Abstract
The Golgi apparatus is known to modify and sort newly synthesized secretory proteins. However, fundamental mysteries remain about the structure, operation, and dynamics of this organelle. Important insights have emerged from studying the Golgi in yeasts. For example, yeasts have provided direct evidence for Golgi cisternal maturation, a mechanism that is likely to be broadly conserved. Here, we highlight features of the yeast Golgi as well as challenges that lie ahead.
Keywords: yeast, Golgi, secretory pathway, cisternal maturation, transitional ER
1. Introduction
Eukaryotic cells contain an “endomembrane system” that includes organelles of the secretory and endocytic pathways. In the secretory pathway, newly synthesized secretory cargo proteins are exported from the endoplasmic reticulum (ER) to the Golgi apparatus, which consists of disk-shaped membranes called cisternae [1]. In most cells, these cisternae are present in ordered stacks. Newly synthesized proteins arrive at the cis face of the stack, pass through medial cisternae, and then arrive at the trans face of the stack. During this time, glycoproteins are processed by an ordered sequence of resident Golgi enzymes. These enzymes show a polarized distribution in which early-acting enzymes are concentrated in cis cisternae while late-acting enzymes are concentrated in trans cisternae [2]. Finally, the fully processed proteins are sorted into transport carriers at the trans-Golgi network (TGN).
Although this general picture is well established, important issues remain unresolved. A central question is how proteins traverse the Golgi stack [3]. We will focus on the cisternal maturation concept, which can explain a variety of observations [4,5]. Morphological studies suggest that cisternae form at the cis face of the Golgi, progress through the stack to the trans face, and ultimately dissipate [6]. The implication is that cisternae are transient compartments that act as forward carriers for newly synthesized proteins. This model can explain how the Golgi transports large cargoes that remain within the cisternae, such as scales in algae [7] and procollagen bundles in mammalian fibroblasts [8]. Indeed, detailed studies provided strong evidence that procollagen-containing cisternae progress from the cis to the trans side of the stack [8,9]. Because trans cisternae have a different resident protein composition than cis cisternae, the implication is that cis cisternae mature into trans cisternae. However, this idea has not been directly tested in mammalian cells because the Golgi cisternae are too closely associated with one another to be resolved by current live-cell microscopy techniques.
Another key question is the role of vesicles in the secretory pathway. Export from the ER is thought to be mediated by COPII-coated transport vesicles [10,11], which form at specialized ER domains known as transitional ER (tER) sites or ER exit sites [1,12]. Some researchers have visualized individual COPII vesicles in mammalian and plant cells [13,14], but others have argued that vesicle formation may not be the primary role of COPII [15,16]. A second class of carriers called COPI vesicles is thought to mediate retrograde traffic within the Golgi stack and from the Golgi to the ER [17]. COPI vesicles have been proposed to recycle resident Golgi enzymes, thereby keeping these enzymes in the organelle during cisternal maturation [18], but the content and directionality of COPI vesicles are still controversial [3].
Finally, there has been a lively debate about whether the Golgi is an independent organelle or a dynamic outgrowth of the ER [19,20]. Mammalian Golgi stacks can evidently form de novo under some experimental conditions [21]. One view is that membrane flux from the ER is the major determinant of the formation and reorganization of the Golgi [22], while an alternative view is that the Golgi exhibits considerable autonomy [23].
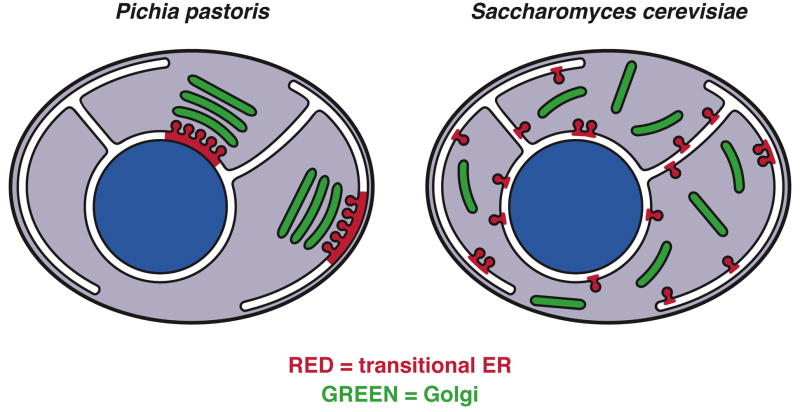
Yeasts are an attractive system for tackling these issues because the yeast secretory pathway is relatively simple and can be studied using genetics, biochemistry, and microscopy. Basic molecular aspects of the secretory pathway are conserved between yeasts and mammals [24]. Two budding yeasts have been studied in detail (Figure 1). Saccharomyces cerevisiae has been used to define many of the components and mechanisms of the secretory pathway. The Golgi in this organism has an unusual structure, consisting of individual cisternae that are scattered throughout the cytoplasm [25–27]. More recently, Pichia pastoris has attracted attention because it is closely related to S. cerevisiae and yet contains ordered Golgi stacks similar to those seen in higher eukaryotes [28,29]. As described below, these two yeasts have complementary advantages for exploring the biogenesis and operation of the Golgi.
Figure 1. Diagram of tER and Golgi organization in two budding yeasts.
In P. pastoris, ordered Golgi stacks are located next to stable tER sites. A typical P. pastoris cell contains 2–5 tER-Golgi units. In S. cerevisiae, the tER sites are smaller and more numerous, and individual Golgi cisternae are scattered throughout the cytoplasm.
2. Yeast Golgi organization
In S. cerevisiae, secretory cargo proteins exit the ER from many small tER sites [29,30], and then undergo a series of processing steps analogous to those that occur in the mammalian Golgi [24]. Golgi compartments can be visualized by immunofluorescence as scattered spots [25,29], and by electron microscopy as individual disk-shaped or fenestrated cisternae that only occasionally associate with one another [27,31]. Resident enzymes that act at different steps in cargo processing are found in distinct cisternae. A typical cell contains on the order of 20 cisternae, which can be characterized as cis, medial, trans or TGN based on their resident protein content [32]. Certain mutant strains accumulate Golgi structures that show a stack-like organization [24], but these structures are probably exaggerated TGN compartments rather than ordered stacks of biochemically distinct cisternae [29].
In Pichia pastoris, Golgi stacks are readily seen by electron microscopy [29]. A typical cell contains 2–5 stacks of about 4 cisternae each. These stacks are biochemically polarized, with homologues of S. cerevisiae early and late Golgi markers concentrated near the cis and trans faces, respectively [28]. Golgi stacks in P. pastoris are tightly associated with long-lived tER sites located on the nuclear envelope or cortical ER [28,29,33] (Figure 2). Video fluorescence microscopy revealed that Golgi stacks and the associated tER sites arise de novo throughout the P. pastoris cell cycle [33].
Figure 2. Tomographic reconstruction of a tER-Golgi unit in P. pastoris.
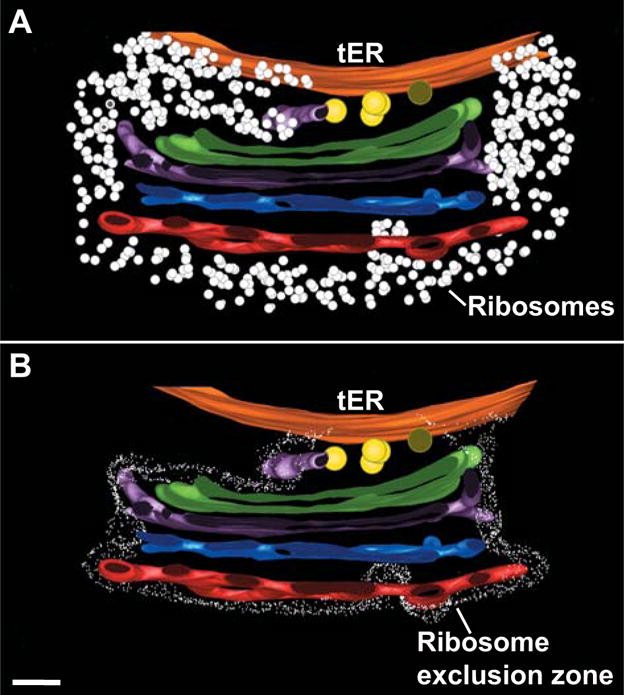
This figure was taken with permission from Ref. 28. (A) In this cross-section view, the ER membrane is orange while the cis, medial, trans, and TGN cisternae are green, purple, blue, and red, respectively. Yellow circles are presumptive COPII vesicles at the tER-Golgi interface, and white dots are ribosomes. The cisternae are attached to each other and to the tER site, and the ribosome-free tER site is continuous with the ribosome-excluding Golgi matrix. (B) Same view as in panel A, except that the border of the ribosome exclusion zone is outlined. Scale bar, 100 nm.
Why is the secretory pathway organized differently in S. cerevisiae and P. pastoris? Presumably, P. pastoris has retained Golgi stacking from a common ancestor while S. cerevisiae has not, but the reason for this divergence is unknown. Interestingly, we recently found that when ER export is slowed in S. cerevisiae, tER sites and cis-Golgi cisternae show a close association that resembles the association seen in P. pastoris (S. Levi, manuscript in preparation). It therefore seems likely that the mechanisms of membrane traffic and compartmental organization are actually quite similar in the two yeasts. This interpretation implies that cisternal stacking is dispensable for the major pathways of Golgi membrane traffic.
Studies of the Golgi in budding yeasts are highlighting conserved structural features. For example, the P. pastoris Golgi shows the following similarities to mammalian and plant Golgi organelles: (i) P. pastoris Golgi stacks are surrounded by a “matrix” that seems to glue the cisternae together [28] (Figure 2). A similar Golgi matrix has been described in mammalian and plant cells [13,23]. (ii) Cisternae at the trans face of P. pastoris Golgi stacks apparently escape from the Golgi matrix and peel off from the stacks, consistent with a maturation mechanism [28]. The same phenomenon has been documented in other cell types [6]. (iii) Golgi cisternae in P. pastoris are fenestrated and have tubular extensions [28]. The same is true for mammalian Golgi cisternae [34,35]. In P. pastoris, fenestrations seem to arise at the rims of cis/medial cisternae [28], and in mammalian cells, fenestrations are often found near the cisternal rims [35].
Budding yeasts lack certain nonconserved features that complicate analysis of the Golgi in other cell types. Most notably, the Golgi stacks in P. pastoris remain physically separate, whereas mammalian Golgi membranes undergo microtubule-dependent centripetal movement followed by assembly into a linked Golgi ribbon [35]. In molecular terms, budding yeasts have homologs of key Golgi structural and trafficking proteins, but the relevant protein families typically have fewer members in budding yeasts than in mammalian cells [36,37]. Thus, budding yeasts contain “stripped down” Golgi organelles that display many of the basic properties seen in other eukaryotes.
The fission yeast Schizosaccharomyces pombe is evolutionarily distant from budding yeasts and therefore provides a complementary experimental system [38]. S. pombe has multiple Golgi organelles, which bear some resemblance to higher plant Golgi stacks with regard to motility and the lack of a clearly visible tER-Golgi association [39,40]. Although Golgi cisternae in S. pombe form stacks, the stacking seems to be relatively weak because isolated early and late cisternae can be detected by fluorescence microscopy [39,41].
3. Yeast tER sites
tER sites were first identified morphologically as vesiculating ER regions devoid of bound ribosomes [1], and were later shown to be sites of COPII concentration [12,42]. A typical mammalian cell has several hundred tER sites [43]. In many protists, Golgi stacks are juxtaposed to tER sites [7]. This same type of organization is seen in P. pastoris, where electron tomography revealed that each tER site is linked to a Golgi stack by a continuous ribosome-excluding matrix [28] (Figure 2). P. pastoris tER sites can be visualized by fluorescence microscopy as 2–5 discrete spots that are located within the ER and next to Golgi organelles [29,33]. In S. cerevisiae and S. pombe, tER sites have not been seen by electron microscopy, but are visible by fluorescence microscopy as many small spots distributed throughout the ER [29,41]. We originally proposed that S. cerevisiae lacked conventional tER sites and that individual COPII vesicles could form throughout the entire ER [29], but a more likely interpretation is that S. cerevisiae has tER sites that are smaller and more numerous than those in most other eukaryotes (Figure 1).
Budding yeasts have been used to characterize tER dynamics. Video microscopy of P. pastoris revealed that tER sites are long-lived structures that form de novo, fuse upon collision, and undergo growth and shrinkage to maintain a steady-state size [33]. Similar behavior has been seen for mammalian tER sites [44]. These data suggest that tER sites are self-organizing membrane domains that are maintained by a balance between growth and shrinkage [33]. The relatively small size of tER sites in S. cerevisiae might reflect kinetic parameters different from those in P. pastoris. In support of this idea, some of the tER sites in S. cerevisiae become larger when ER export is inhibited [30] (S. Levi, unpublished observations).
Little is known about how tER sites are established or how COPII vesicle formation is restricted to these sites. This issue was addressed by identifying a P. pastoris mutant in which the tER sites are smaller and more numerous [45]. The mutation lies in Sec16 [45], a large peripheral ER membrane protein of unknown function that interacts with multiple COPII components [46]. Sec16 homologues were also found to be important for tER organization in Drosophila melanogaster and mammalian cells [47–49]. One possible function of Sec16 is to serve as a scaffold for localizing COPII components [45,48,49]. In addition, our recent work indicates that Sec16 affects tER dynamics by regulating the rate of ER export (Y. Liu, unpublished observations). These findings are beginning to reveal molecular mechanisms for the biogenesis and maintenance of tER sites.
As described above, tER sites are associated with cis-Golgi cisternae in many organisms, including S. cerevisiae. This observation fits with the idea that COPII vesicles fuse homotypically to nucleate the formation of new cis-Golgi cisternae [4,31,50]. In turn, cis-Golgi cisternae might stabilize the associated tER sites. An exciting challenge will be to identify the components that link tER sites to the cis-Golgi.
Golgi stacking may be influenced by the tER-Golgi relationship. The scattered Golgi in S. cerevisiae correlates with an unusually fragmented tER [29] (Figure 1). Intriguingly, when tER organization was perturbed in P. pastoris by mutating Sec16, Golgi stacking was also disrupted [45]. It seems likely that Golgi stacking in yeasts requires two conditions: stable tER sites that repeatedly generate new cisternae, and tethers that attach these cisternae to each other and to the tER sites.
4. Maturation of the yeast Golgi
Features of the P. pastoris Golgi can be interpreted in light of the cisternal maturation model. In particular, the close association of Golgi stacks with tER sites suggests that new Golgi cisternae are constantly being generated near tER sites [19], and the apparent peeling off of cisternae from the trans face of the stack may reflect the terminal maturation of Golgi cisternae into transport carriers [28,33]. Similar interpretations have long been favored by morphologists studying other cell types [6,7].
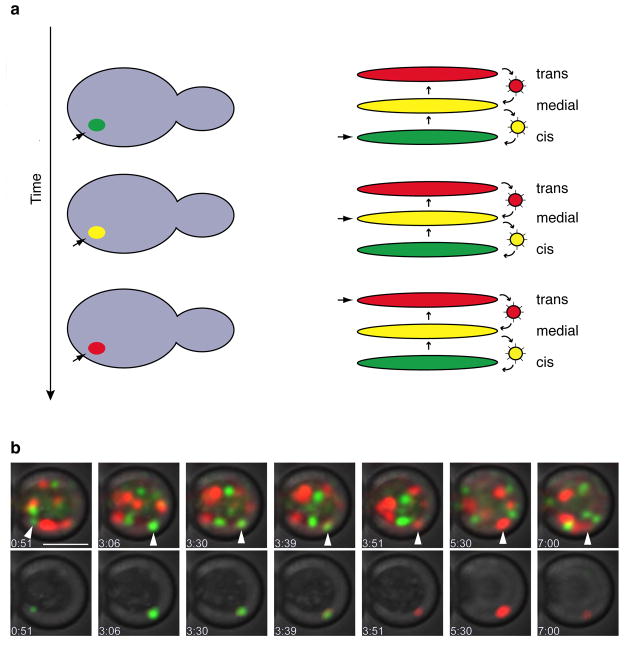
The nonstacked Golgi in S. cerevisiae offers a unique opportunity for a direct test of the cisternal maturation model. Unlike Golgi cisternae in most other eukaryotes, the Golgi cisternae in S. cerevisiae are individually resolvable by fluorescence microscopy [51]. Therefore, if resident proteins of the early and late Golgi are marked with different fluorescent tags, the early cisternae should be observed to change color as they mature into late cisternae (Figure 3a). This prediction was confirmed by two research groups using both peripheral and transmembrane Golgi markers [52,53] (Figure 3b). The apparent lifetime of a Golgi cisterna was similar to the time required for secretory cargo proteins to traverse the Golgi, consistent with the idea that cisternal maturation is the primary mode of anterograde Golgi membrane traffic in S. cerevisiae [52]. These results complement studies indicating that mammalian Golgi cisternae act as forward carriers for secretory cargo proteins such as procollagen [8,9]. The combined data suggest that cisternal maturation is a conserved mechanism for Golgi function.
Figure 3. Visualizing Golgi cisternal maturation in S. cerevisiae.
This figure was adapted with permission from Ref. 52. (a) Predicted dynamics of a maturing cisterna. Green represents a resident early Golgi protein, and red represents a resident late Golgi protein. The cisterna marked with the arrow matures by the recycling of resident Golgi proteins from older to younger cisternae. On the right is a schematic diagram of this process. On the left is a representation of how a maturing cisterna should be visualized as a fluorescent spot that changes color from green to yellow to red. (b) Experimental confirmation of the predicted behavior. Early Golgi cisternae were labeled with GFP-Vrg4, and late Golgi cisternae were labeled with Sec7-DsRed. Displayed are time points from a 4D movie of a single cell. In the panels at the bottom, the cisterna marked with the arrowhead is shown in isolation as it matures. Scale bar, 2 μm.
Despite these successes, the cisternal maturation model is probably oversimplified and certainly requires further testing. For example, an analysis of secretory cargo exit from the mammalian Golgi argued against a simple “conveyor belt” model of maturing cisternae [54]. Exit from the Golgi followed exponential kinetics, possibly indicating that secretory cargoes pass through a long-lived post-Golgi compartment such as the TGN or recycling endosomes [5,54]. This prediction should be testable using video fluorescence microscopy of Golgi and post-Golgi compartments in S. cerevisiae.
5. COPII function in yeast
COPII components were first identified in S. cerevisiae as proteins that are essential for ER-to-Golgi transport [24]. In vitro incubation of microsomes with yeast COPII produces coated vesicles that selectively package secretory cargo proteins [55]. However, COPII coated vesicles have not been visualized by electron microscopy of S. cerevisiae cells, and their presence in mammalian and plant cells has been controversial [13–16]. These findings have led to the suggestion that the main role of COPII in ER export may not actually be to form coated vesicles, but rather to create functionally specialized domains on the ER membrane [15,16].
By contrast, data from P. pastoris suggest that COPII does form coated vesicles in vivo. Electron tomography revealed multiple coated buds emerging from tER sites, as well as coated or partially coated vesicles that accumulated between tER sites and cis-Golgi cisternae [28]. These images presumably represent different stages in the biogenesis of COPII vesicles. It seems likely that COPII vesicles in P. pastoris form at tER sites and then remain locally tethered prior to fusion with each other or with cis-Golgi cisternae. However, such observations do not rule out additional functions for COPII, particularly in cell types that must export large secretory cargoes from the ER.
Studies of S. cerevisiae have revealed diversity in the pathways for COPII-dependent export. For example, most secretory cargo proteins are recognized by direct or indirect interactions with the Sec24 subunit of the COPII coat [11], but the plasma membrane ATPase Pma1 is recognized by the Sec24 homologue Lst1 [56]. The principle that different Sec24 family members recognize distinct cargo proteins has been generalized to other organisms [57]. In a process that is less well understood, yeast proteins with a glycosylphosphatidylinositol (GPI) anchor show different requirements for ER export than other secretory cargo proteins and may actually be exported from distinct tER sites [30]. The combined results indicate that yeast COPII employs sophisticated mechanisms to package the wide range of secretory cargo proteins that leave the ER.
6. COPI function in yeast
COPI was first characterized as a protein complex that generates coated vesicles from mammalian Golgi cisternae, and was proposed to mediate forward traffic through the Golgi stack [58]. S. cerevisiae homologues of the COPI subunits were identified as being important for secretion, but were later shown to function in retrograde Golgi-to-ER transport [59,60]. These and other data have been interpreted to mean that COPI vesicles mediate two processes: the recycling of Golgi proteins from older to younger cisternae during cisternal maturation, and the recycling of components from the Golgi to the ER [51]. Candidates for these two classes of retrograde COPI vesicles have been visualized by tomographic analysis of plant and algal cells [13].
In S. cerevisiae, COPI components are found on Golgi cisternae, but COPI vesicle formation has not been visualized. In P. pastoris, coated buds are occasionally seen on Golgi cisternae, and putative COPI vesicles are found between tER sites and cis-Golgi cisternae outside the regions that contain accumulated COPII vesicles [28]. Thus, P. pastoris tER sites may be bidirectional portals that mediate COPII-dependent ER-to-Golgi traffic as well as COPI-dependent Golgi-to-ER recycling.
The functions of COPI in intra-Golgi traffic are not yet clear, partly due to conflicting data about the content and directionality of COPI vesicles [3]. S. cerevisiae should be suitable for resolving some of these discrepancies. Analysis of yeast COPI mutants supported a model in which COPI affects anterograde traffic indirectly, perhaps by recycling components that are needed to export secretory cargoes from the ER [59]. A role for COPI in cisternal maturation is still tentative. In a temperature-sensitive COPI mutant strain, cisternal maturation was slowed but not arrested at the nonpermissive temperature [53]. This result could mean that COPI contributes to cisternal maturation but is not essential for this process. Alternatively, the existing mutant alleles of COPI might not completely block function at the nonpermissive temperature. A resolution of this issue would be facilitated by improved methods for inactivating yeast proteins [61].
As with COPII, the COPI coat may help to define membrane domains in addition to generating coated vesicles [62]. Hints of such a function come from studies of the ARF GTPase that recruits COPI to the Golgi. When ARF levels are depleted in S. cerevisiae, the cells contain a few large Golgi cisternae instead of many smaller cisternae [63]. Similar effects are seen in mutants defective in recruiting ARF to the early Golgi [64]. The enlarged Golgi cisternae probably result from a reduction in membrane-associated COPI, perhaps indicating that COPI normally constrains the size of Golgi cisternae by inhibiting homotypic fusion. This idea is speculative, but it illustrates the potential value of studying COPI function in yeast.
7. Proliferation and inheritance of the yeast Golgi
Unlike the mammalian Golgi, which breaks down extensively during mitosis [23], the yeast Golgi remains intact and functional throughout the cell cycle [27,65]. This phenomenon is especially clear in P. pastoris, where the number and structure of Golgi stacks is unchanged in mitotic cells [29]. P. pastoris Golgi stacks form de novo in association with newly forming tER sites [33], suggesting that entire tER-Golgi units arise by a process of self-organization.
Golgi membranes in S. cerevisiae are present in the bud from very early in the cell cycle [27,65], so by examining how small buds inherit Golgi cisternae, we can learn about principles of Golgi biogenesis. Because ER membranes are also present in the bud from very early in the cell cycle [66], ER export might nucleate the formation of new Golgi cisternae. Support for this hypothesis came from a demonstration that the presence of early Golgi cisternae in small buds correlated strongly with the presence of ER membranes in the buds [67]. For late Golgi cisternae, the inheritance mechanisms are more complex. Late Golgi cisternae are present in small buds and are often concentrated at sites of polarized growth [65]. Some of the late Golgi cisternae in the buds presumably arise by the maturation of bud-localized early cisternae [67], but in addition, the actin cytoskeleton and the class V myosin Myo2 play a role in transporting late cisternae to the bud and retaining them there [65,68].
Insight into the role of Myo2 in Golgi inheritance came from the discovery that the small GTPase Ypt11 links Myo2 to the Ret2 subunit of the COPI coat [69]. Ypt11 seems to have a key role in transporting late Golgi cisternae to the bud [69]. In addition, Ypt11 has been implicated in the inheritance of the ER and mitochondria [70], suggesting that the inheritance of different organelles may be coordinately regulated.
These studies have focused on yeast-specific pathways for Golgi formation and inheritance, but they may illuminate a general principle. It is attractive to think that in both mammalian cells and yeast, Golgi biogenesis is nucleated by membranes exported from the ER, but also depends on input from existing Golgi compartments [19,67].
8. Suitability of yeasts for studying the Golgi
An important question is whether the Golgi in yeasts might be fundamentally different from that in other eukaryotes [71]. In particular, can the results observed for the nonstacked Golgi in S. cerevisiae be generalized to other organisms? Encouraging data in this regard come from a study of S. pombe, in which isolated early Golgi cisternae were observed to mature into late Golgi cisternae just as in S. cerevisiae [41]. These two yeasts are separated by approximately a billion years of evolution [38], implying that cisternal maturation is an ancient process.
More generally, our view is that differences between cell types often turn out to be superficial variations on a common theme. For example, early suggestions that the Golgi operates differently in algae than in other organisms [1] have not stood the test of time. Several considerations indicate that results obtained with yeasts will be of broad significance for understanding the Golgi:
Molecular studies have revealed strong conservation between the yeast and mammalian secretory pathways [24].
S. cerevisiae is closely related to P. pastoris, which has ordered Golgi stacks [28,29]. Secretory pathway function in these two yeasts is probably very similar. Thus, cisternal stacking may be an “optional” property of the Golgi [72].
As described above, studies of procollagen transport in mammalian cells implied that Golgi cisternae mature. That work provided the intellectual framework for the yeast studies that directly visualized cisternal maturation. It would be a surprising coincidence if the mammalian data inspired the discovery of a yeast-specific phenomenon.
None of these arguments is airtight, and some researchers are more inclined to emphasize the differences between mammalian cells and yeasts. It is important to keep that perspective in mind and to be duly cautious when extrapolating results from yeast systems to other eukaryotes. Nevertheless, we are confident that yeasts will continue to be superb model organisms for exploring conserved properties of the secretory pathway.
Acknowledgments
This work was supported by grants from the March of Dimes (#1-FY07-465) and the NIH (R01 GM061156).
Abbreviations
- ER
endoplasmic reticulum
- tER
transitional ER
- TGN
trans-Golgi network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farquhar MG, Palade GE. The Golgi apparatus (complex) - (1954–1981) - from artifact to center stage. J Cell Biol. 1981;91:77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunphy WG, Rothman JE. Compartmental organization of the Golgi stack. Cell. 1985;42:13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- 3.Rabouille C, Klumperman J. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol. 2005;6:812–817. doi: 10.1038/nrm1735. [DOI] [PubMed] [Google Scholar]
- 4.Glick BS, Malhotra V. The curious status of the Golgi apparatus. Cell. 1998;95:883–889. doi: 10.1016/s0092-8674(00)81713-4. [DOI] [PubMed] [Google Scholar]
- 5.Glick BS, Nakano A. Membrane traffic within the Golgi stack. Annu Rev Cell Dev Biol. 2009 doi: 10.1146/annurev.cellbio.24.110707.175421. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mollenhauer HH, Morré DJ. Perspectives on Golgi apparatus form and function. J Electron Microsc Tech. 1991;17:2–14. doi: 10.1002/jemt.1060170103. [DOI] [PubMed] [Google Scholar]
- 7.Becker B, Melkonian M. The secretory pathway of protists: spatial and functional organization and evolution. Microbiol Rev. 1996;60:697–721. doi: 10.1128/mr.60.4.697-721.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonfanti L, et al. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- 9.Mironov AA, et al. Small cargo proteins and large aggregates can traverse the Golgi by a common mechanism without leaving the lumen of cisternae. J Cell Biol. 2001;155:1225–1238. doi: 10.1083/jcb.200108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes H, Stephens DJ. Assembly, organization, and function of the COPII coat. Histochem Cell Biol. 2008;129:129–151. doi: 10.1007/s00418-007-0363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MC, Miller EA. Molecular mechanisms of COPII vesicle formation. Semin Cell Dev Biol. 2007;18:424–434. doi: 10.1016/j.semcdb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Bannykh SI, Balch WE. Membrane dynamics at the endoplasmic reticulum-Golgi interface. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staehelin LA, Kang BH. Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol. 2008;147:1454–1468. doi: 10.1104/pp.108.120618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeuschner D, Geerts WJ, van Donselaar E, Humbel BM, Slot JW, Koster AJ, Klumperman J. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat Cell Biol. 2006;8:377–383. doi: 10.1038/ncb1371. [DOI] [PubMed] [Google Scholar]
- 15.Mironov AA, et al. ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev Cell. 2003;5:583–594. doi: 10.1016/s1534-5807(03)00294-6. [DOI] [PubMed] [Google Scholar]
- 16.Sparkes IA, Frigerio L, Tolley N, Hawes C. The plant endoplasmic reticulum: a cell-wide web. Biochem J. 2009;423:145–155. doi: 10.1042/BJ20091113. [DOI] [PubMed] [Google Scholar]
- 17.Béthune J, Wieland F, Moelleken J. COPI-mediated transport. J Membr Biol. 2006;211:65–79. doi: 10.1007/s00232-006-0859-7. [DOI] [PubMed] [Google Scholar]
- 18.Glick BS, Elston T, Oster G. A cisternal maturation mechanism can explain the asymmetry of the Golgi stack. FEBS Lett. 1997;414:177–181. doi: 10.1016/s0014-5793(97)00984-8. [DOI] [PubMed] [Google Scholar]
- 19.Glick BS. Can the Golgi form de novo? Nat Rev Mol Cell Biol. 2002;3:615–619. doi: 10.1038/nrm877. [DOI] [PubMed] [Google Scholar]
- 20.Lowe M, Barr FA. Inheritance and biogenesis of organelles in the secretory pathway. Nat Rev Mol Cell Biol. 2007;8:429–439. doi: 10.1038/nrm2179. [DOI] [PubMed] [Google Scholar]
- 21.Puri S, Linstedt AD. Capacity of the Golgi apparatus for biogenesis from the endoplasmic reticulum. Mol Biol Cell. 2003;14:5011–5018. doi: 10.1091/mbc.E03-06-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippincott-Schwartz J, Roberts TH, Hirschberg K. Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol. 2000;16:557–589. doi: 10.1146/annurev.cellbio.16.1.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- 24.Duden R, Schekman R. Insights into Golgi function through mutants in yeast and animal cells. In: Berger EG, Roth J, editors. The Golgi Apparatus. Birkhäuser Verlag; Basel: 1997. pp. 219–246. [Google Scholar]
- 25.Franzusoff A, Redding K, Crosby J, Fuller RS, Schekman R. Localization of components involved in protein transport and processing through the yeast Golgi apparatus. J Cell Biol. 1991;112:27–37. doi: 10.1083/jcb.112.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano A. Yeast Golgi apparatus. In: Mironov AA, Pavelka M, editors. The Golgi Apparatus. Springer; Vienna: 2008. pp. 623–629. [Google Scholar]
- 27.Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol Biol Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogelsvang S, Gomez-Ospina N, Soderholm J, Glick BS, Staehelin LA. Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris. Mol Biol Cell. 2003;14:2277–2291. doi: 10.1091/mbc.E02-10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossanese OW, Soderholm J, Bevis BJ, Sears IB, O’Connor J, Williamson EK, Glick BS. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J Cell Biol. 1999;145:69–81. doi: 10.1083/jcb.145.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillon GA, Watanabe R, Taylor M, Schwabe TM, Riezman H. Concentration of GPI-anchored proteins upon ER exit in yeast. Traffic. 2009;10:186–200. doi: 10.1111/j.1600-0854.2008.00857.x. [DOI] [PubMed] [Google Scholar]
- 31.Morin-Ganet MN, Rambourg A, Deitz SB, Franzusoff A, Képès F. Morphogenesis and dynamics of the yeast Golgi apparatus. Traffic. 2000;1:56–68. doi: 10.1034/j.1600-0854.2000.010109.x. [DOI] [PubMed] [Google Scholar]
- 32.Brigance WT, Barlowe C, Graham TR. Organization of the yeast Golgi complex into at least four functionally distinct compartments. Mol Biol Cell. 2000;11:171–182. doi: 10.1091/mbc.11.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevis BJ, Hammond AT, Reinke CA, Glick BS. De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat Cell Biol. 2002;4:750–756. doi: 10.1038/ncb852. [DOI] [PubMed] [Google Scholar]
- 34.Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rambourg A, Clermont Y. Three-dimensional structure of the Golgi apparatus in mammalian cells. In: Berger EG, Roth J, editors. The Golgi Apparatus. Birkhäuser Verlag; Basel: 1997. pp. 37–61. [Google Scholar]
- 36.Gillingham AK, Munro S. Long coiled-coil proteins and membrane traffic. Biochim Biophys Acta. 2003;1641:71–85. doi: 10.1016/s0167-4889(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 37.Segev N. Ypt/Rab GTPases: regulators of protein trafficking. Sci STKE 2001. 2001:re11. doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- 38.Hedges SB. The origin and evolution of model organisms. Nat Rev Genet. 2002;3:838–849. doi: 10.1038/nrg929. [DOI] [PubMed] [Google Scholar]
- 39.Ayscough K, Hajibagheri NMA, Watson R, Warren G. Stacking of Golgi cisternae in Schizosaccharomyces pombe requires intact microtubules. J Cell Sci. 1993;106:1227–1237. doi: 10.1242/jcs.106.4.1227. [DOI] [PubMed] [Google Scholar]
- 40.Glick BS. To stack or not to stack: the yeast Golgi apparatus. In: Robinson DG, editor. The Golgi Apparatus and the Plant Secretory Pathway. Vol. 9. Blackwell/CRC Press; 2003. pp. 1–15. Annual Plant Reviews. [Google Scholar]
- 41.Vjestica A, Tang XZ, Oliferenko S. The actomyosin ring recruits early secretory compartments to the division site in fission yeast. Mol Biol Cell. 2008;19:1125–1138. doi: 10.1091/mbc.E07-07-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang BL, Peter F, Krijnse-Locker J, Low S, Griffiths G, Hong W. The mammalian homolog of yeast Sec13p is enriched in the intermediate compartment and is essential for protein transport from the endoplasmic reticulum to the Golgi apparatus. Mol Cell Biol. 1997;17:256–266. doi: 10.1128/mcb.17.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammond AT, Glick BS. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol Biol Cell. 2000;11:3013–3030. doi: 10.1091/mbc.11.9.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephens DJ. De novo formation, fusion and fission of mammalian COPII-coated endoplasmic reticulum exit sites. EMBO Rep. 2003;4:210–217. doi: 10.1038/sj.embor.embor736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS. Sec16 is a determinant of transitional ER organization. Curr Biol. 2005;15:1439–1447. doi: 10.1016/j.cub.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 46.Gimeno RE, Espenshade P, Kaiser CA. COPII coat subunit interactions: Sec24p and Sec23p bind to adjacent regions of Sec16p. Mol Biol Cell. 1996;7:1815–1823. doi: 10.1091/mbc.7.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharyya D, Glick BS. Two mammalian Sec16 homologs have nonredundant functions in ER export and transitional ER organization. Mol Biol Cell. 2007;18:839–849. doi: 10.1091/mbc.E06-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes H, et al. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci. 2009;122:2924–2934. doi: 10.1242/jcs.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivan V, de Voer G, Xanthakis D, Spoorendonk KM, Kondylis V, Rabouille C. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol Biol Cell. 2008;19:4352–4365. doi: 10.1091/mbc.E08-03-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai H, et al. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- 51.Wooding S, Pelham HRB. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol Biol Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;22:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- 53.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;22:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- 54.Patterson GH, Hirschberg K, Polishchuk RS, Gerlich D, Phair RD, Lippincott-Schwartz J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barlowe C. COPII and selective export from the endoplasmic reticulum. Biochim Biophys Acta. 1998;1404:67–76. doi: 10.1016/s0167-4889(98)00047-0. [DOI] [PubMed] [Google Scholar]
- 56.Roberg KJ, Crotwell M, Espenshade P, Gimeno R, Kaiser CA. LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. J Cell Biol. 1999;145:659–672. doi: 10.1083/jcb.145.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 2008;27:2918–2928. doi: 10.1038/emboj.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 59.Gaynor EC, Graham TR, Emr SD. COPI in ER/Golgi and intra-Golgi transport: do yeast COPI mutants point the way? Biochim Biophys Acta. 1998;1404:33–51. doi: 10.1016/s0167-4889(98)00045-7. [DOI] [PubMed] [Google Scholar]
- 60.Letourneur F, Gaynor EC, Hennecke S, Démolière C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 61.Haruki H, Nishikawa J, Laemmli UK. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 2008;31:925–932. doi: 10.1016/j.molcel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 62.Kweon HS, et al. Golgi enzymes are enriched in perforated zones of Golgi cisternae but are depleted in COPI vesicles. Mol Biol Cell. 2004;15:4710–4724. doi: 10.1091/mbc.E03-12-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaynor EC, Chen CY, Emr SD, Graham TR. ARF is required for maintenance of yeast Golgi and endosome structure and function. Mol Biol Cell. 1998;9:653–670. doi: 10.1091/mbc.9.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peyroche A, Courbeyrette R, Rambourg A, Jackson CL. The ARF exchange factors Gea1p and Gea2p regulate Golgi structure and function in yeast. J Cell Sci. 2001;114:2241–2253. doi: 10.1242/jcs.114.12.2241. [DOI] [PubMed] [Google Scholar]
- 65.Rossanese OW, Reinke CA, Bevis BJ, Hammond AT, Sears IB, O’Connor J, Glick BS. A role for actin, Cdc1p and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J Cell Biol. 2001;153:47–61. doi: 10.1083/jcb.153.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Preuss D, Mulholland J, Kaiser CA, Orlean P, Albright C, Rose MD, Robbins PW, Botstein D. Structure of the yeast endoplasmic reticulum: localization of ER proteins using immunofluorescence and immunoelectron microscopy. Yeast. 1991;7:891–911. doi: 10.1002/yea.320070902. [DOI] [PubMed] [Google Scholar]
- 67.Reinke CA, Kozik P, Glick BS. Golgi inheritance in Saccharomyces cerevisiae depends on ER inheritance. Proc Natl Acad Sci USA. 2004;101:18018–18023. doi: 10.1073/pnas.0408256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Losev E, Papanikou E, Rossanese OW, Glick BS. Cdc1p is an endoplasmic reticulum-localized putative lipid phosphatase that affects Golgi inheritance and actin polarization by activating Ca2+ signaling. Mol Cell Biol. 2008;28:3336–3343. doi: 10.1128/MCB.00567-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arai S, Noda Y, Kainuma S, Wada I, Yoda K. Ypt11 functions in bud-directed transport of the Golgi by linking Myo2 to the coatomer subunit Ret2. Curr Biol. 2008;18:987–991. doi: 10.1016/j.cub.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 70.Pon LA. Golgi inheritance: rab rides the coat-tails. Curr Biol. 2008;18:R743–R745. doi: 10.1016/j.cub.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Pelham HR, Rothman JE. The debate about transport in the Golgi--two sides of the same coin? Cell. 2000;102:713–719. doi: 10.1016/s0092-8674(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 72.Dacks JB, Davis LA, Sjögren AM, Andersson JO, Roger AJ, Doolittle WF. Evidence for Golgi bodies in proposed ‘Golgi-lacking’ lineages. Proc Biol Sci. 2003;270(Suppl 2):S168–S171. doi: 10.1098/rsbl.2003.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]