Abstract
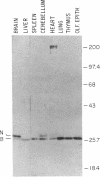
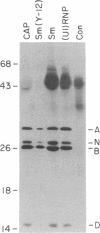
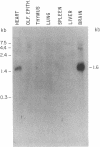
Sera from some patients with systemic lupus erythematosus and other autoimmune diseases have antibodies against nuclear antigens. An example is anti-Sm sera, which recognize proteins associated with small nuclear RNA molecules [small nuclear ribonucleoprotein (snRNP) particles]. In this paper anti-Sm sera were used to probe immunoblots of various rat tissues. A previously unidentified Mr 28,000 polypeptide was recognized by these anti-Sm sera. This polypeptide, referred to as "N," is expressed in a tissue-specific manner, being most abundant in rat brain, less so in heart, and undetectable in the other tissues examined. Immunoprecipitation experiments using antibodies directed against the cap structure of small nuclear RNAs have demonstrated that N is a snRNP-associated polypeptide. Anti-Sm serum was also used to isolate a partial cDNA clone (lambda rb91) from a rat brain phage lambda gt11 cDNA expression library. On RNA blots, the 450-base-pair cDNA insert of this clone hybridized to a 1600-nucleotide mRNA species with an identical tissue distribution to N, suggesting that lambda rb91 encodes at least part of N. A longer cDNA clone was obtained by rescreening the library with lambda rb91. In vitro transcription and subsequent translation of this subcloned, longer insert (pGMA2) resulted in a protein product with the same electrophoretic and immunological properties as N, confirming that pGMA2 encodes N. The tissue distribution of N and the involvement of snRNP particles in nuclear pre-mRNA processing may imply a role for N in tissue-specific pre-mRNA splicing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Billings P. B., Hoch S. O. Characterization of U small nuclear RNA-associated proteins. J Biol Chem. 1984 Oct 25;259(20):12850–12856. [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Bringmann P., Lührmann R. Purification of the individual snRNPs U1, U2, U5 and U4/U6 from HeLa cells and characterization of their protein constituents. EMBO J. 1986 Dec 20;5(13):3509–3516. doi: 10.1002/j.1460-2075.1986.tb04676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Chabot B., Black D. L., LeMaster D. M., Steitz J. A. The 3' splice site of pre-messenger RNA is recognized by a small nuclear ribonucleoprotein. Science. 1985 Dec 20;230(4732):1344–1349. doi: 10.1126/science.2933810. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crenshaw E. B., 3rd, Russo A. F., Swanson L. W., Rosenfeld M. G. Neuron-specific alternative RNA processing in transgenic mice expressing a metallothionein-calcitonin fusion gene. Cell. 1987 May 8;49(3):389–398. doi: 10.1016/0092-8674(87)90291-1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Goridis C., Hirn M., Santoni M. J., Gennarini G., Deagostini-Bazin H., Jordan B. R., Kiefer M., Steinmetz M. Isolation of mouse N-CAM-related cDNA: detection and cloning using monoclonal antibodies. EMBO J. 1985 Mar;4(3):631–635. doi: 10.1002/j.1460-2075.1985.tb03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski P. J., Sharp P. A. Affinity chromatography of splicing complexes: U2, U5, and U4 + U6 small nuclear ribonucleoprotein particles in the spliceosome. Science. 1986 Sep 19;233(4770):1294–1299. doi: 10.1126/science.3638792. [DOI] [PubMed] [Google Scholar]
- Habets W. J., Sillekens P. T., Hoet M. H., Schalken J. A., Roebroek A. J., Leunissen J. A., van de Ven W. J., van Venrooij W. J. Analysis of a cDNA clone expressing a human autoimmune antigen: full-length sequence of the U2 small nuclear RNA-associated B" antigen. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2421–2425. doi: 10.1073/pnas.84.8.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberger M., Pettersson I., Steitz J. A. Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J Biol Chem. 1983 Feb 25;258(4):2604–2613. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kinlaw C. S., Robberson B. L., Berget S. M. Fractionation and characterization of human small nuclear ribonucleoproteins containing U1 and U2 RNAs. J Biol Chem. 1983 Jun 10;258(11):7181–7189. [PubMed] [Google Scholar]
- Kitamura N., Takagaki Y., Furuto S., Tanaka T., Nawa H., Nakanishi S. A single gene for bovine high molecular weight and low molecular weight kininogens. Nature. 1983 Oct 6;305(5934):545–549. doi: 10.1038/305545a0. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987 Jun 19;49(6):763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T. Multiple factors including the small nuclear ribonucleoproteins U1 and U2 are necessary for pre-mRNA splicing in vitro. Cell. 1985 Oct;42(3):725–736. doi: 10.1016/0092-8674(85)90269-7. [DOI] [PubMed] [Google Scholar]
- Krämer A., Keller W., Appel B., Lührmann R. The 5' terminus of the RNA moiety of U1 small nuclear ribonucleoprotein particles is required for the splicing of messenger RNA precursors. Cell. 1984 Aug;38(1):299–307. doi: 10.1016/0092-8674(84)90551-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Evans R. M., Rosenfeld M. G. Splice commitment dictates neuron-specific alternative RNA processing in calcitonin/CGRP gene expression. Cell. 1987 Feb 13;48(3):517–524. doi: 10.1016/0092-8674(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lelay-Taha M. N., Reveillaud I., Sri-Widada J., Brunel C., Jeanteur P. RNA-protein organization of U1, U5 and U4-U6 small nuclear ribonucleoproteins in HeLa cells. J Mol Biol. 1986 Jun 5;189(3):519–532. doi: 10.1016/0022-2836(86)90321-9. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R., Mathey-Prevot B., Bernards A., Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987 Jul 24;237(4813):411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Murray B. A., Hemperly J. J., Gallin W. J., MacGregor J. S., Edelman G. M., Cunningham B. A. Isolation of cDNA clones for the chicken neural cell adhesion molecule (N-CAM). Proc Natl Acad Sci U S A. 1984 Sep;81(17):5584–5588. doi: 10.1073/pnas.81.17.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa H., Kotani H., Nakanishi S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature. 1984 Dec 20;312(5996):729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- Pettersson I., Hinterberger M., Mimori T., Gottlieb E., Steitz J. A. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984 May 10;259(9):5907–5914. [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillekens P. T., Habets W. J., Beijer R. P., van Venrooij W. J. cDNA cloning of the human U1 snRNA-associated A protein: extensive homology between U1 and U2 snRNP-specific proteins. EMBO J. 1987 Dec 1;6(12):3841–3848. doi: 10.1002/j.1460-2075.1987.tb02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Wolin S. L., Rinke J., Pettersson I., Mount S. M., Lerner E. A., Hinterberger M., Gottlieb E. Small ribonucleoproteins from eukaryotes: structures and roles in RNA biogenesis. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):893–900. doi: 10.1101/sqb.1983.047.01.103. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Theissen H., Etzerodt M., Reuter R., Schneider C., Lottspeich F., Argos P., Lührmann R., Philipson L. Cloning of the human cDNA for the U1 RNA-associated 70K protein. EMBO J. 1986 Dec 1;5(12):3209–3217. doi: 10.1002/j.1460-2075.1986.tb04631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieben E. D., Rohleder A. M., Nenninger J. M., Pederson T. cDNA cloning of a human autoimmune nuclear ribonucleoprotein antigen. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7914–7918. doi: 10.1073/pnas.82.23.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in U1 snRNA suppresses a 5' splice site mutation. Cell. 1986 Sep 12;46(6):827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]