Abstract
Bone marrow stromal cells (MSCs) increase vascular endothelial growth factor (VEGF) expression and promote angiogenesis after stroke. Angiopoietin-1 (Ang1) and its receptor Tie2 mediate vascular integrity and angiogenesis as does VEGF and its receptors. In this study, we tested whether MSC treatment of stroke increases Ang1/Tie2 expression, and whether Ang1/Tie2 with VEGF/vascular endothelial growth factor receptor 2 (VEGFR2) (Flk1), in combination, induced by MSCs enhances angiogenesis and vascular integrity. Male Wistar rats were subjected to middle cerebral artery occlusion (MCAo) and treated with or without MSCs. Marrow stromal cell treatment significantly decreased blood–brain barrier (BBB) leakage and increased Ang1, Tie2, and occludin (a tight junction protein) expression in the ischemic border compared with MCAo control. To further test the mechanisms of MSC-induced angiogenesis and vascular stabilization, cocultures of MSCs with mouse brain endothelial cells (MBECs) or astrocytes were performed. Supernatant derived from MSCs cocultured with MBECs significantly increased MBEC expression of Ang1/Tie2 and Flk1 compared with MBEC alone. Marrow stromal cells cocultured with astrocytes also significantly increased astrocyte VEGF and Ang1/Tie2 expression compared with astrocyte culture alone. Conditioned media from MSCs alone, and media from cocultures of MSCs with astrocytes or MBECs, all significantly increased capillary tube-like formation of MBEC compared with control Dulbecco’s modified Eagle’s medium media. Inhibition of Flk1 and/or Ang1 significantly decreased MSC-induced MBEC tube formation. Knockdown of Tie2 expression in MBECs significantly inhibited MSC-induced tube formation. Our data indicate MSC treatment of stroke promotes angiogenesis and vascular stabilization, which is at least partially mediated by VEGF/Flk1 and Ang1/Tie2.
Keywords: angiopoietin1 (Ang1), marrow stromal cell, middle cerebral artery occlusion (MCAo), Tie2, vascular endothelial growth factor (VEGF), vascular stabilization
Introduction
Re-establishment of functional microvasculature promotes stroke recovery. During angiogenesis, the initial vascular plexus forms mature vessels by sprouting, branching, pruning, differential growth of endothelial cells, and recruitment of supporting cells, such as pericytes and smooth muscle cells (Folkman and D’Amore, 1996; Risau, 1997). Angiogenesis and vascular maturation are regulated by vascular endothelial growth factor (VEGF), its receptors, and the angiopoietin-1 (Ang1)/Tie2 system (Patan, 2004). Tie2 and VEGF receptor 2 (Flk1) are tyrosine kinases that play essential roles in angiogenesis.
The blood–brain barrier (BBB) is essential for central nervous system physiological homeostasis. Vascular maturation and stabilization are required for functional angiogenesis (Hirschi et al, 1997). Angiopoietin-1 signaling promotes angiogenesis and remodeling of blood vessels through its receptor tyrosine kinase Tie2, which is expressed on blood vessel endothelial cells. Angiopoietin-1 reduces endothelial permeability and enhances vascular stabilization and maturation (Pfaff et al, 2006; Suri et al, 1996). Transgenic overexpression of Ang1 increases vascular stabilization (Suri et al, 1998), prevents plasma leakage in the ischemic brain, and consequently decreases ischemic lesion volume (Thurston et al, 2000; Zhang et al, 2002). Angiopoietin- 1 decreases VEGF-induced BBB permeability, which is associated with a decrease of MMP-9 activity (Valable et al, 2005). Angiopoietin-1 and VEGF in combination induce a synergistic angiogenic effect, and promote the formation of mature neovessels without the side effects on BBB permeability (Valable et al, 2005).
Bone marrow-derived marrow stromal cells increase endothelial cells proliferation and migration, and contribute to collateral vascular formation through cell incorporation into new or remodeled vessels (Kinnaird et al, 2004). Marrow stromal cells (MSCs) when administered intravenously after stroke selectively migrate to the ischemic area in the brain, and increase parenchymal VEGF and promote angiogenesis in the ischemic brain (Chen et al, 2003; Li et al, 2001). However, whether MSC treatment regulates BBB permeability and vascular stabilization has not been investigated. In this study, we tested the hypothesis that MSC treatment of stroke promotes vascular stabilization and decreases BBB leakage, by increasing Ang1/Tie2 and VEGF/Flk1 expression, and both together promote angiogenesis and vascular maturation after stroke.
Materials and methods
Animal Middle Cerebral Artery Occlusion Model and Marrow Stromal Cell Transplantation
Adult male Wistar rats weighing 270 to 300 g were employed in all experiments. Transient right middle cerebral artery occlusion (MCAo) was induced for 2 h by advancing a 4 to 0 surgical nylon suture (18.5 to 19.5mm) with an expanded (heated) tip from the external carotid artery into the lumen of the internal carotid artery to block the origin of the MCA (Chen et al, 2001). At 1 day after ischemia, randomly selected animals received either MSCs or phosphate-buffered saline (PBS, n = 14/group). Approximately, 3×106 MSCs in 1ml total fluid volume PBS were injected into a tail vein at 24 h after stroke. We have shown previously that treatment of stroke in rat with dead MSCs or with rat liver fibroblasts provides no functional benefit compared with PBS-treated rats (Li et al, 2002). Therefore, this study employs PBS as the control treatment. Rats (n = 6/group) were killed at 4 days after stroke for BBB leakage measurement. Rats (n = 8/group) were killed at 14 days after MCAo for immunostaining.
Quantitative Evaluation of Evans Blue Dye Extravasation
Rats (n = 6/group) were treated with or without MSCs. Evans blue dye of 2% in saline was injected intravenously as a BBB permeability tracer at 4 h before killing at 4 days after stroke. Coronal brain sections from bregma −1 to 1mm were divided into the right and left hemispheres. Evans blue dye fluorescence intensity was determined by a microplate fluorescence reader (excitation 620nm and emission 680 nm). Calculations were based on the external standards dissolved in the same solvent. The amount of extravasated Evans blue dye was quantified as micrograms per ischemic hemisphere (Asahi et al, 2001; Zhang et al, 2002).
Immunohistochemical Staining
Rats (n = 8/group) were killed 14 days after stroke. The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. A standard paraffin block was obtained from the center of the lesion (bregma −1mm to + 1 mm). A series of 6-μm-thick sections were cut from the block. Every 10th coronal section for a total five sections was used for immunohistochemical staining. Antibody against Ang1 (rabbit polyclonal immunoglobulin G, 1:2000, Abecam), Tie2 (rabbit polyclonal immunoglobulin G antibody, 1:80 dilution, Santa Cruz) and occludin (Mouse monoclonal immunoglobulin G antibody, 1:200 dilution, Zymed) were employed, respectively. Control experiments consisted of staining brain coronal tissue sections as outlined above, but the primary antibodies were omitted as described previously (Li et al, 1998).
Double Immunofluorescence Staining
To specifically identify Ang1 or Tie2-reactive cells colocalized with endothelial cells, astrocytes, or pericytes, double immunofluorescence staining was employed. von Willebrand factor (vWF) is a marker for endothelial cells. Glial fibrillary acidic protein (GFAP) is a marker for astrocytes. α-smooth muscle actin (α-SMA) is a pericyte marker. Double immunofluorescence labeling for Ang1 or Tie2 with vWF, GFAP, or α-SMA were performed. A monoclonal antibody against vWF (11:400, DAKO, Carpinteria, CA, USA), a polyclonal antibody against GFAP (1:1000, DAKO, Carpinteria, CA, USA) and a monoclonal antibody against α-SMA (1:100, DAKO, Carpinteria, CA, USA) were used, respectively. Fluorescein isothiocyanate (Calbiochem) and cyanine-5.18 (CY5, Jackson Immunoresearch) were used for double-label immunoreactivity. Each coronal section was first treated with the primary anti-Ang1 or anti-Tie2 antibody with CY3, and was then followed by anti-vWF, anti-GFAP, or anti-α-SMA with fluorescein isothiocyanate staining. Control experiments consisted of staining brain coronal tissue sections as outlined above, but omitted the primary antibodies, as described previously (Li et al, 1998).
Quantitation
For quantitative measurements of Ang1, Tie2, and occludin, five slides from each brain, with each slide containing eight fields from the ischemic penumbra zone were digitized under a ×20 objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD interfaced with an MCID image analysis system (Imaging Research, St Catharines, Canada) (Chen et al, 2003). The data are presented as a percentage of positive area in each field.
Coculture
Mouse brain endothelial cells (MBEC, ATCC, CRL-2299), astrocytes (Astrocyte Type 1 clone, ATCC, CRL-2541), and MSCs were cultured in Dulbecco’s modified Eagle’s medium with sodium pyruvate (Gibco #11995-065) with 10% fetal bovine serum, 1% antibiotic/antimyotic. MBECs (0.4×106) or 0.4×106 astrocytes were added to the lower chamber of the six-well plate, with 0.2×106 MSCs added in the upper chamber of a Falcon 0.4 μm cell-culture insert, in a total of 3ml media (n = 3/group). Cells were left overnight before discarding the insert and harvesting RNA/protein from cells in the lower chamber of six-well plates.
Real-Time Polymerase Chain Reaction
Cultured cells were harvested and total RNA was isolated from treated cells with TRIzol (Invitrogen), following a standard protocol (Livak and Schmittgen, 2001). Quantitative PCR was performed using the SYBR Green real-time polymerase chain reaction method. Quantitative PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA, USA) using three-stage program parameters provided by the manufacturer as follows; 2 mins at 50°C, 10 mins at 95°C, and then 40 cycles of 15 secs at 95°C and 1 min at 60°C. Each sample was tested in triplicate, and relative gene expression data were analyzed using the 2−ΔΔCT method. The following primers for real-time PCR were designed using Primer Express software (ABI). Angiopoietin-1: FWD: TAT TTT GTG ATT CTG GTG ATT; REV: GTT TCG CTT TAT TTT TGT AATG. Tie2: FWD: CGG CCA GGT ACA TAG GAG GAA; REV: TCA CAT CTC CGA ACA ATC AGC. Vascular endothelial growth factor: FWD: GAA AAT CAC TCT GAG CCT TGT; REV: TGC AAG TAC GTT CGT TTA ACTC. Flk1: FWD: ACT CAC AGT TCC CAG AGT GGTT; REV: GAA TGG TGA CCT GTG ATC TTGA. Glyceryldehyde-3- phosphate dehydrogenase: FWD: AGA ACA TCA TCC CTG CAT CC; REV: CAC ATT GGG GGT AGG AAC AC.
Western Blot
Protein was isolated from cultured cells with TRIzol (Invitrogen) following standard protocol. Protein concentrations were determined by a DC protein assay kit (Bio-Rad, Hercules, CA, USA). Protein samples were electrophoresed on gradient sodium dodecyl sulfate–polyacrylamide gel (Bio-Rad) and subsequently electrotransferred to nitrocellulose membranes. Membranes were treated with blocking buffer (5% skimmed milk in 25mm Tris-HCl, pH 8.0, 125mm NaCl, 0.1% Tween 20) for 1 h at room temperature, followed by incubation with primary antibodies for anti-β-actin (1:2000; Sigma, St Louis, MO, USA), anti-Ang1 (1:500 dilution), and anti-Tie2 (1:500 dilution), anti-VEGF (1:1000 dilution, Abcam), anti-Flk1 (1:500 dilution, Santa Cruz) for 16 h at 4°C. The membranes were washed with blocking buffer without milk, and then incubated with horseradish peroxidase-conjugated secondary antibody in blocking buffer.
Capillary-Like Tube Formation Assay
Haralabopoulos et al (1994). Briefly, 0.1 ml growth factor reduced Matrigel (Becton Dickinson) was added per well of a 96-well plate, and then MBECs (2×104 cells) were added and incubated in (1) regular cell-culture medium (Dulbecco’s modified Eagle’s medium) for control, (2) MSC condition media alone, (3) astrocyte condition media alone, (4) MSCs coculture with astrocytes conditioned media, (5) MSCs coculture with MBECs conditioned media, (6) MSC condition media with neutralized Ang1 antibody (1 μg/mL, rabbit anti-Ang1 affinity-purified polycolonal antibody, Chemicon), and (7) MSC conditioned media with neutralized Flk1 antibody (DC101, 1 μg/mL) for 24 h. All assays were performed in n = 6/group. For quantitative measurements of capillary tube formation, Matrigel wells were digitized under a ×4 objective (Olympus BX40) for measurement of total tube length of capillary tube formation using a video camera (Sony DXC- 970MD) interfaced with the MCID image analysis system (Imaging Research, St Catharines, Canada) at 5 and 24 h. Tracks of endothelial cells organized into networks of cellular cords (tubes) were counted and averaged in randomly selected three microscopic fields (Rikitake et al, 2002).
Tie2 small interfering RNA for Mouse Brain Endothelial Cells
Tie2 siRNA (Santa Cruz Biotech) was transfected using Lipofectamine 2000 (Invitrogen) following standard protocol. Briefly, cells were plated out in 10cm plates and allowed to culture until they were 80% confluent. They were then transfected with 8 μg Tie2 siRNA in serum-free media for 6 h. Afterwards, Dulbecco’s modified Eagle’s medium +20% fetal bovine serum was added and cells incubated overnight. The following day, media was changed and 24 h later cells were passaged for the following experiments. Tie2 mRNA and protein expression were measured by real-time PCR and Western blot. In addition, capillary tube formation assay was performed. The experimental groups include (1) normal MBECs control; (2) MBECs treated with MSC supernatant; (3) Tie2 knockdown MBECs (Tie2/MBECs) control; and (4) Tie2/MBECs treated with MSC supernatant for 24 h. All assays were performed in n = 6/group. Total length of capillary tube formation was measured with the MCID image analysis system at 5 h after culture.
Statistical Analysis
Two-sample t-test was used to test for Ang1, Tie2, and occludin expression and tube formation between groups with and without MSCs treatment after stroke at the significance level of 0.05. Data are presented as mean±s.e.
Results
Marrow Stromal Cell Treatment Decreases Blood– Brain Barrier Leakage after Stroke in Rats
To test whether MSC treatment of stroke rats decreases BBB leakage, vascular permeability was quantitatively evaluated by fluorescent detection of extravasated Evans blue dye (Asahi et al, 2001; Zhang et al, 2002). Figures 1A to 1C shows that MSC treatment of stroke significantly decreases BBB leakage (B and C) compared with control MCAo rats (A and C).
Figure 1.
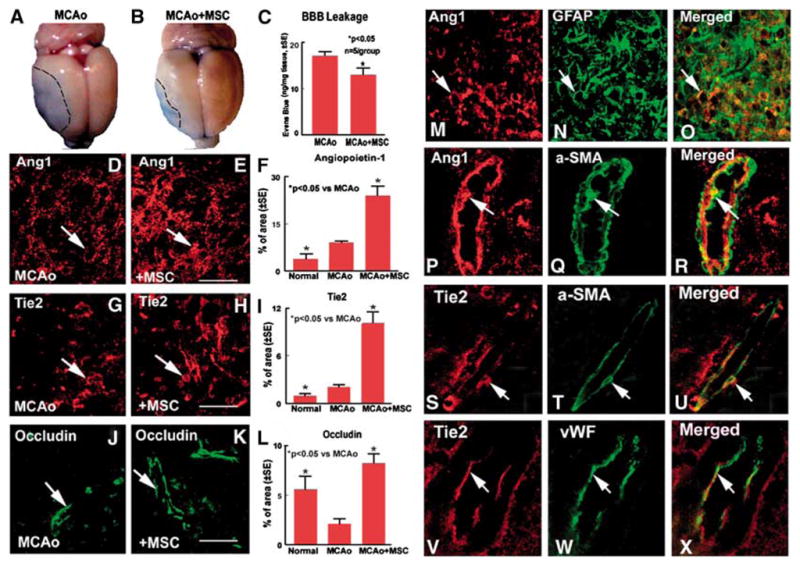
Marrow stromal cell treatment of stroke rats decrease BBB leakage and increase Ang1, Tie2, and occludin expression in the ischemic brain: MCAo rats were treated with or without MSC after stroke. (A, D, G, and J) BBB leakage measured by Evans blue (A), and Ang1 (D), Tie2 (G), and occludin (J) expression in MCAo control rats, respectively. (B, E, H, and K) Evans blue (B), Ang1 (E), Tie2 (H), and occludin (K) expression in the MSC-treated rats, respectively. (C, F, I, and L) Quantitative data of Evans blue level in the ischemic brain (C), and Ang1 (F), Tie2 (I), and occludin (L)-positive cells percentage of area in the ischemic border. Marrow stromal cell treatment stroke animals significant decrease BBB leakage and increase the expression of Ang1, Tie2, and occludin compared with control MCAo animals. Scale bar (E, H, and K)=50μm. (M to R) Double immunostaining of Ang1 with astrocyte (M, Ang1; N, GFAP; and O, Ang1 with GFAP merged); Ang1 with pericyte (P, Ang1; Q, α-SMA; R, Ang1 with α-SMA merged). (S to X) Double immunostaining Tie2 with pericyte (S, Tie2; T, α-SMA; and U, Tie2 with á-SMA merged); Tie2 with brain endothelial cell (V, Tie2;W, vWF; and X, Tie2 with vWF merged), respectively.
Marrow Stromal Cell Treatment Increases Angiopoietin-1/Tie2 and Occludin Expression and Promotes Vascular Stabilization after Stroke
The Ang1/Tie2 system promotes angiogenesis and vascular stability. Occludin, is a tight junction protein, and an index of microvascular integrity (Mark and Davis, 2002). To identify the mechanism by which MSC treatment decreases BBB leakage, Ang1/Tie2 and occludin expression were measured in the ischemic brain. Figure 1 shows that MCAo increases Ang1 (F), Tie2 (I), and decreases occludin (L) expression in the ischemic border area compared with sham control animals. Angiopoietin-1, Tie2, and occludin expression all decrease in the ischemic core after MCAo compared with normal control brain (data not shown). Marrow stromal cell treatment significantly increases Ang1 (E and F), Tie2 (H and I), and occuldin (K and L) expression in the ischemic border compared with control MCAo animals (D for Ang1, G for Tie2, J for occludin). Angiopoietin-1 positive cells primarily colocalize with GFAP (astrocyte, M to O) and α-SMA (pericyte, P to R) reactive cells. Tie2-positive cells colocalize with vWF (endothelial cell, S to U) and α-SMA (pericyte, V to X) reactive cells.
Marrow Stromal Cells Enhance Astrocyte and Brain Endothelial Cell Angiogenic Factor Gene and Protein Expression
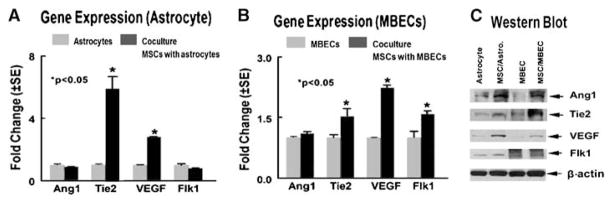
To gain insight into the mechanism by which MSCs promote angiogenesis and vascular stabilization, using a transwell chamber coculture model, MSCs were cocultured with atrocytes or MBECs. Figure 2 shows that MSCs cocultured with astrocytes, significantly increases astrocyte VEGF and Tie2 gene expression (A, P < 0.05) and upregulate Ang1, Tie2, and VEGF protein (C) expression compared with astrocytes culture alone (A and C). Marrow stromal cells cocultured with MBECs significantly promote MBECs Tie2, VEGF, and Flk1 gene (B, P < 0.05), and Ang1, Tie2, and VEGF protein (C) expression compared with MBECs control alone (B and C).
Figure 2.
Marrow stromal cells cocultured with MBECs or astrocytes regulates MBECs or astrocytes Ang1/Tie2 and VEGF/Flk1 gene and protein expression. (A and B) Gene expression measured by real-time PCR. (C)Protein expression measured by Western blot at 24 h. (D and E)Quantitative data of Western blot.
Conditioned Media from Marrow Stromal Cells Cocultured with Astrocytes Increase Angiogenesis and Capillary Tube Formation
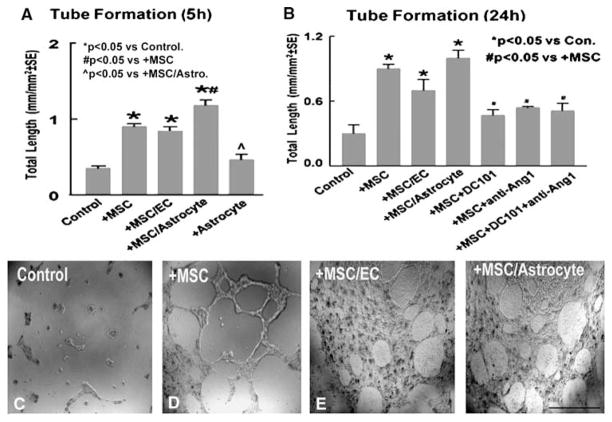
To test whether MSCs regulate angiogenesis and vascular stabilization via the VEGF/Flk1 and Ang1/Tie2 systems, capillary tube-like formation assays were performed in vitro. Marrow stromal cells were cocultured with or without astrocytes or MBECs for 3 days. The conditioned media from MSC culture alone, astrocyte culture alone, coculture of MSCs with astrocytes (MSCs/astrocytes), or coculture of MSCs with MBECs (MSCs/MBECs) were used in tube formation assay. Figure 3A shows capillary tube formation after 5 h in culture. Three conditioned media (MSCs alone, MSCs/astrocytes, or MSCs/MBECs) significantly increase capillary tube formation compared with control at 5 h after culture (P < 0.05). Astrocyte-conditioned media does not significantly increase endothelial cell tube formation compared with control media (). Conditioned media from MSCs cocultured with astrocytes significantly increase capillary tube formation compared with conditioned media from MSCs alone (145%), MSCs cocultured with MBECs (155%), and from astrocyte-alone conditioned media (250%). To test capillary tube-like network stabilization, tube formation was measured at 24 h after culture. Figures 3B to 3F show that these three conditioned media (MSCs alone (D), MSCs/MBECs (E), or MSCs/astrocytes (F), all significantly increase capillary tube-like formation compared with control media at 24 h (C and B, P < 0.05). Inhibition of Flk1 or/and Ang1 significantly attenuates MSCs condition media induced tube formation at 24 h (B, P < 0.05). Therefore, these data indicate that MSC promotion of angiogenesis and tube-like network stabilization are regulated by VEGF/Flk1 and Ang1/Tie2 systems.
Figure 3.
Marrow stromal cells promotes capillary tube formation in vitro. (A) Quantitative data of capillary tube formation at 5 h after culture. (B to F) Tube formation in conditioned media from control (C), MSCs culture alone (D), MSCs cocultured with MBECs (E), and MSCs cocultured with astrocytes (F). (B) quantitative data of capillary tube formation at 24 h after culture. Scale bar in (F)=100 μm.
Knockdown of Tie2 Gene Expression in Mouse Brain Endothelial Cells Inhibits Marrow Stromal Cell- Induced Capillary Tube-Like Formation
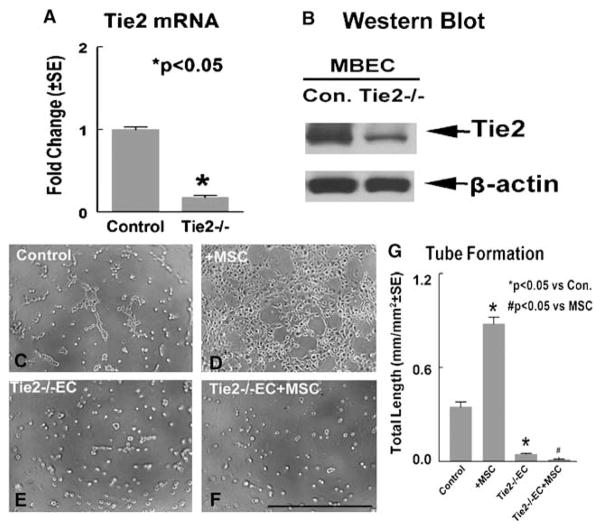
To test the effect of Tie2 on MSC-induced angiogenesis and vascular stabilization, knockdown of Tie2 gene expression in MBECs was performed using Tie2 siRNA. Figure 4 shows that Tie2 siRNA significantly decreases (about 80%) Tie2 gene (A) and protein (B) expression in MBECs compared with normal control MBECs. Capillary tube formation data show that knockdown Tie2 expression in MBECs (E) significantly decreases capillary tube formation compared with control MBECs (C). In addition, MSC conditioned media-induced capillary tube formation was significantly decreased in Tie2 knockdown MBECs (F) compared with normal MBECs treated with MSC conditioned media (D). These data suggest Tie2 plays an important role in MSC-induced angiogenesis.
Figure 4.
Knockdown Tie2 expression in MBECs decreases Tie2 gene and protein expression and attenuates MSC-induced capillary tube formation. (A) Tie2 mRNA expression in MBECs measured by real-time PCR. (B) Tie2 protein expression in MBECs measured by Western blot. (C to F)Capillary tube-like formation in normal MBECs (C), normal MBECs treated with MSC-conditioned media (D), Tie2 knockdown MBECs control (E), and Tie2 knockdown MBECs treated with conditioned MSC media (F). (G)Quantitative analysis of capillary-like tube formation. Scale bar in (F)=100 μm.
Discussion
The major new findings of the present study are (1) MSC treatment of stroke decreases BBB leakage and promotes vascular stabilization. (2) MSC treatment increases endogenous brain Ang1/Tie2 expression as well as promotes tight junction protein, occludin, expression in the ischemic brain. (3) Conditioned media obtained from coculture of MSCs with astrocytes induces more capillary tube-like formation than MSC or astrocyte culture alone. (4) Inhibition of Flk1 or Ang1 significantly attenuates MSC-induced prolonged capillary tube formation. (5) Knockdown of brain endothelial cell Tie2 expression significantly inhibits MSC-induced capillary tube formation. Therefore, VEGF/Flk1 and Ang1/Tie2 appear to mediate MSC-induced angiogenesis, and Ang1/Tie2 may play a critical role in MSC-induced vascular integrity.
MSCs induce endogenous brain astrocyte cross talk with brain endothelial cells and promote angiogenesis and vascular stabilization. Bone marrow- derived cells contribute to revascularization via vessel remodeling after cerebral ischemia (Kokovay et al, 2005). Previous studies have shown that MSC treatment of stroke increases VEGF expression and promotes angiogenesis in the ischemic brain (Chen et al, 2003). In this study, we found that MSCs stimulate VEGF expression primarily in brain astrocytes. Vascular endothelial growth factor is an integral factor in central nervous system wound healing, which is essential for vascular endothelial proliferation and survival, and is also necessary for astrocyte proliferation and maintenance during the repair of brain injury (Krum and Khaibullina, 2003). Neutralization of native VEGF decreases angiogenic activity, astrocyte proliferation, and results in large wound cavities in injured brain (Krum and Khaibullina, 2003). Astrocytes are potent regulators of brain capillary endothelial cell function and have a profound influence on the morphogenetic events underlying the organization of the vessel wall (Ramsauer et al, 2002). The astrocyte network strongly affects vascular patterning (Fruttiger et al, 1996). Endothelial cells at the leading edge of the vascular plexus possess long filopodia that closely follow the underlying astrocyte scaffold (Dorrell et al, 2002; Gerhardt and Betsholtz, 2003). Interaction of astrocytes with capillary-like structures of brain microvessel endothelium plays an important role in regulating angiogenesis and BBB leakage (Jiang et al, 2005). Marrow stromal cell treatment enhances endogenous brain astrocyte VEGF, Ang1, and Tie2 expression, which may promote astrocyte interaction with brain endothelial cells, and stimulate expression of their respective receptors Flk1 and Tie2 in brain endothelial cells. Coculture MSCs with astrocytes shows a synergistic effect on the induction of endothelial cell tube formation. The crosstalk between astrocytes and endothelial cells may regulate angiogenesis and vascular stabilization in the ischemic brain after MSC treatment. There may be an intermediary signal accounting for the observation. We cannot exclude the possibility that an intermediate signal derived from MSC supernatant cell-culture system is responsible for angiogenesis and vascular stabilization. In addition, the in vitro experiments do not necessarily mean that the complex relationships and effects seen in the intact brain are mediated.
Marrow Stromal Cells Increase Vascular Endothelial Growth Factor/Flk-1 and Angiopoietin-1/Tie2 Expression
Marrow stromal cell treatment of stroke in rat upregulates VEGF/Flk1 expression in the ischemic brain (Chen et al, 2003). In this study, we found MSC treatment stroke also increase Ang1/Tie2 expression in the ischemic border. Hyperstimulation both of VEGF and Ang1 expression in mouse brain increases microvessel density while maintaining ZO-1 protein expression (Zhu et al, 2006). A combination of submaximal doses of Ang1 and VEGF enhances angiogenesis and is more potent than the maximal dose of either alone (Chae et al, 2000). Exogenous VEGF-A and Ang-1 stimulate Tie2 expression in the bone marrow vasculature (Kopp et al, 2005). Tie2 is expressed predominantly in endothelial cells and is essential for blood vessel formation and maintenance. Our data show that knockdown Tie2 expression in brain endothelial cells significantly attenuates MSC-induced capillary tube formation. Therefore, Tie2 expression in brain endothelial cells is essential for MSC-induced angiogenesis. Marrow stromal cells behave as small biochemical and molecular ‘factories,’ producing trophic factors (e.g., VEGF/Flk1 and Ang1/Tie2) that may affect the compromised brain tissue angiogenesis in the ischemic brain.
Marrow Stromal Cell Treatment Increases Angiopoietin-1/Tie2 Expression, which Promotes Vascular Stabilization and Decreases Blood–Brain Barrier Leakage after Stroke
Vascular endothelial permeability is maintained by the regulated apposition of adherent and tight junctional proteins (Aurrand-Lions et al, 2002; Bazzoni and Dejana, 2004). The tight junctions of the endothelial and epithelial regions of the BBB are regulated by interactions with the neighboring tissue. Angiopoietin-1 expression colocalizes with pericyte reactive cells (α-SMA) in the ischemic brain. A pericyte-derived Ang1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie2 activation in vitro (Hori et al, 2004). The Ang1/Tie2 system controls pericyte recruitment, endothelial cell survival, and is implicated in blood vessel formation and vascular stabilization (Iurlaro et al, 2003). Marrow stromal cell treatment of stroke increases Ang1/Tie2 expression and upregulates ischemic brain occludin expression as well as decreases BBB leakage.
Conclusion
Marrow stromal cell treatment stroke promotes angiogenesis, vascular stabilization, and decreases BBB leakage. Vascular endothelial growth factor/Flk1 and Ang1/Tie2 likely contribute to this MSC treatment effect.
Acknowledgments
We thank Qinge Lu for technical assistance.
This work was supported by NINDS grants PO1 NS23393, RO1 NS047682.
References
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metallo-proteinase- 9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–32. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurrand-Lions M, Johnson-Leger C, Lamagna C, Ozaki H, Kita T, Imhof BA. Junctional adhesion molecules and interendothelial junctions. Cells Tissues Organs. 2002;172:152–60. doi: 10.1159/000066967. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Chae JK, Kim I, Lim ST, Chung MJ, Kim WH, Kim HG, Ko JK, Koh GY. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–8. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–8. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–10. [PubMed] [Google Scholar]
- Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–5. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Calver AR, Kruger WH, Mudhar HS, Michalovich D, Takakura N, Nishikawa S, Richardson WD. PDGF mediates a neuron–astrocyte interaction in the developing retina. Neuron. 1996;17:1117–31. doi: 10.1016/s0896-6273(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial–pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Haralabopoulos GC, Grant DS, Kleinman HK, Lelkes PI, Papaioannou SP, Maragoudakis ME. Inhibitors of basement membrane collagen synthesis prevent endothelial cell alignment in matrigel in vitro and angiogenesis in vivo. Lab Invest. 1994;71:575–82. [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, D’Amore PA. Cell–cell interactions in vessel assembly: a model for the fundamentals of vascular remodelling. Transpl Immunol. 1997;5:177–8. doi: 10.1016/s0966-3274(97)80034-2. [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–13. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- Iurlaro M, Scatena M, Zhu WH, Fogel E, Wieting SL, Nicosia RF. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–43. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- Jiang S, Khan MI, Lu Y, Werstiuk ES, Rathbone MP. Acceleration of blood–brain barrier formation after transplantation of enteric glia into spinal cords of rats. Exp Brain Res. 2005;162:56–62. doi: 10.1007/s00221-004-2119-3. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2005 doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Shmelkov SV, Ramos CA, Zhang F, Rafii S. Tie2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood. 2005;106:505–13. doi: 10.1182/blood-2004-11-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum JM, Khaibullina A. Inhibition of endogenous VEGF impedes revascularization and astroglial proliferation: roles for VEGF in brain repair. Exp Neurol. 2003;181:241–57. doi: 10.1016/s0014-4886(03)00039-6. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neurosci Lett. 2001;316:67–70. doi: 10.1016/s0304-3940(01)02384-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–80. doi: 10.1161/01.str.29.9.1972. discussion 1980–1971. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia –reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004;117:3–32. doi: 10.1007/978-1-4419-8871-3_1. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Fiedler U, Augustin HG. Emerging roles of the Angiopoietin-Tie and the ephrin-Eph systems asregulators of cell trafficking. J Leukoc Biol. 2006 doi: 10.1189/jlb.1105652. [DOI] [PubMed] [Google Scholar]
- Ramsauer M, Krause D, Dermietzel R. Angiogenesis of the blood–brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002;16:1274–6. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, Inoue N, Yokoyama M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–14. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin- 1. Science. 1998;282:468–71. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, Petit E. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin- 1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–7. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Shwe Y, Du R, Chen Y, Shen FX, Young WL, Yang GY. Effects of angiopoietin-1 on vascular endothelial growth factor-induced angiogenesis in the mouse brain. Acta Neurochir Suppl. 2006;96:438–43. doi: 10.1007/3-211-30714-1_90. [DOI] [PubMed] [Google Scholar]