Abstract
Activation of certain classes of G protein-coupled receptors (GPCRs) can lead to alterations in the actin cytoskeleton, gene transcription, cell transformation, and other processes that are known to be regulated by Rho family small-molecular-weight GTPases. Although these responses can occur indirectly via cross-talk from canonical heterotrimeric G protein cascades, it has recently been demonstrated that Dbl family Rho guanine nucleotide exchange factors (RhoGEFs) can serve as the direct downstream effectors of heterotrimeric G proteins. Heterotrimeric Gα12/13, Gαq, and Gβγ subunits are each now known to directly bind and regulate RhoGEFs. Atomic structures have recently been determined for several of these RhoGEFs and their G protein complexes, providing fresh insight into the molecular mechanisms of signal transduction between GPCRs and small molecular weight G proteins. This review covers what is currently known about the structure, function, and regulation of these recently recognized effectors of heterotrimeric G proteins.
Heterotrimeric G proteins are master regulators of cell homeostasis. By coordinating signaling between the ∼800 G protein-coupled receptors (GPCRs) in the human genome and a relatively small handful of effector enzymes and channels in the cell, they control processes such as muscle contractility, glycogen metabolism, neurotransmission, and the concentration of intracellular ions. Their profound impact on nearly all cellular processes and their therapeutic potential have rendered them one of the most intensely studied signal transduction paradigms at the biochemical and molecular level (Sprang et al., 2007).
When heterotrimeric G proteins are in their inactive, GDP-bound state, they exist as an inert complex composed of α, β, and γ subunits (Gαβγ). In this state, they are substrates for activated GPCRs, which catalyze nucleotide exchange on the α subunit (Gα). When bound to GTP, Gα releases the effector binding surface of the β and γ heterodimer (Gβγ) so that both Gα and Gβγ can interact with and modulate the activity of specific downstream enzymes and channels. The Gα subunit has weak guanine nucleotide triphosphatase (GTPase) activity that slowly returns the G protein to its GDP-bound state. Gα·GDP then becomes resequestered by Gβγ.
Beyond serving as conduits for extracellular signals, heterotrimeric G proteins contribute to the fidelity, duration, and amplitude of GPCR signaling. A given class of heterotrimeric G protein can typically recognize only a subset of GPCRs, and can only interact with one or a few downstream effector targets, ensuring the specificity of signaling from receptor to effector. The rate of GTP hydrolysis on Gα dictates the length of time that its signal is in play, and this rate can be dramatically accelerated by either the effector target or regulators of G protein signaling (RGS) proteins, which serve as GAPs for some classes of Gα subunits (Ross and Wilkie, 2000). Finally, if the activated receptor is not uncoupled from the G protein, such as via phosphorylation by GPCR kinases and binding of arrestins, then Gαβγ can undergo multiple rounds of activation. Thus, regulating the relative rates of G protein activation and deactivation can control the amplitude of signals initiated by GPCRs.
Crystallographic studies have uncovered the molecular mechanism by which activated Gα and Gβγ subunits engage their effector targets. In the case of Gα, the binding of GTP induces a conformational change in structural elements that interact with the γ-phosphate of GTP, known as switch I, II, and III (Fig. 1a). This conformational change lowers the affinity of Gα for Gβγ but increases its affinity for effectors. Upon binding GTP, a shallow hydrophobic canyon is created between switch II and the α3 helix of Gα that is used for binding all the effectors that have been structurally characterized thus far (Tesmer et al., 1997b, 2005; Slep et al., 2001; Chen et al., 2005, 2008; Lutz et al., 2007). There is sufficient variability in the residues that line the perimeter of this effector docking site to explain the exquisite specificity of Gα for its downstream targets. In the case of Gβγ, its ability to bind effectors depends on the unmasking of a broad, relatively hydrophobic surface that is sequestered in the Gαβγ complex. Conformational changes between the Gα- and effector-bound states of Gβγ have thus far proved relatively minor. Although the binding surface for the effectors of Gβγ is believed to overlap with that of Gα, they are believed to involve distinct regions in each case (Ford et al., 1998; Davis et al., 2005).
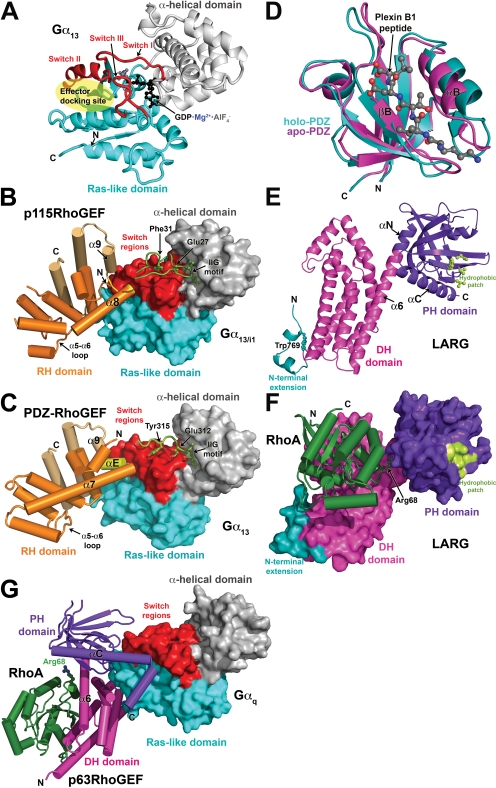
Fig. 1.
Structural studies of heterotrimeric G protein-regulated RhoGEFs. a, structure of Gα13 in its active, GDP-AlF4−-bound conformation. The Ras-like domain is colored cyan, and the α-helical domain is gray. The three nucleotide-dependent switch regions (switch I–III) are red. The canonical effector docking site, a shallow canyon formed between switch II and the α3 helix, is indicated by the transparent yellow ellipse. b, the p115RhoGEF rgRGS domain in complex with Gα13/i1·GDP·AlF4−. The Gα subunit is shown as a molecular surface and is colored as in a. The p115RhoGEF RH domain (orange) binds to the effector docking site of the Gα13/i1 chimera with its α8-α9 loop (yellow). Noncanonical helices that extend the C terminus of the RH domain are colored in gold. The N-terminal motif responsible for GAP activity (green) binds to the α-helical domain with its IIG motif, followed by residues that stabilize catalytic residues in the GTPase active site. c, the rgRGS domain of PDZ-RhoGEF in complex with Gα13·GDP·AlF4−. A short α helix (αE, colored yellow) binds in the effector docking site, which is expanded relative to the effector docking site in the Gα13/i1-p115RhoGEF complex. The N-terminal motif follows the same path as in the complex with p115RhoGEF, but there is no productive interaction with catalytic residues, explaining the absence of GAP activity. d, the LARG PDZ domain. The plexin B1 C-terminal octapeptide binds in a cleft formed between the βB strand and the αB helix, only the last five residues of the peptide being well defined. Ligand binding induces an atypical conformational change in the domain that expands the ligand binding cleft, particularly at the base of the domain (toward bottom of panel). e, the structure of the tandem DH/PH domains of LARG in their RhoA-bound conformation. Elements characteristic of the Lbc subfamily (Fig. 2a) include an N-terminal extension (teal) that coalesces around a buried tryptophan side chain (Trp769) and a hydrophobic patch on the surface of the PH domain (light green) that was recently shown to be critical for RhoA signaling in cells (Aittaleb et al., 2009). The α6 helix of the DH domain is fused to the αN helix of the PH domain to create a continuous, flexible helix that links the two domains. Residues in the α6-αN linker region form critical contacts with switch II of RhoA. f, the LARG DH/PH-RhoA complex. The tandem DH/PH domain is shown as a molecular surface and is colored as in e. RhoA binds principally to the DH domain, although the PH domain also contributes a small amount of buried surface area. Arg68 from switch II of RhoA (ball and stick model) interacts with residues in the α6-αN hinge. The complex is oriented such that the expected plane of the membrane is along the top of the panel. g, atomic structure of the Gαq-p63RhoGEF-RhoA complex. Gαq is shown as a molecular surface, and its domains are colored as for Gα13 in a. The DH (magenta) and PH (purple) domains both contact Gαq, the extended αC helix of the PH domain binding in the effector docking site. Nucleotide-free RhoA binds on the opposite surface of the DH domain from Gαq.
A Role for GPCRs in Cell Migration and Transformation
GPCRs are traditionally thought of as regulators of cellular metabolism or as mediators of sensory perception, such as vision, smell, and taste. The canonical effector enzymes involved in these processes include adenylyl cyclase, cGMP phosphodiesterase, and phospholipase Cβ (PLCβ). The Gα subunits that couple receptors to these effectors represent three of the four subfamilies of human heterotrimeric Gα subunits: Gαs, Gαi/t, and Gαq, respectively. However, GPCRs can also serve as potent oncogenes in a manner more typical of tyrosine kinase growth factor receptors (Whitehead et al., 2001). Indeed, constitutively activated Gα subunits, in particular members of the Gα12/13 subfamily, can lead to strong cell proliferative responses (Dhanasekaran et al., 1998). The mechanism by which this occurs began to be understood by the observation that lysophosphatidic acid (LPA) and bombesin, known ligands for GPCRs, can activate the small-molecular-weight G protein RhoA and induce the formation of stress fibers and focal adhesions (Ridley and Hall, 1992). In subsequent years, numerous studies lent additional support to the idea that Rho GTPases were activated downstream of many GPCRs (Seasholtz et al., 1999; Sah et al., 2000).
The Rho GTPases are a family of peripheral membrane proteins that regulate essential cellular processes, including cell shape, cell migration, cell cycle progression, and gene transcription (Etienne-Manneville and Hall, 2002; Wennerberg and Der, 2004). Like their larger Gα subunit homologs, they cycle between an inactive GDP-bound and an active GTP-bound state that can interact with specific downstream effector targets, depending on the Rho GTPase. In vivo, Rho GTPases require an upstream guanine nucleotide exchange factor (GEF) for activation. The largest and best characterized RhoGEF family is characterized by a catalytic Dbl homology (DH) domain, an extended helical domain of ∼200 amino acids that forms the principal binding site for the nucleotide-free GTPase. The DH domain is almost always immediately followed in the primary sequence by a pleckstrin homology (PH) domain (Schmidt and Hall, 2002; Rossman et al., 2005). In some DH-family RhoGEFs, the PH domain inhibits the intrinsic GEF activity of the DH domain (Das et al., 2000; Welch et al., 2002; Bellanger et al., 2003; Lutz et al., 2007), a constraint that is presumably released upon the interaction of the PH domain with phospholipids or other proteins. In other Dbl family members, the PH domain can play a positive signaling role (Liu et al., 1998; Reuther et al., 2001; Rossman et al., 2002).
GPCRs could regulate Rho GTPase signaling either directly via the interaction of activated heterotrimeric G proteins with a RhoGEF, or indirectly via, for example, activation of protein kinase A (PKA) or PKC, which can then phosphorylate RhoGEFs, their upstream regulators, or their downstream targets. In 1998, it was shown that Gα13 subunits bind directly to p115RhoGEF and stimulate its activity (Hart et al., 1998), thereby revealing a simple, direct path from LPA or thrombin receptors to RhoA (Wang et al., 2004). In 2005, it was shown that Gαq/11 could activate the activity of another RhoA-selective RhoGEF, p63RhoGEF, providing another direct pathway, this time initiated by bombesin, muscarinic, and angiotensin receptors (Lutz et al., 2005). Gβγ is also now known to directly bind and activate RhoGEFs, such as phosphatidylinositol (3,4,5)-triphosphate (PIP3)-dependent Rac exchanger 1 (P-Rex1), which links Gi-coupled chemokine receptors in neutrophils to the activation of Rac2 (Welch et al., 2002).
In this review, we examine what is currently known about the structure and function of Dbl-family RhoGEFs that are directly regulated by heterotrimeric G proteins (Tables 1 and 2, Fig. 2). We focus primarily on three aspects relevant to molecular pharmacology: 1) the structures of RhoGEF domains involved in signal transduction, 2) the specific details of how heterotrimeric G proteins are known to interact with these domains, and 3) the potential mechanisms by which these interactions lead to RhoGEF activation.
TABLE 1.
Heterotrimeric G proteins that directly regulate Dbl family RhoGEFs
| Heterotrimeric G Protein Family & RhoGEF | Known Physiological Roles | Rho Family Substrate |
|---|---|---|
| Gα12/13 | ||
| p115RhoGEF/Lsc | Neutrophil migration, leukocyte homeostasis (Girkontaite et al., 2001; Francis et al., 2006); regulation of apoptosis in thymocytes (Harenberg et al., 2005) | Rho |
| LARG | Signal transduction downstream of thrombin receptors (Wang et al., 2004); growth cone collapse (Swiercz et al., 2002; Hata et al., 2009); induction of salt-induced hypertension in vascular smooth muscle (Ying et al., 2006; Wirth et al., 2008) | Rho |
| PDZ-RhoGEF | Signal transduction downstream of LPA receptors (Wang et al., 2004); Ca2+ sensitization in smooth muscle (Derewenda et al., 2004); neutrophil polarization (Wong et al., 2007); neurite retraction (Togashi et al., 2000); angiotensin II induced contraction in vascular smooth muscle cells (Hilgers et al., 2007; Ying et al., 2009) | Rho |
| Lbc | Promotion of cardiac hypertrophy (Appert-Collin et al., 2007; Carnegie et al., 2008) | Rho |
| Gαq/11 | ||
| p63RhoGEF | Gαq/11-dependent activation of RhoA in smooth muscle cells (S. Lutz, personal communication) | Rho |
| TrioC | In C. elegans, the C-terminal DH/PH domain of UNC-73 promotes acetylcholine vesicle release at neuromuscular junctions (Williams et al., 2007) and regulation of pharynx pumping, speed of locomotion, and egg-laying (Steven et al., 2005) | Rho |
| KalirinC | Neurite extension and neuronal morphology (Penzes et al., 2001) | Rho |
| Gβγ | ||
| P-Rex1 | ROS generation and rate of chemotaxis in neutrophils (Dong et al., 2005; Welch et al., 2005); Purkinje cell morphology and cerebellar function (Donald et al., 2008) | Rac |
| P-Rex2 | Purkinje cell morphology and cerebellar function (Donald et al., 2008) | Rac |
| p114RhoGEF | Unknown | Rho/Raca |
| Clg/PLEKHG2 | Unknown | Rac/Cdc42a |
| Tim/Arhgef5 | Dendritic cell chemotaxis, allergenic airway inflammation (Wang et al., 2009) | Rho |
GTPase specificity has not been demonstrated in vitro.
TABLE 2.
Structural analysis of heterotrimeric g protein-regulated RhoGEFs
| RhoGEF & Domain(s) | Functional Insights | Protein Data Bank Entry |
|---|---|---|
| p115RhoGEF | ||
| RH domain | Along with 1htj, confirmed structural homology of the RhoGEF RH domain to those of RGS proteins | 1iap |
| Gαi/13-rgRGS domain complex | Showed bipartite interaction of the rgRGS domain with an activated chimeric Gα subunit, with the RH domain docking in the effector binding pocket (unlike RGS proteins), and the N-terminal GAP motif interacting primarily with Switch I (like RGS proteins). | 1shz |
| LARG | ||
| DH/PH domains | Along with 1x86, demonstrated flexibility in the α6-αN linker between the DH and PH domains and, along with 1xcg, revealed unique, potentially regulatory features. | 1txd |
| DH/PH-RhoA complex | Along with 1xcg, showed that both DH and PH domains of RH-RhoGEFs directly engage RhoA, helping to explain the positive signaling role of the PH domain in this subfamily. | 1x86 |
| PDZ domain | Along with 2dls, confirmed expected PDZ domain fold in the RH-RhoGEF subfamily. | 2omj(NMR) |
| PDZ domain-Plexin B1 C-terminal octapeptide | Described interaction between the PDZ domain and a C-terminal peptide from one of its receptor targets, and the conformational changes induced by ligand binding. | 2os6(NMR) |
| PDZ-RhoGEF | ||
| RH domain | Along with 1iap, confirmed structural homology of the RhoGEF RH domain with those of RGS proteins. | 1htj |
| Gα13-rgRGS domain complex | Although the Gα binding motifs of PDZ-RhoGEF and p115RhoGEF are structurally distinct, the effector docking site of Gα13 changes shape to accommodate either. The GAP motif of PDZ-RhoGEF is shown to interact differently with Gα13 in its GDP, GDP·AlF4−, and guanosine 5′-O-(3-thio)triphosphate-bound states. An explanation for PDZ-RhoGEF's lack of GAP activity was provided. | 3cx6, 3cx7, 3cx8 |
| DH/PH-RhoA complex | Along with 1x86, showed that both DH and PH domains of RH-RhoGEFs directly engage RhoA, helping to explain the positive signaling role of the PH domain in this subfamily. | 1xcg |
| PDZ domain | Along with 2omj, confirmed expected PDZ domain fold in the RH-RhoGEF subfamily. | 2dls (NMR) |
| p63RhoGEF | ||
| Gαq-DH/PH-RhoA complex | Revealed that Gαq interacts with both the DH and PH domains of TrioC subfamily RhoGEFs. The αC helix of the PH domain binds in the effector docking site of Gαq, whereas additional contacts mediated by the DH domain appear required for activation. | 2rgn |
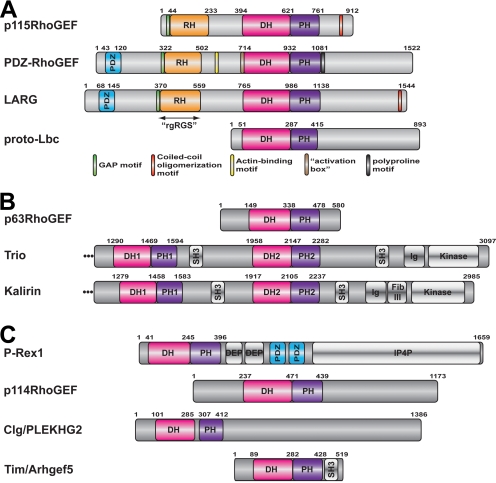
Fig. 2.
Domain structure of RhoGEFs reported to be directly regulated by heterotrimeric G proteins. The amino acid numbers shown above each protein correspond to the human ortholog. For domains that have not been structurally characterized, domain boundaries were assigned based on sequence alignment with their closest homologs of known structure unless otherwise indicated. a, RhoGEFs regulated by Gα12/13 subunits. p115RhoGEF, LARG, and PDZ-RhoGEF constitute the RH-RhoGEF subfamily and are characterized by an N-terminal RH domain. Short sequence motifs that seem to play functional roles are shown as small bars and are color-coded according to the key at the bottom of the panel. b, RhoGEFs regulated by Gαq subunits. All three RhoGEFs have closely related DH/PH domains that constitute the TrioC subfamily. c, RhoGEFs regulated by Gβγ subunits. None of these RhoGEFs are closely related, although p114RhoGEF belongs to the Lbc subfamily of RhoGEFs; hence, its DH/PH domains are closely related to those of the RH-RhoGEFs and Lbc. The boundaries for the PDZ domains and the domains shown in gray were assigned based on the GenPept entry for each protein.
Gα12/13-Regulated RhoGEFs
There are currently four RhoGEFs believed to be directly stimulated by the Gα12/13 subfamily of heterotrimeric G proteins: p115-RhoGEF, leukemia-associated RhoGEF (LARG), postsynaptic density 95, disk large, zona occludens-1 (PDZ)-RhoGEF, and lymphoid blast crisis (Lbc) (Siehler, 2009; Suzuki et al., 2009a) (Table 1, Fig. 2a). Although these enzymes all belong to the Lbc subfamily of RhoGEFs, it is not yet clear whether their homology correlates with an ability to be regulated by heterotrimeric G proteins. The supporting data are weakest for Lbc, which has not yet been shown to bind or to be activated by Gα12/13 subunits using purified proteins in vitro.
The RH-RhoGEFs
Background.
Members of the Gα12/13 subfamily are potent regulators of the actin cytoskeleton (Buhl et al., 1995) and are important for vascular development and chemotaxis (Offermanns et al., 1997). Given that Rho GTPases were also well established regulators of these responses, the hunt was on for RhoGEFs that could operate directly downstream of Gα12/13. One candidate was p115RhoGEF, a RhoA-selective GEF containing a region with very weak homology to the catalytic RGS homology (RH) domain characteristic of RGS proteins (Tesmer, 2009). Indeed, this proved to be the binding site for activated Gα12 and Gα13 subunits, although only Gα13 could stimulate GEF activity (Hart et al., 1998; Kozasa et al., 1998; Mao et al., 1998). Soon thereafter, PDZ-RhoGEF (Fukuhara et al., 1999), also known as GTRAP48 (Jackson et al., 2001), and LARG (Fukuhara et al., 2000) were identified as RH-domain containing RhoGEFs. These three proteins form the RH-RhoGEF subfamily, a small subfamily of Gα13-regulated RhoGEFs. LARG is the only one currently known to be activated by Gα12, although this seems to require phosphorylation of the RhoGEF by Tec tyrosine kinase (Suzuki et al., 2003). All three RH-RhoGEFs are widely expressed in mammals (Hart et al., 1996; Fukuhara et al., 1999; Kourlas et al., 2000; Kuner et al., 2002), but p115RhoGEF and PDZ-RhoGEF are most abundantly expressed in hematopoietic cells and the central nervous system, respectively.
In resting cells, RH-RhoGEFs are predominantly localized to the cytosol, with some presence at the cell membrane or bound to actin. Upon activation of Gα12/13 coupled receptors or coexpression of constitutively active mutants of Gα12 or Gα13, RH-RhoGEFs translocate to the membrane surface (Siehler, 2009; Suzuki et al., 2009a). RH-RhoGEFs also exhibit some receptor selectivity. In HEK293 and PC3 cells, RhoA is activated by PDZ-RhoGEF via LPA receptors and by LARG via thrombin receptors (Wang et al., 2004). The mechanism by which this selectivity occurs is not known, but it is not apparently due to differences in their PDZ domains, because both PDZ-RhoGEF and LARG can bind to the C termini of LPA receptors (Yamada et al., 2005).
The RH RhoGEFs are complex proteins with multiple signaling domains and functional motifs hidden within the low-complexity regions that bracket them. There is considerable evidence that the mechanism of activation is also complex, involving not only recruitment to specific sites at the membrane surface, but also multiple intra- and intermolecular interactions that mediate inhibition of basal activity as well as allosteric activation (Bhattacharyya and Wedegaertner, 2003; Bhattacharyya et al., 2009; Suzuki et al., 2009b; Zheng et al., 2009). The specific details vary among subfamily members (e.g., p115RhoGEF lacks a PDZ domain) and context (e.g., LPA versus thrombin receptors). In the following sections, we summarize what is known about the structure and function of the major domains and other functional motifs of RH-RhoGEFs, highlighting their potential roles in regulating the activity of the DH/PH domains. We end by describing recent solution-based studies of LARG and PDZ-RhoGEF that provide new insights into the molecular mechanism of RH-RhoGEF activation.
The RhoGEF RH Domain.
As the principal binding site for Gα12/13 subunits, structural analysis of the RhoGEF RH domain was anticipated to explain the molecular basis for selectivity and GAP activity and to shed some light on the mechanism of regulation by heterotrimeric G proteins. The canonical RH domain is a bundle of nine helices that forms a relatively flat domain with two lobes (Tesmer, 2009). In RGS proteins, the α5-α6 loop of the domain interacts with catalytic residues in switch I and II of Gα to accelerate GAP activity (Tesmer et al., 1997a). In contrast, the RH domains of p115RhoGEF and LARG do not have GAP activity. Instead, a short sequence motif positioned immediately N-terminal to the RH domain is required for stimulating GTP hydrolysis in p115RhoGEF and LARG (Chen et al., 2003). The region spanning this N-terminal motif and the RH domain has been termed the “rgRGS” domain (Chen et al., 2001) (Fig. 2a).
Crystal structures of the p115RhoGEF (Chen et al., 2001) and the PDZ-RhoGEF (Longenecker et al., 2001) RH domains confirmed that they indeed retain most of the canonical RH domain fold, albeit with a ∼65-residue extension that contributes three extra α helices to the C terminus (Fig. 1, b and c). However, these initial structures did not reveal an obvious mode of interaction with Gα12/13 subunits, in part because the Gα-interacting residues of RGS proteins are not conserved in the RhoGEF RH domain (Tesmer, 2009). Although the entire rgRGS protein of p115RhoGEF was crystallized, the N-terminal GAP motif was not ordered.
The structure of the p115RhoGEF rgRGS domain in complex with a chimeric Gα13/i1 protein (Chen et al., 2005) revealed a surprising bipartite interaction mediated by the N-terminal GAP motif and the RH domain (Fig. 1b). The Gα13/i1 chimera used in this study was created to circumvent problems in expressing functional Gα13 in Escherichia coli and contains the α helical domain and switch I and II elements from Gα13, with the remainder from Gαi1. As a consequence, the effector docking site contains residues that are not native to Gα13, including some that directly contact the RH domain. It is noteworthy that this chimera was never shown to activate p115RhoGEF. The N-terminal motif (residues 22-37) begins with an IIG sequence motif that anchors the peptide on the α-helical domain of Gα13/i1 and ends with an acidic sequence in which Glu27 and Phe31 contact Gα13/i1-Arg200 in switch I and Gln226 in switch II, respectively, stabilizing them in a more transition state-like conformation. The structure thereby provides a molecular explanation for the mild GAP activity exhibited by p115RhoGEF. The RH domain (residues 44-233), as if supplanted from switch I by the N-terminal GAP motif, binds to the canonical effector docking site of Gα13/i1 (Fig. 1b). Instead of using the α5-α6 loop to bind Gα, the interaction is mediated by structural elements from the unique C-terminal extension of the RH domain, in particular the α8-α9 loop, which bears hydrophobic residues that pack into the effector docking site. Consequently, the canonical core of the RhoGEF RH domain can be thought of as a scaffold that positions unique functional motifs/extensions at its N and C termini that are required for high-affinity binding, recognition of the activated conformation of the Gα subunit, and, in p115RhoGEF and LARG, GAP activity.
Recent crystal structures of complexes between a different Gα13 chimera (all but the N-terminal helix was native to Gα13) and the PDZ-RhoGEF rgRGS domain recapitulate the major findings of the Gα13/i1-p115RhoGEF complex (Chen et al., 2008). As before, the N-terminal motif of the RhoGEF anchors to the α-helical domain of Gα13 using an IIG sequence motif and interacts with the switch regions (Fig. 1c). In this case, however, Glu312 and Tyr315 (analogous to Glu27 and Phe32 in p115RhoGEF) do not make productive interactions with residues in the active site of Gα13, explaining the lack of GAP activity as well as the observation that PDZ-RhoGEF has similar affinity for the guanosine 5′-O-(3-thio)triphosphate- and GDP·AlF4−-bound states of Gα13 (Chen et al., 2008). It is noteworthy that residues in the RH domain of p115RhoGEF that bind to the effector docking site of Gα13 are not conserved in PDZ-RhoGEF (Fig. 1, b and c). Despite this difference, a similar complex is formed, with the effector docking site expanding to accommodate the larger “αE” helix that occupies the position analogous to the α8-α9 loop of p115RhoGEF. However, it is not clear whether the differences in the structure of the effector docking site are a consequence of differences in the chimeric Gα13 subunits used, differences in the RH domains, or both. The structure of the PDZ-RhoGEF rgRGS domain in complex with deactivated Gα13·GDP was also determined, in which switch II retains its active conformation despite the presence of GDP. Thus, the duration of signaling via RH-RhoGEFs could be regulated not only by the rate of GTP hydrolysis but also by the rate at which Gα13·GDP is sequestered by Gβγ (Chen et al., 2008).
The PDZ Domain.
PDZ-RhoGEF and LARG can also be activated by receptors other than GPCRs via interactions mediated by the PDZ domains found at their N termini. PDZ domains are common structural domains that typically bind the C termini of proteins found at the cell membrane (Nourry et al., 2003). Compared with other PDZ domains, the RH-RhoGEF PDZ domain binds to surprisingly diverse biological targets (for review, see Smietana et al., 2008). Perhaps the best characterized is plexin B1, a semaphorin 4D receptor that mediates RhoA activation and axonal growth cone collapse (Aurandt et al., 2002; Hirotani et al., 2002; Perrot et al., 2002; Swiercz et al., 2002). Both RhoGEFs also bind to LPA-1 and -2 receptors, which couple primarily with Gα12/13 subunits. Interaction between the C termini of these receptors and the PDZ domain was reported to be required for RhoA activation (Yamada et al., 2005).
NMR structures of the LARG PDZ domain alone and in complex with a plexin-B1 C-terminal octapeptide have been reported (Liu et al., 2008) (Fig. 1d). A solution structure of the PDZ-RhoGEF PDZ domain is also available (Protein Data Bank entry 2dls). They retain the typical PDZ domain fold, which consists of six antiparallel strands (βA-βF) and two helices (αA and αB). Peptide ligands dock in an extended conformation into a groove located between βB and αB, where they form a β-sheet like interaction with the βB strand. The LARG PDZ domain undergoes an atypical conformational change upon ligand binding relative to other characterized PDZ domains (Fig. 1d), and the apo form exhibits a high degree of conformational flexibility (Liu et al., 2008). These characteristics may facilitate the binding of these PDZ domains to a wide variety of targets.
DH/PH Domain Structures.
The canonical DH domain is composed of 10 or more helices that assemble into an oblong shape reminiscent of a chaise lounge (Worthylake et al., 2000). The last helix (called α6) transverses the longest dimension of the domain and often forms a continuous helix with αN, the first helix of the PH domain (Fig. 1e). The PH domain consists of a flattened seven-stranded antiparallel β-barrel, capped at one end by αC, the hallmark C-terminal helix found in all PH domains. In some PH domains, loops on the opposite end of the barrel from αC are used to bind specific phospholipid head groups. The αN helix is characteristic of PH domains found in DH/PH tandems.
Structures have been determined for the LARG DH/PH domains, both alone and in complex with RhoA (Kristelly et al., 2004) and for the PDZ-RhoGEF DH/PH-RhoA complex (Derewenda et al., 2004). Both RhoA complexes exhibit similar domain organization and intersubunit contacts (Fig. 1f). As in Dbs, another RhoGEF in which the PH domain contributes positively to GEF activity (Rossman et al., 2002, 2003), direct contacts are formed between RhoA and the PH domain. Although this interface accounts for only ∼8% of the total buried surface area in the LARG DH/PH-RhoA complex, mutation of residues in the PH domain-RhoA interface diminished GEF activity to the level of the DH domain alone (Kristelly et al., 2004). Mutation of residues in the PDZ-RhoGEF interface did not lead to significant differences in vitro (Oleksy et al., 2006), and hence the functional importance of the RhoA-PH domain interface seems to vary among RH-RhoGEFs.
Examination of the DH/PH structures reveals several structural elements that could play a role in regulation. The first is a flexible, helical extension at the N terminus of the DH domain (Fig. 1e). Either truncation of the extension or mutation of a tryptophan (LARG-Trp769) buried between the extension and the DH domain reduced GEF activity (Kristelly et al., 2004). However, the LARG-W769D mutation exhibited only a minor defect in serum response element transcription assays in cells (Aittaleb et al., 2009). Sequence analysis predicts that the extension exists in all Lbc subfamily RhoGEFs, suggesting that it is not involved in regulation specific to the RH-RhoGEFs. Instead, recent data suggest that the N-terminal extension could be more important for autoinhibition of basal activity (see Solution Studies).
The second notable feature is a relatively long helical linker between the DH and PH domains that allows for considerable conformational flexibility between the two domains. For example, there is a ∼30° difference in orientation of the PH domain relative to the DH domain between the LARG DH/PH and DH/PH-RhoA structures, the hinge occurring at the junction of the α6 and αN helices. The four unique DH/PH domains in the LARG DH/PH-RhoA structure also exhibit conformational differences in the α6-αN linker (Kristelly et al., 2004). Because the mutation of residues in the PH domain that directly interact with RhoA inhibits GEF activity (Kristelly et al., 2004), these contacts were proposed to alter the conformation of the α6-αN linker region in a manner that facilitates the binding of RhoA. Indeed, residues at the end of α6 make critical interactions with switch II of the bound GTPase (Aghazadeh et al., 1998). Interactions with other proteins or phospholipids that likewise influence the relative orientation of the DH and PH domains could likewise affect LARG activity. Solution studies of the PDZ-RhoGEF DH/PH domains found no evidence for an analogous large conformational change in the linker region (Cierpicki et al., 2009), suggesting that in PDZ-RhoGEF the PH domain may simply serve to stabilize the α6 helix of the DH domain.
Finally, in all three DH/PH structures, a solvent-exposed patch of conserved hydrophobic residues is observed in the PH domain (Fig. 1e,f). Depending on the crystal structure, the patch forms either a 2-fold crystallographic or a quasi-2-fold molecular interface that buries ∼800 Å2 of surface area. Located on the side of the PH domain β barrel, the patch would be in position to bind adjacent peripheral membrane proteins or domains at the cell membrane. Mutation of residues in the hydrophobic patch of LARG had no effect on nucleotide exchange activity in vitro but abrogated the ability of LARG to induce RhoA activation and stress fiber formation in cells (Aittaleb et al., 2009). Because the activity of these mutants could be rescued by fusion with nonspecific membrane targeting motifs, the hydrophobic patch seems to contribute to the proper localization of LARG by interacting with unknown target(s) at the cell membrane. However, membrane targeting by constitutively activated Gα13 did not rescue activity, suggesting that the hydrophobic patch also plays an essential role in GPCR-mediated regulation, although it does not yet seem to involve direct interactions with activated Gα13 (Aittaleb et al., 2009). The hydrophobic patch is conserved among all Lbc subfamily members, once again indicating a common functional role. Indeed, mutation of the hydrophobic patch of Lbc likewise abolished its ability to promote RhoA-mediated transcriptional activation in cells (Aittaleb et al., 2009).
Other Functional Motifs.
p115RhoGEF, LARG, and PDZ-RhoGEF undergo homo- and heterodimerization mediated by their C-terminal tails, and specific coiled-coil motifs have been identified in p115RhoGEF and LARG (Eisenhaure et al., 2003; Chikumi et al., 2004; Grabocka and Wedegaertner, 2007) (Fig. 2a). Deletion of the C terminus augments the ability of RH-RhoGEFs to induce RhoA activation in cells, suggesting a negative regulatory role for dimerization, presumably via the recruitment of unknown inhibitory factors (Eisenhaure et al., 2003; Chikumi et al., 2004). A proline-rich motif positioned immediately C-terminal to the PDZ-RhoGEF PH domain (residues 1081-1119) has been shown to be essential for plasma membrane localization of PDZ-RhoGEF and is required for PDZ-RhoGEF induced cortical actin reorganization and cell rounding (Togashi et al., 2000). The RH-DH/PH linker region also contains functional elements. In addition to an acidic autoinhibitory motif (Zheng et al., 2009), the RH-DH/PH linker of PDZ-RhoGEF contains a unique actin-binding motif (Banerjee and Wedegaertner, 2004; Banerjee et al., 2009).
Solution Studies.
Two recent solution-based studies have provided new insights into RH-RhoGEF regulation (Fig. 3a). Using surface plasmon resonance, the first study showed that Gα13 could interact not only with the RH but also with the DH/PH and C-terminal regions of LARG (Suzuki et al., 2009b), consistent with earlier observations using coimmunoprecipitation (Wells et al., 2002; Bhattacharyya and Wedegaertner, 2003). The presence of all three domains (RH, DH, and PH) was required for formation of the highest affinity complex, and the K204A mutation of Gα13, which abrogates binding to the RH domain (Nakamura et al., 2004; Grabocka and Wedegaertner, 2005), did not eliminate binding to the DH/PH domain. Thus, Gα13 seems to exhibit at least two LARG binding surfaces: one for the rgRGS domain that is dependent on the activation status of Gα13 and another for the DH/PH domains that uses activation-independent structural elements, potentially the C-terminal region of the Ras-like domain (Kreutz et al., 2007).
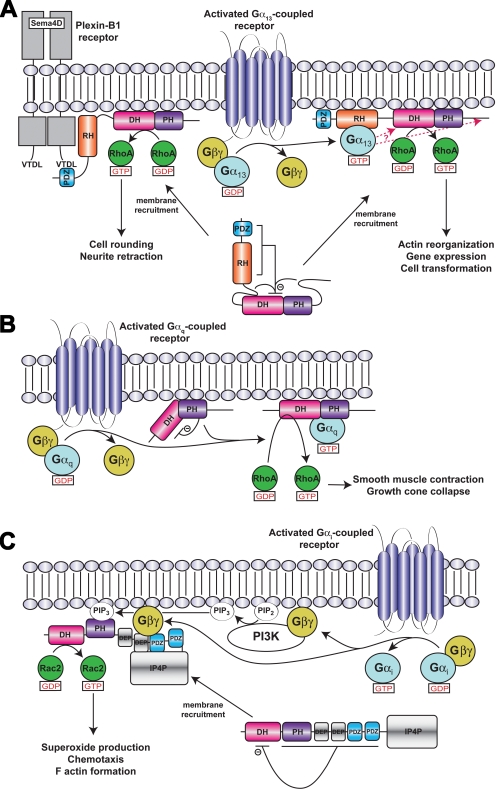
Fig. 3.
Models of RhoGEF activation. a, LARG/PDZ-RhoGEF activation. Membrane recruitment and RhoA activation is mediated by receptors or other proteins that interact with the PDZ domain, such as the plexin B1 receptor, or by activated Gα13 subunits, which interact with the RH domain. Plexin B1 signaling to RhoA does not seem to require Gα13 (Perrot et al., 2002). Although membrane recruitment is one aspect of signal transduction to RH-RhoGEFs, additional interactions at the cell membrane involving the various structural domains and sequence motifs of the RH-RhoGEF are probably required for full activation (Bhattacharyya and Wedegaertner, 2003; Bhattacharyya et al., 2009). Indeed, there is evidence that Gα13 interacts with other regions in addition to the RH domain (indicated by red arrows) to stabilize a high-affinity complex and stimulate GEF activity (Suzuki et al., 2009b). Autoinhibition of basal activity is believed to be imposed by the cooperative actions of the N-terminal region and C-terminal tail of the RhoGEF, as well as by an acidic sequence in the RH-DH linker region (Zheng et al., 2009). Not included in this schematic is the hydrophobic patch of the PH domain, which is required for RhoA activation in cells and probably mediates protein-protein interactions at the membrane surface (Aittaleb et al., 2009). b, p63RhoGEF/TrioC subfamily activation. Unlike in RH-RhoGEFs, p63RhoGEF seems to be membrane-associated and the PH domain of p63RhoGEF autoinhibits basal activity, in part because of unfavorable interactions between switch II of RhoA and residues in the α6-αN linker region of p63RhoGEF. The bridging interactions of the Gα subunit are proposed not only to fix the PH domain in a less inhibitory conformation but also to activate the DH domain allosterically. c, Activation of P-Rex1 by Gβγ subunits in neutrophils. In neutrophils, P-Rex1 is a Rac2-specific GEF (Dong et al., 2005; Welch et al., 2005) that can be fully activated by GPCRs because of the presence of class IB PI3K (Donald et al., 2004), which are likewise activated by Gβγ subunits. The mechanism of activation shown is based on that proposed by Urano et al. (2008) with Gβγ binding to an intramolecular complex formed by the second DEP, first PDZ, and IP4P domains. Binding to Gβγ is expected to relieve the inhibition imposed by the DEP and PDZ domains (Hill et al., 2005) and recruit the enzyme to the cell surface.
Truncation studies of PDZ-RhoGEF identified a conserved acidic motif (residues 706-712) a few amino acids upstream of the N-terminal helical extension of the DH domain that serves an autoinhibitory role (Zheng et al., 2009). NMR spectroscopy comparing the chemical shifts in DH/PH fragments with or without this acidic motif suggests that it interacts with basic residues on the DH domain, in particular Arg867 and Arg868. Because these residues also interact with the bound GTPase, their interaction with the acidic motif would form an autoinhibited state reminiscent of that of Vav (Aghazadeh et al., 2000). Indeed, charge-reversing mutations of four of the acidic residues in the motif (the “activation box”; Fig. 2a) enhanced GEF activity ∼20-fold in a DH/PH fragment of PDZ-RhoGEF. The enhancement was curiously only ∼2-fold for the same mutants in the context of a larger fragment that also contained the PDZ and RH domain. This may indicate that the acidic motif and the PDZ and RH domains act synergistically to autoinhibit activity. Acidic motifs also occur in the RH-DH linker regions of p115RhoGEF and LARG, although it remains to be seen if they function similarly. If the activation box is used in all Lbc subfamily members to regulate basal activity, it may provide a functional explanation for the N-terminal extension of the DH domain, which folds in such a manner that it would juxtapose the acidic motif of PDZ-RhoGEF with Arg867 and Arg868 on the surface of the DH domain.
Lbc-RhoGEF
Lbc RhoGEF was originally isolated as a transforming oncogene (onco-Lbc) from patients with myeloid leukemia (Toksoz and Williams, 1994) and is the founding member of the Lbc subfamily of RhoGEFs. No atomic structures are currently available for this enzyme. Beyond the DH/PH domains, proto-Lbc contains a predicted α-helical domain and a proline rich motif in its C terminus that have been shown to act as negative regulators of proto-Lbc transforming activity and localize the enzyme to the membrane fraction of cells (Sterpetti et al., 1999). Onco-Lbc lacks this C-terminal region. The Lbc-RhoGEF splice variant AKAP-Lbc, or AKAP13, is highly expressed in the heart and functions both as a cAMP-dependent protein kinase (PKA)-scaffolding protein and a GEF for RhoA (Diviani et al., 2001). Similar to the RH-RhoGEFs, there is evidence that AKAP-Lbc can undergo homo-oligomerization through a leucine zipper motif in the C-terminal region. Deletion of this C-terminal region in AKAP-Lbc enhances its GEF activity, suggesting a negative regulatory role for oligomerization (Baisamy et al., 2005).
Proto-Lbc and AKAP-Lbc have been shown to be specifically stimulated by Gα12 as well as via activation of the LPA receptor (Majumdar et al., 1999; Diviani et al., 2001; Dutt et al., 2004). The activation seems direct, because Lbc can be coimmunoprecipitated from HEK293 cells with constitutively active Gα12 (Diviani et al., 2001; Dutt et al., 2004). However, it should be noted that Gαq can also be precipitated with Lbc, although with no obvious effect on RhoA activation (Sagi et al., 2001; Pi et al., 2002). The binding site for Gα12 is not known, but a region C-terminal to the PH domain with debatable homology to RH domains has been identified (Dutt et al., 2004). This region overlaps with the leucine zipper motif shown to mediate homodimerization (Baisamy et al., 2005).
Gαq-Regulated RhoGEFs
The involvement of Gαq in Rho-mediated signaling has been recognized for more than a decade. Dominant-negative RhoA and C3 endotoxin were used to demonstrate the existence of a unique Rho-dependent pathway in response to the activation of Gq-coupled receptors in cardiac myocytes (Sah et al., 1996). Later, the development of cell-based, RhoA pulldown assays enabled a direct demonstration of an increase in RhoA·GTP in response to the activation of Gαq-coupled receptors in HEK293 cells, establishing that Gαq operated upstream of RhoA, at least in some signaling contexts (Chikumi et al., 2002). Studies of mouse embryonic fibroblasts deficient in either Gα12/Gα13 or Gαq/Gα11 subunits confirmed that these G protein families activate RhoA via independent pathways, although RhoA activation by Gα12/Gα13 subunits was more robust (Vogt et al., 2003).
A number of RhoGEFs have been reported to interact with, and are thus potentially regulated by, Gαq. These include LARG (Booden et al., 2002; Chikumi et al., 2002; Vogt et al., 2003) and Lbc (Sagi et al., 2001; Pi et al., 2002). In neither case has a direct interaction between the RhoGEF and Gαq been demonstrated with purified components, and no Gαq-mediated stimulation of GEF activity has been reconstituted in vitro. Furthermore, reports conflict on whether or not the RH domain of LARG is responsible for binding Gαq.
p63RhoGEF
p63RhoGEF, which is expressed predominantly in the brain and heart, has been shown to be activated by Gq-coupled receptors in a manner that directly competes with the activation of PLCβ, the canonical effector of Gαq (Lutz et al., 2005). A splice variant lacking the first 106 amino acids, known as GEFT, was reported to activate Cdc42 and Rac1 in cell-based assays and with purified proteins in vitro (Guo et al., 2003). Although there is the possibility of cross-talk in cells, GEF activity toward Cdc42 and Rac1 has not been demonstrated by other groups (Souchet et al., 2002; Lutz et al., 2004; Rojas et al., 2007). Primary sequence analysis also strongly suggests that p63RhoGEF and its splice variants are selective for RhoA.
The primary sequence of p63RhoGEF lacked an obvious RH domain that could serve as a binding site for Gαq. Initial truncation studies instead localized the binding site to a fragment of p63RhoGEF that includes the PH domain (Lutz et al., 2005). Subsequently, more detailed truncation studies, site-directed mutagenesis, and structure determination revealed that the C-terminal helix of the PH domain formed the principal binding site for activated Gαq (Lutz et al., 2007; Rojas et al., 2007). The closely related, C-terminal set of DH/PH domains in Trio and Kalirin were also shown to bind and be activated by Gαq in vitro (Lutz et al., 2007; Rojas et al., 2007) and in cells (Lutz et al., 2007). Although the physiological role of p63RhoGEF in mammals probably involves smooth muscle function (S. Lutz, personal communication), UNC-73E, the p63RhoGEF homolog in Cenorhabditis elegans, is known to serve as a bona fide downstream effector of Gαq and works in conjunction with PLCβ to regulate the release of acetylcholine vesicles at neuromuscular synapses (Williams et al., 2007). Its knockout, in combination with a PLCβ-null allele, generates the same phenotype as knockouts of Gαq, confirming the necessary involvement of both pathways downstream of Gαq (Williams et al., 2007). Finally, the kinase domain of mixed lineage kinase 3 was reported to bind to p63RhoGEF and inhibit its interaction with Gαq, thereby regulating directed cell migration (Swenson-Fields et al., 2008). Although it is not yet known what moiety of p63RhoGEF could be involved in binding mixed lineage kinase 3, the PH domain would be an obvious candidate. It is noteworthy that Gα16, a divergent member of the Gαq subfamily, binds but fails to activate p63RhoGEF, suggesting that Gα16 serves as a general inhibitor of Gαq/11 signaling through p63RhoGEF, just as Gα12 may inhibit Gα13 signaling through p115RhoGEF (Yeung and Wong, 2009). The idea of Gα16 serving as a p63RhoGEF “trap” is also supported by at least one other study (Moepps et al., 2008).
Structural Studies of p63RhoGEF.
After delineation of the minimal fragment of p63RhoGEF required for high-affinity binding to Gαq, the Gαq-DH/PH-RhoA complex was isolated and crystallized. Its structure determination revealed that the C-terminal helix of the p63RhoGEF PH domain was unusually long (Fig. 1g), the hydrophobic residues on the extension of this helix binding directly into the effector docking site of Gαq (Lutz et al., 2007). Mutation of these hydrophobic residues abrogates binding (Lutz et al., 2007; Rojas et al., 2007). Other residues, principally from the β1-β4 sheet of the PH domain, form specific contacts that help to dictate specificity for the Gαq/11 subfamily. The interface is highly reminiscent of the interface between the PH domain of GRK2 and Gβγ (Lodowski et al., 2003), establishing a theme for how PH domains serve as protein-protein interaction modules. It is noteworthy that the manner in which p63RhoGEF engages Gαq is distinct from how the rgRGS domain engages Gα13 in that the GAP binding site on switch I is left freely accessible to RGS proteins. p63RhoGEF lacks significant GAP activity on its own but can allosterically regulate the binding of RGS proteins (Shankaranarayanan et al., 2008).
Although the principal interaction is via the PH domain, the C-terminal region of the Gα protein, including its C-terminal α5 helix, forms direct interactions with the DH domain and residues in the DH/PH domain interface. Mutation of some of these residues in p63RhoGEF leads to a loss of Gαq activation in vitro but had only minor effects on binding, indicating that these contacts are most important for regulation of activity (Lutz et al., 2007). More recently, mutation of Tyr356 in the C terminus of Gαq has also been shown to abrogate activation of p63RhoGEF (A. Shankaranarayanan, C. A. Boguth, S. Lutz, C. Vettel, F. Uhleman, M. Aittaleb, T. Wieland, and Tesmer JJ, submitted). Because the equivalent residue in Gα16 is isoleucine, this specific contact may help explain why Gα16 binds yet fails to activate p63RhoGEF.
Compared with structures of other characterized DH/PH tandem domains, the PH domain of p63RhoGEF in complex with Gαq is held in an unusual orientation relative to the DH domain, being rotated ∼50° around the axis of the α6 helix relative to those of Dbs and TrioN, the closest homologs of known structure (Snyder et al., 2002; Chhatriwala et al., 2007). The PH domain makes no direct contacts with the substrate RhoA. Although the structure of p63RhoGEF in the absence of Gαq is not yet available, it is anticipated that the domain-bridging interactions of Gαq help to constrain the DH and PH domains in this conformation and that the DH/PH domains adopt a more Dbs-like conformation in the basal state.
Mechanism of Activation.
Unlike the RH-RhoGEFs, p63RhoGEF is predominantly found localized to actin-rich structures (Souchet et al., 2002), and overexpressed p63RhoGEF is localized to the cell surface (A. Shankaranarayanan, C. A. Boguth, S. Lutz, C. Vettel, F. Uhleman, M. Aittaleb, T. Wieland, and Tesmer JJ, submitted). Therefore, membrane translocation is not expected to play a major role in activation. In addition, unlike RH-RhoGEFs and Dbs, the PH domain of p63RhoGEF is autoinhibitory, the isolated DH/PH fragment having far less activity than the isolated DH domain (Lutz et al., 2004, 2007; Rojas et al., 2007). Thus, the PH domain must somehow interfere with the binding of RhoA. Indeed, the binding of Gαq greatly enhances the affinity of RhoA for the DH/PH domains (A. Shankaranarayanan, C. A. Boguth, S. Lutz, C. Vettel, F. Uhleman, M. Aittaleb, T. Wieland, and Tesmer JJ, submitted). Because the PH domain does not inhibit GEF activity when added in trans (A. Shankaranarayanan, C. A. Boguth, S. Lutz, C. Vettel, F. Uhleman, M. Aittaleb, T. Wieland, and Tesmer JJ, submitted), the inhibition mediated by the PH domain seems to require a covalent linkage between the DH and PH domains, once again implicating the α6-αN linker as a region of regulatory importance.
Inspection of residues in the α6-αN linker region indicate that Arg341 of p63RhoGEF might sterically and electrostatically repel Arg68 in switch II of RhoA if the PH domain were in a more Dbs-like orientation relative to the DH domain. An R341A mutation indeed enhances the basal activity of p63RhoGEF but, surprisingly, does not eliminate activation by Gαq (A. Shankaranarayanan, C. A. Boguth, S. Lutz, C. Vettel, F. Uhleman, M. Aittaleb, T. Wieland, and Tesmer JJ, submitted). In fact, the R341A mutation allows Gαq to enhance activity to levels greater than those of the DH domain alone, indicating that Gαq can activate p63RhoGEF not only by removing an autoinhibitory restraint but also by activating the DH domain allosterically. This is presumably carried out via the interactions of the C-terminal region of Gαq with the DH domain, as observed in the crystal structure, which in turn alters the conformation of the α6-αN linker (A. Shankaranarayanan, C. A. Boguth, S. Lutz, C. Vettel, F. Uhleman, M. Aittaleb, T. Wieland, and Tesmer JJ, submitted). A schematic model for Gαq-mediated activation of p63RhoGEF and related DH/PH domains is shown in Fig. 3b.
Trio and Kalirin
Trio and Kalirin are closely related proteins that contain two sets of DH/PH domains and a much more complex domain structure than p63RhoGEF (Fig. 2b). Both proteins play critical roles in neurite growth and neuronal morphology (Table 1). The second set of DH/PH domains in Trio (TrioC) is closely related to that of p63RhoGEF and is likewise selective for RhoA. The first set, TrioN, which is selective for RhoG and Rac, has been structurally characterized (Liu et al., 1998; Skowronek et al., 2004; Chhatriwala et al., 2007). The PH domain of TrioC likewise inhibits GEF activity (Bellanger et al., 2003), and the regulation of the TrioC and KalirinC DH/PH domains by Gαq is expected to be similar to that of p63RhoGEF (Lutz et al., 2007; Rojas et al., 2007). It is not known how the other domains in these complex proteins will affect regulation by heterotrimeric G proteins, especially in light of the fact that downstream signaling from each set of DH/PH domains has the potential to antagonize each other. There are, however, isoforms of Trio and Kalirin in mammals (Kawai et al., 1999; McPherson et al., 2005) and of UNC-73 in C. elegans (Steven et al., 2005) that contain only the C-terminal DH/PH domains. These splice variants are thus expected to act in a manner analogous to that of p63RhoGEF in cells.
Gβγ-Regulated RhoGEFs
As yet, there are no reported structures of Gβγ in complex with a RhoGEF, and compared with the Gα12/13- and Gαq-regulated RhoGEFs, relatively little is known about the specific residues involved in binding or the molecular mechanisms involved. In two of the four examples covered here, the physiological roles of the enzyme are unknown (Table 1). The example with the strongest experimental support is P-Rex1, in which Gβγ-mediated regulation has been demonstrated by a number of different groups, and a direct interaction was quantitatively measured in vitro using surface plasmon resonance (Urano et al., 2008). Gβγ is also reported to regulate directional sensing mediated by the RhoGEF PIXα via complex formation with p21-associated protein kinase (Li et al., 2003). However, because the interaction of Gβγ with PIXα is thought to be indirect, it is not discussed further in this review.
The P-Rex Subfamily
The P-Rex subfamily of RhoGEFs is made up of P-Rex1 (Welch et al., 2002) and P-Rex2 (Donald et al., 2004; Rosenfeldt et al., 2004). P-Rex1 and P-Rex2 are of similar size (∼185 kDa) and have high sequence identity (59%) (Donald et al., 2004). Their domain architecture begins with the characteristic DH/PH tandem domains, followed by two DEP domains, two PDZ domains, and a C-terminal domain that exhibits homology to inositol polyphosphate-4-phosphatase (IP4P) (Welch et al., 2002) (Fig. 2c). The IP4P-like domain does not seem to have phosphatase activity (Hill et al., 2005). P-Rex2b, a splice variant of P-Rex2, lacks the IP4P domain (Rosenfeldt et al., 2004). P-Rex1, P-Rex2, and P-Rex2b are all directly regulated by Gβγ subunits and PIP3 (Welch et al., 2002; Donald et al., 2004; Li et al., 2005).
P-Rex1 is highly expressed in white blood cells and the brain. In neutrophils, P-Rex1 couples GPCRs to Rac-dependent formation of reactive oxygen species (Welch et al., 2002). Upon binding chemokines, Gi-coupled GPCRs release Gβγ subunits, which increase the concentration of PIP3 by activating class IB phosphatidyl inositol-3 kinases (PI3Ks). Thus, in neutrophils, the activation of Rac can occur independently from activation of tyrosine kinase receptors. P-Rex2 has wide tissue distribution but is most strongly expressed in skeletal muscle, small intestine, and placenta, whereas the splice variant P-Rex2b is expressed highly in the heart (Donald et al., 2004; Rosenfeldt et al., 2004). Neither isoform of P-Rex2 is expressed in neutrophils. Because the cells in which P-Rex2 and P-Rex2b are expressed generally lack class IB PI3Ks, it is believed that P-Rex2 integrates signals generated from both GPCRs and tyrosine kinase receptors, the latter of which activates the more ubiquitously expressed class IA PI3Ks (Donald et al., 2004).
Mechanism of Activation.
In cells, P-Rex1 is activated when both Gβγ and PI3K are overexpressed; in vitro, PIP3 and Gβγ act synergistically to activate the enzyme (Welch et al., 2002). Not all Gβγ subunit isoforms can activate P-Rex1 equally, although the ones with the most activity seem to be the same ones that activate PI3K the most (Mayeenuddin et al., 2006). Gγ1 and Gγ11, two of the Gγ subunits that were less effective at activating P-Rex1, are farnesylated instead of geranylgeranylated, suggesting that their ability to stably associate with the cell membrane is important for regulation by Gβγ. Phosphorylation of P-Rex1 by PKA inhibits activation by Gβγ by approximately 50-fold (Mayeenuddin and Garrison, 2006), and thus P-Rex1 can be regulated by GPCRs via both direct (Gβγ) and indirect (PIP3 and PKA) pathways.
To delineate the regions that are important for Gβγ and PIP3 binding, truncation studies of P-Rex1 were conducted. The isolated DH/PH tandem domain fragment exhibited higher activity than the full-length protein, indicating that other domains of the protein are involved in autoinhibition of basal activity. Of all the domains, the PH domain was found to be most important for PIP3 activation of P-Rex1, although it lacks obvious sequence signatures for phospholipid binding found in other PIP3-binding PH domains, and there was evidence for at least one other PIP3 binding site in the protein (Hill et al., 2005).
Identification of the Gβγ binding-site has not been as clear-cut. The isolated DH/PH tandem domain of P-Rex1 was reportedly activated by Gβγ, as was a variant with the PH domain deleted, suggesting that the DH domain, being the only domain in common between the constructs, was sufficient for binding and regulation by Gβγ (Hill et al., 2005). However, direct activation of the isolated DH domain was not shown. Coimmunoprecipitation assays using various fragments of P-Rex2b suggested instead that the PH domain contained the binding site for Gβγ (Li et al., 2005). A more recent study of P-Rex1, again using coimmunoprecipitation, indicated that the DH domain does not directly bind Gβγ. Rather, an intramolecular complex formed by the second DEP, first PDZ, and IP4P domains creates a binding site for Gβγ. PKA phosphorylation, presumably at consensus sites found in these domains, weakened these interdomain contacts and thus inhibited Gβγ binding (Urano et al., 2008). This latter result, however, is difficult to reconcile with the fact that P-Rex2b seems to be regulated by Gβγ and altogether lacks an IP4P domain (Li et al., 2005) and that the DH/PH tandem domains of P-Rex1 are sufficient to observe PIP3- and Gβγ-mediated membrane translocation (Barber et al., 2007). Thus, although PIP3-mediated membrane recruitment represents one component of the activation mechanism for P-Rex subfamily RhoGEFs, the molecular basis for allosteric control of enzymatic activity by Gβγ remains obscure, except that it probably results in the release of the inhibition mediated by domains C-terminal to the DH domain (Fig. 3c).
p114RhoGEF
p114RhoGEF is another member of the Lbc subfamily of RhoGEFs that can be activated via stimulation of LPA or M3-muscarinic receptors. Beyond the characteristic tandem DH/PH module, the protein contains no other recognized structural domains but does contain a C-terminal proline-rich region (Blomquist et al., 2000). The protein is widely expressed (Blomquist et al., 2000; Niu et al., 2003), and a shorter variant was detected in placenta and smooth muscle (Niu et al., 2003). There are conflicting reports on whether it is selective for RhoA (Blomquist et al., 2000; Nagata and Inagaki, 2005) or both RhoA and Rac1 (Niu et al., 2003). However, it should be noted that its DH/PH domains are closely related in sequence to those of other Lbc subfamily RhoGEFs, which are all believed to be specific for RhoA. The physiological roles of p114RhoGEF are unknown, but it has been identified as a binding partner for septin 9b, a protein involved in cytokinesis. This interaction is believed to be mediated by regions C-terminal to the DH/PH domains (Nagata and Inagaki, 2005).
Initial studies indicated that p114RhoGEF activity is directly regulated by Gβγ and not by Gα12/13 subunits (Niu et al., 2003). A subsequent study, however, found no evidence that the enzyme could be activated by Gβγ (Ueda et al., 2008). The reason for this discrepancy is not clear. Truncations of p114RhoGEF demonstrated that the DH/PH module has higher basal GEF activity than wild type, suggesting that the C-terminal region is inhibitory (Niu et al., 2003); hence, Gβγ binding would be anticipated to release autoinhibition. To identify the Gβγ binding site, an N-terminal fragment containing the DH/PH domains and C-terminal fragments (with and without the proline-rich region) were coexpressed in the presence or absence of Gβγ in NIH3T3 cells (Niu et al., 2003). Although the N-terminal catalytic fragment (residues 1–647) was as fully responsive to Gβγ activation as WT in driving SRF-mediated transcription, coimmunoprecipitation from cell lysates suggested that Gβγ could bind to either N- or C-terminal fragments.
Clg
Common-site lymphoma/leukemia GEF (Clg; also known as PLEKHG2 or FLJ00018) is a widely expressed GEF that is selective for Cdc42, although some activity for Rac1 has been demonstrated (Himmel et al., 2002; Ueda et al., 2008). The enzyme was singled out in a screen designed to identify RhoGEF enzymes for which SRF-mediated transcription could be enhanced by the coexpression of Gβγ subunits. The existence of such an enzyme could help explain how Gi-coupled receptors induce cell-spreading in NIH3T3 fibroblasts (Ueda et al., 2001). In addition to the DH/PH tandem domains, putative formin homology and PDZ domains are predicted in the C terminus of Clg. The importance of these motifs is not known, although the C terminus of the enzyme clearly plays a role in inhibition of basal GEF activity (Ueda et al., 2008). Constructs of Clg that contain only the DH domain do not activate transcription, indicating that the PH domain is necessary for activity in cells, perhaps via the direct interactions of this domain with the Rho GTPase or the cell membrane. Treatment with wortmannin demonstrated that Clg activity is not dependent on PIP3, which is known to interact with the PH domain of P-Rex1.
To identify the binding site for Gβγ, a series of fragments of Clg with increasing portions of the C terminus removed were expressed, purified, and tested for their ability to bind Gβγ (Ueda et al., 2008). A DH domain fragment was also tested. All fragments except the DH domain fragment could bind Gβγ in a GST-pulldown assay. The smallest fragment that retained nearly full ability to bind Gβγ spans residues 1 to 134, which includes a small portion of the DH domain. This fragment could inhibit the ability of Clg to promote SRF-dependent gene transcription and induce cell spreading in a concentration dependent manner, supporting its identification as the Gβγ binding site (Ueda et al., 2008).
Tim/Arhgef5
Tim, also known as Arhgef5, was first identified in a screen for focus-forming genes in NIH3T3 cells (Chan et al., 1994) and more recently in a small interfering RNA based screen for enzymes essential for macrophage chemotaxis (Wang et al., 2009). The enzyme is selective for RhoA and related GTPases (Snyder et al., 2002; Xie et al., 2005; Wang et al., 2009). Tim is a relatively simple RhoGEF, having in addition to the DH/PH domains a C-terminal SH3 domain that interacts with an N-terminal proline-rich region of the protein (Yohe et al., 2008). In addition, a helix N-terminal to the DH domain is predicted to bind directly to the DH domain to autoinhibit activity (Yohe et al., 2007). Both release of the SH3 domain (e.g., via competition with other proline rich motifs) and tyrosine phosphorylation of the N-terminal helix are postulated to be required for full activation of Tim (Yohe et al., 2008).
Because Gβγ subunits are known to be involved in leukocyte chemotaxis, the ability of Gβγ to bind and regulate the activity of Tim was tested. In coimmunoprecipitation assays, Gβγ was shown to bind to fragments that contained the PH domain and the preceding DH/PH linker region. Pulldown assays using purified proteins demonstrated that the interaction between Tim and Gβγ was direct. Gβγ also activated Tim in a serum response element reporter assay as well as in vitro using purified proteins (Wang et al., 2009). It remains to be seen how regulation by Gβγ is integrated into the layers of autoinhibition already mediated by the SH3 domain and N-terminal helix motif, or if it simply provides a mechanism to bypass them. It also remains to be seen whether other members of the Tim subfamily of RhoGEFs, which includes ephexin/NGEF, neuroblastoma, WGEF, SGEF, and Vsm-RhoGEF (Rossman et al., 2005), are also regulated by Gβγ.
Conclusions
Crystal structures and other biophysical studies of RH-RhoGEFs and p63RhoGEF have yielded substantial new insights into the molecular mechanisms governing their regulation by heterotrimeric G proteins. The current data suggest that in most cases, heterotrimeric G proteins interact directly with the catalytic core of the enzyme to stimulate activity. For example, the Gα13-RH domain complex is expected to bind to the DH/PH domain in RH-RhoGEFs, and Gαq forms direct contacts with both DH and PH domains of p63RhoGEF in the Gαq-p63RhoGEF-RhoA crystal structure. The binding of heterotrimeric G proteins does not always have to be stimulatory, as it has been proposed that Gα12 and Gα16 can antagonize signaling by their closely related paralogs. These proteins bind with high affinity to the same sites as Gα13 and Gαq, respectively, yet somehow fail to induce a conformational change that leads to RhoGEF activation. The molecular basis for this failure to activate is not yet understood.
Based on the available structural data from the RH-RhoGEFs and p63RhoGEF, the conformation of the α6-αN linker, and hence the relative orientation of their DH and PH domains (Fig. 1, e–g), is expected to play a role in tuning activity. In RH-RhoGEFs, the PH domain augments the activity of the adjacent DH domain, even in the absence of heterotrimeric G proteins, by trapping the linker region in a more optimal conformation. In this case, it is tempting to speculate that the role of heterotrimeric G proteins may primarily be to displace autoinhibitory elements from the DH/PH core domain. In TrioC RhoGEFs, the PH domain and linker adopt a conformation that instead inhibits the binding of RhoA in the basal state. Confronted with this scenario, heterotrimeric G proteins must bind directly to the DH/PH core and constrain the DH and PH domains in a manner that relieves inhibition, that does not block the binding of RhoA, and that allosterically activates nucleotide exchange (Fig. 1g).
As in other Gα-effector complexes, the effector docking site of Gα forms the principal binding site for downstream RhoGEFs. The effector docking site of Gα13 seems to be conformationally flexible, not only in response to GTP loading and hydrolysis but also when challenged by the unique docking motifs presented by p115RhoGEF and PDZ-RhoGEF (Chen et al., 2008) (Fig. 1, b and c). Thus, even closely related effectors need not necessarily have well conserved Gα-binding motifs. Contacts made with Gα outside of the effector docking site mandate specificity and can also contribute to regulation, as in the case of RH-RhoGEFs and p63RhoGEF.
Unfortunately, the structural data reported thus far are still not definitive with regard to the mechanism of signal transduction for any heterotrimeric G protein-regulated RhoGEF. In no case are structures known for both the inactive and active forms of these enzymes, and future efforts are likely to focus on obtaining other snapshots of these enzymes along their reaction coordinates. Structures of RH-RhoGEFs that include the RH domain, in addition to the DH/PH domains, and of apo-p63RhoGEF would be especially informative. This is a challenging task, especially when considering domains that are connected by long, unstructured linkers. High-resolution spectroscopic studies, such as those employed by Zheng et al. (2009), hold promise in these situations. Our understanding of the molecular basis for regulation of the Gβγ-regulated RhoGEFs lags even further behind those that are regulated by Gα subunits. Structural analysis of these enzymes and their Gβγ complexes will be important for rapidly advancing our understanding of the molecular mechanisms that control their activity.
Acknowledgments
We thank Drs. Thomas Wieland (University of Heidelberg) and Tomek Cierpicki (University of Michigan, Ann Arbor, MI) for critical reading of the manuscript.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL086865, HL071818].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.061234
- Dbs
- Dbl's big sister
- DEP
- Disheveled/EGL10/Pleckstrin
- DH
- Dbl homology
- GAP
- GTPase-activating protein
- GEF
- guanine nucleotide exchange factor
- GPCR
- G protein-coupled receptor
- HEK
- human embryonic kidney
- IP4P
- inositol polyphosphate-4-phosphatase
- LARG
- leukemia-associated RhoGEF
- Lbc
- lymphoid blast crisis
- LPA
- lysophosphatidic acid
- PDZ
- postsynaptic density 95, disk large, zona occludens-1
- PH
- pleckstrin homology
- PI3K
- phosphatidyl inositol-3 kinase
- PIP3
- phosphatidylinositol (3,4,5)-triphosphate
- PKA
- cAMP-dependent protein kinase
- PLC
- phospholipase C
- P-Rex
- PIP3-dependent Rac exchanger
- rgRGS
- RhoGEF fragment containing both N-terminal GAP motif and RH domain
- RGS
- regulators of G protein signaling
- RhoGEF
- Rho guanine nucleotide exchange factor
- RH
- RGS homology
- SH3
- Src homology 3
- SRF
- serum response factor.
References
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK. (2000) Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102: 625–633 [DOI] [PubMed] [Google Scholar]
- Aghazadeh B, Zhu K, Kubiseski TJ, Liu GA, Pawson T, Zheng Y, Rosen MK. (1998) Structure and mutagenesis of the Dbl homology domain. Nat Struct Biol 5: 1098–1107 [DOI] [PubMed] [Google Scholar]
- Aittaleb M, Gao G, Evelyn CR, Neubig RR, Tesmer JJ. (2009) A conserved hydrophobic surface of the LARG pleckstrin homology domain is critical for RhoA activation in cells. Cell Signal 21: 1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T, Diviani D. (2007) The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A 104: 10140–10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL. (2002) The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci U S A 99: 12085–12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisamy L, Jurisch N, Diviani D. (2005) Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J Biol Chem 280: 15405–15412 [DOI] [PubMed] [Google Scholar]
- Banerjee J, Fischer CC, Wedegaertner PB. (2009) The amino acid motif L/IIxxFE defines a novel actin-binding sequence in PDZ-RhoGEF. Biochemistry 48: 8032–8043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee J, Wedegaertner PB. (2004) Identification of a novel sequence in PDZ-RhoGEF that mediates interaction with the actin cytoskeleton. Mol Biol Cell 15: 1760–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber MA, Donald S, Thelen S, Anderson KE, Thelen M, Welch HC. (2007) Membrane translocation of P-Rex1 is mediated by G protein betagamma subunits and phosphoinositide 3-kinase. J Biol Chem 282: 29967–29976 [DOI] [PubMed] [Google Scholar]
- Bellanger JM, Estrach S, Schmidt S, Briançon-Marjollet A, Zugasti O, Fromont S, Debant A. (2003) Different regulation of the Trio Dbl-Homology domains by their associated PH domains. Biol Cell 95: 625–634 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya R, Banerjee J, Khalili K, Wedegaertner PB. (2009) Differences in Galpha12- and Galpha13-mediated plasma membrane recruitment of p115-RhoGEF. Cell Signal 21: 996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya R, Wedegaertner PB. (2003) Characterization of Gα13-dependent plasma membrane recruitment of p115RhoGEF. Biochem J 371: 709–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomquist A, Schwörer G, Schablowski H, Psoma A, Lehnen M, Jakobs KH, Rümenapp U. (2000) Identification and characterization of a novel Rho-specific guanine nucleotide exchange factor. Biochem J 352: 319–325 [PMC free article] [PubMed] [Google Scholar]
- Booden MA, Siderovski DP, Der CJ. (2002) Leukemia-associated Rho guanine nucleotide exchange factor promotes Gαq-coupled activation of RhoA. Mol Cell Biol 22: 4053–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl AM, Johnson NL, Dhanasekaran N, Johnson GL. (1995) Gα12 and Gα13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem 270: 24631–24634 [DOI] [PubMed] [Google Scholar]
- Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, et al. (2008) AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell 32: 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AM, McGovern ES, Catalano G, Fleming TP, Miki T. (1994) Expression cDNA cloning of a novel oncogene with sequence similarity to regulators of small GTP-binding proteins. Oncogene 9: 1057–1063 [PubMed] [Google Scholar]
- Chen Z, Singer WD, Danesh SM, Sternweis PC, Sprang SR. (2008) Recognition of the activated states of Gα13 by the rgRGS domain of PDZRhoGEF. Structure 16: 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Singer WD, Sternweis PC, Sprang SR. (2005) Structure of the p115RhoGEF rgRGS domain-Gα13/i1 chimera complex suggests convergent evolution of a GTPase activator. Nat Struct Mol Biol 12: 191–197 [DOI] [PubMed] [Google Scholar]
- Chen Z, Singer WD, Wells CD, Sprang SR, Sternweis PC. (2003) Mapping the Gα13 binding interface of the rgRGS domain of p115RhoGEF. J Biol Chem 278: 9912–9919 [DOI] [PubMed] [Google Scholar]
- Chen Z, Wells CD, Sternweis PC, Sprang SR. (2001) Structure of the rgRGS domain of p115RhoGEF. Nat Struct Biol 8: 805–809 [DOI] [PubMed] [Google Scholar]
- Chhatriwala MK, Betts L, Worthylake DK, Sondek J. (2007) The DH and PH domains of Trio coordinately engage Rho GTPases for their efficient activation. J Mol Biol 368: 1307–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikumi H, Barac A, Behbahani B, Gao Y, Teramoto H, Zheng Y, Gutkind JS. (2004) Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene 23: 233–240 [DOI] [PubMed] [Google Scholar]
- Chikumi H, Vázquez-Prado J, Servitja JM, Miyazaki H, Gutkind JS. (2002) Potent activation of RhoA by Gαq and Gq-coupled receptors. J Biol Chem 277: 27130–27134 [DOI] [PubMed] [Google Scholar]
- Cierpicki T, Bielnicki J, Zheng M, Gruszczyk J, Kasterka M, Petoukhov M, Zhang A, Fernandez EJ, Svergun DI, Derewenda U, et al. (2009) The solution structure and dynamics of the DH-PH module of PDZRhoGEF in isolation and in complex with nucleotide-free RhoA. Protein Sci 18: 2067–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Shu X, Day GJ, Han J, Krishna UM, Falck JR, Broek D. (2000) Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J Biol Chem 275: 15074–15081 [DOI] [PubMed] [Google Scholar]
- Davis TL, Bonacci TM, Sprang SR, Smrcka AV. (2005) Structural and molecular characterization of a preferred protein interaction surface on G protein beta gamma subunits. Biochemistry 44: 10593–10604 [DOI] [PubMed] [Google Scholar]
- Derewenda U, Oleksy A, Stevenson AS, Korczynska J, Dauter Z, Somlyo AP, Otlewski J, Somlyo AV, Derewenda ZS. (2004) The crystal structure of RhoA in complex with the DH/PH fragment of PDZRhoGEF, an activator of the Ca2+ sensitization pathway in smooth muscle. Structure 12: 1955–1965 [DOI] [PubMed] [Google Scholar]
- Dhanasekaran N, Tsim ST, Dermott JM, Onesime D. (1998) Regulation of cell proliferation by G proteins. Oncogene 17: 1383–1394 [DOI] [PubMed] [Google Scholar]
- Diviani D, Soderling J, Scott JD. (2001) AKAP-Lbc anchors protein kinase A and nucleates Gα12-selective Rho-mediated stress fiber formation. J Biol Chem 276: 44247–44257 [DOI] [PubMed] [Google Scholar]
- Donald S, Hill K, Lecureuil C, Barnouin R, Krugmann S, John Coadwell W, Andrews SR, Walker SA, Hawkins PT, Stephens LR, et al. (2004) P-Rex2, a new guanine-nucleotide exchange factor for Rac. FEBS Lett 572: 172–176 [DOI] [PubMed] [Google Scholar]
- Donald S, Humby T, Fyfe I, Segonds-Pichon A, Walker SA, Andrews SR, Coadwell WJ, Emson P, Wilkinson LS, Welch HC. (2008) P-Rex2 regulates Purkinje cell dendrite morphology and motor coordination. Proc Natl Acad Sci U S A 105: 4483–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D. (2005) P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol 15: 1874–1879 [DOI] [PubMed] [Google Scholar]
- Dutt P, Nguyen N, Toksoz D. (2004) Role of Lbc RhoGEF in Gα12/13-induced signals to Rho GTPase. Cell Signal 16: 201–209 [DOI] [PubMed] [Google Scholar]
- Eisenhaure TM, Francis SA, Willison LD, Coughlin SR, Lerner DJ. (2003) The Rho guanine nucleotide exchange factor Lsc homo-oligomerizes and is negatively regulated through domains in its carboxyl terminus that are absent in novel splenic isoforms. J Biol Chem 278: 30975–30984 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. (2002) Rho GTPases in cell biology. Nature 420: 629–635 [DOI] [PubMed] [Google Scholar]
- Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, et al. (1998) Molecular basis for interactions of G protein βγ subunits with effectors. Science 280: 1271–1274 [DOI] [PubMed] [Google Scholar]
- Francis SA, Shen X, Young JB, Kaul P, Lerner DJ. (2006) Rho GEF Lsc is required for normal polarization, migration, and adhesion of formyl-peptide-stimulated neutrophils. Blood 107: 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Chikumi H, Gutkind JS. (2000) Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G12 family to Rho. FEBS Lett 485: 183–188 [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. (1999) A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem 274: 5868–5879 [DOI] [PubMed] [Google Scholar]
- Girkontaite I, Missy K, Sakk V, Harenberg A, Tedford K, Pötzel T, Pfeffer K, Fischer KD. (2001) Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat Immunol 2: 855–862 [DOI] [PubMed] [Google Scholar]
- Grabocka E, Wedegaertner PB. (2005) Functional consequences of Gα13 mutations that disrupt interaction with p115RhoGEF. Oncogene 24: 2155–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabocka E, Wedegaertner PB. (2007) Disruption of oligomerization induces nucleocytoplasmic shuttling of leukemia-associated rho Guanine-nucleotide exchange factor. Mol Pharmacol 72: 993–1002 [DOI] [PubMed] [Google Scholar]
- Guo X, Stafford LJ, Bryan B, Xia C, Ma W, Wu X, Liu D, Songyang Z, Liu M. (2003) A Rac/Cdc42-specific exchange factor, GEFT, induces cell proliferation, transformation, and migration. J Biol Chem 278: 13207–13215 [DOI] [PubMed] [Google Scholar]
- Harenberg A, Girkontaite I, Giehl K, Fischer KD. (2005) The Lsc RhoGEF mediates signaling from thromboxane A2 to actin polymerization and apoptosis in thymocytes. Eur J Immunol 35: 1977–1986 [DOI] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. (1998) Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science 280: 2112–2114 [DOI] [PubMed] [Google Scholar]
- Hart MJ, Sharma S, elMasry N, Qiu RG, McCabe P, Polakis P, Bollag G. (1996) Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J Biol Chem 271: 25452–25458 [DOI] [PubMed] [Google Scholar]
- Hata K, Kaibuchi K, Inagaki S, Yamashita T. (2009) Unc5B associates with LARG to mediate the action of repulsive guidance molecule. J Cell Biol 184: 737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers RH, Todd J, Jr, Webb RC. (2007) Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J Hypertens 25: 1687–1697 [DOI] [PubMed] [Google Scholar]
- Hill K, Krugmann S, Andrews SR, Coadwell WJ, Finan P, Welch HC, Hawkins PT, Stephens LR. (2005) Regulation of P-Rex1 by phosphatidylinositol (3,4,5)-trisphosphate and Gβγ subunits. J Biol Chem 280: 4166–4173 [DOI] [PubMed] [Google Scholar]
- Himmel KL, Bi F, Shen H, Jenkins NA, Copeland NG, Zheng Y, Largaespada DA. (2002) Activation of clg, a novel dbl family guanine nucleotide exchange factor gene, by proviral insertion at evi24, a common integration site in B cell and myeloid leukemias. J Biol Chem 277: 13463–13472 [DOI] [PubMed] [Google Scholar]
- Hirotani M, Ohoka Y, Yamamoto T, Nirasawa H, Furuyama T, Kogo M, Matsuya T, Inagaki S. (2002) Interaction of plexin-B1 with PDZ domain-containing Rho guanine nucleotide exchange factors. Biochem Biophys Res Commun 297: 32–37 [DOI] [PubMed] [Google Scholar]
- Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, Lin CI, Bowers WJ, Federoff HJ, Sternweis PC, Rothstein JD. (2001) Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature 410: 89–93 [DOI] [PubMed] [Google Scholar]
- Kawai T, Sanjo H, Akira S. (1999) Duet is a novel serine/threonine kinase with Dbl-Homology (DH) and Pleckstrin-Homology (PH) domains. Gene 227: 249–255 [DOI] [PubMed] [Google Scholar]
- Kourlas PJ, Strout MP, Becknell B, Veronese ML, Croce CM, Theil KS, Krahe R, Ruutu T, Knuutila S, Bloomfield CD, et al. (2000) Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: evidence for its fusion with MLL in acute myeloid leukemia. Proc Natl Acad Sci U S A 97: 2145–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. (1998) p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science 280: 2109–2111 [DOI] [PubMed] [Google Scholar]
- Kreutz B, Hajicek N, Yau DM, Nakamura S, Kozasa T. (2007) Distinct regions of Gα13 participate in its regulatory interactions with RGS homology domain-containing RhoGEFs. Cell Signal 19: 1681–1689 [DOI] [PubMed] [Google Scholar]
- Kristelly R, Gao G, Tesmer JJ. (2004) Structural determinants of RhoA binding and nucleotide exchange in leukemia-associated Rho guanine-nucleotide exchange factor. J Biol Chem 279: 47352–47362 [DOI] [PubMed] [Google Scholar]
- Kuner R, Swiercz JM, Zywietz A, Tappe A, Offermanns S. (2002) Characterization of the expression of PDZ-RhoGEF, LARG and Gα12/Gα13 proteins in the murine nervous system. Eur J Neurosci 16: 2333–2341 [DOI] [PubMed] [Google Scholar]
- Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, et al. (2003) Directional sensing requires Gβγ-mediated PAK1 and PIXα-dependent activation of Cdc42. Cell 114: 215–227 [DOI] [PubMed] [Google Scholar]
- Li Z, Paik JH, Paik JH, Wang Z, Hla T, Wu D. (2005) Role of guanine nucleotide exchange factor P-Rex-2b in sphingosine 1-phosphate-induced Rac1 activation and cell migration in endothelial cells. Prostaglandins Other Lipid Mediat 76: 95–104 [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang J, Yang Y, Huang H, Shen W, Hu Q, Wang X, Wu J, Shi Y. (2008) Conformational change upon ligand binding and dynamics of the PDZ domain from leukemia-associated Rho guanine nucleotide exchange factor. Protein Sci 17: 1003–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang H, Eberstadt M, Schnuchel A, Olejniczak ET, Meadows RP, Schkeryantz JM, Janowick DA, Harlan JE, Harris EA, et al. (1998) NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell 95: 269–277 [DOI] [PubMed] [Google Scholar]
- Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. (2003) Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science 300: 1256–1262 [DOI] [PubMed] [Google Scholar]
- Longenecker KL, Lewis ME, Chikumi H, Gutkind JS, Derewenda ZS. (2001) Structure of the RGS-like domain from PDZ-RhoGEF: linking heterotrimeric G protein-coupled signaling to Rho GTPases. Structure (Camb) 9: 559–569 [DOI] [PubMed] [Google Scholar]
- Lutz S, Freichel-Blomquist A, Rümenapp U, Schmidt M, Jakobs KH, Wieland T. (2004) p63RhoGEF and GEFT are Rho-specific guanine nucleotide exchange factors encoded by the same gene. Naunyn Schmiedebergs Arch Pharmacol 369: 540–546 [DOI] [PubMed] [Google Scholar]
- Lutz S, Freichel-Blomquist A, Yang Y, Rümenapp U, Jakobs KH, Schmidt M, Wieland T. (2005) The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem 280: 11134–11139 [DOI] [PubMed] [Google Scholar]
- Lutz S, Shankaranarayanan A, Coco C, Ridilla M, Nance MR, Vettel C, Baltus D, Evelyn CR, Neubig RR, Wieland T, et al. (2007) Structure of Gαq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science 318: 1923–1927 [DOI] [PubMed] [Google Scholar]
- Majumdar M, Seasholtz TM, Buckmaster C, Toksoz D, Brown JH. (1999) A rho exchange factor mediates thrombin and Gα12-induced cytoskeletal responses. J Biol Chem 274: 26815–26821 [DOI] [PubMed] [Google Scholar]
- Mao J, Yuan H, Xie W, Wu D. (1998) Guanine nucleotide exchange factor GEF115 specifically mediates activation of Rho and serum response factor by the G protein α subunit Gα13. Proc Natl Acad Sci U S A 95: 12973–12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeenuddin LH, Garrison JC. (2006) Phosphorylation of P-Rex1 by the cyclic AMP-dependent protein kinase inhibits the phosphatidylinositiol (3,4,5)-trisphosphate and Gβγ-mediated regulation of its activity. J Biol Chem 281: 1921–1928 [DOI] [PubMed] [Google Scholar]
- Mayeenuddin LH, McIntire WE, Garrison JC. (2006) Differential sensitivity of P-Rex1 to isoforms of G protein βγ dimers. J Biol Chem 281: 1913–1920 [DOI] [PubMed] [Google Scholar]
- McPherson CE, Eipper BA, Mains RE. (2005) Multiple novel isoforms of Trio are expressed in the developing rat brain. Gene 347: 125–135 [DOI] [PubMed] [Google Scholar]
- Moepps B, Tulone C, Kern C, Minisini R, Michels G, Vatter P, Wieland T, Gierschik P. (2008) Constitutive serum response factor activation by the viral chemokine receptor homologue pUS28 is differentially regulated by Gαq/11 and Gα16. Cell Signal 20: 1528–1537 [DOI] [PubMed] [Google Scholar]
- Nagata K, Inagaki M. (2005) Cytoskeletal modification of Rho guanine nucleotide exchange factor activity: identification of a Rho guanine nucleotide exchange factor as a binding partner for Sept9b, a mammalian septin. Oncogene 24: 65–76 [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kreutz B, Tanabe S, Suzuki N, Kozasa T. (2004) Critical role of lysine 204 in switch I region of Gα13 for regulation of p115RhoGEF and leukemia-associated RhoGEF. Mol Pharmacol 66: 1029–1034 [DOI] [PubMed] [Google Scholar]
- Niu J, Profirovic J, Pan H, Vaiskunaite R, Voyno-Yasenetskaya T. (2003) G protein βγ subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production. Circ Res 93: 848–856 [DOI] [PubMed] [Google Scholar]
- Nourry C, Grant SG, Borg JP. (2003) PDZ domain proteins: plug and play! Sci STKE (179): re7. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Mancino V, Revel JP, Simon MI. (1997) Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science 275: 533–536 [DOI] [PubMed] [Google Scholar]
- Oleksy A, Opaliński Ł, Derewenda U, Derewenda ZS, Otlewski J. (2006) The molecular basis of RhoA specificity in the guanine nucleotide exchange factor PDZ-RhoGEF. J Biol Chem 281: 32891–32897 [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Kambampati V, Mains RE, Eipper BA. (2001) Distinct roles for the two Rho GDP/GTP exchange factor domains of kalirin in regulation of neurite growth and neuronal morphology. J Neurosci 21: 8426–8434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot V, Vazquez-Prado J, Gutkind JS. (2002) Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem 277: 43115–43120 [DOI] [PubMed] [Google Scholar]
- Pi M, Spurney RF, Tu Q, Hinson T, Quarles LD. (2002) Calcium-sensing receptor activation of rho involves filamin and rho-guanine nucleotide exchange factor. Endocrinology 143: 3830–3838 [DOI] [PubMed] [Google Scholar]
- Reuther GW, Lambert QT, Booden MA, Wennerberg K, Becknell B, Marcucci G, Sondek J, Caligiuri MA, Der CJ. (2001) Leukemia-associated Rho guanine nucleotide exchange factor, a Dbl family protein found mutated in leukemia, causes transformation by activation of RhoA. J Biol Chem 276: 27145–27151 [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70: 389–399 [DOI] [PubMed] [Google Scholar]
- Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J. (2007) Gαq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem 282: 29201–29210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeldt H, Vázquez-Prado J, Gutkind JS. (2004) P-REX2, a novel PI-3-kinase sensitive Rac exchange factor. FEBS Lett 572: 167–171 [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. (2000) GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem 69: 795–827 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Cheng L, Mahon GM, Rojas RJ, Snyder JT, Whitehead IP, Sondek J. (2003) Multifunctional roles for the PH domain of Dbs in regulating Rho GTPase activation. J Biol Chem 278: 18393–18400 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6: 167–180 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Worthylake DK, Snyder JT, Siderovski DP, Campbell SL, Sondek J. (2002) A crystallographic view of interactions between Dbs and Cdc42: PH domain-assisted guanine nucleotide exchange. EMBO J 21: 1315–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi SA, Seasholtz TM, Kobiashvili M, Wilson BA, Toksoz D, Brown JH. (2001) Physical and functional interactions of Gαq with Rho and its exchange factors. J Biol Chem 276: 15445–15452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah VP, Hoshijima M, Chien KR, Brown JH. (1996) Rho is required for Gαq and α1-adrenergic receptor signaling in cardiomyocytes. Dissociation of Ras and Rho pathways. J Biol Chem 271: 31185–31190 [DOI] [PubMed] [Google Scholar]
- Sah VP, Seasholtz TM, Sagi SA, Brown JH. (2000) The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol 40: 459–489 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. (2002) Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 16: 1587–1609 [DOI] [PubMed] [Google Scholar]
- Seasholtz TM, Majumdar M, Brown JH. (1999) Rho as a mediator of G protein-coupled receptor signaling. Mol Pharmacol 55: 949–956 [DOI] [PubMed] [Google Scholar]
- Shankaranarayanan A, Thal DM, Tesmer VM, Roman DL, Neubig RR, Kozasa T, Tesmer JJ. (2008) Assembly of high order Gαq-effector complexes with RGS proteins. J Biol Chem 283: 34923–34934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehler S. (2009) Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J Pharmacol 158: 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronek KR, Guo F, Zheng Y, Nassar N. (2004) The C-terminal basic tail of RhoG assists the guanine nucleotide exchange factor trio in binding to phospholipids. J Biol Chem 279: 37895–37907 [DOI] [PubMed] [Google Scholar]
- Slep KC, Kercher MA, He W, Cowan CW, Wensel TG, Sigler PB. (2001) Structural determinants for regulation of phosphodiesterase by a G protein at 2.0 Å. Nature 409: 1071–1077 [DOI] [PubMed] [Google Scholar]
- Smietana K, Kasztura M, Paduch M, Derewenda U, Derewenda ZS, Otlewski J. (2008) Degenerate specificity of PDZ domains from RhoA-specific nucleotide exchange factors PDZRhoGEF and LARG. Acta Biochim Pol 55: 269–280 [PubMed] [Google Scholar]
- Snyder JT, Worthylake DK, Rossman KL, Betts L, Pruitt WM, Siderovski DP, Der CJ, Sondek J. (2002) Structural basis for the selective activation of Rho GTPases by Dbl exchange factors. Nat Struct Biol 9: 468–475 [DOI] [PubMed] [Google Scholar]
- Souchet M, Portales-Casamar E, Mazurais D, Schmidt S, Léger I, Javré JL, Robert P, Berrebi-Bertrand I, Bril A, Gout B, et al. (2002) Human p63RhoGEF, a novel RhoA-specific guanine nucleotide exchange factor, is localized in cardiac sarcomere. J Cell Sci 115: 629–640 [DOI] [PubMed] [Google Scholar]
- Sprang SR, Chen Z, Du X. (2007) Structural basis of effector regulation and signal termination in heterotrimeric Gα proteins. Adv Protein Chem 74: 1–65 [DOI] [PubMed] [Google Scholar]
- Sterpetti P, Hack AA, Bashar MP, Park B, Cheng SD, Knoll JH, Urano T, Feig LA, Toksoz D. (1999) Activation of the Lbc Rho exchange factor proto-oncogene by truncation of an extended C terminus that regulates transformation and targeting. Mol Cell Biol 19: 1334–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven R, Zhang L, Culotti J, Pawson T. (2005) The UNC-73/Trio RhoGEF-2 domain is required in separate isoforms for the regulation of pharynx pumping and normal neurotransmission in C. elegans. Genes Dev 19: 2016–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Hajicek N, Kozasa T. (2009a) Regulation and physiological functions of G12/13-mediated signaling pathways. Neurosignals 17: 55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Nakamura S, Mano H, Kozasa T. (2003) Gα12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci U S A 100: 733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Tsumoto K, Hajicek N, Daigo K, Tokita R, Minami S, Kodama T, Hamakubo T, Kozasa T. (2009b) Activation of leukemia-associated RhoGEF by Gα13 with significant conformational rearrangements in the interface. J Biol Chem 284: 5000–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson-Fields KI, Sandquist JC, Rossol-Allison J, Blat IC, Wennerberg K, Burridge K, Means AR. (2008) MLK3 limits activated Gαq signaling to Rho by binding to p63RhoGEF. Mol Cell 32: 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. (2002) Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 35: 51–63 [DOI] [PubMed] [Google Scholar]
- Tesmer JJ. (2009) Structure and function of regulator of G protein signaling homology domains. Prog Mol Biol Transl Sci 86: 75–113 [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Berman DM, Gilman AG, Sprang SR. (1997a) Structure of RGS4 bound to AlF4−-activated Giα1: stabilization of the transition state for GTP hydrolysis. Cell 89: 251–261 [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. (1997b) Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα·GTPγS. Science 278: 1907–1916 [DOI] [PubMed] [Google Scholar]
- Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. (2005) Snapshot of activated G proteins at the membrane: the Gαq-GRK2-Gβγ complex. Science 310: 1686–1690 [DOI] [PubMed] [Google Scholar]
- Togashi H, Nagata K, Takagishi M, Saitoh N, Inagaki M. (2000) Functions of a rho-specific guanine nucleotide exchange factor in neurite retraction. Possible role of a proline-rich motif of KIAA0380 in localization. J Biol Chem 275: 29570–29578 [DOI] [PubMed] [Google Scholar]
- Toksoz D, Williams DA. (1994) Novel human oncogene lbc detected by transfection with distinct homology regions to signal transduction products. Oncogene 9: 621–628 [PubMed] [Google Scholar]
- Ueda H, Morishita R, Yamauchi J, Itoh H, Kato K, Asano T. (2001) Regulation of Rac and Cdc42 pathways by Gi during lysophosphatidic acid-induced cell spreading. J Biol Chem 276: 6846–6852 [DOI] [PubMed] [Google Scholar]
- Ueda H, Nagae R, Kozawa M, Morishita R, Kimura S, Nagase T, Ohara O, Yoshida S, Asano T. (2008) Heterotrimeric G protein βγ subunits stimulate FLJ00018, a guanine nucleotide exchange factor for Rac1 and Cdc42. J Biol Chem 283: 1946–1953 [DOI] [PubMed] [Google Scholar]
- Urano D, Nakata A, Mizuno N, Tago K, Itoh H. (2008) Domain-domain interaction of P-Rex1 is essential for the activation and inhibition by G protein βγ subunits and PKA. Cell Signal 20: 1545–1554 [DOI] [PubMed] [Google Scholar]
- Vogt S, Grosse R, Schultz G, Offermanns S. (2003) Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J Biol Chem 278: 28743–28749 [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu M, Kozasa T, Rothstein JD, Sternweis PC, Neubig RR. (2004) Thrombin and lysophosphatidic acid receptors utilize distinct rhoGEFs in prostate cancer cells. J Biol Chem 279: 28831–28834 [DOI] [PubMed] [Google Scholar]
- Wang Z, Kumamoto Y, Wang P, Gan X, Lehmann D, Smrcka AV, Cohn L, Iwasaki A, Li L, Wu D. (2009) Regulation of immature dendritic cell migration by RhoA guanine nucleotide exchange factor Arhgef5. J Biol Chem 284: 28599–28606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. (2002) P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell 108: 809–821 [DOI] [PubMed] [Google Scholar]
- Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, Okkenhaug K, Coadwell WJ, Andrews SR, Thelen M, et al. (2005) P-Rex1 regulates neutrophil function. Curr Biol 15: 1867–1873 [DOI] [PubMed] [Google Scholar]
- Wells CD, Liu MY, Jackson M, Gutowski S, Sternweis PM, Rothstein JD, Kozasa T, Sternweis PC. (2002) Mechanisms for reversible regulation between G13 and Rho exchange factors. J Biol Chem 277: 1174–1181 [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. (2004) Rho-family GTPases: it's not only Rac and Rho (and I like it). J Cell Sci 117: 1301–1312 [DOI] [PubMed] [Google Scholar]
- Whitehead IP, Zohn IE, Der CJ. (2001) Rho GTPase-dependent transformation by G protein-coupled receptors. Oncogene 20: 1547–1555 [DOI] [PubMed] [Google Scholar]
- Williams SL, Lutz S, Charlie NK, Vettel C, Ailion M, Coco C, Tesmer JJ, Jorgensen EM, Wieland T, Miller KG. (2007) Trio's Rho-specific GEF domain is the missing Gαq effector in C. elegans. Genes Dev 21: 2731–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horváth B, Maser-Gluth C, Greiner E, et al. (2008) G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14: 64–68 [DOI] [PubMed] [Google Scholar]
- Wong K, Van Keymeulen A, Bourne HR. (2007) PDZRhoGEF and myosin II localize RhoA activity to the back of polarizing neutrophil-like cells. J Cell Biol 179: 1141–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthylake DK, Rossman KL, Sondek J. (2000) Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature 408: 682–688 [DOI] [PubMed] [Google Scholar]
- Xie X, Chang SW, Tatsumoto T, Chan AM, Miki T. (2005) TIM, a Dbl-related protein, regulates cell shape and cytoskeletal organization in a Rho-dependent manner. Cell Signal 17: 461–471 [DOI] [PubMed] [Google Scholar]
- Yamada T, Ohoka Y, Kogo M, Inagaki S. (2005) Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs). J Biol Chem 280: 19358–19363 [DOI] [PubMed] [Google Scholar]
- Yeung WW, Wong YH. (2009) The RhoA-specific guanine nucleotide exchange factor p63RhoGEF binds to activated Gα16 and inhibits the canonical phospholipase Cβ pathway. Cell Signal 21: 1317–1325 [DOI] [PubMed] [Google Scholar]
- Ying Z, Giachini FR, Tostes RC, Webb RC. (2009) PYK2/PDZ-RhoGEF links Ca2+ signaling to RhoA. Arterioscler Thromb Vasc Biol 29: 1657–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Jin L, Palmer T, Webb RC. (2006) Angiotensin II up-regulates the leukemia-associated Rho guanine nucleotide exchange factor (RhoGEF), a regulator of G protein signaling domain-containing RhoGEF, in vascular smooth muscle cells. Mol Pharmacol 69: 932–940 [DOI] [PubMed] [Google Scholar]
- Yohe ME, Rossman K, Sondek J. (2008) Role of the C-terminal SH3 domain and N-terminal tyrosine phosphorylation in regulation of Tim and related Dbl-family proteins. Biochemistry 47: 6827–6839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohe ME, Rossman KL, Gardner OS, Karnoub AE, Snyder JT, Gershburg S, Graves LM, Der CJ, Sondek J. (2007) Auto-inhibition of the Dbl family protein Tim by an N-terminal helical motif. J Biol Chem 282: 13813–13823 [DOI] [PubMed] [Google Scholar]
- Zheng M, Cierpicki T, Momotani K, Artamonov MV, Derewenda U, Bushweller JH, Somlyo AV, Derewenda ZS. (2009) On the mechanism of autoinhibition of the RhoA-specific nucleotide exchange factor PDZRhoGEF. BMC Struct Biol 9: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]