaesthetized with ketamine/xylazine and the left carotid artery was exposed. Circulating leukocytes were labeled by i.v. injection of rhodamine 6G. Ly6C+ inflammatory monocytes were labeled with fluorescent latex beads as previously described (Soehnlein et al., 2008). The antibody to IL-10 (clone JES5.2A) was injected twice i.p. (500 µg/mouse), 24 and 2 h before intravital microscopy. Recordings were made using a microscope (BX51; Olympus) equipped with a saline-immersion 20× objective.
Statistics.
Data are presented as mean ± SEM. Data were analyzed by a nonparametric Mann-Whitney U test or Welch-corrected t test, as appropriate, using Prism 4 software (GraphPad Software, Inc.). P-values <0.05 were considered significant.
Online supplemental material.
Fig. S1 shows additional phenotypic features of the atherosclerotic plaques of CD40−/−Apoe−/− mice (classification of Virmani et al. [2000], Caspase 3 and FoxP3 levels). Fig. S2 shows MMP-2 and MMP-9 levels in plaques of CD40−/−Apoe−/− mice, as well as gelatinase activity and TIMP-1 levels in their BM-derived macrophages. Fig. S3 shows the extended immune phenotyping of splenocytes in all the experimental groups. Fig. S4 shows the balanced effector T cell phenotype in absence of CD40-TRAF2/3/5 interactions. Fig. S5 shows MMP-2 and MMP-9 levels in plaques of the CD40-TRAF mice, as well as gelatinase activity and TIMP-1 levels in their BM-derived macrophages. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091293/DC1.
Acknowledgments
This work was supported by the Humboldt Foundation (Sofja Kovalevskaja grant to E. Lutgens), the Netherlands Organization for Scientific Research (VIDI grant to E. Lutgens and M. P. de Winther), the Netherlands Heart Foundation (Dr. E. Dekker grant to E. Lutgens and D. Lievens, established investigator grant to E. Lutgens and M.P. de Winther), and the Deutsche Forschungsgemeinschaft (DFG FOR809, ZE827/1-1 to A. Zernecke and WE1913/11-1 to C. Weber).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- Apoe
- apolipoprotein E
- CD40L
- CD40 ligand
- Ldlr
- low-density lipoprotein receptor
- mRNA
- messenger RNA
- SMA
- smooth muscle actin
- SMC
- smooth muscle cell
- TIMP
- tissue inhibitor of metalloproteinase
- TRAF
- TNF receptor-associated factor
References
- Ahonen C., Manning E., Erickson L.D., O'Connor B., Lind E.F., Pullen S.S., Kehry M.R., Noelle R.J. 2002. The CD40-TRAF6 axis controls affinity maturation and the generation of long-lived plasma cells. Nat. Immunol. 3:451–456 10.1038/ni792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Oufella H., Salomon B.L., Potteaux S., Robertson A.K., Gourdy P., Zoll J., Merval R., Esposito B., Cohen J.L., Fisson S., et al. 2006. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12:178–180 10.1038/nm1343 [DOI] [PubMed] [Google Scholar]
- André P., Prasad K.S., Denis C.V., He M., Papalia J.M., Hynes R.O., Phillips D.R., Wagner D.D. 2002. CD40L stabilizes arterial thrombi by a beta3 integrin—dependent mechanism. Nat. Med. 8:247–252 10.1038/nm0302-247 [DOI] [PubMed] [Google Scholar]
- Au P.Y., Yeh W.C. 2007. Physiological roles and mechanisms of signaling by TRAF2 and TRAF5. Adv. Exp. Med. Biol. 597:32–47 10.1007/978-0-387-70630-6_3 [DOI] [PubMed] [Google Scholar]
- Auffray C., Sieweke M.H., Geissmann F. 2009. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27:669–692 10.1146/annurev.immunol.021908.132557 [DOI] [PubMed] [Google Scholar]
- Beckers L., Heeneman S., Wang L., Burkly L.C., Rousch M.M., Davidson N.O., Gijbels M.J., de Winther M.P., Daemen M.J., Lutgens E. 2007. Disruption of hedgehog signalling in ApoE−/− mice reduces plasma lipid levels, but increases atherosclerosis due to enhanced lipid uptake by macrophages. J. Pathol. 212:420–428 10.1002/path.2193 [DOI] [PubMed] [Google Scholar]
- Bishop G.A., Xie P. 2007. Multiple roles of TRAF3 signaling in lymphocyte function. Immunol. Res. 39:22–32 10.1007/s12026-007-0068-1 [DOI] [PubMed] [Google Scholar]
- Donners M.M., Beckers L., Lievens D., Munnix I., Heemskerk J., Janssen B.J., Wijnands E., Cleutjens J., Zernecke A., Weber C., et al. 2008. The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood. 111:4596–4604 10.1182/blood-2007-05-088906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie F.H., Fava R.A., Foy T.M., Aruffo A., Ledbetter J.A., Noelle R.J. 1993. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 261:1328–1330 10.1126/science.7689748 [DOI] [PubMed] [Google Scholar]
- Engel D., Seijkens T., Poggi M., Sanati M., Thevissen L., Beckers L., Wijnands E., Lievens D., Lutgens E. 2009. The immunobiology of CD154-CD40-TRAF interactions in atherosclerosis. Semin. Immunol. 21:308–312 10.1016/j.smim.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Gerritse K., Laman J.D., Noelle R.J., Aruffo A., Ledbetter J.A., Boersma W.J., Claassen E. 1996. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA. 93:2499–2504 10.1073/pnas.93.6.2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35 10.1038/nri978 [DOI] [PubMed] [Google Scholar]
- Hansson G.K. 2005. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352:1685–1695 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- Hansson G.K., Libby P. 2006. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 6:508–519 10.1038/nri1882 [DOI] [PubMed] [Google Scholar]
- Hostager B.S. 2007. Roles of TRAF6 in CD40 signaling. Immunol. Res. 39:105–114 10.1007/s12026-007-0082-3 [DOI] [PubMed] [Google Scholar]
- Kanters E., Pasparakis M., Gijbels M.J., Vergouwe M.N., Partouns-Hendriks I., Fijneman R.J., Clausen B.E., Förster I., Kockx M.M., Rajewsky K., et al. 2003. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 112:1176–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Andrews D., Colvin R.B., Sachs D.H., Cosimi A.B. 2000. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat. Med. 6:114 10.1038/72162 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Walsh P.T., Walsh M.C., Speirs K.M., Chiffoleau E., King C.G., Hancock W.W., Caamano J.H., Hunter C.A., Scott P., et al. 2003. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 19:353–363 10.1016/S1074-7613(03)00230-9 [DOI] [PubMed] [Google Scholar]
- Koenen R.R., von Hundelshausen P., Nesmelova I.V., Zernecke A., Liehn E.A., Sarabi A., Kramp B.K., Piccinini A.M., Paludan S.R., Kowalska M.A., et al. 2009. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat. Med. 15:97–103 10.1038/nm.1898 [DOI] [PubMed] [Google Scholar]
- Lievens D., Eijgelaar W.J., Biessen E.A., Daemen M.J., Lutgens E. 2009. The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thromb. Haemost. 102:206–214 [DOI] [PubMed] [Google Scholar]
- Lu L.F., Ahonen C.L., Lind E.F., Raman V.S., Cook W.J., Lin L.L., Noelle R.J. 2007. The in vivo function of a noncanonical TRAF2-binding domain in the C-terminus of CD40 in driving B-cell growth and differentiation. Blood. 110:193–200 10.1182/blood-2006-07-038414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens E., Gorelik L., Daemen M.J., de Muinck E.D., Grewal I.S., Koteliansky V.E., Flavell R.A. 1999. Requirement for CD154 in the progression of atherosclerosis. Nat. Med. 5:1313–1316 10.1038/15271 [DOI] [PubMed] [Google Scholar]
- Lutgens E., Cleutjens K.B., Heeneman S., Koteliansky V.E., Burkly L.C., Daemen M.J. 2000. Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype. Proc. Natl. Acad. Sci. USA. 97:7464–7469 10.1073/pnas.97.13.7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens E., Lutgens S.P., Faber B.C., Heeneman S., Gijbels M.M., de Winther M.P., Frederik P., van der Made I., Daugherty A., Sijbers A.M., et al. 2006. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 113:98–107 10.1161/CIRCULATIONAHA.105.561449 [DOI] [PubMed] [Google Scholar]
- Lutgens E., Lievens D., Beckers L., Donners M., Daemen M. 2007. CD40 and its ligand in atherosclerosis. Trends Cardiovasc. Med. 17:118–123 10.1016/j.tcm.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Mach F., Schönbeck U., Sukhova G.K., Atkinson E., Libby P. 1998. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 394:200–203 10.1038/28204 [DOI] [PubMed] [Google Scholar]
- MacKenna DA, Omens J.H. 1996. A semi-automated method for measuring collagen area fraction and size distribution using picrosirius red. Cardiovascular Pathobiology. 1:104–117 [Google Scholar]
- Mantovani A., Garlanda C., Locati M. 2009. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler. Thromb. Vasc. Biol. 29:1419–1423 10.1161/ATVBAHA.108.180497 [DOI] [PubMed] [Google Scholar]
- Martinez F.O., Helming L., Gordon S. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27:451–483 10.1146/annurev.immunol.021908.132532 [DOI] [PubMed] [Google Scholar]
- Mosser D.M., Edwards J.P. 2008. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8:958–969 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan L., Bishop G.A., Head K.Z., Zhang L., Wahl L.M., Suttles J. 2005. TNF receptor-associated factor 6 is an essential mediator of CD40-activated proinflammatory pathways in monocytes and macrophages. J. Immunol. 174:1081–1090 [DOI] [PubMed] [Google Scholar]
- Schönbeck U., Sukhova G.K., Shimizu K., Mach F., Libby P. 2000. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc. Natl. Acad. Sci. USA. 97:7458–7463 10.1073/ pnas.97.13.7458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Liu Y.J. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161 10.1038/nri746 [DOI] [PubMed] [Google Scholar]
- Soehnlein O., Zernecke A., Eriksson E.E., Rothfuchs A.G., Pham C.T., Herwald H., Bidzhekov K., Rottenberg M.E., Weber C., Lindbom L. 2008. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 112:1461–1471 10.1182/blood-2008-02-139634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F.K., Libby P., Aikawa E., Alcaide P., Luscinskas F.W., Weissleder R., Pittet M.J. 2007. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117:195–205 10.1172/JCI29950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F.K., Weissleder R., Pittet M.J. 2009. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29:1424–1432 10.1161/ATVBAHA.108.180521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F., Alvarez D., Kaplan T.J., Jakubzick C., Spanbroek R., Llodra J., Garin A., Liu J., Mack M., van Rooijen N., et al. 2007. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117:185–194 10.1172/JCI28549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto N., Kobayashi N., Azuma S., Yamamoto T., Inoue J. 1999. Two differently regulated nuclear factor kappaB activation pathways triggered by the cytoplasmic tail of CD40. Proc. Natl. Acad. Sci. USA. 96:1234–1239 10.1073/pnas.96.4.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C., Mallat Z., Tedgui A., Clauss M., Zeiher A.M., Dimmeler S. 2001. Upregulation of TRAF-3 by shear stress blocks CD40-mediated endothelial activation. J. Clin. Invest. 108:1451–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S., Matsuzawa A., Zhang W., Tseng P.H., Keats J.J., Wang H., Vignali D.A., Bergsagel P.L., Karin M. 2008. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat. Immunol. 9:1364–1370 10.1038/ni.1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani R., Kolodgie F.D., Burke A.P., Farb A., Schwartz S.M. 2000. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20:1262–1275 [DOI] [PubMed] [Google Scholar]
- Vowinkel T., Anthoni C., Wood K.C., Stokes K.Y., Russell J., Gray L., Bharwani S., Senninger N., Alexander J.S., Krieglstein C.F., et al. 2007. CD40-CD40 ligand mediates the recruitment of leukocytes and platelets in the inflamed murine colon. Gastroenterology. 132:955–965 10.1053/j.gastro.2006.12.027 [DOI] [PubMed] [Google Scholar]
- Weber C., Zernecke A., Libby P. 2008. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 8:802–815 10.1038/nri2415 [DOI] [PubMed] [Google Scholar]
- Zapata J.M. 2003. TNF-receptor-associated factors as targets for drug development. Expert Opin. Ther. Targets. 7:411–425 10.1517/14728222.7.3.411 [DOI] [PubMed] [Google Scholar]
- Zirlik A., Bavendiek U., Libby P., MacFarlane L., Gerdes N., Jagielska J., Ernst S., Aikawa M., Nakano H., Tsitsikov E., Schönbeck U. 2007a. TRAF-1, -2, -3, -5, and -6 are induced in atherosclerotic plaques and differentially mediate proinflammatory functions of CD40L in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 27:1101–1107 10.1161/ATVBAHA.107.140566 [DOI] [PubMed] [Google Scholar]
- Zirlik A., Maier C., Gerdes N., MacFarlane L., Soosairajah J., Bavendiek U., Ahrens I., Ernst S., Bassler N., Missiou A., et al. 2007b. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 115:1571–1580 10.1161/CIRCULATIONAHA.106.683201 [DOI] [PubMed] [Google Scholar]