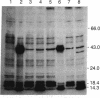
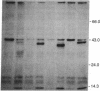
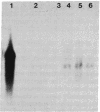
Abstract
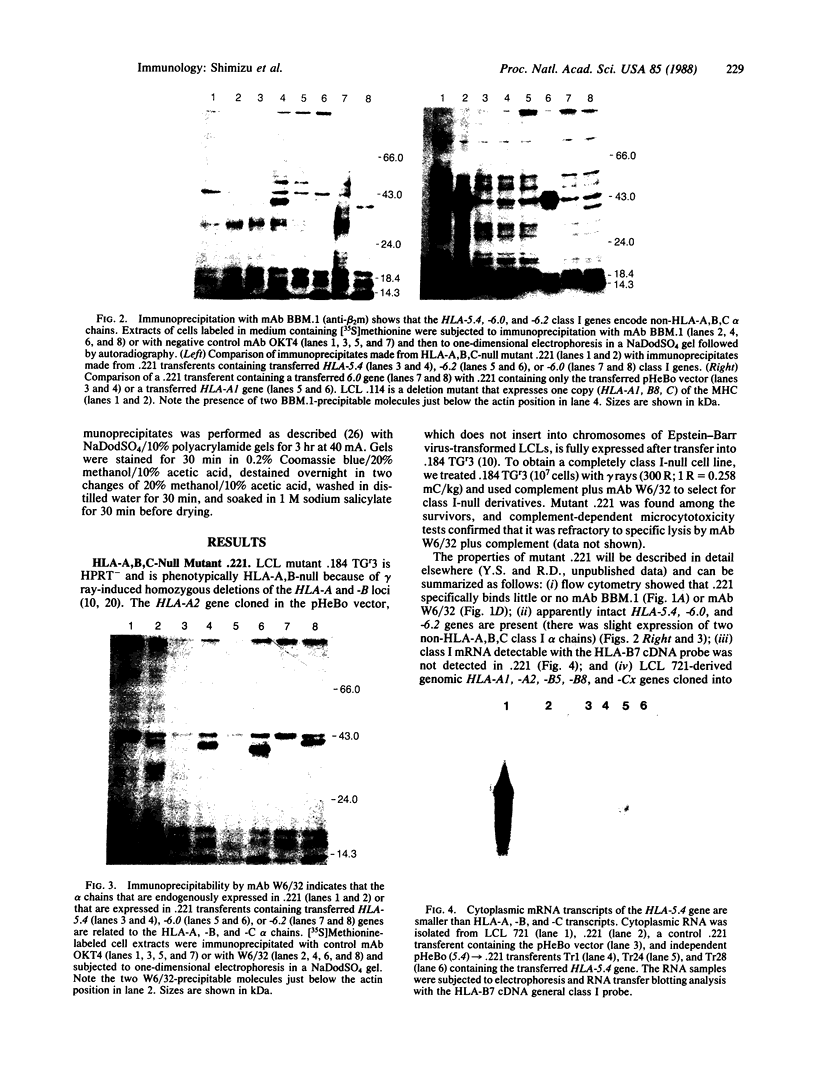
The HLA-A, -B, and -C class I human histocompatibility antigens and the genes that encode them have been isolated and characterized. Apparently complete class I non-HLA-A, B, C genes have been identified on HindIII-generated 5.4-kilobase (kb), 6.0-kb, and 6.2-kb DNA fragments derived from lymphoblastoid cell line (LCL) 721. We studied the expressibility of these genes by subcloning them into the nonintegrating pHeBo vector and transferring the chimeric plasmids into mutant LCL 721.221. This mutant was derived from LCL 721 by means of immunoselections following gamma-ray mutagenesis that eliminated expressions of the HLA-A, -B, and -C alpha chains. The HLA-A, B, C-null phenotype of mutant 721.221 made it possible to monitor the expression of class I genes transferred into it by assaying cell surface binding of monoclonal antibodies BBM.1 and W6/32, which recognize beta 2-microglobulin and HLA class I alpha-chain epitopes, respectively. Increased binding of BBM.1 and W6/32 was clearly observed in transferents containing the class I gene of the 6.0-kb DNA fragment but not in transferents containing the class I genes of the 5.4- and 6.2-kb DNA fragments. However, one-dimensional gel electrophoresis of BBM.1 and W6/32 immunoprecipitates made with [35S]methionine-labeled cell lysates showed that transfer of each non-HLA-A, B, C class I gene into 721.221 resulted in the appearance of an alpha chain that coprecipitated with beta 2-microglobulin. The three previously unreported alpha chains differed from each other in size and were smaller than HLA-A, -B, and -C alpha chains. These observations clearly show that these three cloned, nonallelic, non-HLA-A, B, C class I genes encode alpha chains that can be expressed in human cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., Fraser J., Flyer D., Calvin S., Flavell R. Beta 2-microglobulin is not required for cell surface expression of the murine class I histocompatibility antigen H-2Db or of a truncated H-2Db. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7447–7451. doi: 10.1073/pnas.83.19.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiot M., Bernard A., Raynal B., Knapp W., Deschildre C., Boumsell L. Heterogeneity of the first cluster of differentiation: characterization and epitopic mapping of three CD1 molecules on normal human thymus cells. J Immunol. 1986 Mar 1;136(5):1752–1758. [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Brennand J., Konecki D. S., Caskey C. T. Expression of human and Chinese hamster hypoxanthine-guanine phosphoribosyltransferase cDNA recombinants in cultured Lesch-Nyhan and Chinese hamster fibroblasts. J Biol Chem. 1983 Aug 25;258(16):9593–9596. [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Calabi F., Milstein C. A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. Nature. 1986 Oct 9;323(6088):540–543. doi: 10.1038/323540a0. [DOI] [PubMed] [Google Scholar]
- Chatterjee-Das S., Lillehoj E. P., Hernandez D. M., Coligan J. E., Sachs D. H. Analysis of the D-region products of H-2q using monoclonal antibodies reveals the expression of a new class I-like molecule. Immunogenetics. 1987;25(1):7–14. doi: 10.1007/BF00768827. [DOI] [PubMed] [Google Scholar]
- DeMars R., Rudersdorf R., Chang C., Petersen J., Strandtmann J., Korn N., Sidwell B., Orr H. T. Mutations that impair a posttranscriptional step in expression of HLA-A and -B antigens. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8183–8187. doi: 10.1073/pnas.82.23.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchet R., Boscher M., Bouhallier O., Merdrignac G., Genetet B., Turmel P., Charron D. J. New class I in man: serological and molecular characterization. Hum Immunol. 1986 Sep;17(1):3–20. doi: 10.1016/0198-8859(86)90069-8. [DOI] [PubMed] [Google Scholar]
- Ferrara G. B., Longo A., Tosi R., Biagini A., Mennucci M., Carminati G. HLA segregation of human T-cell subpopulation markers. Hum Immunol. 1981 May;2(3):213–223. doi: 10.1016/0198-8859(81)90013-6. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Burkly L. C., Sherman D. H., Waneck G. L., Widera G. Molecular biology of the H-2 histocompatibility complex. Science. 1986 Jul 25;233(4762):437–443. doi: 10.1126/science.3726537. [DOI] [PubMed] [Google Scholar]
- Francke U., Pellegrino M. A. Assignment of the major histocompatibility complex to a region of the short arm of human chromosome 6. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1147–1151. doi: 10.1073/pnas.74.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit E., Gil R., Altshuler R. Lymphocytes modulated by beta interferon express new non-HLA-A,B,C class I antigens. Hum Immunol. 1987 Jan;18(1):53–63. doi: 10.1016/0198-8859(87)90112-1. [DOI] [PubMed] [Google Scholar]
- Gazit E., Gothelf Y., Gil R., Orgad S., Pitman T. B., Watson A. L., Yang S. Y., Yunis E. J. Alloantibodies to PHA-activated lymphocytes detect human Qa-like antigens. J Immunol. 1984 Jan;132(1):165–169. [PubMed] [Google Scholar]
- Gazit E., Terhorst C., Mahoney R. J., Yunis E. J. Alloantigens of the human T (HT) genetic region of the HLA linkage group. Hum Immunol. 1980 Sep;1(2):97–109. doi: 10.1016/0198-8859(80)90097-x. [DOI] [PubMed] [Google Scholar]
- Geraghty D. E., Koller B. H., Orr H. T. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbase de Lima M., Wollman E. E., Lepage V., Degos L., Dausset J. Alloantigens expressed on activated human T cells different from HLA-A, B, C, and DR antigens. Immunogenetics. 1981;13(6):529–537. doi: 10.1007/BF00343720. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Jones E. A., Van Heyningen V., Solomon E., Bobrow M., Miggiano V., Bodmer W. F. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975 Mar 20;254(5497):267–269. doi: 10.1038/254267a0. [DOI] [PubMed] [Google Scholar]
- Goverman J., Hunkapiller T., Hood L. A speculative view of the multicomponent nature of T cell antigen recognition. Cell. 1986 May 23;45(4):475–484. doi: 10.1016/0092-8674(86)90279-5. [DOI] [PubMed] [Google Scholar]
- Kahn-Perles B., Wietzerbin J., Caillol D. H., Lemonnier F. Delineation of three subsets of class I human T antigens (HTA) on Molt-4 cells: serologic and regulatory relationship to HLA class I antigens. J Immunol. 1985 Mar;134(3):1759–1765. [PubMed] [Google Scholar]
- Kavathas P., Bach F. H., DeMars R. Gamma ray-induced loss of expression of HLA and glyoxalase I alleles in lymphoblastoid cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4251–4255. doi: 10.1073/pnas.77.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Christiansen F. T., Gosar I., Silver D. M., Pollack M. S., Dupont B. Frequency of alloantibodies reacting with PHA-activated T lymphocytes, unexplainable by known HLA activities. Hum Immunol. 1980 Dec;1(4):347–355. doi: 10.1016/0198-8859(80)90110-x. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Geraghty D., Orr H. T., Shimizu Y., DeMars R. Organization of the human class I major histocompatibility complex genes. Immunol Res. 1987;6(1-2):1–10. doi: 10.1007/BF02918100. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Orr H. T. Cloning and complete sequence of an HLA-A2 gene: analysis of two HLA-A alleles at the nucleotide level. J Immunol. 1985 Apr;134(4):2727–2733. [PubMed] [Google Scholar]
- Koller B. H., Sidwell B., DeMars R., Orr H. T. Isolation of HLA locus-specific DNA probes from the 3'-untranslated region. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5175–5178. doi: 10.1073/pnas.81.16.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch P. G., van de Rijn M., Smart J. E., Knowles R. W., Terhorst C. Isolation and purification of the human thymocyte antigens T6 and M241. Mol Immunol. 1986 Feb;23(2):131–139. doi: 10.1016/0161-5890(86)90034-9. [DOI] [PubMed] [Google Scholar]
- Moore G. E., Gerner R. E., Franklin H. A. Culture of normal human leukocytes. JAMA. 1967 Feb 20;199(8):519–524. [PubMed] [Google Scholar]
- Nadon N., Sekhon G., Brown L. J., Korn N., Petersen J. W., Strandtmann J., Chang C., DeMars R. Derepression of HPRT locus on inactive X chromosome of human lymphoblastoid cell line. Somat Cell Mol Genet. 1986 Nov;12(6):541–554. doi: 10.1007/BF01671940. [DOI] [PubMed] [Google Scholar]
- Olive D., Dubreuil P., Mawas C. Two distinct TL-like molecular subsets defined by monoclonal antibodies on the surface of human thymocytes with different expression on leukemia lines. Immunogenetics. 1984;20(3):253–264. doi: 10.1007/BF00364207. [DOI] [PubMed] [Google Scholar]
- Orr H. T., DeMars R. Mapping of class I DNA sequences within the human major histocompatibility complex. Immunogenetics. 1983;18(5):489–502. doi: 10.1007/BF00364390. [DOI] [PubMed] [Google Scholar]
- Paul P., Fauchet R., Boscher M. Y., Sayagh B., Masset M., Medrignac G., Dausset J., Cohen D. Isolation of a human major histocompatibility complex class I gene encoding a nonubiquitous molecule expressed on activated lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2872–2876. doi: 10.1073/pnas.84.9.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Koller B., Geraghty D., Orr H., Shaw S., Kavathas P., DeMars R. Transfer of cloned human class I major histocompatibility complex genes into HLA mutant human lymphoblastoid cells. Mol Cell Biol. 1986 Apr;6(4):1074–1087. doi: 10.1128/mcb.6.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Chorney M. J., Lawrance S. K., Pan J., Smith Z., Smith C. L., Weissman S. M. Structure, expression, and molecular mapping of a divergent member of the class I HLA gene family. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4224–4228. doi: 10.1073/pnas.84.12.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada N., Tanigaki N., Pressman D. Human cell membrane components bound to beta2-microglobulin in T cell-type cell lines. J Immunol. 1978 Feb;120(2):513–519. [PubMed] [Google Scholar]
- Tanigaki N., Tokuyama H., Fukunishi T., Minowada J., Pressman D. Human cell membrane components dominant in T cell lineage: identification and characterization of human TL-like antigens. J Immunol. 1979 Dec;123(6):2906–2914. [PubMed] [Google Scholar]
- Vasilov R. G., Hahn A., Mölders H., van Rood J. J., Breuning M., Ploegh H. L. Analysis of human class I antigens by two-dimensional gel electrophoresis. I. Polymorphism, evidence for additional (non-HLA-A, B, C) gene products, and identification of variant HLA-A, B antigens. Immunogenetics. 1983;17(4):333–356. doi: 10.1007/BF00372454. [DOI] [PubMed] [Google Scholar]
- Williams D. B., Swiedler S. J., Hart G. W. Intracellular transport of membrane glycoproteins: two closely related histocompatibility antigens differ in their rates of transit to the cell surface. J Cell Biol. 1985 Sep;101(3):725–734. doi: 10.1083/jcb.101.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen A., Giphart M. J., de Groot G., Morolli B., Festenstein H., Nijenhuis L. E., van Rood J. J. Two different T-cell systems in humans, one of which is probably equivalent to Qa or Tla in mice. Hum Immunol. 1985 Apr;12(4):235–246. doi: 10.1016/0198-8859(85)90339-8. [DOI] [PubMed] [Google Scholar]
- van de Rijn M., Lerch P. G., Knowles R. W., Terhorst C. The thymic differentiation markers T6 and M241 are two unusual MHC class I antigens. J Immunol. 1983 Aug;131(2):851–855. [PubMed] [Google Scholar]