Abstract
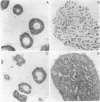
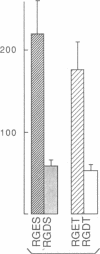
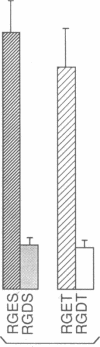
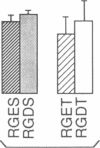
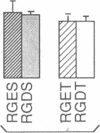
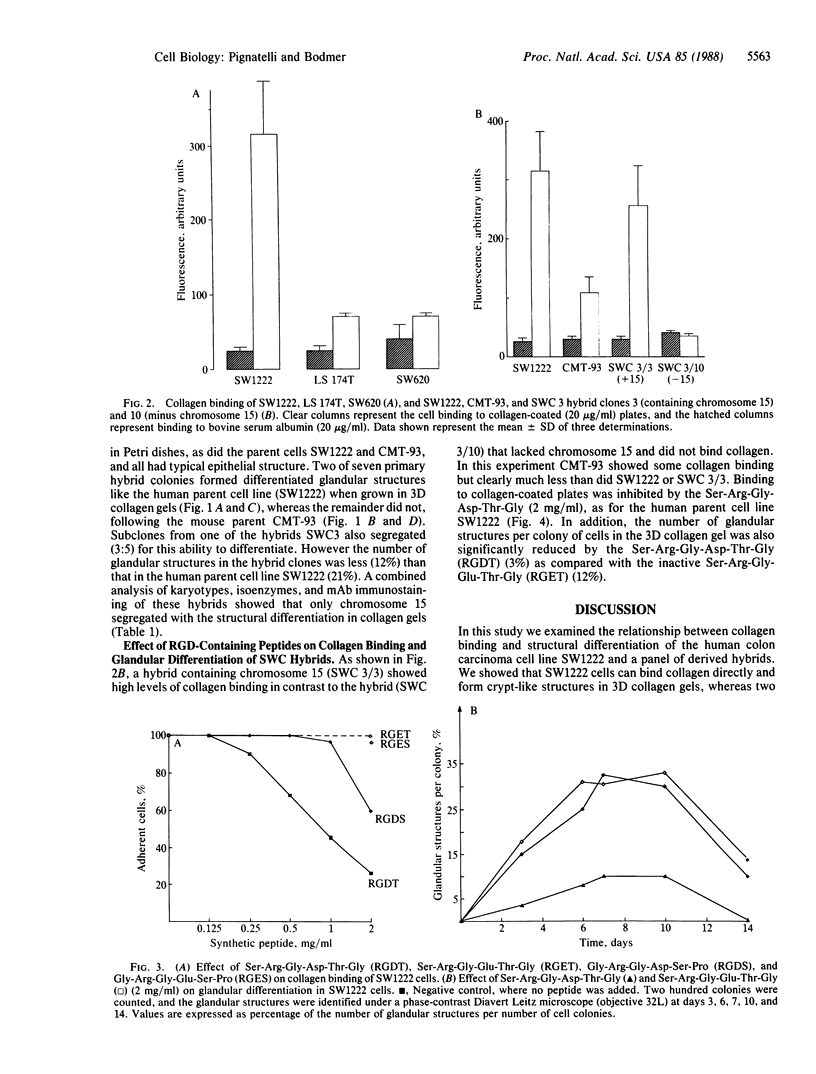
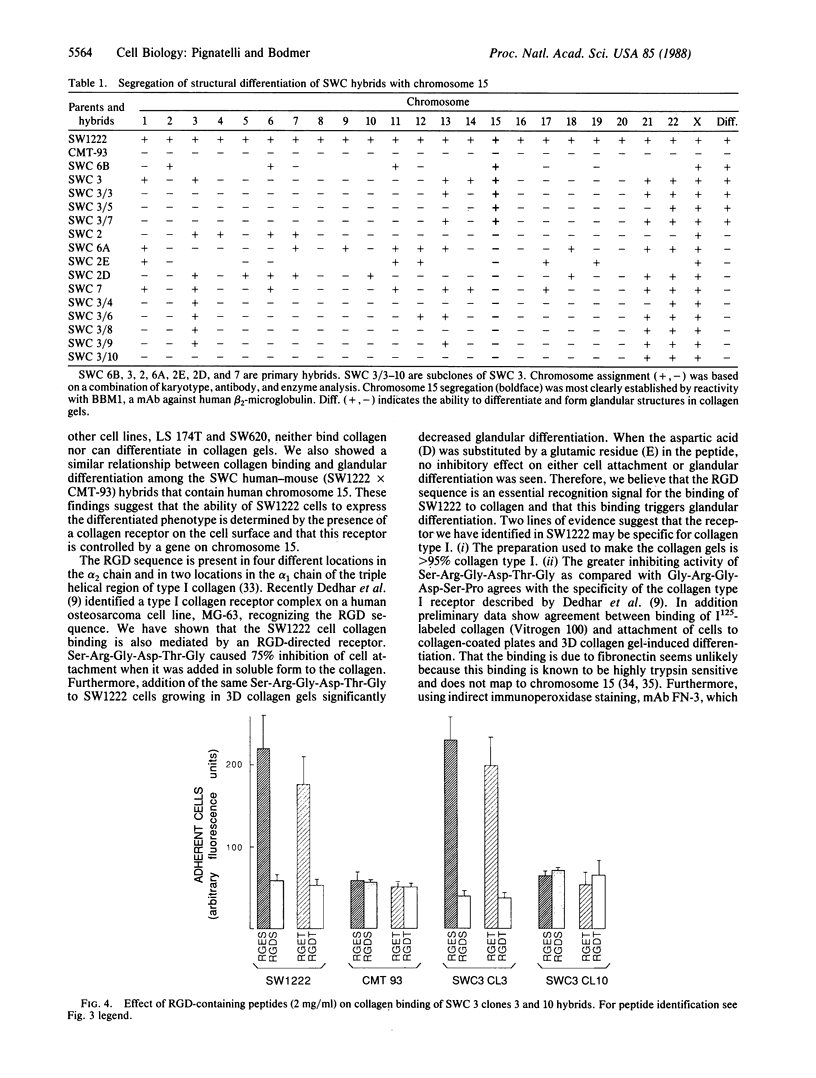
We have examined the interaction between collagen binding and epithelial differentiation by using a human colon carcinoma cell line (SW1222) that can differentiate structurally when grown in a three-dimensional collagen gel to form glandular structures. As much as 66% inhibition of glandular differentiation can be achieved by addition to the culture of a synthetic peptide (2 mg/ml) containing the Arg-Gly-Asp-Thr (RGDT) sequence, which is a cell recognition site found in collagen. Arg-Gly-Asp-Thr also inhibited the cell attachment to collagen-coated plates. A control peptide containing the Arg-Gly-Glu-Thr (RGET) sequence had no effect on cell adhesion or cell differentiation. Chromosome 15 was found in all human-mouse hybrid clones [from a cross between SW1222 and a mouse rectal carcinoma cell line (CMT-93)] that could differentiate in the collagen gel and bind collagen. Both binding to collagen and glandular differentiation of the hybrid cells were also inhibited by Arg-Gly-Asp-Thr as for the parent cell line SW1222. The ability of SW1222 cells to express the differentiated phenotype appears, therefore, to be determined by an Arg-Gly-Asp-directed collagen receptor on the cell surface that is controlled by a gene on chromosome 15.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Knowles B. B., Goodfellow P. N. A human cell-surface antigen defined by a monoclonal antibody and controlled by a gene on chromosome 12. Somatic Cell Genet. 1981 Jul;7(4):435–443. doi: 10.1007/BF01542988. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Knowles B. B., Parkar M., Pym B., Stanley K., Goodfellow P. N. A human cell-surface antigen defined by a monoclonal antibody and controlled by a gene on human chromosome 1. Ann Hum Genet. 1985 Jan;49(Pt 1):31–39. doi: 10.1111/j.1469-1809.1985.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Bai Y., Sheer D., Hiorns L., Knowles R. W., Tunnacliffe A. A monoclonal antibody recognizing a cell surface antigen coded for by a gene on human chromosome 17. Ann Hum Genet. 1982 Oct;46(Pt 4):337–347. doi: 10.1111/j.1469-1809.1982.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bobrow M., Cross J. Differential staining of human and mouse chromosomes in interspecific cell hybrids. Nature. 1974 Sep 6;251(5470):77–79. doi: 10.1038/251077a0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Lomakka G., Zech L. The 24 fluorescence patterns of the human metaphase chromosomes - distinguishing characters and variability. Hereditas. 1972;67(1):89–102. doi: 10.1111/j.1601-5223.1971.tb02363.x. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- DUKES C. E., BUSSEY H. J. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958 Sep;12(3):309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., Ephrussi B., Yamamoto K. Regulation of pigment synthesis in mammalian cells, as studied by somatic hybridization. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1437–1440. doi: 10.1073/pnas.56.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S., Ruoslahti E., Pierschbacher M. D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987 Mar;104(3):585–593. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRMANN R. L., GEY G. O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956 Jun;16(6):1375–1403. [PubMed] [Google Scholar]
- Franks L. M., Hemmings V. J. A cell line from an induced carcinoma of mouse rectum. J Pathol. 1978 Jan;124(1):35–38. doi: 10.1002/path.1711240108. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Sutherland R., Greaves M., Solomon E., Povey S. Expression of human transferrin receptor is controlled by a gene on chromosome 3: assignment using species specificity of a monoclonal antibody. Somatic Cell Genet. 1982 Mar;8(2):197–206. doi: 10.1007/BF01538677. [DOI] [PubMed] [Google Scholar]
- Goodfellow P., Banting G., Levy R., Povey S., McMichael A. A human X-linked antigen defined by a monoclonal antibody. Somatic Cell Genet. 1980 Nov;6(6):777–787. doi: 10.1007/BF01538976. [DOI] [PubMed] [Google Scholar]
- Hall H. G., Farson D. A., Bissell M. J. Lumen formation by epithelial cell lines in response to collagen overlay: a morphogenetic model in culture. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4672–4676. doi: 10.1073/pnas.79.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ruoslahti E. Detachment of cells from culture substrate by soluble fibronectin peptides. J Cell Biol. 1985 Jun;100(6):1948–1954. doi: 10.1083/jcb.100.6.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Fietzek P. P., Kühn K. Comparative analysis of the sequences of the three collagen chains alpha 1(I), alpha 2 and alpha 1(III) Functional and genetic aspects. J Mol Biol. 1980 Aug 15;141(3):293–314. doi: 10.1016/0022-2836(80)90182-5. [DOI] [PubMed] [Google Scholar]
- Keen J., Chang S. E., Taylor-Papadimitriou J. Monoclonal antibodies that distinguish between human cellular and plasma fibronectin. Mol Biol Med. 1984 Feb;2(1):15–27. [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics of human cancer. Annu Rev Genet. 1986;20:231–251. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- Koch G. A., Schoen R. C., Klebe R. J., Shows T. B. Assignment of a fibronection gene to human chromosome 2 using monoclonal antibodies. Exp Cell Res. 1982 Oct;141(2):293–302. doi: 10.1016/0014-4827(82)90217-8. [DOI] [PubMed] [Google Scholar]
- Leibovitz A., Stinson J. C., McCombs W. B., 3rd, McCoy C. E., Mazur K. C., Mabry N. D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976 Dec;36(12):4562–4569. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pontecorvo G. Production of mammalian somatic cell hybrids by means of polyethylene glycol treatment. Somatic Cell Genet. 1975 Oct;1(4):397–400. doi: 10.1007/BF01538671. [DOI] [PubMed] [Google Scholar]
- Richman P. I., Tilly R., Jass J. R., Bodmer W. F. Colonic pericrypt sheath cells: characterisation of cell type with new monoclonal antibody. J Clin Pathol. 1987 Jun;40(6):593–600. doi: 10.1136/jcp.40.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Court J. Different mechanisms in the attachment of cells to native and denatured collagen. J Cell Sci. 1979 Aug;38:267–281. doi: 10.1242/jcs.38.1.267. [DOI] [PubMed] [Google Scholar]
- Seabright M. The use of proteolytic enzymes for the mapping of structural rearrangements in the chromosomes of man. Chromosoma. 1972;36(2):204–210. doi: 10.1007/BF00285214. [DOI] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Spurr N. K., Durbin H., Sheer D., Parkar M., Bobrow L., Bodmer W. F. Characterization and chromosomal assignment of a human cell surface antigen defined by the monoclonal antibody AUAI. Int J Cancer. 1986 Nov 15;38(5):631–636. doi: 10.1002/ijc.2910380503. [DOI] [PubMed] [Google Scholar]
- Tom B. H., Rutzky L. P., Jakstys M. M., Oyasu R., Kaye C. I., Kahan B. D. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976 Mar;12(3):180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Mayes E. L., Stroobant P., Bennet P. L., Young S., Goodfellow P. N., Banting G. S., Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20(2):149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]
- Woodroofe M. N., Tunnacliffe A., Pym B., Goodfellow P. N., Walsh F. S. Human muscle cell surface antigen 16.3A5 is encoded by a gene on chromosome 11. Somat Cell Mol Genet. 1984 Sep;10(5):535–540. doi: 10.1007/BF01534858. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Akiyama S. K., Hasegawa T., Hasegawa E., Humphries M. J., Kennedy D. W., Nagata K., Urushihara H., Olden K., Chen W. T. Recent advances in research on fibronectin and other cell attachment proteins. J Cell Biochem. 1985;28(2):79–97. doi: 10.1002/jcb.240280202. [DOI] [PubMed] [Google Scholar]
- Yang J., Richards J., Bowman P., Guzman R., Enami J., McCormick K., Hamamoto S., Pitelka D., Nandi S. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3401–3405. doi: 10.1073/pnas.76.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]