Abstract
Divergent Toll-like receptor 7 (TLR7) and TLR9 signaling has been proposed to distinguish pathogenic from nonpathogenic simian immunodeficiency virus infection in primate models. We demonstrate here that increased expression of type I interferon in pathogenic rhesus macaques compared to nonpathogenic African green monkeys was associated with the recruitment of plasmacytoid dendritic cells in the lymph nodes and the presence of an inflammatory environment early after infection, instead of a difference in the TLR7/9 response.
Type I interferons (IFNs) are synthesized in response to a variety of pathogens, including bacteria, protozoa, and viruses. DNA and RNA viruses induce type I IFN expression by plasmacytoid dendritic cells (pDCs) through stimulation of Toll-like receptor 7 (TLR7) and TLR9 (46). The IFN response is an early event in simian immunodeficiency virus (SIV)-infected monkeys (1, 21, 28, 41). SIV infection of nonhuman primates remains an invaluable animal model for studies of AIDS pathogenesis, therapeutics, and vaccines. In particular, understanding the basis of pathogenic and nonpathogenic host-virus relationships in nonhuman primates is likely to provide important clues regarding AIDS pathogenesis (20). It has recently been reported that pDCs from the nonpathogenic model of SIV infection, sooty mangabey, produce markedly less IFN-α in response to SIV and other TLR7 and TLR9 ligands than pDCs from pathogenic rhesus macaques (RMs) of Indian origin (29). Therefore, it has been proposed that chronic stimulation of pDCs by SIV and human immunodeficiency virus (HIV) in non-natural hosts may drive the unrelenting immune system activation and dysfunction associated with AIDS progression (29). However, the detailed dynamics of IFN-α production in these monkeys was not addressed. Furthermore, a peak of plasma levels of type I IFN has been observed early after SIV infection in African green monkeys (12)—a nonpathogenic model of SIV infection—in contrast to the previous hypothesis. In addition, transcriptional gene profiling in nonpathogenic SIV-infected monkeys has revealed the presence of genes related to type I and type II IFN responses (23).
pDCs migrate through high endothelial venules during viral and bacterial infections following the release of inflammatory cytokines and chemokines (4). Therefore, we have analyzed more thoroughly the question of changes in IFN-α synthesis and the relationship with dynamics of pDC accumulation and viral replication in peripheral lymph nodes (LNs) during the acute phase of different models of SIV infection in nonhuman primates (9). Indeed, few studies have focused on LNs. We performed a longitudinal study of 8 Indian and 20 Chinese RMs experimentally infected with the SIVmac251 strain (among these latter 7 were noncontrollers [NC-RMs] and 13 controllers [C-RMs]). In addition, six Chinese RMs were experimentally infected with a nef deletion SIVmac251 strain (SIVΔnef), and six African green monkeys were infected with SIVagm-sab.
MATERIALS AND METHODS
Monkeys, virus, and samples.
RMs (Macaca mulatta) were seronegative for simian T leukemia virus type 1, SRV-1 (type D retrovirus), herpesvirus B, and SIVmac. Animals were housed and cared for in compliance with existing French regulations (9). Macaques were inoculated intravenously with 10 50% animal infectious doses of the SIVmac251 strain (provided by A. M. Aubertin, INSERM U74, Strasbourg, France). Axillary and inguinal LNs were collected before infection and at days 7, 11, 14, and 60 postinfection. Half of the LNs were frozen and stored at −70°C for subsequent analysis by in situ hybridization or immunohistochemistry, while the other half was processed for flow cytometric analyses, as previously described (9). Six African green monkeys (AGMs) (Chlorocebus aethiops sabeus) were experimentally infected with 300 50% tissue culture infective doses of the SIVagm.sab92018 strain. This virus preparation was never passaged in vitro.
Quantitative analysis of productively infected cells and IFN-expressing cells.
Productively infected cells (SIV+ RNA cells) were assessed in LNs by in situ hybridization as previously described (9). Infected cells were detected and counted in the paracortical zone on a minimum of three sections by using a Nikon-FXA microscope. IFN-α1 gene expression (which is one of the main IFN-α subtypes produced) was detected by in situ hybridization as described previously (21). Briefly, a fragment of 600 bp of the IFN-α1 gene specific for RMs and AGMs was introduced into a plasmid (pSP65), while the antisense riboprobes were generated from the SP6 promoter. The plasmids were then labeled with 35S-labeled UTP. To enhance the penetration of the probes into tissue sections, the 35S-labeled RNA were subjected to mild alkaline hydrolysis in order to obtain a majority of fragments in the 150- to 200-nucleotide range. The number of positive cells was then divided by the surface of the entire LN section, and the results were expressed as the number of positive cells per 2-mm2 section. The mean count was calculated for three slides of the same LNs obtained in a blinded fashion by two different investigators.
Immunophenotyping of pDCs.
LN cells were incubated with a cocktail of monoclonal antibodies for 30 min at 4°C in the dark. We used a cocktail of fluorescein isothiocyanate (FITC)-conjugated antibodies, namely, lineage (Lin) (6, 45), comprising anti-CD3 (FN-18), anti-CD14 (MφP9), anti-CD20 (L27), in combination with phycoerythrin (PE)-conjugated anti-CD123 (7G3) antibodies, with peridinin-chlorophyll protein (PerCp)-conjugated anti-human leukocyte antigen (HLA)-DR (L243), and finally with biotinylated CD11c (3.9) antibodies. All monoclonal antibodies were purchased from BD Biosciences, except for the anti-CD3 and anti-CD11c antibodies obtained from Biosource (Nivelles, Belgium) and eBioscience (Clinisciences, Montrouge, France), respectively. Cells were washed and incubated in the presence of allophycocyanin (APC)-coupled streptavidin for 30 min in the dark at 4°C and then fixed. At least 500,000 events corresponding to mononuclear cells by forward- and side-scatter characteristics were acquired by using a FACSCalibur and further analyzed by using CellQuest analysis software (BD Biosciences). However, pDCs from SIV-infected RMs of Indian origin were not assessed due to import restriction of these species for research in Europe. Fresh cells from RMs of Indian origin and AGMs to test cytokines were kindly provided by F. Wanert, Centre de Primatologie, Université de Strasbourg.
Ex vivo PBMC stimulation with TLR agonists.
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation of peripheral blood from SIV− monkeys. PBMC (500,000 cells per well) were incubated in duplicate wells with 10 ng of lipopolysaccharide (LPS; TLR4 agonist)/ml, 1 μg of CLO97 (TLR7)/ml, and 1 μM ODN M362 (TLR9; InvivoGen) for 18 h at 37°C. After stimulation, supernatants were collected and assessed for cytokine detection. PBMC after stimulation were incubated with brefeldin A (5 μg/ml; Sigma-Aldrich) and then surface stained for CD123, CD11c, and Lin and fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS). Cells were then permeabilized with 0.5% saponin in PBS-fetal calf serum, followed by incubation with FITC-labeled anti-IFN-α2 antibody (eBiosciences clone 225.C) as previously described (6) and as described in other reports (29). Finally, the cells were washed and analyzed by flow cytometry.
Quantification of IFN-α and IL-8.
Type I IFN was measured by an enzyme-linked immunosorbent assay (ELISA; PBL Biomedical laboratories) as previously described (29, 45) and, as shown here, was able to detect type I IFN levels in both RMs and AGMs. Interleukin-8 (IL-8) was measured by using an inflammatory cytokine cytometric bead array kit (BD Biosciences).
Statistical analyses.
Friedman test with Dunn's correction was used to determine whether differences in means from sequential samples were significant. Correlations were evaluated by using the Spearman's test. Statistical significance between the different groups was analyzed by the nonparametric Mann-Whitney test. Prism version 3.0 (GraphPad Software) was used for statistical analyses.
RESULTS
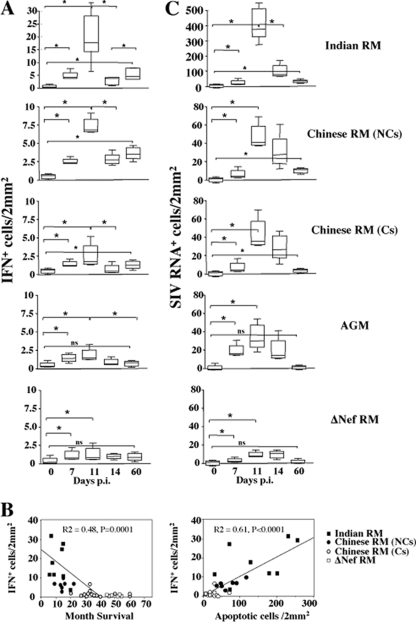
By in situ hybridization, we found that cells expressing IFN-α1 (IFN-α1+), one of the major subtypes of type I IFN, expanded at days 7 and 11 and then declined at day 14 (Fig. 1A and 2A). Whereas the dynamics were qualitatively similar between the different groups analyzed, the magnitude of the response was clearly distinct. RMs of Indian origin (RMIs) infected by SIV produced, at the peak (day 11), higher levels of IFN-α1 (median, 16 IFN+ cells/2 mm2) than that observed in NC-RMs of Chinese origin (7.5 IFN+ cells/2 mm2; RMIs versus NC-RMs, P = 0.04), and C-RMs (2.5 IFN+ cells/2 mm2; RMIs versus C-RMs, P = 0.0006; NC-RMs versus C-RMs, P = 0.003) compared to uninfected monkeys (0.5 IFN+ cells/2 mm2). The levels at the peak were less than 2.0 IFN+ cells/2 mm2 in SIVΔnef-infected RMs and in SIV-infected AGMs. At 2 months (Fig. 2A), the numbers of IFN-α1+ cells were higher in monkeys progressing faster to AIDS (RMIs [4.8 IFN+ cells/2 mm2] and NC-RMs of Chinese origin [3.2 IFN+ cells/2 mm2]; RMIs versus NC-RMs, P = 0.02) than that observed in nonprogressors (C-RMs [0.98 IFN+ cells/2 mm2] and AGMs [0.4 IFN+ cells/2 mm2]; NC-RMs versus C-RMs [P = 0.0003] and C-RMs versus AGMs [P = 0.01]). The levels of type I IFN detected in the plasma (Fig. 3) were consistent with the dynamics of IFN-α1+ cells in LNs and revealed at the peak higher levels in animals progressing faster to AIDS compared to nonprogressors (NC-RMs versus C-RMs [P = 0.017] and NC-RMs versus AGMs [P = 0.001]). Moreover, no significance difference was observed between C-RMs and AGMs (P = not significant). Our data also revealed a significant correlation between the numbers of IFN-α1+ cells at the peak and disease outcome (months of survival) (Fig. 2B). In order to analyze whether the Indian group had an impact on the correlation, we excluded this group, and found a positive correlation between the expression of type I IFN and the months of survival, including only NC- and C-RMs (r2 = 0.53, P = 0.0002). Animals have been followed up for disease outcome and killed at different time points postinfection corresponding to a wasting syndrome as previously described (9, 10, 33, 35, 36). The extent of apoptosis—a predictive factor of disease outcome (20, 49)—quantified in the tissues at the peak, also correlated with the levels of IFN-α1+ cells (Fig. 2B).
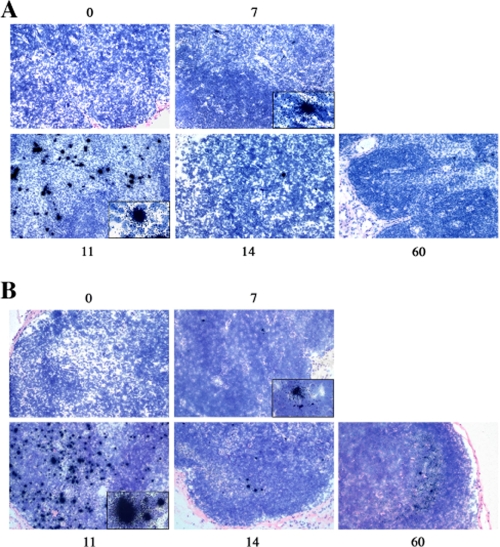
FIG. 1.
Detection of IFNα1+ cells and productive SIV infection in LNs from RMs of Indian origin. (A) Detection of IFN-α1+ cells in LNs from one RM of Indian origin at 7, 11, 14, and 60 days after infection by in situ hybridization of (magnification, ×200). (B) Detection of virus-replicating cells (SIV RNA+ cells) in LNs at 7, 11, 14, and 60 days after infection by in situ hybridization of the same monkey as shown above (magnification, ×200).
FIG. 2.
Comparison of IFN-α1+ cells expression in pathogenic and nonpathogenic SIV models. (A) Dynamics of IFN-α1+ cells in LNs during primary SIV infection. IFN-α1-expressing cells (shown as IFN-α1+ cells/2 mm2) were detected by in situ hybridization at days 0, 7, 11, 14, and 60 in the LNs of Indian RMs, Chinese RMs (noncontrollers, NCs; controllers, Cs), AGMs, and Δnef SIV-infected RMs. IFN-α1+ cells were quantified, and means ± the standard deviations (SD) are shown. Statistical significance: *, P < 0.05; ns, not significant. (B) IFN-α1-expressing cells in LNs predict AIDS correlation between the number of IFN-α1+ cells at day 11 and survival (months of survival) and the extent of apoptosis quantified by the TUNEL method at day 14. Each symbol represents one individual. (C) Dynamics of productive SIV infection in LNs during primary SIV infection. Virus-replicating cells (shown as SIV+ cells/2 mm2) were detected by in situ hybridization in the LNs of Indian RMs, Chinese RMs (noncontrollers, NCs; controllers, Cs), AGMs, and Δnef SIV-infected RMs at 7, 11, 14, and 60 days after SIVmac251 infection. SIV-RNA+ cells were quantified, and means ± the SD are shown. Statistical significances: *, P < 0.05; ns, not significant.
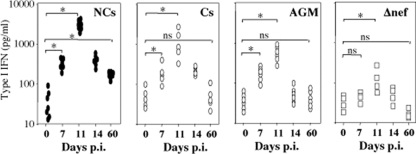
FIG. 3.
Detection of type I IFN in the blood of SIV-infected monkeys. Type I IFN-α was assessed by ELISA. Each symbol represent an individual monkey. Statistical significance: *, P < 0.05; ns, not significant.
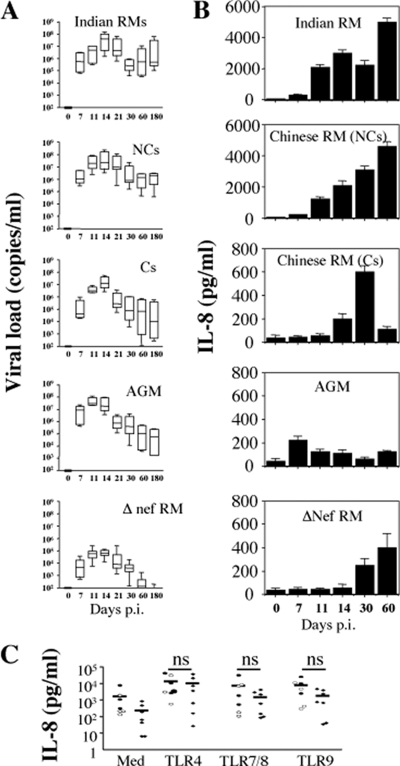
A straightforward explanation would be that increasing viral replication in these tissues induced higher levels of IFN-α1+ cells. Although we found that RMIs displayed higher numbers of SIV+ RNA cells compared to RMs of Chinese origin at the peak (∼10-fold higher, Fig. 2A), there was no significant difference in the extent of SIV+ cells, as well as in plasma viral load between NC-RMs, C-RMs, and SIV-infected AGMs at the same time (Fig. 2C). Lower levels of SIV+ cells and lower plasma viral load compared to the other groups of macaques were detected only in SIVΔnef-infected RMs. Consistent with previous reports (9, 27), these findings suggest that the level of viral replication at the peak does not discriminate between pathogenic and nonpathogenic primate models of SIV infection (RMIs nevertheless showed higher frequencies of infected cells and faster disease progression than Chinese RMs). These results also indicate that the extent of viral replication detected in the LNs was not the only factor driving higher levels of IFN-α1+ cells in the tissues at the peak of viral replication between RMs and AGMs.
Thereafter, the viral load declined. Thus, at day 60, the numbers of SIV+ RNA cells were higher in Indian than in Chinese RMs (15.9/2 and 7.4/2 mm2, P = 0.001) (Fig. 2C). However, it should be noted that at day 60, the number of SIV+ RNA cells in LNs was extremely low in AGMs (0.5/2 mm2), despite a detectable viral load (see Fig. 6A). Viral load in the blood also declined in the blood to reach a steady state (days 60 to 180). Higher levels in NC-RMs than in C-RMs and AGM (P = 0.001) were observed. Thus, the level of IFN-α1+ cells detected in the LNs at day 60 is more consistent with the notion that persistence of SIV replication in LNs was associated with higher levels of IFN-α1+ cells. These results are consistent with another report indicating that the expression of genes related to the IFN response in peripheral LNs was higher in progressors than in nonprogressors RMs (32).
FIG. 6.
Dynamics of IL-8 during primary SIV-infection. (A) Viral load was quantified in peripheral blood of SIV-infected Indian RMs, Chinese NC- and C-RMs, SIV-infected AGMs, and SIVΔnef-infected RMs at different days postinfection. (B) The levels of IL-8 were detected in the sera by ELISA at days 0, 7, 14, 21, 30, and 60 after inoculation. Values are means ± the SD (n = 6). (C) IL-8 production after TLRs stimulation. PBMC isolated from healthy monkeys (solid ovals, Indian RMs; open ovals, Chinese RMs; solid diamonds, AGMs [n = 7]) were stimulated overnight in the absence (Med) or presence of the following TLR agonists: LPS, 10 ng/ml (TLR4); CLO97, 1 μg/ml (TLR7); and CpG-DNA, 1 μM (TLR9). Supernatants were collected and assessed for the detection of IL-8 by ELISA. Each symbol represents one individual.
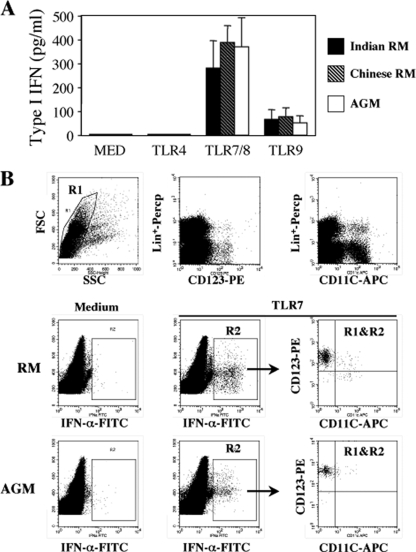
Differential IFN-α1+ expression in pathogenic and nonpathogenic models, despite similar levels of viral replication during the acute phase, suggests an alternative explanation such as a difference in TLR signaling. However, in contrast to the previous report on sooty mangabeys (29), we found that in vitro stimulation of PBMC with TLR7 and TLR9 ligands induced similar levels of type I IFN-α as detected by ELISA from PBMC between both RMs of Indian and Chinese origins and AGMs (Fig. 4A).
FIG. 4.
IFN-α production after TLRs stimulation. (A) PBMC isolated from healthy monkeys were stimulated overnight in the absence (Med) or presence of the TLR agonists: LPS, 10 ng/ml (TLR4); CLO97, 1 μg/ml (TLR7); and CpG-DNA, 1 μM (TLR9). Supernatants were collected and assessed for the detection of type I IFN-α by ELISA. Values are means ± the SD (n = 7). (B) Phenotype of IFN-α2a-expressing cells by flow cytometry. PBMC isolated from monkeys (RM versus AGM) were stimulated overnight in the absence (Med) or presence of CLO97 at 1 μg/ml (TLR7). Cells were stained with antibodies directed against IFN-α2a, CD11c, and CD123 and included a lineage marker (Lin+). The gating strategy is shown. Similar data have been obtained with four healthy monkeys of each species.
Consistent with the notion that CD123+ and CD11c− pDCs are potent producers of type I IFNs (4), we observed, by flow cytometry, that the predominant source of IFN-α2 was pDCs both in healthy RMs of Chinese origin and in AGMs (Fig. 4B).
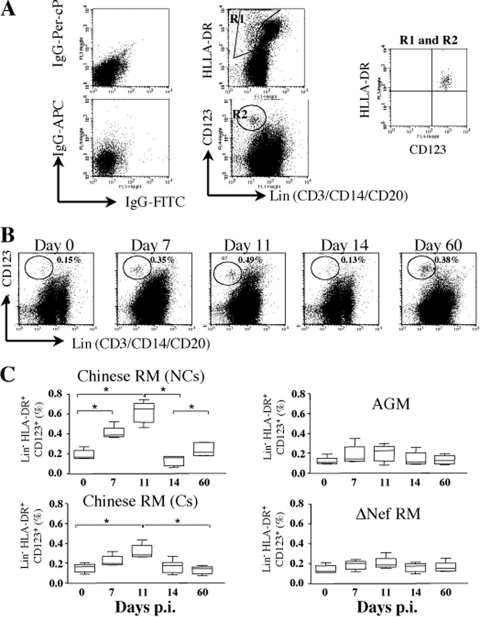
Therefore, we next examined the dynamics of pDCs (Fig. 5) (6, 45). The percentage of pDCs in healthy RMs exhibited a mean of 0.17% (range, 0.057 to 0.30%) in the LNs. This percentage increased early after infection by days 7 and 11 coincident with the peak of type I IFN-expressing cells. The percentage of pDCs then drastically decreased and returned to baseline levels at day 14. Interestingly, the percentages of pDCs were higher in the LNs of NC-RMs than in LNs of C-RMs both at day 11 (0.6% ± 0.11% versus 0.31% ± 0.07%, P = 0.005) and at day 60 (0.25% ± 0.07% versus 0.11% ± 0.06%, P = 0.01), a finding consistent with the dynamics of IFN-α+ cells. In contrast, we found no major changes in the dynamic of pDCs in LNs both in SIV-infected AGMs and SIVΔnef-infected RMs. We cannot exclude that other cells produce type I IFN in the LNs, such as macrophages which may produce 10-fold less type I IFN than pDCs. However, during the acute phase, IFN-α+ cells were essentially localized in the paracortical T-cell zone (Fig. 1) but not in the germinal centers, which contain a majority of B cells (37), and most of the IFN-α+ cells were CD123+ as determined by immunochemistry (data not shown), which is consistent with another report (25).
FIG. 5.
Dynamics of pDCs in LNs during primary SIV infection. (A) Gating strategy used to analyze pDCs in LNs as defined by Lin− CD123+ HLA-DR+ cells. (B) The results for one representative RM analyzed at different time points after infection are shown. (C) pDCs were defined as Lin− CD123+ HLA-DR+ cells and analyzed by flow cytometry. Means ± the SD are shown in NC-RMs (NCs, n = 6) and C-RMs (Cs, n = 6), SIVΔnef-infected RMs (n = 6), and SIV-infected AGMs (n = 6). Statistical significance: *, P < 0.05; ns, not significant.
The production of chemokines and cytokines in an inflammatory environment participates in the recruitment of pDCs. Interestingly, the level of IL-8, which is a general marker of inflammation, was increased early after infection at day 7 in both RMs and AGMs (RMs of Indian origin, 300 ± 36 pg/ml; RMs of Chinese origin, 200 ± 25 pg/ml; AGMs, 220 ± 35 pg/ml) (Fig. 6B). Thereafter, IL-8 was highly expressed only in pathogenic SIV-infected RMIs (3,000 ± 232 pg/ml at day 14), as well of NC-RMs of Chinese origin (2,100 ± 270 pg/ml at day 14), compared to C-RMs (200 ± 45 pg/ml at day 14, P = 0.0003) and nonpathogenic SIV models (AGMs, 110 ± 29 pg/ml at day 14, P = 0.0006) (Fig. 6B). Other reports have also shown an increased level of IL-8 early after infection of RMs of Indian origin (5, 25). At day 60, the levels of IL-8 remained higher in monkeys progressing faster to AIDS (5,000 ± 269 pg/ml in RMIs, and 4,600 ± 320 pg/ml in NC-RMs) compared to AGMs (120 ± 14 pg/ml, P = 0.0003) and C-RMs (110 ± 23 pg/ml, P = 0.0006). However, the absence of IL-8 detection in the nonpathogenic AGM model was not related to defective TLR signaling (Fig. 6C). Thus, the inflammatory response is uncoupled from the extent of viral replication at the peak but characterizes pathogenic models of SIV infection.
DISCUSSION
This study showed an early elevation of IFN-α1 expression in peripheral LNs, as well type I IFN in the blood during the acute phase of SIV infections in both pathogenic and nonpathogenic primates. The magnitude during the acute phase and the persistent expression of IFN-α1 at the set point is associated with a more rapid progression toward AIDS. The extent of viral replication in the tissues may participate in the direct elevation of type I IFN-α1; in fact, RMs of Indian origin exhibited increased expression of type I IFN-α1 and increased levels of viral replication compared to RMs of Chinese origin and macaques infected with the Δnef SIVmac251 strain. However, despite similar levels of viral replication within LNs at the peak in AGMs and C- and NC-RMs of Chinese origin, the level of IFN-α1 expression was higher only in RMs that progress toward AIDS, compared to C-RMs and AGMs. This discrepancy was not due to a defect in TLR signaling between the different species, in contrast to an earlier report in sooty mangabey (29). Our data revealed that recruitment of pDCs, which represent the main cell type producing type I IFN (4), occurs early after SIV infection concomitantly with the peak of type I IFN. Higher recruitment of pDCs within LNs is observed in monkeys progressing more rapidly to AIDS and associated with greater inflammatory response (as measured by the presence of circulating IL-8). Recently, the examination of global gene expression changes of pathogenic (pigtail monkeys) and nonpathogenic (AGMs) SIV infections (23) has revealed in LNs that the greatest differential regulation of genes is related to (i) genes associated with cell death, which is consistent with the extent of apoptosis detected by the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) method in the tissues of SIV-infected monkeys (9, 14, 33, 43, 49); (ii) inflammatory and chemotaxis genes, which is consistent with the detection of IL-8 and other reports (1, 25, 40, 42); (iii) higher levels of immune activation consistent with previous studies showing that in NCs the extent of CD8 activation is greater than in CS RMs of Chinese origin (34, 36); and (iv) genes associated with the IFN response (1, 21, 41). Consistent with our data, Milush et al. (32) found that expression of type I IFN expression in LNs was more highly expressed in NC-RMs than in C-RMs. However, these authors observed that genes associated with the IFN response were differently expressed in mucosal tissues and peripheral LNs. Thus, these genes were more highly expressed in the LNs of RMs that do not control infection (NC-RMs), whereas the genes were expressed less in the colon, and vice versa, in nonprogressors (C-RMs). Lederer et al. (23) also observed higher levels of IFN gene in the colon of nonpathogenic AGMs compared to pathogen SIV-infected pigtail monkeys. More recently, mRNA expression of four immune modulators—IFN-α, oligoadenylate synthetase, CXCL9, and CXCL10—was positively associated with disease progression within the LN tissues of SIV-infected RMs of Indian origin (13) and consistent with the profile of mRNAs observed in lymphatic tissue of untreated HIV-infected persons during the acute phase (26). Thus, depending on the anatomical location, the levels of expression of IFN-associated genes may distinguish pathogenic and nonpathogenic infections.
A further observation was the persistence of IFN-α expression and pDC recruitment in the peripheral LNs of animals progressing to AIDS. These findings provide evidence that productive infection persists despite a prolonged IFN-α response within peripheral LNs. This was consistent with gene expression (23), since most of the significant changes between days 10 and 45 postinfection in the LNs and the colon involved genes associated with IFN responses and were significantly attenuated during nonpathogenic SIV infection (AGMs) compared to pathogenic SIV infection (pigtail monkeys). The decreased expression of these genes and the lower levels of IFN-α1+ cells detected in the LNs of AGMs may in part be due to decreased viral replication in these LNs, despite similar levels of viral load in the blood. However, we do not know whether this also occurs in the colon. Thus, it has been assumed that the strong and early IFN signaling in the colons of SIV-infected AGMs should be a mechanism for limiting SIV pathogenesis, and such early attenuation of the inflammatory response in AGMs may help to reduce damage (23). Similar observations have been reported in macaques that progress slowly (32). Thus, depending on the anatomical location, the production of type I IFN should be beneficial or detrimental, controlling or favoring an inflammatory response.
Activities and therapeutic uses of type I IFNs have clearly established a wide breadth of biological activities. In particular, it has been reported in the past that low-dose IFN-α treatment exerts an anti-inflammatory control action associated with a significant reduction in tissue infiltration of neutrophils, macrophages, and lymphocytes. In contrast, high doses can directly induce proinflammatory and pyretic responses in humans, and a common adverse reaction is lymphopenia associated with flulike symptoms (for reviews, see references 2 and 38).
The notion that type I IFN could be more detrimental than beneficial during HIV infection was originally proposed a decade ago; in fact, it has been demonstrated that type I IFN in the sera of chronically HIV-infected patients was associated with disease progression, instead of resolution (17, 22), and the loss of uninfected T cells in vitro due to HIV-1 is mediated by IFN-α (50). It has also been reported that IFN-α derived from pDCs, following in vitro exposure to HIV, was implicated in the killing of T cells (19). Thus, type I IFNs may lead to immune deviation by altering CD4 Th-cell differentiation through the promotion of CD4 T-cell apoptosis during the acute phase (9, 14, 24, 33, 43, 49). Indeed, the events occurring early after infection play a key role in conditioning further disease evolution in particular the loss of memory CD4 T cells (24, 30, 35, 39, 44, 48). However, it remains to be determined whether pDCs from SIV-infected monkeys are capable of inducing CD4 T-cell death.
Moreover, models of viral infection in mice, such as infection with lymphocytic choriomeningitis virus or measles virus, have established that there is induction of a generalized immune suppression in their natural hosts. This occurs early after infection concomitantly with a change in the balance of type I IFN and IL-12 expression (3, 11, 18) in which type I IFN antagonizes IL-12 production (8, 31). IL-12 is a cytokine essential in the development of cell-mediated immunity (47) and cell survival (7, 15, 16). The peak of type I IFN observed in our study early after infection may lead to decreased IL-12 expression in LNs during the acute phase. Thus, the recruitment of pDCs in the peripheral LNs during the acute phase of SIV infection could have a detrimental indirect effect on CD4 T-cell function. It remains to be determined whether the balance of IL-12 expression is affected during the acute phase in both pathogenic and nonpathogenic primate models.
In conclusion, our data demonstrate that the increased number of IFN-α1+ cells in the peripheral LNs of RMs of Chinese origin pathogenically infected with SIV is at least related to an early elevation in the level of recruitment of pDCs and associated with an increased inflammatory environment during the acute phase. Conversely, reduced recruitment of pDCs and reduced expression of IFN-α1 in the peripheral LNs is correlated with nonpathogenic disease outcome, despite an intense peak of viral replication.
Acknowledgments
M.L. and A.B. were supported by grants from Sidaction. L.C.-G. was supported by grant from MRET of PARIS XI University. Funding from the ANRS and the FRM to J.E. supported this work, and part of this study has been supported by EUPRIM-Net under EU contract RII3-026155 of the 6th Framework Program. J.C. acknowledges the support of the Canada Research Chair program. J.Z. is partly supported by grants from the Australian NHMRC.
We thank J. M. Panaud (Institut Pasteur) for assistance with microphotographs.
Footnotes
Published ahead of print on 25 November 2009.
REFERENCES
- 1.Abel, K., M. J. Alegria-Hartman, K. Rothaeusler, M. Marthas, and C. J. Miller. 2002. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J. Virol. 76:8433-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadori, M. 2007. The role of IFN-alpha as homeostatic agent in the inflammatory response: a balance between danger and response? J. Interferon Cytokine Res. 27:181-189. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, P., C. F. Evans, and M. B. Oldstone. 1995. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69:1059-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. [DOI] [PubMed] [Google Scholar]
- 5.Choi, Y. K., B. A. Fallert, M. A. Murphey-Corb, and T. A. Reinhart. 2003. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood 101:1684-1691. [DOI] [PubMed] [Google Scholar]
- 6.Chung, E., S. B. Amrute, K. Abel, G. Gupta, Y. Wang, C. J. Miller, and P. Fitzgerald-Bocarsly. 2005. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin. Diagn. Lab. Immunol. 12:426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clerici, M., A. Sarin, R. L. Coffman, T. A. Wynn, S. P. Blatt, C. W. Hendrix, S. F. Wolf, G. M. Shearer, and P. A. Henkart. 1994. Type 1/type 2 cytokine modulation of T-cell programmed cell death as a model for human immunodeficiency virus pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 91:11811-11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousens, L. P., J. S. Orange, H. C. Su, and C. A. Biron. 1997. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc. Natl. Acad. Sci. U. S. A. 94:634-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cumont, M. C., O. Diop, B. Vaslin, C. Elbim, L. Viollet, V. Monceaux, S. Lay, G. Silvestri, R. Le Grand, M. Muller-Trutwin, B. Hurtrel, and J. Estaquier. 2008. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J. Virol. 82:1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumont, M. C., V. Monceaux, L. Viollet, S. Lay, R. Parker, B. Hurtrel, and J. Estaquier. 2007. TGF-beta in intestinal lymphoid organs contributes to the death of armed effector CD8 T cells and is associated with the absence of virus containment in rhesus macaques infected with the simian immunodeficiency virus. Cell Death Differ. 14:1747-1758. [DOI] [PubMed] [Google Scholar]
- 11.Dalod, M., T. P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-Paturel, F. Briere, G. Trinchieri, and C. A. Biron. 2002. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diop, O. M., M. J. Ploquin, L. Mortara, A. Faye, B. Jacquelin, D. Kunkel, P. Lebon, C. Butor, A. Hosmalin, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2008. Plasmacytoid dendritic cell dynamics and alpha interferon production during simian immunodeficiency virus infection with a nonpathogenic outcome. J. Virol. 82:5145-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durudas, A., J. M. Milush, H. L. Chen, J. C. Engram, G. Silvestri, and D. L. Sodora. 2009. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. J. Virol. 83:12229-12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estaquier, J., T. Idziorek, F. de Bels, F. Barre-Sinoussi, B. Hurtrel, A. M. Aubertin, A. Venet, M. Mehtali, E. Muchmore, P. Michel, et al. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. U. S. A. 91:9431-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estaquier, J., T. Idziorek, W. Zou, D. Emilie, C. M. Farber, J. M. Bourez, and J. C. Ameisen. 1995. T helper type 1/T helper type 2 cytokines and T-cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J. Exp. Med. 182:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estaquier, J., M. Tanaka, T. Suda, S. Nagata, P. Golstein, and J. C. Ameisen. 1996. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood 87:4959-4966. [PubMed] [Google Scholar]
- 17.Fahey, J. L., J. M. Taylor, R. Detels, B. Hofmann, R. Melmed, P. Nishanian, and J. V. Giorgi. 1990. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N. Engl. J. Med. 322:166-172. [DOI] [PubMed] [Google Scholar]
- 18.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbeuval, J. P., A. W. Hardy, A. Boasso, S. A. Anderson, M. J. Dolan, M. Dy, and G. M. Shearer. 2005. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 102:13974-13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurtrel, B., F. Petit, D. Arnoult, M. Muller-Trutwin, G. Silvestri, and J. Estaquier. 2005. Apoptosis in SIV infection. Cell Death Differ. 12(Suppl. 1):979-990. [DOI] [PubMed] [Google Scholar]
- 21.Khatissian, E., M. G. Tovey, M. C. Cumont, V. Monceaux, P. Lebon, L. Montagnier, B. Hurtrel, and L. Chakrabarti. 1996. The relationship between the interferon alpha response and viral burden in primary SIV infection. AIDS Res. Hum. Retrovir. 12:1273-1278. [DOI] [PubMed] [Google Scholar]
- 22.Lane, H. C., V. Davey, J. A. Kovacs, J. Feinberg, J. A. Metcalf, B. Herpin, R. Walker, L. Deyton, R. T. Davey, Jr., J. Falloon, et al. 1990. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection: a randomized, placebo-controlled trial. Ann. Intern. Med. 112:805-811. [DOI] [PubMed] [Google Scholar]
- 23.Lederer, S., D. Favre, K. A. Walters, S. Proll, B. Kanwar, Z. Kasakow, C. R. Baskin, R. Palermo, J. M. McCune, and M. G. Katze. 2009. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 5:e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 25.Li, Q., J. D. Estes, P. M. Schlievert, L. Duan, A. J. Brosnahan, P. J. Southern, C. S. Reilly, M. L. Peterson, N. Schultz-Darken, K. G. Brunner, K. R. Nephew, S. Pambuccian, J. D. Lifson, J. V. Carlis, and A. T. Haase. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Q., A. J. Smith, T. W. Schacker, J. V. Carlis, L. Duan, C. S. Reilly, and A. T. Haase. 2009. Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J. Immunol. 183:1975-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling, B., R. S. Veazey, A. Luckay, C. Penedo, K. Xu, J. D. Lifson, and P. A. Marx. 2002. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS 16:1489-1496. [DOI] [PubMed] [Google Scholar]
- 28.Malleret, B., B. Maneglier, I. Karlsson, P. Lebon, M. Nascimbeni, L. Perie, P. Brochard, B. Delache, J. Calvo, T. Andrieu, O. Spreux-Varoquaux, A. Hosmalin, R. Le Grand, and B. Vaslin. 2008. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood 112:4598-4608. [DOI] [PubMed] [Google Scholar]
- 29.Mandl, J. N., A. P. Barry, T. H. Vanderford, N. Kozyr, R. Chavan, S. Klucking, F. J. Barrat, R. L. Coffman, S. I. Staprans, and M. B. Feinberg. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14:1077-1087. [DOI] [PubMed] [Google Scholar]
- 30.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 31.McRae, B. L., R. T. Semnani, M. P. Hayes, and G. A. van Seventer. 1998. Type I IFNs inhibit human dendritic cell IL-12 production and Th1 cell development. J. Immunol. 160:4298-4304. [PubMed] [Google Scholar]
- 32.Milush, J. M., K. Stefano-Cole, K. Schmidt, A. Durudas, I. Pandrea, and D. L. Sodora. 2007. Mucosal innate immune response associated with a timely humoral immune response and slower disease progression after oral transmission of simian immunodeficiency virus to rhesus macaques. J. Virol. 81:6175-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monceaux, V., J. Estaquier, M. Fevrier, M. C. Cumont, Y. Riviere, A. M. Aubertin, J. C. Ameisen, and B. Hurtrel. 2003. Extensive apoptosis in lymphoid organs during primary SIV infection predicts rapid progression toward AIDS. Aids 17:1585-1596. [DOI] [PubMed] [Google Scholar]
- 34.Monceaux, V., R. Ho Tsong Fang, M. C. Cumont, B. Hurtrel, and J. Estaquier. 2003. Distinct cycling CD4+- and CD8+-T-cell profiles during the asymptomatic phase of simian immunodeficiency virus SIVmac251 infection in rhesus macaques. J. Virol. 77:10047-10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monceaux, V., L. Viollet, F. Petit, M. C. Cumont, G. R. Kaufmann, A. M. Aubertin, B. Hurtrel, G. Silvestri, and J. Estaquier. 2007. CD4+ CCR5+ T-cell dynamics during simian immunodeficiency virus infection of Chinese rhesus macaques. J. Virol. 81:13865-13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monceaux, V., L. Viollet, F. Petit, R. Ho Tsong Fang, M. C. Cumont, J. Zaunders, B. Hurtrel, and J. Estaquier. 2005. CD8+ T-cell dynamics during primary simian immunodeficiency virus infection in macaques: relationship of effector cell differentiation with the extent of viral replication. J. Immunol. 174:6898-6908. [DOI] [PubMed] [Google Scholar]
- 37.Nascimbeni, M., L. Perie, L. Chorro, S. Diocou, L. Kreitmann, S. Louis, L. Garderet, B. Fabiani, A. Berger, J. Schmitz, J. P. Marie, T. J. Molina, J. Pacanowski, J. P. Viard, E. Oksenhendler, S. Beq, O. Abehsira-Amar, R. Cheynier, and A. Hosmalin. 2009. Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients, but barely participate in interferon alpha expression. Blood 113:6112-6119. [DOI] [PubMed] [Google Scholar]
- 38.Pestka, S. 2007. The interferons: 50 years after their discovery, there is much more to learn. J. Biol. Chem. 282:20047-20051. [DOI] [PubMed]
- 39.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reinhart, T. A., B. A. Fallert, M. E. Pfeifer, S. Sanghavi, S. Capuano III, P. Rajakumar, M. Murphey-Corb, R. Day, C. L. Fuller, and T. M. Schaefer. 2002. Increased expression of the inflammatory chemokine CXC chemokine ligand 9/monokine induced by interferon-gamma in lymphoid tissues of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. Blood 99:3119-3128. [DOI] [PubMed] [Google Scholar]
- 41.Sanghavi, S. K., and T. A. Reinhart. 2005. Increased expression of TLR3 in lymph nodes during simian immunodeficiency virus infection: implications for inflammation and immunodeficiency. J. Immunol. 175:5314-5323. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer, T. M., C. L. Fuller, S. Basu, B. A. Fallert, S. L. Poveda, S. K. Sanghavi, Y. K. Choi, D. E. Kirschner, E. Feingold, and T. A. Reinhart. 2006. Increased expression of interferon-inducible genes in macaque lung tissues during simian immunodeficiency virus infection. Microbes Infect. 8:1839-1850. [DOI] [PubMed] [Google Scholar]
- 43.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 44.Sun, Y., S. R. Permar, A. P. Buzby, and N. L. Letvin. 2007. Memory CD4+ T-lymphocyte loss and dysfunction during primary simian immunodeficiency virus infection. J. Virol. 81:8009-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teleshova, N., J. Kenney, J. Jones, J. Marshall, G. Van Nest, J. Dufour, R. Bohm, J. D. Lifson, A. Gettie, and M. Pope. 2004. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN-γ-secreting simian immunodeficiency virus-specific T cells. J. Immunol. 173:1647-1657. [DOI] [PubMed] [Google Scholar]
- 46.Tough, D. F. 2004. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk. Lymphoma 45:257-264. [DOI] [PubMed] [Google Scholar]
- 47.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 48.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T-cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 49.Viollet, L., V. Monceaux, F. Petit, R. Ho Tsong Fang, M. C. Cumont, B. Hurtrel, and J. Estaquier. 2006. Death of CD4+ T cells from lymph nodes during primary SIVmac251 infection predicts the rate of AIDS progression. J. Immunol. 177:6685-6694. [DOI] [PubMed] [Google Scholar]
- 50.Zagury, D., A. Lachgar, V. Chams, L. S. Fall, J. Bernard, J. F. Zagury, B. Bizzini, A. Gringeri, E. Santagostino, J. Rappaport, M. Feldman, A. Burny, and R. C. Gallo. 1998. Interferon alpha and Tat involvement in the immunosuppression of uninfected T cells and C-C chemokine decline in AIDS. Proc. Natl. Acad. Sci. U. S. A. 95:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]