Abstract
The viral genome-linked protein, VPg, of potyviruses is a multifunctional protein involved in viral genome translation and replication. Previous studies have shown that both eukaryotic translation initiation factor 4E (eIF4E) and eIF4G or their respective isoforms from the eIF4F complex, which modulates the initiation of protein translation, selectively interact with VPg and are required for potyvirus infection. Here, we report the identification of two DEAD-box RNA helicase-like proteins, PpDDXL and AtRH8 from peach (Prunus persica) and Arabidopsis (Arabidopsis thaliana), respectively, both interacting with VPg. We show that AtRH8 is dispensable for plant growth and development but necessary for potyvirus infection. In potyvirus-infected Nicotiana benthamiana leaf tissues, AtRH8 colocalizes with the chloroplast-bound virus accumulation vesicles, suggesting a possible role of AtRH8 in viral genome translation and replication. Deletion analyses of AtRH8 have identified the VPg-binding region. Comparison of this region and the corresponding region of PpDDXL suggests that they are highly conserved and share the same secondary structure. Moreover, overexpression of the VPg-binding region from either AtRH8 or PpDDXL suppresses potyvirus accumulation in infected N. benthamiana leaf tissues. Taken together, these data demonstrate that AtRH8, interacting with VPg, is a host factor required for the potyvirus infection process and that both AtRH8 and PpDDXL may be manipulated for the development of genetic resistance against potyvirus infections.
Plant viruses are obligate intracellular parasites that infect many agriculturally important crops and cause severe losses each year. One of the common characteristics of plant viruses is their relatively small genome that encodes a limited number of viral proteins, making them dependent on host factors to fulfill their infection cycles (Maule et al., 2002; Whitham and Wang, 2004; Nelson and Citovsky, 2005; Decroocq et al., 2006). In order to establish a successful infection, the invading virus must recruit an array of host proteins (host factors) to translate and replicate its genome and to move locally from cell to cell via the plasmodesmata and systemically via the vascular system. It has been suggested that down-regulation or mutation of some of the required host factors may result in recessively inherited resistance to viruses (Kang et al., 2005b).
Potyviruses, belonging to the genus Potyvirus in the family Potyviradae, constitute the largest group of plant viruses (Rajamäki et al., 2004). Potyviruses have a single positive-strand RNA genome approximately 10 kb in length, with a viral genome-linked protein (VPg) covalently attached to the 5′ end and a poly(A) tail at the 3′ end (Urcuqui-Inchima et al., 2001; Rajamäki et al., 2004). The viral genome contains a single open reading frame (ORF) that translates into a polypeptide with a molecular mass of approximately 350 kD, which is cleaved into 10 mature proteins by viral proteases (Urcuqui-Inchima et al., 2001). Recently, a novel viral protein resulting from a frameshift in the P3 cistron has been reported (Chung et al., 2008). Of the 11 viral proteins, VPg is a multifunctional protein and the only other viral protein present in the viral particles (virions) besides the coat protein and the cylindrical inclusion protein (CI; Oruetxebarria et al., 2001; Puustinen et al., 2002; Gabrenaite-Verkhovskaya et al., 2008). The nonstructural protein is linked to the viral RNA by a phosphodiester bond between the 5′ terminal uridine residue of the RNA and the O4-hydroxyl group of amino acid Tyr (Murphy et al., 1996; Oruetxebarria et al., 2001; Puustinen et al., 2002). Mutation of the Tyr residue that links VPg to the viral RNA abolishes virus infectivity completely (Murphy et al., 1996). In infected cells, VPg and its precursor NIa are present in the nucleus and in the membrane-associated virus replication vesicles in the cytoplasm (Carrington et al., 1993; Rajamäki and Valkonen, 2003; Cotton et al., 2009). As a component of the replication complex, VPg may serve as a primer for viral RNA replication (Puustinen and Mäkinen, 2004) and as an analog of the m7G cap of mRNAs for the viral genome to recruit the translation complex for translation (Michon et al., 2006; Beauchemin et al., 2007; Khan et al., 2008). Furthermore, VPg has been suggested to be an avirulence factor for recessive resistance genes in diverse plant species (Moury et al., 2004; Kang et al., 2005b; Bruun-Rasmussen et al., 2007). Thus, VPg plays a pivotal role in the virus infection process. The molecular identification of VPg-interacting host proteins and the subsequent functional characterization of such interactions may advance knowledge of the intricate virus replication mechanisms and help develop novel antiviral strategies.
Previous studies have shown that VPg and its precursor NIa interact with several host proteins, including three essential components of the host protein translation apparatus (Thivierge et al., 2008). The first protein is the cellular translation initiation factor eIF4E or its isoform eIF(iso)4E, identified through a yeast two-hybrid screen using VPg as a bait (Wittmann et al., 1997; Schaad et al., 2000). The protein complex of VPg and eIF4E is an essential component for virus infectivity (Robaglia and Caranta, 2006). Mutations and knockout of eIF4E or eIF(iso)4E confer resistance to infection (Lellis et al., 2002; Ruffel et al., 2002; Nicaise et al., 2003; Gao et al., 2004; Kang et al., 2005a; Ruffel et al., 2005; Decroocq et al., 2006; Bruun-Rasmussen et al., 2007). It is well known that potyviruses recruit selectively one of the eIF4E isoforms, depending on specific virus-host combinations (German-Retana et al., 2008). For instance, in Arabidopsis (Arabidopsis thaliana), eIF(iso)4E is required for infection by Turnip mosaic virus (TuMV), Plum pox virus (PPV), and Lettuce mosaic virus, while eIF4E is indispensable for infection by Clover yellow vein virus (Duprat et al., 2002; Lellis et al., 2002; Sato et al., 2005; Decroocq et al., 2006). The second cellular protein interacting with VPg is another translation initiation factor, eIF4G. Analysis of Arabidopsis knockout mutants for eIF4G or its isomers eIF(iso)4G1 and eIF(iso)4G2 has yielded results supporting the idea that the recruitment of eIF4G for potyvirus infection is also isoform dependent (Nicaise et al., 2007). Recently, poly(A)-binding protein (PABP), the translation initiation factor that bridges the 5′ and 3′ termini of the mRNA into proximity, has been proposed to be essential for efficient multiplication of TuMV (Dufresne et al., 2008). PABP was previously documented to interact with NIa, a VPg precursor containing both VPg and the proteinase NIa-Pro (Léonard et al., 2004). As the translation factors eIF(iso)4E and PABP have been found to be internalized in virus-induced vesicles, it has been suggested that the interactions between VPg and these translation factors are crucial for viral RNA translation and/or replication (Beauchemin and Laliberté, 2007; Beauchemin et al., 2007; Cotton et al., 2009). Besides these three translation factors, a Cys-rich plant protein, potyvirus VPg-interaction protein, was also found to associate with VPg (Dunoyer et al., 2004). This plant-specific VPg-interacting host protein contains a PHD finger domain and acts as an ancillary factor to support potyvirus infection and movement (Dunoyer et al., 2004).
In this study, we describe the identification of an Arabidopsis DEAD-box RNA helicase (DDX), AtRH8, and a peach (Prunus persica) DDX-like protein, PpDDXL, both interacting with the potyviral VPg protein. Using the atrh8 mutant, we demonstrate that AtRH8 is not required for plant growth and development in Arabidopsis but is necessary for infection by two plant potyviruses, PPV and TuMV. Furthermore, we present evidence that AtRH8 colocalizes with the virus accumulation complex in potyvirus-infected leaf tissues, which reveals a possible role of AtRH8 in virus infection. Finally, we have identified the VPg-binding region (VPg-BR) of AtRH8 and PpDDX and show that overexpression of the VPg-BR either from AtRH8 or PpDDXL suppresses virus accumulation.
RESULTS
Identification of a VPg-Interacting DEAD Box RNA Helicase-Like Protein from Peach and Arabidopsis
To identify VPg-interacting host proteins in plants, a yeast two-hybrid cDNA library screen was carried out. The library was constructed from PPV-infected peach leaf tissues in order to search VPg-interacting host candidates in its natural host during virus infection. A total of 1.3 × 106 transformed cDNA clones were tested against the PPV VPg as bait. The resulting positive clones were rescued and isolated for sequencing. Based on the results of BLASTX searches (E value ≤ 1 × 10−10), a total of 85 peach proteins were identified. Of these positive clones, five contained a stretch of the same cDNA sequence. The predicted peptide from the longest clone shares 96% to 98% sequence similarity to a number of proteins, including the ATP-dependent RNA helicase and eIF4A, that belong to the DDX family. Thus, this gene is designated PpDDXL. Based on the multiple occurrences of PpDDXL in the screen, the fact that RNA helicase is part of the eIF4F translation complex, and the assumption that host RNA helicases may be involved in viral genome replication, PpDDXL was chosen for further molecular and functional characterizations.
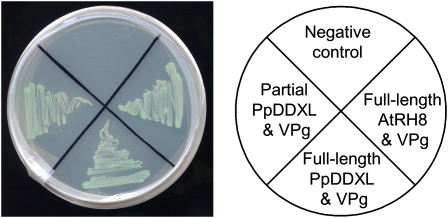
The full-length cDNA of PpDDXL was obtained using RACE PCR techniques and deposited into GenBank (accession no. GQ865547). The interactions between the partial or full-length PpDDXL proteins and the PPV VPg were confirmed in yeast (Fig. 1). The full-length cDNA of PpDDXL is 1,692 bp, with a 5′ untranslated region of 293 bp, an ORF of 1,242 bp, and a 3′ untranslated region of 157 bp (Supplemental Fig. S1). It encodes a polypeptide of 413 amino acids with a predicted molecular mass of 47 kD and a pI of 5.48. The corresponding genomic DNA sequence of PpDDXL was obtained by PCR amplification of genomic DNA (GenBank accession no. GQ865548). Alignment of the cDNA and genomic sequence of PpDDXL indicated that PpDDXL contains three introns and four exons (Supplemental Fig. S1A). Domain analyses using the Pfam program (http://pfam.sanger.ac.uk/) identified the DEAD/DEAH box (amino acids 64–230) signature and helicase-conserved C-terminal (amino acids 298–374) domains (Supplemental Fig. S1, B and C).
Figure 1.
Yeast two-hybrid assay of protein-protein interaction between the PPV VPg and PpDDXL from peach or AtRH8 from Arabidopsis. Yeast cotransformants were grown on the selective medium SD/−Ade/−His/−Leu/−Trp plus X-α-Gal and incubated at 28°C for 4 d. [See online article for color version of this figure.]
Due to the unavailability of an efficient genetic transformation protocol for the characterization of gene functions in Prunus species, Arabidopsis was selected as a model host for exploring the roles of PpDDXL and related RNA helicases in potyvirus infections. BLAST searches against the Arabidopsis database revealed 10 Arabidopsis DDXs that shared high sequence similarity to PpDDXL (Supplemental Fig. S2A). Although the three Arabidopsis eIF4As and two putative eIF4As (AT3G13920, AT1G54270, AT1G72730, AT3G1960, and AT1G51380, respectively) are most similar to PpDDXL, there were no corresponding homozygous knockout T-DNA lines available. Extensive screening of progeny plants from the eight heterozygous eIF4A T-DNA lines (SALK_038072, SALK_072655, SALK_107633, SALK_123728, SALK_135778, SALK_107633, SAIL_755_B08, and WiscDsLox254D02) failed to recover any homozygous plants. These data suggest a possible detrimental effect to the plant when silencing these eIF4As. Indeed, even the heterozygous T-DNA seedlings showed abnormal phenotypes in the number and length of root hair (Supplemental Fig. 2B). Thus, AtRH8 (AT4G00660), the next most related candidate to PpDDXL, was selected for functional characterization. The ORF of AtRH8 was obtained from Arabidopsis wild-type Columbia cDNA using reverse transcription (RT)-PCR with gene-specific primers. The ORF of AtRH8 consists of 1,518 nucleotides encoding a 505-amino acid protein. A yeast two-hybrid assay confirmed a positive interaction between the PPV VPg and AtRH8 (Fig. 1).
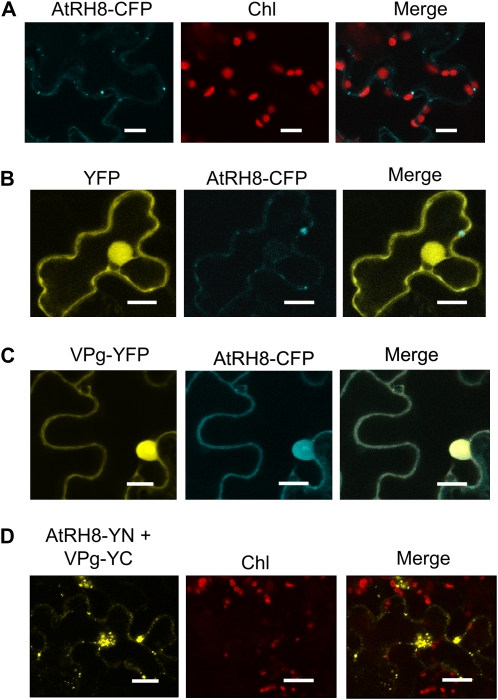
To study if AtRH8 and VPg colocalize in planta, transient expression vectors encoding AtRH8-cyan fluorescent protein (CFP) fusion (AtRH8-CFP) and VPg-yellow fluorescent protein (YFP) fusion (VPg-YFP) were constructed. Transient expression of these chimeric genes was achieved through agroinfiltration. As a control, AtRH8 was expressed alone (Fig. 2A) or coexpressed with YFP (Fig. 2B). The distribution of AtRH8 was found in the cytoplasm (Fig. 2, A and B), whereas YFP was in the cytoplasm and in the nucleus (due to diffusion; Fig. 2B). In addition, AtRH8 also formed some punctate structures in the cytoplasm (Fig. 2, A and B). In Nicotiana benthamiana epidermal cells coexpressing AtRH8-CFP and VPg-YFP, the two proteins colocalized in the nucleus and in the cytoplasm (Fig. 2C). Previously, VPg-YFP was reported to localize mainly in the nucleus when expressed alone (Wei and Wang, 2008). Thus, the VPg-YFP interfered in the distribution of AtRH8-CFP. To further investigate the interaction between VPg and AtRH8 in planta, a bimolecular fluorescence complementation (BiFC) assay was carried out. Several BiFC negative control combinations were set up to ensure the validity of the BiFC results. These combinations included the N-terminal (YN) and C-terminal (YC) fragments of YFP, AtRH8-YN and YC, YN and AtRH8-YC, VPg-YN and YC, and YN and VPg-YC (Supplemental Fig. S3). When AtRH8-YN was coexpressed with VPg-YC in N. benthamiana plants, a strong emission of YFP fluorescence was observed in the cytoplasm and in the nucleus as early as 2 d after agroinfiltration (Fig. 2D). Taken together, these data demonstrate a physical interaction between AtRH8 and VPg.
Figure 2.
In planta interaction between AtRH8 and the PPV VPg. A, Confocal microscopy imaging of AtRH8-CFP expressed in N. benthamiana leaf. Bars = 14 μm. B, Coexpression of YFP and AtRH8 fused with CFP (AtRH8-CFP) in 3-week-old N. benthamiana plants. Agrobacterium EHA105 strain containing YFP and AtRH8-CFP expression plasmids were coinfiltrated into the lower epidermal leaf surface. AtRH8-CFP was distributed in the cytoplasm, and YFP labeled both the cytoplasm and the nucleus (due to diffusion). Bars = 16 μm. C, Coexpression of AtRH8-CFP and VPg fused with YFP (VPg-YFP). VPg-YFP was localized to the cytoplasm as well as the nucleus. AtRH8-CFP colocalized with VPg-YFP. Bars = 16 μm. D, BiFC analysis of AtRH8 and VPg. AtRH8 was fused with the N-terminal fragment of YFP (AtRH8-YN) and coexpressed with the fusion of VPg with the C-terminal fragment of YFP (VPg-YC). Bars = 17 μm. All confocal images were taken 2 d post infiltration. Chl, Chloroplast autofluorescence.
Requirement of AtRH8 for Potyviral Infection
To investigate the functional role of AtRH8 in virus infection, a homozygous T-DNA line of AtRH8, SALK_016830, with a T-DNA insertion in the promoter region was acquired from the Arabidopsis Biological Resource Center (http://www.biosci.ohio-state.edu/pcmb/Facilities/abrc/abrchome.htm). The T-DNA PCR screen on genomic DNA as well as genetic analysis confirmed that there is only one T-DNA insertion (data not shown). RT-PCR analysis of total RNA isolated from leaf tissues of this mutant line and the wild type revealed no expression of AtRH8 in the homozygous T-DNA line (Supplemental Fig. S4). Thus, this line (atrh8) is a true knockout mutant of AtRH8.
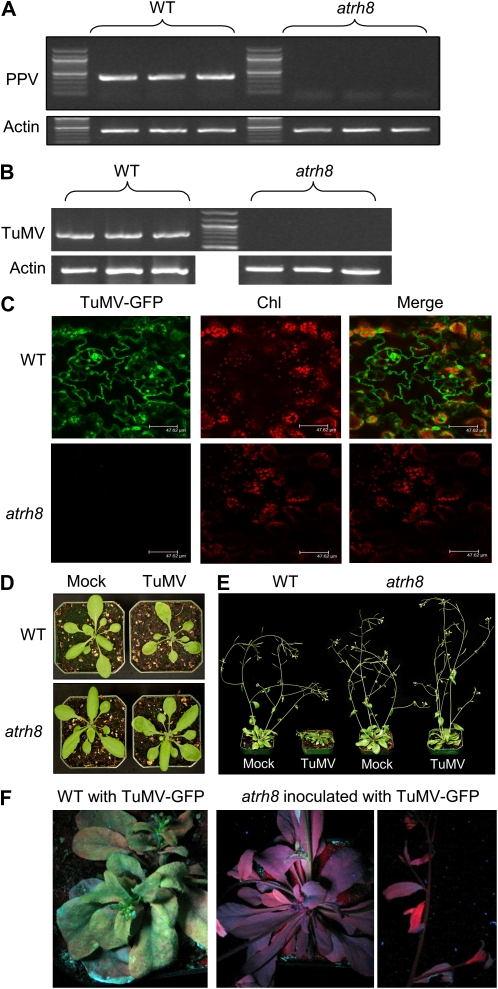
To test if AtRH8 is required for PPV infection, the atrh8 mutant and wild-type plants were mechanically inoculated with a Canadian PPV-D isolate. Total RNA was extracted from the upper newly emerged leaves 14 d post infection (dpi). RT-PCR assays were used to monitor the accumulation of the viral RNA. The PPV genomic RNA was detected only in the wild type (Fig. 3A). Mild disease symptoms such as slight growth retardation were found in the infected wild-type plants, consistent with our previous observation (Babu et al., 2008). In contrast, the atrh8 mutant plants inoculated with PPV did not show any disease symptoms, and no PPV was detectable in these inoculated mutant plants (Fig. 3A). Taken together, these data suggest that atrh8 mutants are resistant to PPV.
Figure 3.
Requirement of AtRH8 for potyvirus infection in Arabidopsis plants. A and B, Results from RT-PCR analysis of PPV and TuMV accumulation, respectively. Wild-type (WT) plants and atrh8 mutants were mechanically inoculated and agroinfiltrated with PPV and TuMV, respectively. Total RNA extracted from upper newly emerged leaves 2 weeks post inoculation was used for cDNA synthesis. The cDNA was amplified using PPV Coat Protein (CP)-specific primers and TuMV HC-Pro-specific primers for the corresponding assay. Actin2 was selected as the endogenous reference gene to serve as an internal control of RT-PCR. C, Confocal imaging of newly emerged leaves of TuMV-infiltrated wild-type and atrh8 mutant plants. Three-week-old Arabidopsis plants were agroinfiltrated with a GFP-tagged TuMV infectious clone and observed 10 d post infiltration. Chl, Chloroplast autofluorescence. Bars = 48 μm. D and E, Phenotypes of wild-type and atrh8 mutant plants 3 and 14 d post infiltration, respectively. Mock, Infiltrated with an empty vector; TuMV, infiltrated with the GFP-tagged TuMV infectious clone. F, Photographs of wild-type and arth8 mutant plants inoculated with the GFP-tagged TuMV infectious clone under UV light (19 dpa).
To test if AtRH8 is also needed by another potyvirus during infection, the wild-type and atrh8 plants were agroinfiltrated with a GFP-tagged TuMV infectious clone (TuMV-GFP). Diagnosis of these plants 14 d post agroinfiltration (dpa) with RT-PCR revealed the presence of the TuMV genomic RNA in the wild-type plants but not in the atrh8 mutants (Fig. 3B). Consistent with our PCR results, a strong emission of GFP fluorescence was observed in the newly emerged leaves of the infiltrated wild-type plants but not the atrh8 plants (Fig. 3C). Phenotypes of the wild-type plants and atrh8 mutants agroinfiltrated with TuMV-GFP or mock infiltrated were closely examined. Under the normal growth conditions without TuMV infiltration, atrh8 mutants showed no developmental differences from wild-type plants (see mock-inoculated wild type and atrh8; Fig. 3, D and E) and displayed normal vegetative growth, flowering development, and seed production. At 3 dpa, the wild-type plants agroinfiltrated with TuMV-GFP began exhibiting yellowing on the surface of the leaves and slight growth stunting. In contrast, no difference was observed between TuMV-infiltrated atrh8 mutants and mock-infiltrated wild-type or atrh8 plants (Fig. 3D). At the later infection stage (14 dpa), the infected wild-type plant displayed the full spectrum of disease symptoms, including mosaic and necrosis on leaves, severe growth retardation, reduced apical dominance, curled bolts, and the typical inflorescence stunting (Fig. 3E), similar to previous descriptions (Lellis et al., 2002). The TuMV-infiltrated atrh8 mutants, however, showed normal growth and fertility with no signs of infection symptoms. When the TuMV-infected plants were exposed under UV light at 19 dpa, the stunted wild-type plants exhibited bright green fluorescent emission from the tagged GFP, but no GFP was exhibited in TuMV-infiltrated atrh8 plants (Fig. 3F). Taken together, these results indicate that AtRH8 is required for both PPV and TuMV infections.
Colocalization of AtRH8 with Virus-Induced Replication-Associated Membranous Vesicles in Planta
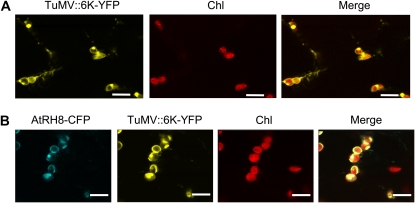
To explore a possible role of AtRH8 in virus infection, the subcellular localization of AtRH8 was examined in planta in the presence of virus infection. Potyviral 6K2 protein is an integral membrane protein that induces the formation of the endoplasmic reticulum-derived vesicles (Schaad et al., 1997). Recently, we have found that these 6K2 vesicles originate at endoplasmic reticulum exit sites and target chloroplasts for virus replication (Fig. 4A; Wei and Wang, 2008; T. Wei and A. Wang, unpublished data). These 6K2 vesicles contain viral replication-associated proteins such as NIa and 6K2-NIa (two VPg precursors), NIb (viral RNA-dependent RNA polymerase), viral RNA (carrying VPg), and host factors such as eIF(iso)4E, PABP2, and eEF1A (Cotton et al., 2009). All of these components are essential for viral genome translation/replication. To visualize the subcellular localization of AtRH8 in virus-infected leaves, the AtRH8-CFP was transiently expressed in N. benthamiana infected with a TuMV infectious clone carrying an additional 6K2 tagged with YFP at the junction of P1 and HC-Pro (TuMV″6K-YFP). In contrast to the distribution of AtRH8 in the cytoplasm when expressed alone or coexpressed with a control protein (Fig. 2, A and B), AtRH8 was strongly localized with chloroplast-associated 6K2 vesicles during TuMV infection (Fig. 4B).
Figure 4.
Colocalization of AtRH8 with the virus accumulation complex in TuMV-infected N. benthamiana epidermal cells. A, Confocal microscopy imaging of N. benthamiana epidermal cells that had been preinfected with a TuMV infectious clone tagged with a 6K2-YFP fusion (TuMV″6K-YFP) for 4 d and then agroinfiltrated with an empty vector. In plant cells infected by a similar infectious clone (TuMV″6K-GFP), 6K2 vesicles contain viral replication-associated proteins such as NIa and 6K2-NIa (two VPg precursors), NIb (viral RNA-dependent RNA polymerase), viral RNA (carrying VPg), and host factors such as eIF(iso)4E, PABP2, and eEF1A (Cotton et al., 2009). These 6K2 vesicles originate at endoplasmic reticulum exit sites (Wei and Wang, 2008) and target chloroplasts for virus replication (T. Wei and A. Wang, unpublished data). Bars = 10 μm. B, AtRH8-CFP was transiently expressed in N. benthamiana leaves that had been preinfected with the TuMV″6K-YFP infectious clone for 4 d. AtRH8-CFP colocalized with the chloroplast-bound 6K2-YFP vesicles. Bars = 10 μm. All confocal images were taken 2 dpa with the empty vector or the AtRH8 expression vector. Chl, Chloroplast autofluorescence.
Determination of VPg-BR in AtRH8 and PpDDXL
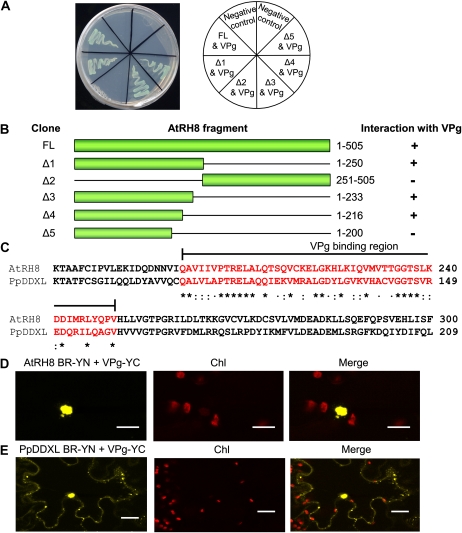
To determine the VPg-BR of AtRH8, a series of deletions were conducted on AtRH8. Initially, the protein was truncated into two moieties, with the N-terminal portion containing 250 amino acids and the C-terminal portion containing 257 amino acids (Fig. 5, A and B). The truncated protein was fused into the pAD-GAL plasmid of the yeast two-hybrid system. The interaction assay was conducted using the PPV VPg as the interaction partner cloned into the pBD-GAL plasmid. Growth of the yeast transformants on selective medium showed that VPg positively interacted exclusively with the N-terminal fragment of AtRH8 and not the C-terminal portion, suggesting that the interaction site resides within the first 250 amino acids of AtRH8. The N-terminal 250 amino acids were subjected to additional sequential deletions (Fig. 5, A and B). Based on the deletion analyses, the VPg-BR of AtRH8 consists of 50 amino acids (amino acids 201–250; Fig. 5, A and B). Protein sequence comparison of the VPg-BR (amino acids 140–189) of PpDDXL and that of AtRH8 indicated a 74% similarity (Fig. 5C). The protein predictor SSpro version 4.5 program from the ExPasy Proteomics Server (http://www.expasy.ch) identified an α-helix in this region in both PpDDXL and AtRH8.
Figure 5.
Identification of VPg-BR in AtRH8 and PpDDXL. A, Protein-protein interaction between the truncated AtRH8 and the PPV VPg in the yeast two-hybrid assay. Yeast cells were cotransformed with the VPg bait vector and prey vectors (AtRH8 deletions represented by diagram of bars in B), plated on the highly stringent selective medium SD/−Ade/−His/−Leu/−Trp plus X-α-Gal, and incubated at 28°C for 4 d. B, Summary of results from A. Clone names and schematic representation of various forms of the truncated AtRH8 are shown (corresponding amino acid positions are indicated). +, Positive interaction; −, negative interaction. C, Protein sequence alignment of the VPg-BR of AtRH8 and that of PpDDXL. The VPg-BR sequences were aligned using the ClustalW program. Amino acids within the binding region are colored in red and compared for sequence similarity. Asterisks indicate identical amino acids; colons indicate strongly related amino acids (belonging to the same group such as polar, nonpolar, basic, and acidic and having side chains sharing a similar chemical structure); and dots indicate weakly related amino acids (belonging to the same group such as polar, nonpolar, basic, and acidic and having side chains with different chemical structures). D, BiFC analysis of the interaction between the VPg-BR of AtRH8 and the PPV VPg 2 d post coinfiltration. Confocal microscopy imaging on the coexpression of AtRH8 VPg-BR fused with the N-terminal fragment of YFP (AtRH8 BR-YN) and the PPV VPg fused with the C-terminal fragment of YFP (VPg-YC) in 3-week-old N. benthamiana lower leaf epidermal cells is shown. Bars = 17 μm. E, BiFC analysis of the interaction between the VPg-BR of PpDDXL and the PPV VPg 2 d post coinfiltration. The VPg-BR was fused with the N-terminal fragment of YFP (PpDDXL BR-YN) and coexpressed with the PPV VPg fused with the C-terminal fragment of YFP (VPg-YC). Bars = 19 μm. Chl, Chloroplast autofluorescence.
In order to verify the interaction between the PPV VPg and the VPg-BR in planta, BiFC assays between the PPV VPg and the VPg-BR of AtRH8 or that of PpDDXL were conducted in N. benthamiana plants. Three-week-old plants were agroinfiltrated to coexpress the BR-YN (the VPg-BR of AtRH8 or PpDDXL attached to the N-terminal fragment of YFP) and the VPg-YC (the PPV VPg fused to the C-terminal fragment of YFP) as well as the reverse combinations. Infiltrated leaf tissues were observed by confocal microscopy 2 dpa. A positive interaction was observed between the VPg-BR of AtRH8 and the PPV VPg mainly in the nucleus (Fig. 5D). The interaction of the VPg-BR of PpDDXL and the PPV VPg was found in the nucleus and in the cytoplasm (Fig. 5E). These data illustrate that the VPg-BR of AtRH8 and PpDDXL is responsible for protein-protein interaction with the PPV VPg.
Suppression of Virus Infection by Transient Overexpression of the VPg-BR of AtRH8 and That of PpDDXL
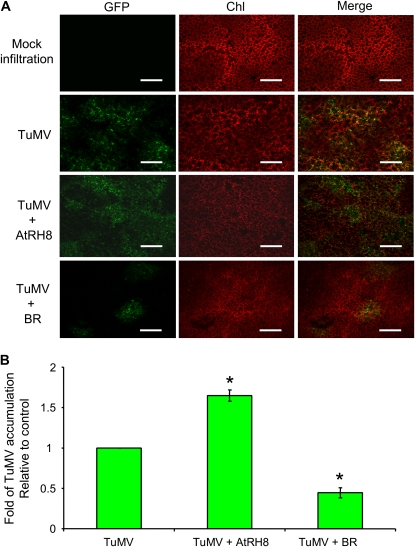
As described above, potyvirus infection requires the presence of AtRH8. To determine if overexpression of AtRH8 affects potyvirus infection, N. benthamiana leaves were coinfiltrated with the TuMV-GFP clone and an empty vector (as a control) or with TuMV-GFP and a plant AtRH8 expression vector. TuMV infection was assessed by real-time PCR analyses 2 dpa and visualized by confocal observation 3 dpa. In comparison with the control leaves (infiltrated with TuMV-GFP and an empty vector), leaves expressing AtRH8 and infected by TuMV-GFP displayed a much stronger green fluorescence intensity (Fig. 6A). Quantification of TuMV using real-time PCR revealed a 1.6-fold increase in virus accumulation in the leaves overexpressing AtRH8 (Fig. 6B).
Figure 6.
Effect of transient overexpression of AtRH8 and its VPg-BR on TuMV accumulation in N. benthamiana plants. A, Ectopic expression of AtRH8 enhances TuMV infection in N. benthamiana. Confocal images of 3-week-old N. benthamiana leaves agroinfiltrated with different combinations of plasmids are shown. The photographs represent the infection pattern in N. benthamiana at 3 d post infiltration. Chl, Chloroplast autofluorescence; GFP, green fluorescence emission from a GFP-tagged TuMV infectious clone; TuMV, coinfiltration of a GFP-tagged TuMV clone with an empty vector; TuMV + AtRH8, coinfiltration of the TuMV-GFP clone and the AtRH8-expressing clone; TuMV + BR, coinfiltration of the TuMV-GFP clone and the AtRH8-BR-expressing clone. Bars = 300 μm. B, Real-time PCR quantification of TuMV accumulation in N. benthamiana at 2 d post infiltration. Three independent experiments were carried out for quantification analyses. In each experiment, three plants were used per treatment. The values represent means of fold change relative to the control (TuMV alone). The asterisks indicate that TuMV accumulation in N. benthamiana expressing AtRH8 (TuMV + AtRH8) or the AtRH8-BR (TuMV + BR) was significantly higher or lower than that in the control (TuMV; P < 0.01). Error bars represent sd.
To assess the effect of transient overexpression of the VPg-BR of AtRH8 on potyvirus infection, the TuMV-GFP infectious clone was coinfiltrated into N. benthamiana with an expression plasmid containing the VPg-BR of AtRH8. Coexpression of the VPg-BR led to a reduction of the virus accumulation relative to the control (coinfiltration of TuMV-GFP clone with an empty vector; Fig. 6A). Quantitative analysis of the viral RNA indicated that viral RNA in the leaves expressing the VPg-BR of AtRH8 accumulated about 31% of that in the control (Fig. 6B). Furthermore, we tested the effect of the VPg-BR of AtRH8 on the infection of Tobacco etch virus (TEV), another member of the Potyvirus genus. Since the GFP-tagged TEV infectious clone was incompatible with the agroinfiltration system (Schaad et al., 1997), mechanical inoculation of TEV was used for infection. The accumulation of TEV in the leaves expressing the VPg-BR of AtRH8 was approximately 34% of that in the control (Supplemental Fig. S5). Confocal visualization of the inoculated tissues revealed a consistent reduction of green fluorescence emission as quantified by real-time PCR results (Supplemental Fig. S5). Similarly, real-time PCR analyses of TEV and TuMV infections in the presence of the VPg-BR of PpDDXL revealed a decrease of virus accumulation by approximately 5- and 3-fold in comparison with the respective controls (Supplemental Fig. S6). These data suggest that overexpression of the VPg-BR of AtRH8 or that of PpDDXL exerts a suppression effect on potyvirus infections.
DISCUSSION
In this study, we have reported the identification of two VPg-interacting plant DDX proteins, AtRH8 from Arabidopsis and PpDDXL from peach (Figs. 1 and 2). These DDX proteins share sequence homology with eIF4A, a component of the eIF4F multiprotein complex. We used the Arabidopsis AtRH8 homozygous T-DNA insertion lines to functionally characterize the requirement of AtRH8 in potyvirus infection. We found that AtRH8 knockout plants (atrh8 mutants) grew and developed as the wild-type plants, indicating that AtRH8 is dispensable for the normal plant growth and development (Fig. 3). But these mutants were unable to support PPV and TuMV infections, suggesting that AtRH8 is required for virus infections (Fig. 3). Therefore, AtRH8 is a host factor that plays an essential role in the virus infection cycle. To our knowledge, this report is the first showing that a plant DDX protein is required for virus infection in plants.
RNA helicases represent a large family of proteins implicated in almost every step of RNA metabolism (de la Cruz et al., 1999; Tanner and Linder, 2001; Lorsch, 2002; Mohr et al., 2002). During the virus infection process, RNA helicases have been suggested to be involved in (1) translation of the viral RNA, (2) selection of the RNA template for translation or replication, (3) recruitment of the viral RNA for replication, (4) RNA synthesis, and/or (5) RNA stability (Li et al., 2009). Previously, it has been shown that in yeast cells, DED1, an eIF4A-like RNA helicase, is essential for the translation of Brome mosaic virus (BMV; bromovirus; Noueiry et al., 2000). A point mutation in DED1 does not affect yeast normal growth but results in the inhibition of BMV replication through selectively blocking the translation of the BMV RNA2 that encodes the viral RNA-dependent RNA polymerase 2a (Noueiry et al., 2000). By a yet unknown mechanism, this mutant also inhibits the replication of Tomato bushy stunt virus (TBSV), a tombusvirus (Jiang et al., 2006). In a recent yeast protein array using protein-RNA interactions, several other RNA helicases bound to BMV and TBSV genomic RNAs have been documented (Li et al., 2009). These results suggest that besides DED1, other RNA helicases may also participate in regulating the translation and/or replication of viral RNAs. In animal cells, it has been reported that Human immunodeficiency virus type 1 and Hepatitis C virus both recruit DEAD-box RNA helicase 3 (DDX3) for viral genome replication (Fang et al., 2004, 2005; Ariumi et al., 2007). It is assumed that DDX3 promotes the translocation of viral RNA through the nuclear pore complex by remodeling the virus replication complex (Yedavalli et al., 2004). Interestingly, DDX3 has also been shown to chaperone a type of mRNA granules for translation in the developing brain of rat embryos (Elvira et al., 2006). These DDX3-containing granules contain the full protein translation apparatus, including both the small and large ribosomal subunits as well as mRNA. In this study, AtRH8 colocalized with the TuMV accumulation complex in virus-infected cells (Fig. 4). The potyvirus replication complex has been shown to contain viral replicase components (such as NIa, 6K2-NIa, and NIb), viral genomic RNA (carrying NIa or VPg), double-stranded RNA, and host translational proteins [such as eIF(iso)4E, PABP2, and eEF1A; Cotton et al., 2009]. It is possible that AtRH8 and PpDDXL both play a role in viral genome translation, as suggested for yeast DED1 and for human DDX3.
The presence of AtRH8 in the virus accumulation complex but not in the nucleus in infected cells (Fig. 4) is consistent with the recent finding that eIF(iso)4E, also a VPg-interacting translation initiation factor, is localized to the TuMV replication complex (Cotton et al., 2009). In infected cells, NIa is the major form of VPg, which, as a viral RNA genome-linked protein, is present in the cytoplasm or, as a nuclear localization signal-containing protein, is translocated into the nucleus (Restrepo-Hartwig and Carrington, 1992; Carrington et al., 1993; Rajamäki and Valkonen, 2003, 2009). It is puzzling that AtRH8 and eIF(iso)4E were mainly found in the cytoplasm but not in the nucleus. One possible explanation is that in the early infection stage, NIa or VPg is mainly intercepted by the virus replication complex for replication, with only a small amount of NIa or VPg transported to the nucleus. Indeed, large amounts of viral RNA (carrying NIa or VPg) are concentrated in the virus replication complex in the early infection stage (a few days after infection; Cotton et al., 2009; T. Wei and A. Wang, unpublished data), whereas high-level accumulation of NIa or VPg in the nucleus has only been shown in the later infection stage (i.e. 20 dpi; Rajamäki and Valkonen, 2003, 2009). Nuclear transport of NIa may be regulated by differential cleavage efficiency (Restrepo-Hartwig and Carrington, 1992). For instance, cleavage at the N terminus of 6K2 in the potyviral polyprotein occurs preferentially to the N terminus of NIa, leading to the transient accumulation of the 6K2-NIa precursor protein (Restrepo-Hartwig and Carrington, 1992). The cytoplasmic 6K2-NIa impedes nuclear localization of NIa (Restrepo-Hartwig and Carrington, 1992) and colocalizes with the TuMV replication complex (Cotton et al., 2009). In addition to different VPg precursors, several posttranslationally modified forms of VPg and NIa have also been found in infected plants (Hafrén and Mäkinen, 2008). These modified forms of VPg and NIa may also be intracellularly differentially distributed. It is possible that different VPg precursors or their modified forms have different binding abilities to AtRH8. Further determination of their subcellular localization over the infection course and analysis of their ability to interact with AtRH8 may help understand the mechanism underlying the recruitment of AtRH8 to the virus accumulation complex.
As discussed above, both PpDDXL and AtRH8 appear to be RNA helicases by sequence comparison (Supplemental Fig. S1). Interestingly, the potyviral CI also contains an RNA-binding domain and has ATPase and RNA helicase activities (Laín et al., 1990, 1991; Eagles et al., 1994). A genetic study on the CI using a TEV infectious clone revealed that CI plays essential dual roles in TEV replication and cell-to-cell movement (Carrington et al., 1998). In agreement with this finding, the potyviral CI has been shown to associate with the TuMV replication complex that contains host factors such as eIF(iso)4E (Cotton et al., 2009) and the pea seed-borne mosaic virus CI form plasmodesmata-associated cone-like structures that mediate the passage of virus into the adjacent cell (Roberts et al., 1998). In virus-infected plants, the potato virus A CI also binds to a subpopulation of viral particles through an interaction with the viral coat protein (Gabrenaite-Verkhovskaya et al., 2008). The importance of CI as a helicase is also implicated in several other studies as well (Jiménez et al., 2006; Abdul-Razzak et al., 2009; Shand et al., 2009). For instance, mutations in the C-terminal portion of the lettuce mosaic virus CI result in overcoming recessive resistance mediated by two eIF4E alleles (Abdul-Razzak et al., 2009), indicating the involvement of CI in the potyvirus-eIF4E interactome. This resistance breakage may be made by restoring the CI-interacting (directly or indirectly) protein network that includes the essential components of the eIF4F complex (Abdul-Razzak et al., 2009). Therefore, the potyviral CI may be directly involved in RNA synthesis through interacting with the nascent genome to regulate translocation and disassembly of the virion (Gabrenaite-Verkhovskaya et al., 2008). Since CI and AtRH8 both are physically present in close proximity to the virus accumulation complex and are functionally reciprocally irreplaceable in virus infection, they may coordinate to provide helicase activities required by different aspects of viral genome expression and replication.
The result in this study showing that atrh8 mutants were resistant to both PPV and TuMV (Fig. 3) is in concordance with the properties of recessive resistance. Recently, Kang et al. (2007) have reported that constitutive overexpression of a pepper (Capsicum annuum) recessive resistance gene, pvr1 (an eIF4E mutant), in tomato (Solanum lycopersicum) generates dominant resistance to TEV and other potyviruses, including Pepper mottle virus and Potato virus Y. In this report, overexpression of the VPg-BR of AtRH8 or that of PpDDXL significantly suppressed virus infection (Fig. 6; Supplemental Figs. S5 and S6). This resistance, resembling a dominant negative effect, is likely due to the interaction between VPg and the overexpressed VPg-BR that impairs the recruitment efficiency of functional AtRH8 or PpDDXL to the virus accumulation vesicles. This finding may open up a novel strategy in the development of genetic resistance against viruses in plants and other organisms. Both recessive and dominant negative approaches are superior to the pathogen-derived resistance strategy currently being widely used to engineer resistance to plant viruses. This strategy functions through RNA silencing direct targeting on the viral genome (Grumet et al., 1987; Baulcombe, 2004; Wang and Metzlaff, 2005; Wang et al., 2006). This type of viral resistance can be overcome in two scenarios: in mixed infections by a strong silencing suppressor from unrelated viruses (Mitter et al., 2001) and through random sequence mutation during virus replication that lacks a proofreading mechanism (Kang et al., 2005b). Thus, our study also provides an immediate interest for agricultural studies and could potentially serve as a feasible solution to viral diseases in crops. However, the broader application of AtRH8 or PpDDXL for viral control will certainly depend on further elucidating the exact role of these DDX-like proteins in the translation, replication, and regulation of the virus infection cycle.
MATERIALS AND METHODS
Yeast Two-Hybrid Screen
The yeast two-hybrid screen was conducted using the Matchmaker Library Construction and Screening Kits (Clontech) following the supplier's instruction manual. The VPg of a PPV-D strain was cloned into the bait vector, pGBKT7, encoding the binding domain to generate plasmid pGBKT7-VPg. The peach (Prunus persica) cDNA library was prepared by inserting cDNA derived from PPV-infected peach leaf tissues into the prey vector pGADT7-rec, encoding the activation domain. Positive clones were isolated and transformed into the Escherichia coli DH5α strain for plasmid preparation and DNA sequencing.
5′ RACE and 3′ RACE for PpDDXL Gene Cloning
To obtain the 5′ terminus of the PpDDXL gene, 5′ RACE was performed using the FirstChoice RLM-RACE kit (Ambion) following the manufacturer's instructions. The 5′ RACE PpDDXL outer primer, 5′ RACE PpDDXL inner primer, 3′ RACE PpDDXL outer primer, and 3′ RACE PpDDXL inner primer (listed in Supplemental Table S1) were used to obtain the full-length PpDDXL cDNA. All PCRs were performed using the Phusion High-Fidelity DNA polymerase (New England Biolabs) at an annealing temperature of 60°C for 30 cycles. The PCR product was cloned with the Zero Blunt TOPO Cloning Kit (Invitrogen) and sequenced. Multiple sequence alignment to homolog proteins in different plant species was done using SeqMan from DNAStar version 6 and ClustalW alignment programs.
T-DNA Mutant Analysis
All the T-DNA insertion lines used in this study were obtained from the Arabidopsis Biological Resource Center. The T-DNA insertion site of the atrh8 mutant was confirmed by PCR using the T-DNA left border-specific primer (LBa1) and AtRH8-specific primers (LP16830 and RP16830). The position of the T-DNA insertion in the AtRH8 gene mutant allele was verified by DNA sequencing of the PCR products. The single T-DNA insertion was confirmed by DNA gel-blot analyses. The expression of AtRH8 was verified by RT-PCR with AtRH8-specific primers (AtRH8-F and AtRH8-R) to confirm the T-DNA line as a true knockout mutant.
Cloning AtRH8, and Deletion Analysis
cDNA encoding the full-length AtRH8 was amplified by PCR with primers AtRH8-F and AtRH8-BamHI-R using cDNA derived from the wild-type Arabidopsis (Arabidopsis thaliana) plants. The PCR products were inserted into the pGADT7 vector to generate plasmid pGAD-T7-AtRH8. AtRH8 deletion analysis fragments were obtained using primers listed in Supplemental Table S1. All constructs were confirmed by sequencing.
Plasmid Construction for Expression in Plants
To construct the TuMV″6K-YFP infectious clone, the infectious clone TuMV″6K-GFP (or pCambiaTunos/6KGFP; Cotton et al., 2009) was digested with SmaI and KpnI, and the resulting GFP-containing DNA fragment was cloned into the pBluescript SK+ vector (Stratagene) to obtain plasmid pBlue-6K2-GFP-HCPro. This plasmid was subjected to three steps of subcloning to replace GFP with YFP that was retrieved from a plasmid previously constructed to express YFP (Wei and Wang, 2008). The resulting plasmid pBlue-6K2-YFP-HCPro clone, upon sequencing confirmation, was cleaved with SmaI and KpnI, and the 6K2-YFP-HCPro fragment was recloned into the corresponding sites of pCambiaTunos/6KGFP to create the TuMV″6K-YFP construct.
Gateway technology (Invitrogen) was used to generate plasmids for expression in plants as described previously (Wei and Wang, 2008; Lu et al., 2009). PCR-amplified DNA segments including AtRH8 (with primers AtRH8-Gate-F and AtRH8-Gate-R), VPg (with primers VPg-Gate-F and VPg-Gate-R) and VPg-BR from AtRH8, and PpDDXL (with primers VPgBR-AtRH8Gate-F, VPgBR-AtRH8Gate-R, VPgBR-PpDDXLGate-F, and VPgBR-PpDDXLGate-R) were transferred by recombination into the entry vector pDONR201 (Invitrogen) using BP Clonase II (Invitrogen) following the manufacturer's protocol. The insert of the resulting pDONR clone was verified by sequencing. The insert was subsequently cloned into the destination vector using LR Clonase II (Invitrogen) to generate plant expression vectors AtRH8-CFP, AtRH8-YN, AtRH8-YC, VPg-YFP, VPg-YN, VPg-YC, VPgBR-AtRH8-YN, VPgBR-AtRH8-YC, VPgBR-PpDDXL-YN, and VPgBR-PpDDXL-YC.
Agroinfiltration and Confocal Microscopy
The binary vectors were introduced into Agrobacterium tumefaciens strain GV3101 or EHA105 by electroporation. The Agrobacterium culture was prepared according to Sparkes et al. (2006). For the TuMV infection assay, 3-week-old Arabidopsis plants were agroinfiltrated with Agrobacterium containing the GFP-tagged TuMV plasmid at an optical density at 500 nm of 0.05. For BiFC and colocalization experiments, the 3-week-old Nicotiana benthamiana plants were agoinfiltrated with the mixture of Agrobacterium cultures (each at an optical density at 600 nm of 1.0) in 1:1 ratio. Confocal microscopy work was carried out following Wei and Wang (2008).
Virus Inoculation
Mechanical inoculation of Arabidopsis with PPV-D and TuMV-GFP was as described (Babu et al., 2008). For TEV inoculation, 3-week-old N. benthamiana plants were rubbed with the TEV inoculum prepared by grinding 1 g of TEV-infected N. benthamiana leaves with 5 mL of 1× phosphate-buffered saline buffer (pH 7.4).
RT-PCR and Real-Time RT-PCR
All RT-PCR and real-time PCR analyses were performed with three biological replicates. Total RNA was prepared following the instructions of the RNeasy Plant Mini Kit (Qiagen). The first-strand synthesis and subsequent PCR amplification of both the internal standard and target gene fragments were performed using the SuperScript two-step RT-PCR system (Invitrogen). For AtRH8 expression analysis, the Arabidopsis Actin2 gene was used as the internal control of RT-PCR using Actin2-specific primers At-actin-F and At-actin-R. For virus detection, newly emerged leaves of virus-inoculated plants were harvested at 14 dpi or 14 dpa. The infection of PPV and TuMV was diagnosed by RT-PCR with PPV-specific primers PPVcp-F and PPVcp-R and TuMV-specific primers HC-Pro-F and HC-Pro-R, respectively.
Real-time PCR preparations were carried out using the LightCycler480 Probes Master Kit (Roche) on a LightCycler480 real-time PCR system (Roche) following the manufacturer's instructions. Three pairs of primers, TEVcp-F and TEVcp-R, TuHC-F and TuHC-R, and NbEF-1α-F and NbEF-1α-R, were used for quantification analyses of TEV, TuMV, and N. benthamiana elongation factor-1α (NbEF-1α), respectively. NbEF-1α served as the internal reference control. The hydrolysis probe designs were based on TaqMan Probe design tutorial guidelines by Beacon Designer and AlleleID. The corresponding hydrolysis probes to TEV, TuMV, and NbEF1α are listed in Supplemental Table S1. The standard curve of each sample was generated to achieve an efficiency of 2.0 prior to the relative quantification analysis. Each sample was assayed in triplicate 20-μL volumes, and data were analyzed using the LightCycler480 software SW1.5 (Roche). The RNA level was calculated using the mean threshold cycle value normalized to that of the reference gene, NbEF-1α.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers GQ865547 and GQ865548.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Analysis of PpDDXL.
Supplemental Figure S2. Analysis of PpDDXL homologous genes in Arabidopsis.
Supplemental Figure S3. Negative controls for the BiFC assay of AtRH8 and the PPV VPg in N. benthamiana.
Supplemental Figure S4. Expression analysis of the Arabidopsis AtRH8 homozygous T-DNA insertion line (SALK_016830).
Supplemental Figure S5. Effect of transient overexpression of the VPg-BR of AtRH8 on TEV accumulation in N. benthamiana plants.
Supplemental Figure S6. Effect of transient overexpression of the VPg-BR of PpDDXL on TEV and TuMV accumulation in N. benthamiana plants.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We are indebted to Jim Carrington (Oregon State University) for providing plasmid TEV-GFP, Yuhai Cui (Agriculture and Agri-Food Canada [AAFC]) for providing a modified BiFC vector, Lorne Stobbs and Antonet Svircev (AAFC) for assistance in PPV infection assays, Alex Molnar (AAFC) for photography, Jamie McNeil (AAFC) for expert technical assistance, and Mark Bernards (University of Western Ontario) for helpful discussion and suggestions. We also acknowledge two anonymous reviewers for their very helpful comments in improving the manuscript.
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada and Agriculture and Agri-Food Canada to A.W. and by scholarships (Ontario Graduate Scholarship and Ontario Graduate Scholarship in Science and Technology) to T.-S.H.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Aiming Wang (aiming.wang@agr.gc.ca).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abdul-Razzak A, Guiraud T, Peypelut M, Walter J, Houvenaghel MC, Candresse T, Le Gall O, German-Retana S (2009) Involvement of the cylindrical inclusion (CI) protein in the overcoming of an eIF4E-mediated resistance against Lettuce mosaic potyvirus. Mol Plant Pathol 10 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariumi Y, Kuroki M, Abe K, Dansako H, Ikeda M, Wakita T, Kato N (2007) DDX3 DEAD-box RNA helicase is required for Hepatitis C virus RNA replication. J Virol 81 13922–13926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu M, Griffiths JS, Huang TS, Wang A (2008) Altered gene expression changes in Arabidopsis leaf tissues and protoplasts in response to Plum pox virus infection. BMC Genomics 9 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D (2004) RNA silencing in plants. Nature 431 356–363 [DOI] [PubMed] [Google Scholar]
- Beauchemin C, Boutet N, Laliberté JF (2007) Visualization of the interaction between the precursors of VPg, the viral protein linked to the genome of Turnip mosaic virus, and the translation eukaryotic initiation factor (iso) 4E in planta. J Virol 81 775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin C, Laliberté JF (2007) The poly(A) binding protein is internalized in virus-induced vesicles or redistributed to the nucleolus during Turnip mosaic virus infection. J Virol 81 10905–10913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun-Rasmussen M, Moller IS, Tulinius G, Hansen JKR, Lund OS, Johansen IE (2007) The same allele of translation initiation factor 4E mediates resistance against two Potyvirus spp. in Pisum sativum. Mol Plant Microbe Interact 9 1075–1082 [DOI] [PubMed] [Google Scholar]
- Carrington JC, Haldeman R, Dolja VV, Restrepo-Hartwig MA (1993) Internal cleavage and trans-proteolytic activities of the VPg-proteinase (NIa) of tobacco etch potyvirus in vivo. J Virol 67 6995–7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington JC, Jensen PE, Schaad MC (1998) Genetic evidence for an essential role for potyvirus CI protein in cell-to-cell movement. Plant J 14 393–400 [DOI] [PubMed] [Google Scholar]
- Chung BY, Miller WA, Atkins JF, Firth AE (2008) An overlapping essential gene in the Potyviridae. Proc Natl Acad Sci USA 105 5897–5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton S, Grangeon R, Thuivierge K, Mathieu I, Ide C, Wei T, Wang A, Laliberté JF (2009) Turnip mosaic virus RNA replication complex vesicles are mobile, align with microfilaments and are each derived from a single viral genome. J Virol 83 10460–10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroocq V, Sicard O, Alamillo JM, Lansac M, Eyquard JP, García JA, Candresse T, Le Gall O, Revers F (2006) Multiple resistance traits control Plum pox virus infection in Arabidopsis thaliana. Mol Plant Microbe Interact 19 541–549 [DOI] [PubMed] [Google Scholar]
- de la Cruz J, Kressler D, Linder P (1999) Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem Sci 24 192–198 [DOI] [PubMed] [Google Scholar]
- Dufresne PJ, Ubalijoro E, Fortin MG, Laliberté JF (2008) Arabidopsis thaliana class II poly(A)-binding proteins are required for efficient multiplication of Turnip mosaic virus. J Gen Virol 89 2339–2348 [DOI] [PubMed] [Google Scholar]
- Dunoyer P, Thomas C, Harrison S, Revers F, Maule A (2004) A cysteine-rich plant protein potentiates potyvirus movement through an interaction with the virus genome-linked protein VPg. J Virol 78 2301–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat A, Caranta C, Revers F, Menand B, Browning KS, Robaglia C (2002) The Arabidopsis eukaryotic initiation factor (iso)4E is dispensable for plant growth but required for susceptibility to potyviruses. Plant J 32 927–934 [DOI] [PubMed] [Google Scholar]
- Eagles RM, Balmori-Melián E, Beck DL, Gardner RC, Forster RLS (1994) Characterization of NTPase, RNA-binding and RNA helicase activities of the cytoplasmic inclusion protein of tamarillo mosaic potyvirus. Eur J Biochem 224 677–684 [DOI] [PubMed] [Google Scholar]
- Elvira G, Wasiak S, Blandford V, Tong XK, Serrano A, Fan X, del Rayo Sánchez-Carbente M, Servant F, Bell AW, Boismenu D, et al (2006) Characterization of an RNA granule from developing brain. Mol Cell Proteomics 5 635–651 [DOI] [PubMed] [Google Scholar]
- Fang J, Acheampong E, Dave R, Wang F, Mukhtar M, Pomerantz RJ (2005) The RNA helicase DDX1 is involved in restricted HIV-1 Rev function in human astrocytes. Virology 336 299–307 [DOI] [PubMed] [Google Scholar]
- Fang J, Kubota S, Yang B, Zhou N, Zhang H, Godbout R, Pomerantz RJ (2004) A DEAD box protein facilitates HIV-1 replication as a cellular co-factor of Rev. Virology 330 471–480 [DOI] [PubMed] [Google Scholar]
- Gabrenaite-Verkhovskaya R, Andrew IA, Kalinina NO, Torrance L, Taliansky ME, Mäkine K (2008) Cylindrical inclusion protein of Potato virus A is associated with a subpopulation of particles isolated from infected plants. J Gen Virol 89 829–838 [DOI] [PubMed] [Google Scholar]
- Gao Z, Johansen E, Eyers S, Thomas CL, Noel Ellis TH, Maule AJ (2004) The potyvirus recessive resistance gene, sbm1, identifies a novel role for translation initiation factor eIF4E in cell-to-cell trafficking. Plant J 40 376–385 [DOI] [PubMed] [Google Scholar]
- German-Retana S, Walter J, Le Gall O (2008) Lettuce mosaic virus: from pathogen diversity to host interactions. Mol Plant Pathol 9 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet R, Sanford JC, Johnston SA (1987) Pathogen-derived resistance to viral infection using a negative regulatory molecule. Virology 161 561–569 [DOI] [PubMed] [Google Scholar]
- Hafrén A, Mäkinen K (2008) Purification of viral genome-linked protein VPg from Potato virus A-infected plants reveals several post-translationally modified forms of the protein. J Gen Virol 89 1509–1518 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Serviene E, Gal J, Panavas T, Nagy PD (2006) Identification of essential host factors affecting tombusvirus RNA replication based on the yeast Tet promoters Hughes Collection. J Virol 80 7394–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez I, López L, Alamillo JM, Valli A, García JA (2006) Identification of a Plum pox virus CI-interacting protein from chloroplast that has a negative effect in virus infection. Mol Plant Microbe Interact 19 350–358 [DOI] [PubMed] [Google Scholar]
- Kang BC, Yeam I, Frantz JD, Murphy JF, Jahn MM (2005)a The pvr1 locus in Capsicum encodes a translation initiation factor eIF4E that interacts with tobacco etch virus VPg. Plant J 42 392–405 [DOI] [PubMed] [Google Scholar]
- Kang BC, Yeam I, Jahn MM (2005. b) Genetics of plant virus resistance. Annu Rev Phytopathol 43 581–621 [DOI] [PubMed] [Google Scholar]
- Kang BC, Yeam I, Li H, Perez KW, Jahn MM (2007) Ectopic expression of a recessive resistance gene generates dominant potyvirus resistance in plants. Plant Biotechnol J 5 526–536 [DOI] [PubMed] [Google Scholar]
- Khan MA, Miyoshi H, Gallie DR, Goss DJ (2008) Potyvirus genome-linked protein, VPg, directly affects wheat germ in vitro translation: interactions with translation initiation factors eIF4F and eIFiso4F. J Biol Chem 283 1340–1349 [DOI] [PubMed] [Google Scholar]
- Laín S, Martín M, Riechmann JL, García JA (1991) Novel catalytic activity associated with positive-strand RNA virus infection: nucleic acid-stimulated ATPase activity of the plum pox potyvirus helicase protein. J Virol 65 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laín S, Riechmann JL, García JA (1990) RNA helicase: a novel activity associated with a protein encoded by a positive strand RNA virus. Nucleic Acids Res 18 7003–7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lellis AD, Kasschau KD, Whitham SA, Carrington JC (2002) Loss-of-susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF(iso)4E during potyvirus infection. Curr Biol 12 1046–1051 [DOI] [PubMed] [Google Scholar]
- Léonard S, Viel C, Beauchemin C, Daigneault N, Fortin MG, Laliberté JF (2004) Interaction of VPg-Pro of Turnip mosaic virus with the translation initiation factor 4E and the poly(A)-binding protein in planta. J Gen Virol 85 1055–1063 [DOI] [PubMed] [Google Scholar]
- Li Z, Pogany J, Panavas T, Xu K, Esposito AM, Kinzy TG, Nagy PD (2009) Translation elongation factor 1A is a component of the tombusvirus replicase complex and affects the stability of the p33 replication co-factor. Virology 385 245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch JR (2002) RNA chaperones exist and DEAD box proteins get a life. Cell 109 797–800 [DOI] [PubMed] [Google Scholar]
- Lu Q, Tang X, Tian G, Wang F, Liu K, Nguyen V, Kohalmi SE, Keller WA, Tsang EWT, Harada JJ, et al (2009) Arabidopsis homolog of the yeast TREX-2 mRNA export complex: components and anchoring nucleoporin. Plant J (in press) [DOI] [PubMed]
- Maule A, Leh V, Lederer C (2002) The dialogue between viruses and hosts in compatible interactions. Curr Opin Plant Biol 5 279–284 [DOI] [PubMed] [Google Scholar]
- Michon T, Estevez Y, Walter J, German-Retana S, Le Gall O (2006) The potyviral virus genome-linked protein VPg forms a ternary complex with the eukaryotic initiation factors eIF4E and eIF4G and reduces eIF4E affinity for a mRNA cap analogue. FEBS J 273 1312–1322 [DOI] [PubMed] [Google Scholar]
- Mitter N, Sulistyowati E, Grahan MW, Dietzgen RG (2001) Suppression of gene silencing: a threat to virus-resistant transgenic plants? Trends Plant Sci 6 246–247 [DOI] [PubMed] [Google Scholar]
- Mohr S, Stryker JM, Lambowitz AM (2002) A DEAD-box protein functions as an ATP-dependent RNA chaperone in group I intron splicing. Cell 109 769–779 [DOI] [PubMed] [Google Scholar]
- Moury B, Morel C, Johansen E, Guilbaud L, Souche S, Ayme V, Caranta C, Palloix A, Jacquemond M (2004) Mutations in Potato virus Y genome-linked protein determine virulence toward recessive resistance in Capsicum annuum and Lycopersicon hirsutum. Mol Plant Microbe Interact 17 322–329 [DOI] [PubMed] [Google Scholar]
- Murphy JF, Klein PG, Hunt AG, Shaw JG (1996) Replacement of the tyrosine residue that links a potyviral VPg to the viral RNA is lethal. Virology 220 535–538 [DOI] [PubMed] [Google Scholar]
- Nelson RS, Citovsky V (2005) Plant viruses: invaders of cells and pirates of cellular pathways. Plant Physiol 138 1809–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise V, Gallois JL, Chafiai F, Allen LM, Schurdi-Levraud V, Browning KS, Candresse T, Caranta C, Le Gall O, German-Retana S (2007) Coordinated and selective recruitment of eIF4E and eIF4G factors for potyvirus infection in Arabidopsis thaliana. FEBS Lett 581 1041–1046 [DOI] [PubMed] [Google Scholar]
- Nicaise V, German-Retana S, Sanjuán R, Dubrana MP, Mazier M, Maisonneuve B, Candresse T, Caranta C, Le Gall O (2003) The eukaryotic translation initiation factor 4E controls lettuce susceptibility to the potyvirus Lettuce mosaic virus. Plant Physiol 132 1272–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noueiry AO, Chen J, Ahlquist P (2000) A mutant allele of essential, general translation initiation factor DED1 selectively inhibits translation of a viral mRNA. Proc Natl Acad Sci USA 97 12985–12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oruetxebarria L, Guo D, Merits A, Mäkinen K, Saarma M, Valkonen JPT (2001) Identification of the genome-linked protein in virion of Potato virus A, with comparison to other members in the genome Potyvirus. Virus Res 73 101–112 [DOI] [PubMed] [Google Scholar]
- Puustinen P, Mäkinen K (2004) Uridylylation of the potyvirus VPg by viral replicase NIb correlates with the nucleotide binding capacity of VPg. J Biol Chem 279 38103–38110 [DOI] [PubMed] [Google Scholar]
- Puustinen P, Rajamäki ML, Ivanov KI, Valkonen JPT, Mäkinen K (2002) Detection of the potyviral genome-linked protein VPg in virions and its phosphorylation by host kinases. J Virol 76 12703–12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamäki ML, Mäki-Valkama T, Mäkinen K, Valkonen JPT (2004) Infection with potyviruses. In N Talbot, ed, Plant-Pathogen Interactions. Blackwell Publishing, Oxford, pp 68–91
- Rajamäki ML, Valkonen JPT (2003) Localization of a potyvirus and the viral genome-linked protein in wild potato leaves at an early stage of systemic infection. Mol Plant Microbe Interact 12 25–34 [DOI] [PubMed] [Google Scholar]
- Rajamäki ML, Valkonen JPT (2009) Control of nuclear and nucleolar localization of nuclear inclusion protein a of picorna-like Potato virus A in Nicotiana species. Plant Cell 21 2485–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo-Hartwig MA, Carrington JC (1992) Regulation of nuclear transport of a plant potyvirus protein by autoproteolysis. J Virol 66 5662–5666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaglia C, Caranta C (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci 11 40–45 [DOI] [PubMed] [Google Scholar]
- Roberts IM, Wang D, Findlay K, Maule AJ (1998) Ultrastructural and temporal observations of the potyvirus cylindrical inclusions (CIs) show that the CI protein acts transiently in aiding virus movement. Virology 245 173–181 [DOI] [PubMed] [Google Scholar]
- Ruffel S, Dussault MH, Palloix A, Moury B, Bendahmane A, Robaglia C, Caranta C (2002) A natural recessive resistance gene against Potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E). Plant J 32 1067–1075 [DOI] [PubMed] [Google Scholar]
- Ruffel S, Gallois JL, Lesage ML, Caranta C (2005) The recessive potyvirus resistance gene pot-1 is the tomato orthologue of the pepper pvr2-eIF4E gene. Mol Genet Genomics 274 346–353 [DOI] [PubMed] [Google Scholar]
- Sato M, Nakahara K, Yoshii M, Ishikawa M, Uyeda I (2005) Selective involvement of members of the eukaryotic initiation factor 4E family in the infection of Arabidopsis thaliana by potyviruses. FEBS Lett 579 1167–1171 [DOI] [PubMed] [Google Scholar]
- Schaad MC, Anderberg RJ, Carrington JC (2000) Strain-specific interaction of the Tobacco etch virus NIa protein with the translation initiation factor eIF4E in the yeast two-hybrid system. Virology 273 300–306 [DOI] [PubMed] [Google Scholar]
- Schaad MC, Jensen PE, Carrington JC (1997) Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J 16 4049–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand K, Theodoropoulos C, Stenzel D, Dale JL, Harrison M (2009) Expression of Potato virus Y cytoplasmic inclusion protein in tobacco results in disorganization of parenchyma cells, distortion of epidermal cells, and induces mitochondrial and chloroplast abnormalities, formation of membrane whorls and atypical lipid accumulation. Micron 40 730–736 [DOI] [PubMed] [Google Scholar]
- Sparkes IA, Runions J, Kearnes A, Hawes C (2006) Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1 2019–2025 [DOI] [PubMed] [Google Scholar]
- Tanner NK, Linder P (2001) DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol Cell 8 251–262 [DOI] [PubMed] [Google Scholar]
- Thivierge K, Cotton S, Dufresne PJ, Mathieu I, Beauchemin C, Ide C, Fortin MG, Laliberté JF (2008) Eukaryotic elongation factor 1A interacts with Turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles. Virology 377 216–225 [DOI] [PubMed] [Google Scholar]
- Urcuqui-Inchima S, Haenni AL, Bernardi F (2001) Potyvirus proteins: a wealth of functions. Virus Res 74 157–175 [DOI] [PubMed] [Google Scholar]
- Wang A, Sanfaçon H, Stobbs LW, James D, Thompson D, Svircev AM, Brown DCW (2006) Plum pox virus in Canada: progress in research and future prospects for disease control. Can J Plant Pathol 28 182–196 [Google Scholar]
- Wang MB, Metzlaff M (2005) RNA silencing and antiviral defence in plants. Curr Opin Plant Biol 8 216–222 [DOI] [PubMed] [Google Scholar]
- Wei T, Wang A (2008) Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J Virol 82 12252–12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham SA, Wang Y (2004) Roles for host factors in plant viral pathogenicity. Curr Opin Plant Biol 7 365–371 [DOI] [PubMed] [Google Scholar]
- Wittmann S, Chatel H, Fortin MG, Laliberté JF (1997) Interaction of the viral protein genome linked of Turnip mosaic potyvirus with the translational eukaryotic initiation factor (iso) 4E of Arabidopsis thaliana using the yeast two-hybrid system. Virology 234 84–92 [DOI] [PubMed] [Google Scholar]
- Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT (2004) Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119 381–392 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.