Abstract
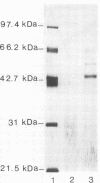
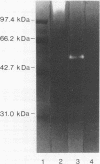
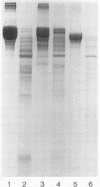
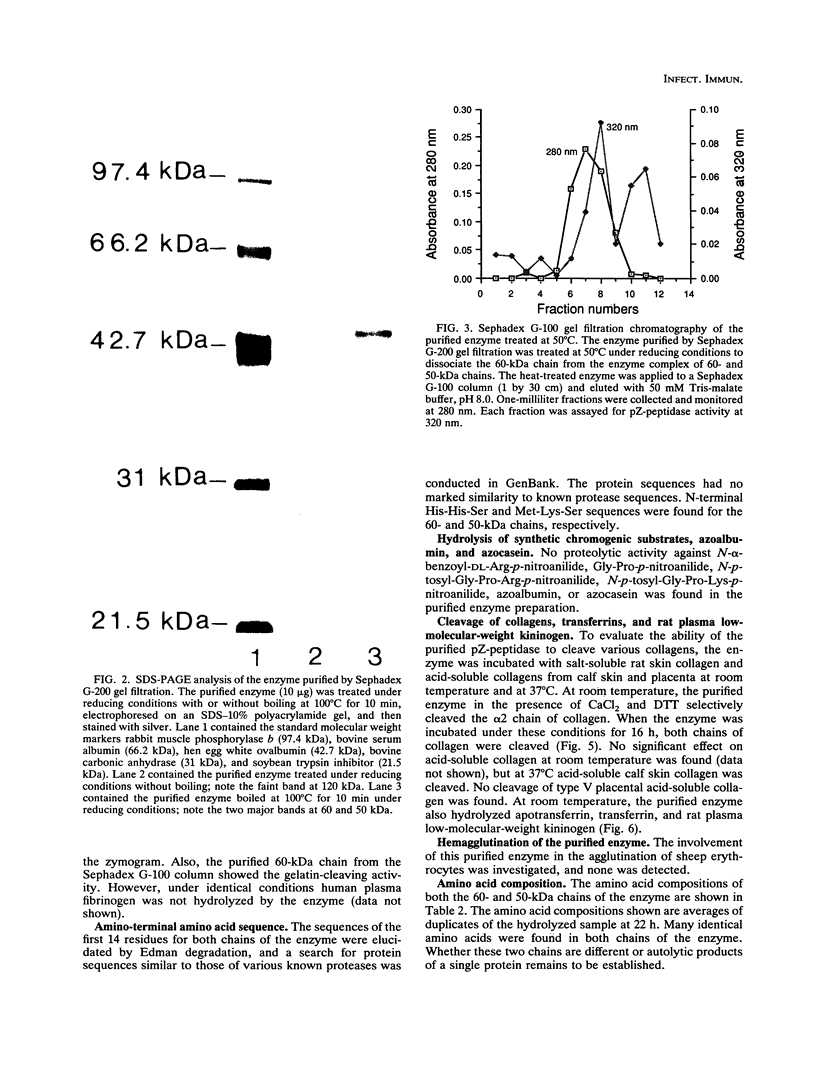
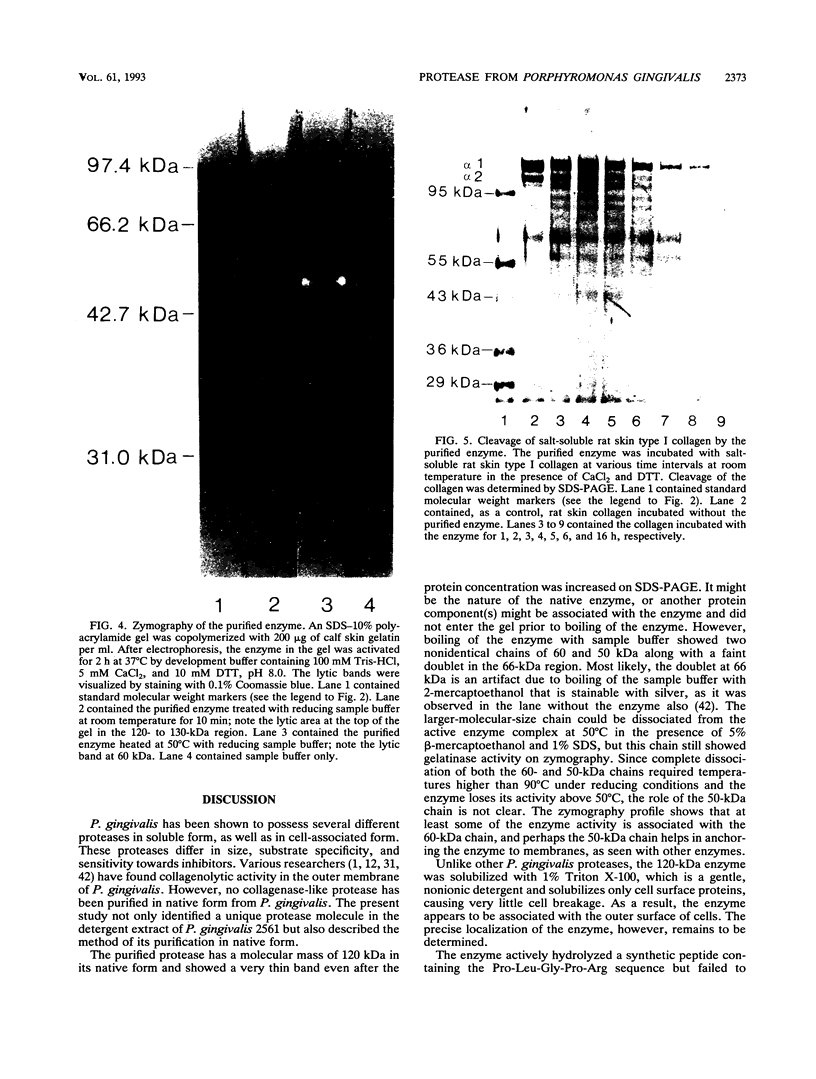
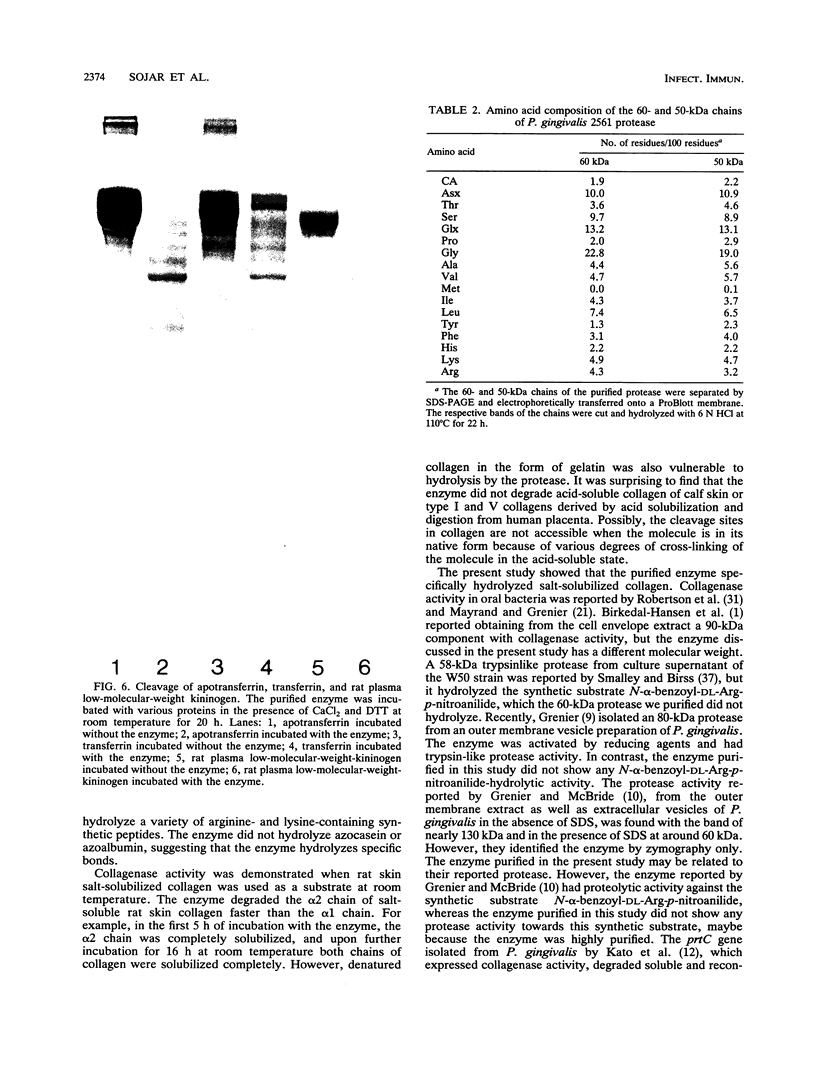
An enzyme capable of hydrolyzing the substrate 4-phenylazobenzyloxycarbonyl-L-prolyl-leucyl-glycyl-prolyl-D-ar gin ine (pZ-peptide), pZ-peptidase, was purified from the oral bacterium Porphyromonas gingivalis. pZ-peptidase hydrolyzed salt-solubilized type I collagen from rat skin, rat plasma low-molecular-weight kininogen, and transferrin at room temperature in the presence of calcium and dithiothreitol. pZ-peptidase did not cleave acid-soluble type I calf skin collagen, type V placental collagen, lysozyme, albumin, or human plasma fibrinogen. Furthermore, the purified enzyme did not hydrolyze N-alpha-benzoyl-DL-Arg-p-nitroanilide, Gly-Pro-p-nitroanilide, N-p-tosyl-Gly-Pro-Arg-p-nitroanilide, N-p-tosyl-Gly-Pro-Lys-p-nitroanilide, azoalbumin, or azocasein. Under reducing conditions, the native enzyme migrated as a single band at 120 kDa on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. However, when heated to 100 degrees C for 10 min in SDS under reducing conditions, the enzyme migrated as a major band at 50 kDa and a minor band at 60 kDa on SDS-polyacrylamide gel electrophoresis. Zymography using calf skin gelatin revealed the gelatin-cleaving activity of the enzyme as evidenced by a diffuse band in the range of 120 to 300 kDa under reducing conditions at room temperature, suggesting that this is the native form of the enzyme. However, incubation at 50 degrees C for 10 min under reducing conditions showed gelatin-cleaving activity at a distinct band of 60 kDa. A minimum temperature of 50 degrees C was required to dissociate the 60-kDa chain from the native complex in active form on gelatin zymography. The ability of the enzyme to cleave other proteins, including kininogen and transferrin, suggests that it has specificity for the Pro-X-Gly sequence found in several proteins, including collagen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkedal-Hansen H., Taylor R. E., Zambon J. J., Barwa P. K., Neiders M. E. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J Periodontal Res. 1988 Jul;23(4):258–264. doi: 10.1111/j.1600-0765.1988.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Bourgeau G., Lapointe H., Péloquin P., Mayrand D. Cloning, expression, and sequencing of a protease gene (tpr) from Porphyromonas gingivalis W83 in Escherichia coli. Infect Immun. 1992 Aug;60(8):3186–3192. doi: 10.1128/iai.60.8.3186-3192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Höfling J. F., Sundqvist G. K. Degradation of albumin, haemopexin, haptoglobin and transferrin, by black-pigmented Bacteroides species. J Med Microbiol. 1984 Aug;18(1):39–46. doi: 10.1099/00222615-18-1-39. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Frandsen E. V., Reinholdt J., Kilian M. Enzymatic and antigenic characterization of immunoglobulin A1 proteases from Bacteroides and Capnocytophaga spp. Infect Immun. 1987 Mar;55(3):631–638. doi: 10.1128/iai.55.3.631-638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Isolation and characterization of a protease from Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):716–720. doi: 10.1128/iai.55.3.716-720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Purification and characterization of a 43-kDa protease of Bacteroides gingivalis. Oral Microbiol Immunol. 1990 Dec;5(6):360–362. doi: 10.1111/j.1399-302x.1990.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Grenier D. Inactivation of human serum bactericidal activity by a trypsinlike protease isolated from Porphyromonas gingivalis. Infect Immun. 1992 May;60(5):1854–1857. doi: 10.1128/iai.60.5.1854-1857.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987 Jan;55(1):111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Kato T., Takahashi N., Kuramitsu H. K. Sequence analysis and characterization of the Porphyromonas gingivalis prtC gene, which expresses a novel collagenase activity. J Bacteriol. 1992 Jun;174(12):3889–3895. doi: 10.1128/jb.174.12.3889-3895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Vail T. A., Switalski L. M., Hook M. Specific cell components of Bacteroides gingivalis mediate binding and degradation of human fibrinogen. J Bacteriol. 1991 Jan;173(2):495–504. doi: 10.1128/jb.173.2.495-504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. Rapid identification of Bacteroides gingivalis. J Clin Microbiol. 1982 Feb;15(2):345–346. doi: 10.1128/jcm.15.2.345-346.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. A., Meyer T. F. Biochemical characterization of Porphyromonas (Bacteroides) gingivalis collagenase. Infect Immun. 1992 Apr;60(4):1524–1529. doi: 10.1128/iai.60.4.1524-1529.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Dor R. H., Warren R. A., Kelln R. A. The relationship of serine protease activity to RNA polymerase modification and sporulation in Bacillus subtilis. J Mol Biol. 1973 May 5;76(1):103–122. doi: 10.1016/0022-2836(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Schmidt E., Morrison E. C. Bacterial profiles of subgingival plaques in periodontitis. J Periodontol. 1985 Aug;56(8):447–456. doi: 10.1902/jop.1985.56.8.447. [DOI] [PubMed] [Google Scholar]
- Lottenberg R., Christensen U., Jackson C. M., Coleman P. L. Assay of coagulation proteases using peptide chromogenic and fluorogenic substrates. Methods Enzymol. 1981;80(Pt 100):341–361. doi: 10.1016/s0076-6879(81)80030-4. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mayrand D., Grenier D. Detection of collagenase activity in oral bacteria. Can J Microbiol. 1985 Feb;31(2):134–138. doi: 10.1139/m85-026. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Morales T. I., Woessner J. F., Howell D. S., Marsh J. M., LeMaire W. J. A microassay for the direct demonstration of collagenolytic activity in Graafian follicles of the rat. Biochim Biophys Acta. 1978 Jun 9;524(2):428–434. doi: 10.1016/0005-2744(78)90180-8. [DOI] [PubMed] [Google Scholar]
- Mortensen S. B., Kilian M. Purification and characterization of an immunoglobulin A1 protease from Bacteroides melaninogenicus. Infect Immun. 1984 Sep;45(3):550–557. doi: 10.1128/iai.45.3.550-557.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T., Hino M., Fuyamada H., Hayakawa T., Sakakibara S. New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem. 1976 Aug;74(2):466–476. doi: 10.1016/0003-2697(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Okuda K., Yamamoto A., Naito Y., Takazoe I., Slots J., Genco R. J. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986 Dec;54(3):659–665. doi: 10.1128/iai.54.3.659-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Endo J., Hinode D., Nagata A., Maehara R., Sato M., Nakamura R. Isolation and characterization of protease from culture supernatant of Bacteroides gingivalis. J Periodontal Res. 1987 Nov;22(6):491–498. doi: 10.1111/j.1600-0765.1987.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Reisner A. H. Gel protein stains: a rapid procedure. Methods Enzymol. 1984;104:439–441. doi: 10.1016/s0076-6879(84)04110-0. [DOI] [PubMed] [Google Scholar]
- Robertson P. B., Lantz M., Marucha P. T., Kornman K. S., Trummel C. L., Holt S. C. Collagenolytic activity associated with Bacteroides species and Actinobacillus actinomycetemcomitans. J Periodontal Res. 1982 May;17(3):275–283. doi: 10.1111/j.1600-0765.1982.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Sato M., Otsuka M., Maehara R., Endo J., Nakamura R. Degradation of human secretory immunoglobulin A by protease isolated from the anaerobic periodontopathogenic bacterium, Bacteroides gingivalis. Arch Oral Biol. 1987;32(4):235–238. doi: 10.1016/0003-9969(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Shah H. N., Gharbia S. E. Lysis of erythrocytes by the secreted cysteine proteinase of Porphyromonas gingivalis W83. FEMS Microbiol Lett. 1989 Oct 1;52(1-2):213–217. doi: 10.1016/0378-1097(89)90199-7. [DOI] [PubMed] [Google Scholar]
- Slots J., Bragd L., Wikström M., Dahlén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986 Jul;13(6):570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Direct hemagglutination technique for differentiating Bacteroides asaccharolyticus oral strains from nonoral strains. J Clin Microbiol. 1979 Sep;10(3):371–373. doi: 10.1128/jcm.10.3.371-373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979 Oct;6(5):351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J. Trypsin-like enzyme activity of the extracellular membrane vesicles of Bacteroides gingivalis W50. J Gen Microbiol. 1987 Oct;133(10):2883–2894. doi: 10.1099/00221287-133-10-2883. [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Shuttleworth C. A., Birss A. J. Collagenolytic activity of the extracellular vesicles of Bacteroides gingivalis W50 and an avirulent variant W50/BE1. Arch Oral Biol. 1989;34(7):579–583. doi: 10.1016/0003-9969(89)90098-8. [DOI] [PubMed] [Google Scholar]
- Sorsa T., Uitto V. J., Suomalainen K., Turto H., Lindy S. A trypsin-like protease from Bacteroides gingivalis: partial purification and characterization. J Periodontal Res. 1987 Sep;22(5):375–380. doi: 10.1111/j.1600-0765.1987.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist G., Carlsson J., Herrmann B., Tärnvik A. Degradation of human immunoglobulins G and M and complement factors C3 and C5 by black-pigmented Bacteroides. J Med Microbiol. 1985 Feb;19(1):85–94. doi: 10.1099/00222615-19-1-85. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Tasheva B., Dessev G. Artifacts in sodium dodecyl sulfate-polyacrylamide gel electrophoresis due to 2-mercaptoethanol. Anal Biochem. 1983 Feb 15;129(1):98–102. doi: 10.1016/0003-2697(83)90057-x. [DOI] [PubMed] [Google Scholar]
- Toda K., Otsuka M., Ishikawa Y., Sato M., Yamamoto Y., Nakamura R. Thiol-dependent collagenolytic activity in culture media of Bacteroides gingivalis. J Periodontal Res. 1984 Jul;19(4):372–381. doi: 10.1111/j.1600-0765.1984.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Kinouchi T., Wakano Y., Ohnishi Y. Purification and characterization of a protease from Bacteroides gingivalis 381. Infect Immun. 1987 Feb;55(2):420–427. doi: 10.1128/iai.55.2.420-427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A. J., van Steenbergen T. J., de Graaff J. The role of black-pigmented Bacteroides in human oral infections. J Clin Periodontol. 1988 Mar;15(3):145–155. doi: 10.1111/j.1600-051x.1988.tb01561.x. [DOI] [PubMed] [Google Scholar]