Abstract
Nutrient overabundance is known to promote cellular hypertrophy, a significant pathological event in diseases like diabetes and cancer, although mechanisms have remained unclear. In this issue of Developmental Cell, Wu and Derynck provide a new model that links metabolism and cell growth by demonstrating that hyperglycemia can increase TGF-β-dependent activation of the mTOR pathway to promote cellular hyperplasia.
Control of cell size is a central feature of tissue homeostasis. If cells atrophy, cell metabolism decreases, and cell size and growth potential are reduced (Rathmell et al., 2000). Conversely, cellular hypertrophy is associated with increased metabolism, which can lead to inflammation, reduced cellular or tissue function, and pathologies like diabetes, obesity, and cancer (Conlon and Raff, 1999). Cell hypertrophy represents an increase in cell size through elevated protein synthesis without DNA duplication and can be induced by growth factors, hormones, extracellular matrix protein accumulation, and hyperglycemia. The phosphatidylinositol-3 kinase (PI3K)/mTOR pathway has been genetically and biochemically shown to regulate cell size (Plas and Thompson, 2005). Activation of this pathway promotes cell growth, while inhibition prevents hypertrophy and can often lead to cellular atrophy.
While mechanisms that control the PI3K/ mTOR pathway in growth-factor-stimulated hypertrophy have been well defined, the mechanisms by which hyperglycemia (elevated glucose levels) promotes hypertrophy are unclear (Wolf and Ziyadeh, 1999). A manuscript by Wu and Derynck (2009) in this issue of Developmental Cell now highlights a vital contribution by transforming growth factor beta (TGF-β) to this process, identifying a role for TGF-β in glucose-induced hypertrophy through activation of matrix metalloproteinases (MMPs) and the Akt/mTOR pathway. This novel connection between glucose over-abundance and cellular pathology may provide new directions in understanding both control of cell growth and the spectrum of pathologies that characterize the metabolic syndrome that is associated with obesity.
TGF-β functions as a pleiotropic cytokine that either suppresses or promotes cell growth and proliferation. In healthy tissues, TGF-β acts as an inhibitory cytokine, but during tumorigenesis TGF-β promotes tumor growth, a flip in function described as the TGF-β paradox. The outcome of TGF-β-induced signaling is, therefore, context dependent. How this switch occurs is poorly understood, but the fact that the TGF-β receptor can initiate multiple signaling pathways may contribute. TGF-β activation leads to phosphorylation and activation of Smad transcription factors, which can directly control target gene expression. In addition, however, TGF-β has Smad-independent effects, such as activation of the PI3K/mTOR pathway (Lamouille and Derynck, 2007). The finding that both hyperglycemia and TGF-β can promote hypertrophy of epithelial cells (Ziyadeh, 2004) led Wu and Derynck to test a possible role for TGF-β and PI3K/mTOR in mediating glucose-stimulated hypertrophy.
In support of this hypothesis, Wu and Derynck show that blocking the activity of TGF-β receptor I (TβRI) kinase by pharmacological or genetic means prevented glucose-induced hypertrophy. Cells cultured in high levels of glucose, representative of uncontrolled hyperglycemia in diabetes, exhibited elevated levels of TGF-β signaling. Glucose increased both Smad-dependent signaling and Smad-independent activation of the PI3K/Akt/ mTOR pathway. The latter pathway was critical for glucose-induced hypertrophy because the mTOR inhibitor rapamycin prevented cell growth. These data suggest that TGF-β and TβRI mediate the response to glucose via the activation of the PI3K/Akt/mTOR pathway.
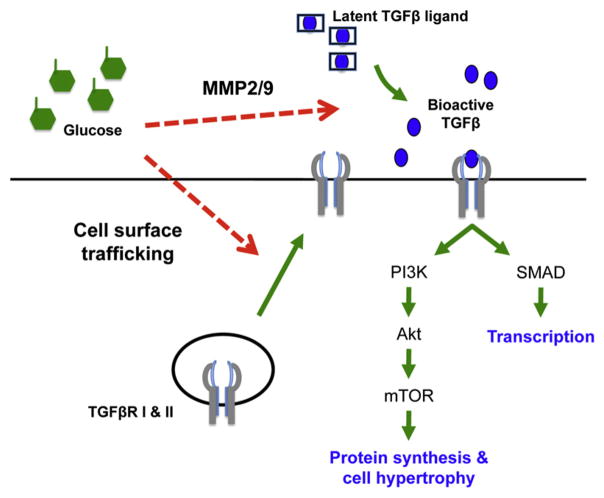
How does high glucose elicit activation of the TGF-β pathway? Nutrient-sensing signaling mechanisms can affect cells in a number of ways, and the authors demonstrate that hyperglycemia activated TGF-β signaling at the levels of both receptor and ligand availability (Figure 1). Although high levels of glucose did not increase the total cellular expression of TGF-β receptors, trafficking (recycling of TGF-β receptors to the cell surface) was selectively increased. A different cell surface receptor, the transferrin receptor, was not affected by changes in glucose. In addition to the TGF-β receptors, increased glucose availability also promoted MMP-2/9-dependent processing and activation of the TGF-β ligand.
Figure 1. Model of Glucose-Stimulated Cellular Hypertrophy.
Hyperglycemia increases TGF-β signaling through two proposed mechanisms (red dashed arrows). Glucose overabundance led to increased cell surface levels of TβRI and RII as well as activation and processing of latent TGF-β by MMP-2 and MMP-9. Cellular hypertrophy is caused by Smad-independent activation of the PI3K/Akt/mTOR pathway.
Each of these processes may be critical for linking cellular nutrient status with cell signaling. Several key mechanistic questions remain, however. One critical issue is whether glucose must be metabolized to elicit TGF-β-dependent cell hypertrophy. Hyperglycemia can directly alter glucose metabolism, as well as cause changes in lipid metabolism and protein glycosylation or oxidation. With these multifaceted effects of high glucose levels, it will be important to establish specific metabolic pathways and metabolites that may mediate the impact on TGF-β and hypertrophy.
Downstream of glucose or glucose metabolism, the primary causes of the increased trafficking of TGF-β receptors and of processing of TGF-β ligand to an active form by MMP-2 and MMP-9 also remain unclear. The TGF-β receptor may be selectively modified. It may associate with distinct proteins in hyperglycemia, or, possibly, more general changes in Rab proteins or lipid microdomains may occur and selectively impact TβRI at high levels of glucose. In either case, it will be interesting in future work to determine if other cell surface receptors are also affected by hyperglycemia—a prospect that could have significant implications for the pathology of diabetes and the metabolic syndrome. Regulation of MMP-2/9 can occur through altering MMP expression or inhibiting MMP inhibitory enzymes known as tissue inhibitors of metalloproteinases (TIMP). The activity of MMPs depends on the ratio between MMPs and TIMPs, and high glucose levels may alter this ratio (Tarallo et al., 2009). How MMPs and TIMPs are regulated by nutrient availability or metabolism, however, remains largely uncertain.
Activation of the Akt/mTOR signaling pathway by TβRI and Smad-independent signaling ultimately leads to a glucose-induced increase in cell size and hypertrophy. This pathway plays a well-established role in glucose uptake and consumption by both normal and cancerous cells (Plas and Thompson, 2005). This reciprocal control between glucose metabolism and Akt/mTOR suggests that additional feedback mechanisms may exist in glucose-driven cell hypertrophy. Given the contradictory roles of TGF-β in alternately suppressing or promoting cell proliferation (Rahimi and Leof, 2007), such feedback mechanisms may play roles in TGF-β-induced hypertrophy. Similarly, pathways that impinge on TGF-β or Akt signaling may be important modulators of glucose-induced hypertrophy.
In a global context, these findings suggest that glucose overavailability can have significant and direct effects on important cell signaling pathways. In addition to signaling pancreatic β-cells to secrete insulin, hyperglycemia can lead to inflammation and Toll-like receptor signaling (Shi et al., 2006), and elevated glucose can inhibit apoptosis (Plas and Thompson, 2005). With the rapidly increasing obesity and diabetes epidemics, understanding how cells respond to changes in glucose availability, for instance during hyperglycemia, is critical. Cellular hypertrophy can promote a variety of pathologies. Glucose-stimulated hypertrophy via activation of TGF-β and the PI3K/Akt/mTOR pathway thus provides another example of nutrient sensing and signaling that is clinically important and may impact a variety of other cellular physiologies through MMPs, TGF-β, and the Akt/mTOR pathway.
References
- Conlon I, Raff M. Cell. 1999;96:235–244. doi: 10.1016/s0092-8674(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Derynck R. J Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DR, Thompson CB. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- Rahimi RA, Leof EB. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarallo S, Beltramo E, Berrone E, Dentelli P, Porta M. Acta Diabetol. 2009 doi: 10.1007/s00592-012-0390-5. in press. Published online April 29, 2009. 10.1007/s00592-009-0124-5. [DOI] [PubMed] [Google Scholar]
- Wolf G, Ziyadeh FN. Kidney Int. 1999;56:393–405. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Wu L, Derynck R. Dev Cell. 2009;17:35–48. doi: 10.1016/j.devcel.2009.05.010. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyadeh FN. J Am Soc Nephrol. 2004;15(Suppl 1):S55–S57. doi: 10.1097/01.asn.0000093460.24823.5b. [DOI] [PubMed] [Google Scholar]