Abstract
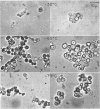
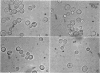
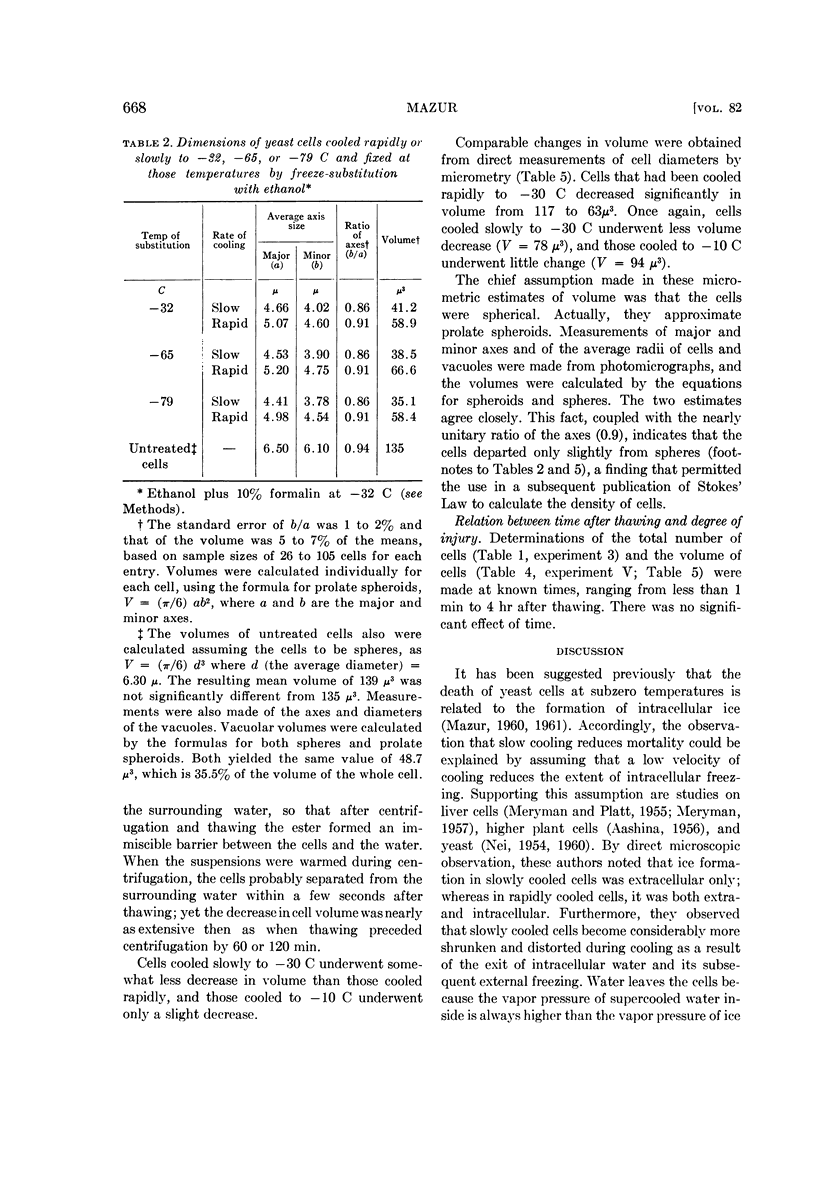
Mazur, Peter (Oak Ridge National Laboratory, Oak Ridge, Tenn.). Manifestations of injury in yeast cells exposed to subzero temperatures. I. Morphological changes in freeze-substituted and in “frozen-thawed” cells. J. Bacteriol. 82:662–672. 1961.—When cells of the yeast Saccharomyces cerevisiae are cooled rapidly to −30 C or below, fewer than 0.01% survive. In contrast, when they are cooled slowly, up to 50% survive. The effect of cooling rate on survival was reflected in the morphological appearance of cells both before and after thawing. Appearance before thawing was observed by fixing the cells at subzero temperatures by freeze-substitution with cold ethanol. Slowly cooled freeze-substituted cells were considerably smaller and more flattened than those cooled rapidly. The differences in appearance and the differences in survival are both consistent with the view that intracellular ice formation occurs more extensively in rapidly cooled cells and is responsible for their higher mortality.
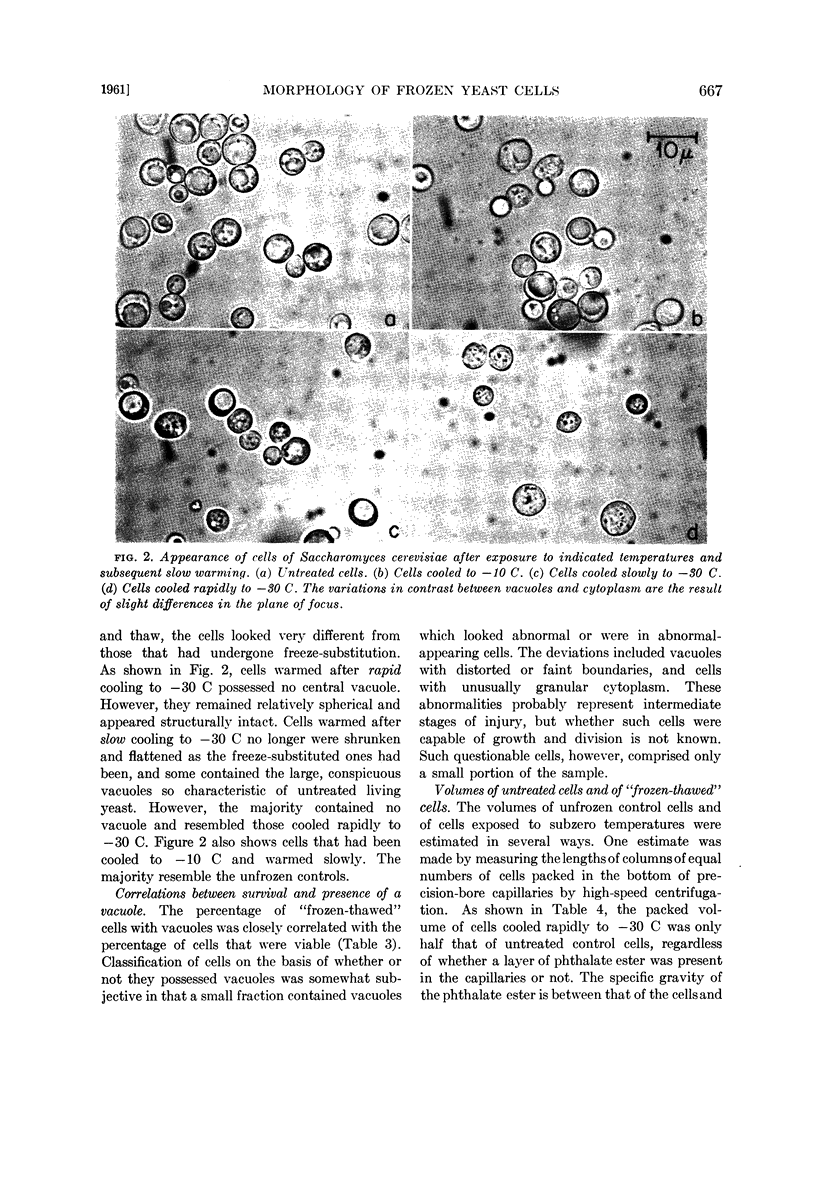
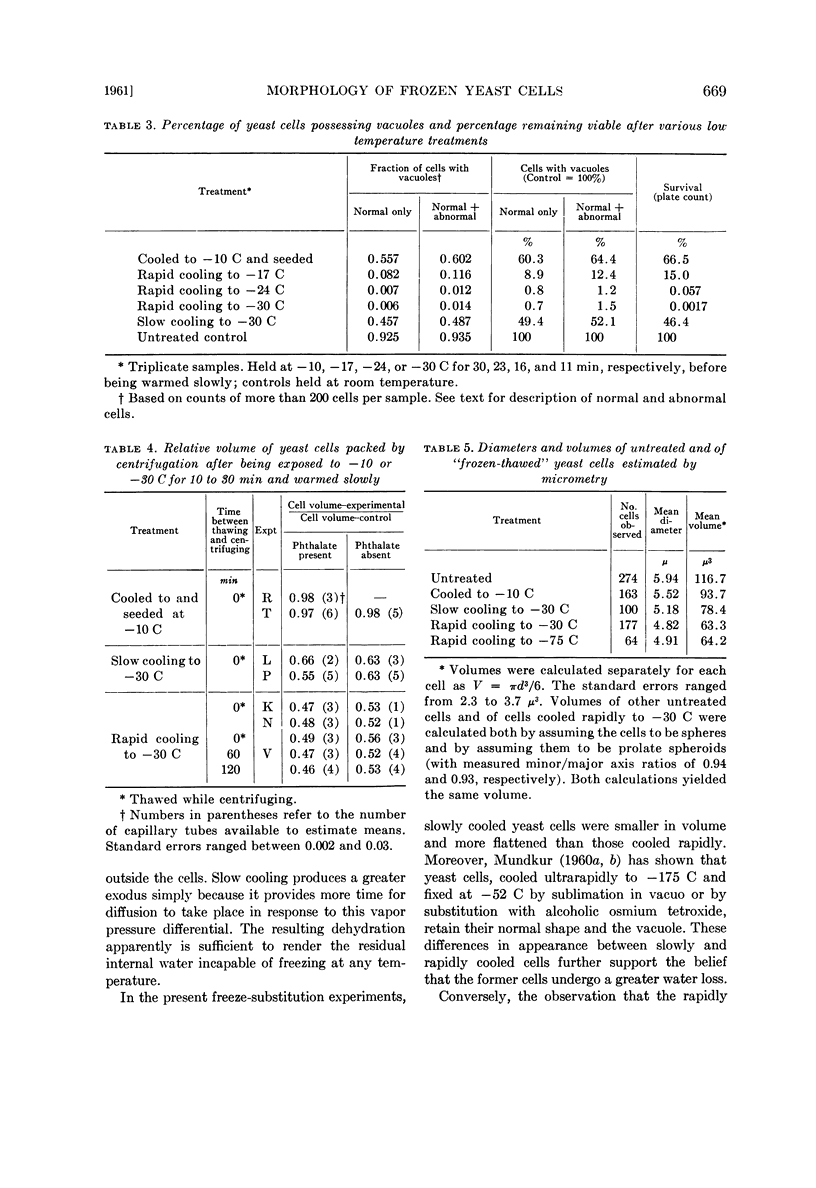
In spite of the high mortality (more than 99.99% killed), rapidly cooled cells remained as intact morphological entities when they were allowed to warm and thaw instead of undergoing freeze-substitution. However, they did differ from normal living yeast in two major respects: Their volume was halved and they lacked the large vacuole found in almost all the untreated living cells. The possession of a vacuole was closely correlated with survival. Suspensions warmed after slow cooling to −30 C contained cells of normal appearance and also nonvacuolate smaller ones. The admixture of morphological types was consistent with the fact that slow cooling yielded a higher percentage of viable cells than did rapid cooling.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FEDER N., SIDMAN R. L. Methods and principles of fixation by freeze-substitution. J Biophys Biochem Cytol. 1958 Sep 25;4(5):593–600. doi: 10.1083/jcb.4.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-MORAN H. Low-temperature preparation techniques for electron microscopy of biological specimens based on rapid freezing with liquid helium II. Ann N Y Acad Sci. 1960 Apr 13;85:689–713. doi: 10.1111/j.1749-6632.1960.tb49990.x. [DOI] [PubMed] [Google Scholar]
- HANSEN I. A., NOSSAL P. M. Morphological and biochemical effects of freezing on yeast cells. Biochim Biophys Acta. 1955 Apr;16(4):502–512. doi: 10.1016/0006-3002(55)90270-5. [DOI] [PubMed] [Google Scholar]
- HEARD B. E. Nuclear crystals in slowlyfrozen tissues at very low temperatures; comparison of normal and ascites tumour cells. Br J Surg. 1955 May;42(176):659–663. doi: 10.1002/bjs.18004217617. [DOI] [PubMed] [Google Scholar]
- HEARD B. E. The histological appearances of some normal tissues at low temperatures. Br J Surg. 1955 Jan;42(174):430–437. doi: 10.1002/bjs.18004217416. [DOI] [PubMed] [Google Scholar]
- LUYET B. On various phase transitions occurring in aqueous solution at low temperatures. Ann N Y Acad Sci. 1960 Apr 13;85:549–569. doi: 10.1111/j.1749-6632.1960.tb49982.x. [DOI] [PubMed] [Google Scholar]
- MAZUR P. Physical and temporal factors involved in the death of yeast at subzero temperatures. Biophys J. 1961 Jan;1:247–264. doi: 10.1016/s0006-3495(61)86887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZUR P. Physical factors implicated in the death of microorganisms at subzero temperatures. Ann N Y Acad Sci. 1960 Apr 13;85:610–629. doi: 10.1111/j.1749-6632.1960.tb49986.x. [DOI] [PubMed] [Google Scholar]
- MAZUR P., RHIAN M. A., MAHLANDT B. G. Survival of Pasteurella tularensis in gelatin-saline after cooling and warming at subzero temperatures. Arch Biochem Biophys. 1957 Sep;71(1):31–51. doi: 10.1016/0003-9861(57)90005-x. [DOI] [PubMed] [Google Scholar]
- MERYMAN H. T. Physical limitations of the rapid freezing method. Proc R Soc Lond B Biol Sci. 1957 Dec 17;147(929):452–459. doi: 10.1098/rspb.1957.0064. [DOI] [PubMed] [Google Scholar]
- MUNDKUR B. Electron microscopical studies of frozen-dried yeast. I. Localization of polysaccharides. Exp Cell Res. 1960 Jun;20:28–42. doi: 10.1016/0014-4827(60)90219-6. [DOI] [PubMed] [Google Scholar]
- MUNDKUR B. Submicroscopic morphology of frozen-dried yeast. Exp Cell Res. 1960 Oct;21:201–205. doi: 10.1016/0014-4827(60)90361-x. [DOI] [PubMed] [Google Scholar]
- PERSIDSKY M. D., LUYET B. J. Low-temperature recrystallization in gelatin gels and its relationship to concentration. Biodynamica. 1959 Dec;8:107–120. [PubMed] [Google Scholar]
- RAPATZ G., LUYET B. Recrystallization at high sub-zero temperatures in gelatin gels subjecte to various cooling treatments. Biodynamica. 1959 Dec;8:85–105. [PubMed] [Google Scholar]
- REY L. R. Studies on the action of liquid nitrogen on cultures in vitro of fibroblasts. Proc R Soc Lond B Biol Sci. 1957 Dec 17;147(929):460–466. doi: 10.1098/rspb.1957.0065. [DOI] [PubMed] [Google Scholar]
- REY L. R. Thermal analysis of eutectics in freezing solutions. Ann N Y Acad Sci. 1960 Apr 13;85:510–534. doi: 10.1111/j.1749-6632.1960.tb49979.x. [DOI] [PubMed] [Google Scholar]
- STEPHENSON J. L. Ice crystal growth during the rapid freezing of tissues. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):45–52. doi: 10.1083/jcb.2.4.45. [DOI] [PMC free article] [PubMed] [Google Scholar]