Abstract
Melanosomes are tissue-specific “lysosome-related” organelles of pigment cells in which melanins are synthesized and stored. Analyses of the trafficking and fate of melanosomal components are beginning to reveal how melanosomes are formed through novel pathways from early endosomal intermediates. These studies unveil generalized structural and functional modifications of the endosomal system in specialized cells, and provide unexpected insights into the biogenesis of multivesicular bodies and how compartmentalization regulates protein refolding. Moreover, genetic disorders that affect the biogenesis of melanosomes and other lysosome-related organelles have shed light into the molecular machinery that controls specialized endosomal sorting events.
Introduction
The endosomal system comprises a complex network of organelles and membrane sub-domains with important functions in signal transduction, nutrient uptake, pathogen destruction and/or propagation, and other essential processes1,2. Although great advances have been made in defining basic mechanisms that underlie membrane dynamics in the secretory pathway, the endosomal system has been more difficult to functionally dissect due to redundancy and compensation by alternative routes. Moreover, the mammalian endocytic network is more complex relative to that of simple eukaryotes such as the yeast Saccharomyces cerevisiae; thus, it is often difficult to infer specific molecular functions in mammals based on the phenotype of mutant yeast strains.
Recent work on the melanosome has provided insights into mammalian endosomal membrane dynamics by revealing how specific trafficking events are exploited to generate tissue-specific organelles. Melanosomes are intracellular organelles that are uniquely generated by pigment cells in the skin and eye, where they function to synthesize and store melanin pigments. Although melanosomes are considered to be lysosome-related organelles (LRO), recent data show that their contents derive from early endosomal membranes. Many new insights have emerged from hereditary diseases in humans and model organisms characterized by reduced or absent pigmentation, often combined with additional seemingly unrelated disorders such as bleeding diathesis, lung fibrosis, and immunodeficiency, due to generalized LRO defects3. Identification of the genes that are mutated in these diseases and functional characterization of their products has dramatically advanced our understanding of pigment cell biology and of the molecular features that underlie melanosome biogenesis. Many of these features have parallels in LROs in vertebrates and primitive eukaryotes, such as the eye pigment granules of Drosophila melanogaster and the gut granules of Caenorhabditis elegans.
This review highlights recent insights into melanosome biogenesis that have advanced our general understanding of organelle formation and endosomal protein sorting. We also discuss important connections between melanosome morphogenesis, neurodegenerative disorders and general mechanisms of endosomal partitioning. Last, we propose models to explain endosomal membrane dynamics required for the formation of conventional and specialized organelles.
Melanosome composition and function
Melanins are complex pigments that provide the skin, hair and eyes of mammals with colour and photoprotection against ionizing radiation. They also function in the development of the optic nervous system and regulate retinal function4. Melanins typically consist of copolymers of black and brown eumelanins and red and yellow pheomelanins5. Melanin synthesis in epidermal and ocular melanocytes and in pigment epithelia of the retina, iris and ciliary body of the eye occurs within melanosomes6,7. Sequestration within melanosomes protects components of the cytosol and other membranous organelles from oxidative attack during melanin synthesis, and concentrates melanins for storage (in eye pigment cells) or cell transfer (from epidermal melanocytes to keratinocytes in the skin and hair). Melanosomes that harbour primarily pheomelanins differ in structure and composition from melanosomes with primarily eumelanins8, are much less well characterized, and will not be discussed further.
Melanosome formation and maturation
Melanosomes mature within the melanocyte or the developing retinal pigment epithelial cell through four morphologically distinct stages6 (FIG. 1). The first two stages lack pigment, but are characterized by intralumenal proteinaceous fibrils that begin to form in stage I and are completed by stage II. Once the fibrous striations are fully formed in ellipsoidal stage II melanosomes, melanin synthesis begins. Melanins deposit on the fibrils, resulting in their thickening and blackening with maturation to stage III until all internal structure is masked in stage IV. In eye pigment cells, stage IV melanosomes are retained and absorb stray light and likely trap free radicals9. In epidermal melanocytes, stage IV melanosomes are translocated along microtubules from the cell center to actin-rich dendritic tips, and then transferred in a still unresolved manner to neighbouring keratinocytes10. Melanosome translocation in these cells involves bidirectional transport along microtubules, facilitated by both dynein and kinesin motors, and peripheral capture on actin filaments11. Capture on actin filaments is mediated at least in part by a complex that consists of myosin Va, the small GTPase RAB27a and the linker protein melanophilin (also known as SLAC2a); this complex is dysregulated in the heritable disease Griscelli syndrome and related animal models11.
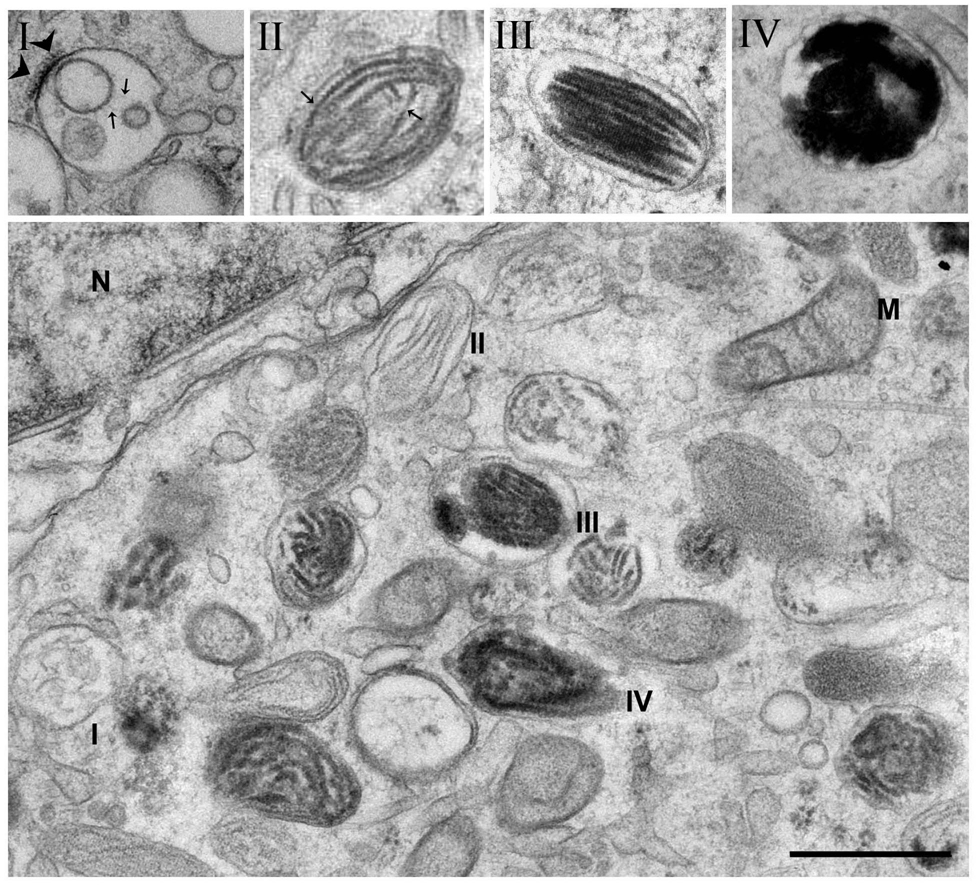
Figure 1. Ultrastructural characterization of melanosomes.
Electron microscopy analyses of MNT-1 human melanoma cells fixed by high pressure freezing before cryosubstitution and epon embedding. a. The four stages of melanosome development. Note the dense bilayered coat (arrowhead) and intralumenal vesicles (arrows) of stage I melanosomes, the proteinaceous fibrils (arrows) of stage II, and the melanin deposition in stages III and IV. b. Typical field of MNT-1 cytoplasm near the nucleus, which contains all four stages of melanosomes. M, mitochondria; N, nucleus. Bar, 0,5 µm.
The enzymatic machinery and structural components of melanosomes (BOX 1; see also ref. 6) are synthesized by pigment cells and must be appropriately targeted to newly forming melanosomes. Most known components are integral membrane proteins that are uniquely expressed in pigment cells, and mutations in the genes that encode them often cause albinism in humans or coat colour dilution in animals12. For example, PMEL17 (also known as gp100 or SILV; referred to here as PMEL) serves as the structural foundation of the fibrils in stage I and II melanosomes, whereas the melanin biosynthetic enzymes tyrosinase, tyrosinase-related protein-1 (TYRP1) and DOPAchrome tautomerase (DCT), are enriched in stage III and IV melanosomes13–15. Melanosomes also harbour ubiquitous components of lysosomes and late endosomes16,17.
BOX 1. Lumenal and membrane components specific to melanosomes
Consistent with their tissue-specific function, melanosomes harbour numerous tissue-specific proteins that function as enzymes, structural scaffolds, and/or ion channels to optimise the synthesis, polymerization and detoxification of melanin intermediates. The identity and function of many of these components has been reviewed previously6,7, and their deficiency results in pigmentation disorders in humans (ocular or oculocutaneous albinism) and/or model organisms. Some of the main components include:
Tyrosinase – the enzyme that initiates melanin synthesis by catalysing the oxidation of tyrosine to DOPA and subsequent oxidation of DOPA to DOPAquinone; at steady state predominantly in stage III and IV melanosomes.
TYRP1 (also known as TRP1 or gp75) – a tyrosinase-related protein (over 50% sequence similarity to tyrosinase) that may have catalytic activity or modulate tyrosinase activity; at steady state predominantly in stage III and IV melanosomes.
DOPAchrome tautomerase (DCT; also known as TRP2) – a tyrosinase-related protein (over 50% sequence similarity to tyrosinase) that catalyses a late step in eumelanin formation; at steady state predominantly in stage III and IV melanosomes
PMEL17 (also known as gp100, ME20 or silver) – structural component of the melanosome fibrillar “matrix” upon which melanins are deposited; it accumulates in stage I and II melanosomes and is masked by melanin in later stages.
MART1 – small integral membrane protein. It regulates fibril formation by PMEL17 and it is present in melanosomes and late endosomes.
OA1 – putative G-protein-coupled receptor on melanosomes and lysosomes.
P (OCA2, pink-eyed dilute) – putative anion transporter, likely present in melanosomes.
SLC45A2 (also known as MATP, OCA4, underwhite) – putative membrane transporter, likely present in melanosomes.
SLC24A5 – putative cation exchanger, likely present in melanosomes.
Although initially considered to be modified lysosomes16 like the cytolytic granules of T cells18, eumelanosomes within skin melanocytes coexist with separate bona fide lysosomes13, a feature they share with a class of LRO-comprising platelet dense granules, lung type II epithelial-cell lamellar bodies, and others19. To generate these LRO, host cells must correctly sort macromolecules that are destined for conventional lysosomes from those destined for the LRO. This sorting is unique to specialized cell types, as most melanosomal proteins localize to late endosomes and lysosomes when expressed ectopically in non-pigment cells20–22, and is uniquely defective in the genetic disease, Hermansky-Pudlak syndrome (HPS)23.
How is such sorting achieved? Recent insights from analyses of “wild-type” and HPS model melanocytes indicate that melanosomes develop by successive protein sorting events from multiple distinct domains of early endosomes. Below, we discuss evidence that supports a model in which early stage melanosomes derive from vacuolar domains of early endosomes, and then mature in a process that requires cargo delivery from tubular endosomal domains.
The formation of premelanosome fibrils
PMEL is the main constituent of the intralumenal proteinaceous fibrils of stage I and II premelanosomes (FIG. 2). The fibrils are immunoreactive for PMEL by microscopy and biochemical analyses24, and expression of PMEL alone in non-melanocytic cells22 or even in vitro25 results in the formation of morphologically similar fibrils (although PMEL fibrillogenesis may be modified by other melanocyte-specific proteins such as MART126 through as yet unknown mechanisms). By contrast, melanosomes from silver mice — in which PMEL is depleted due to a truncation of the cytoplasmic domain (see below) — lack fibrillar structure and are consequently enlarged and rounded27. Thus, PMEL is both necessary and sufficient for premelanosome fibril formation and melanosome shape. The importance of the fibrils in pigment-cell function is supported by pigmentation defects — most likely due to loss of melanocyte viability28,29 — in Pmel-mutant zebrafish30, dogs31, horses32, chickens and mice24.
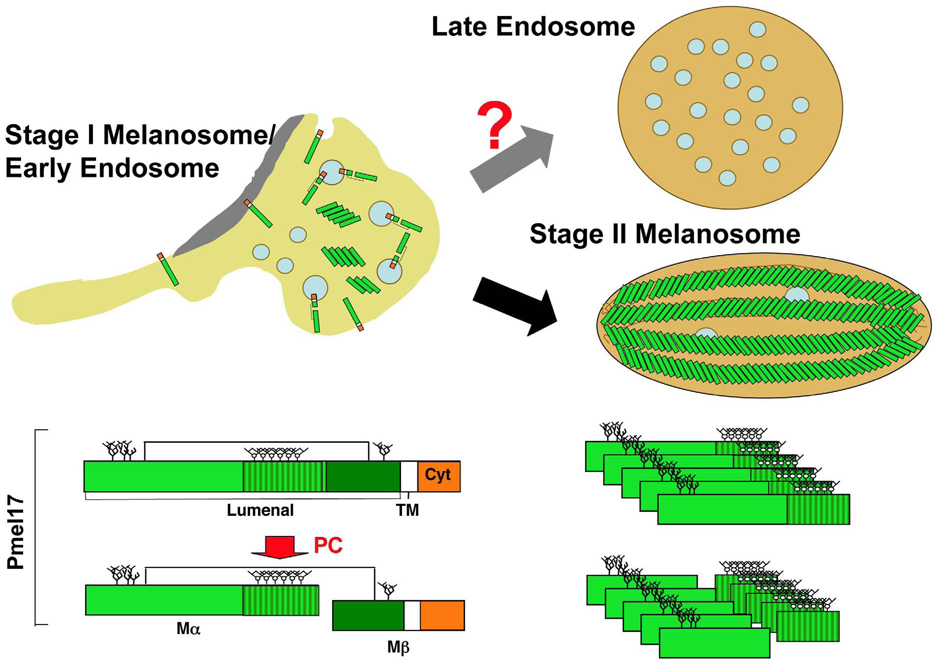
Figure 2. Model for formation of premelanosome fibrils.
The melanocyte-specific protein, Pmel (the primary structure of which is shown at the bottom) is present on the limiting membrane and the internal vesicles of stage I melanosomes/vacuolar early endosomes. Within these structures, Pmel is cleaved to Mα and Mβ fragments by a proprotein convertase (PC; bottom left). Mα fragments that dissociate from membranes begin to form small, irregular fibrils that become fully organized within stage II melanosomes. Only Mα and derived fragments (bottom right) are reproducibly detected in stage II melanosomes, whereas late endosomes are essentially devoid of detectable Pmel fragments. The figure depicts one of several hypothetical models to explain how cargo segregates from stage I melanosomes to both stage II melanosomes and late endosomes, in which each “daughter” organelle bears distinct cohorts of internal membranes. Pmel lumenal, transmembrane (TM) and cytoplasmic (cyt) domains are indicated at bottom. The RPT domain, consisting of partial direct repeats required for fibril formation, is highlighted. Squares and circles represent N- and O-linked glycosylation, respectively.
Mature fibrils copurify with fragments of the PMEL lumenal domain but not with full-length PMEL14,33. The largest of these fragments, known as Mα, encompasses the N-terminal 443 lumenal residues of the largest human splice isoform and rapidly forms fibrils in vitro25. Mα is proteolytically cleaved from the remaining PMEL fragment (Mβ) in a post-Golgi compartment, likely endosomes34, by a proprotein convertase33 (FIG. 2). Inhibition of proprotein convertase activity in melanocytes or elimination of the PMEL cleavage site in transfected HeLa cells blocks the formation of fibrils and of morphologically intact melanosomes33, which indicates that cleavage is required for fibrillogenesis. Similarly, proprotein convertase cleavage of von Willebrand factor — a coagulation regulatory protein— underlies the formation of a distinct type of tubular fibril in Weibel-Palade bodies35.
Similarities with pathological fibrils
The premelanosome fibrils are unique among organelles in forming a polar, intersecting network. However, they resemble the pathological amyloid fibrils that form in neurofibrillary diseases, such as Alzheimer’s and Parkinson’s disease and the spongiform encaphalopathies36. Indeed, purified melanosomes and fibrils that are formed in vitro by purified recombinant Mα fragments can be labelled with amyloid-specific dyes such as Congo Red and thioflavins; these recombinant fibrils have amyloid-like spectroscopic and X-ray diffraction properties25. Like for PMEL, proteolytic cleavage initiates amyloid formation by the Aβ peptide in Alzheimer’s disease and gelsolin in familial amyloidosis of Finnish type36. Moreover, like α-synuclein (the aetiologic agent of Parkinson’s disease37) and sup35 (a yeast prion38), PMEL fibrillogenesis requires a domain within Mα that consists of tandem imperfect repeats34,39. Thus, PMEL appears to be a physiological amyloid, and a model for understanding the mechanisms by which formation of some types of pathological amyloid are regulated in vivo.
PMEL sorting within MVBs en route to premelanosomes
PMEL is synthesized by melanocytes, and thus must navigate the secretory pathway to accumulate in premelanosomes. Nevertheless, unlike the tubular von Willebrand factor-containing fibrils of Weibel-Palade bodies that begin to form in the trans-Golgi network (TGN) of endothelial cells40, PMEL-containing fibrils are not observed in early secretory compartments of melanocytes. How does PMEL access premelanosomes in a pre-fibrillar form? Although a contrasting view of PMEL trafficking and premelanosome formation has been invoked based on immunoreactivity in isolated subcellular fractions14,41, a wealth of evidence supports the notion that endosomes are an obligate intermediate in PMEL processing24.
PMEL sorting to endosomes
The biosynthetic routes by which PMEL accesses premelanosomes are emerging but not yet fully unravelled (FIG. 2). At least a fraction of newly synthesized PMEL traffics through the plasma membrane27. A cytoplasmic di-leucine-based internalization signal facilitates — but is not absolutely required for — PMEL accumulation in endosomes, and thus facilitates efficient downstream sorting and fibril formation. A cytoplasmic domain truncation in the silver mouse eliminates this motif and results in PMEL accumulation on the cell surface and consequent depletion from melanosomes27. The rapid internalization and accumulation of PMEL on endosomal membranes, observed by microscopy13,15, likely explains the copurification of PMEL with the clathrin adaptors AP-1 and AP-2 by subcellular fractionation42. Although di-leucine-dependent endocytosis is likely mediated by AP-243, as yet there is no direct evidence to support a role for AP-1 or AP-2 in physiological sorting of PMEL in melanocytes.
Several reports have suggested that PMEL bypasses the Golgi en route to premelanosomes based on abundant steady state levels of immature forms of PMEL, some of which copurify with stage II melanosomes by subcellular fractionation14,42. However, this is not supported by the requirements for post-Golgi processing in fibril formation22,33 and in generating antibody epitopes detected on fibrils13,22,44 (for a full discussion, see ref. 24).
PMEL sorting within multivesicular endosomes
PMEL fibril formation is initiated in stage I melanosomes. These organelles correspond to vacuolar domains of early endosomes13,34, which are morphologically similar to vacuolar early endosomes that are found in all cells and in which intralumenal vesicles (ILVs) form by invagination of the limiting membrane45 (BOX 2). In melanocytes, PMEL is enriched in the ILVs of stage I melanosomes13 (see also FIG. 2). PMEL also becomes enriched in ILVs of multivesicular bodies (MVBs) when expressed in non-melanocytic cells22. Thus, the PMEL sorting mechanism to ILVs is not limited to specialized cell types.
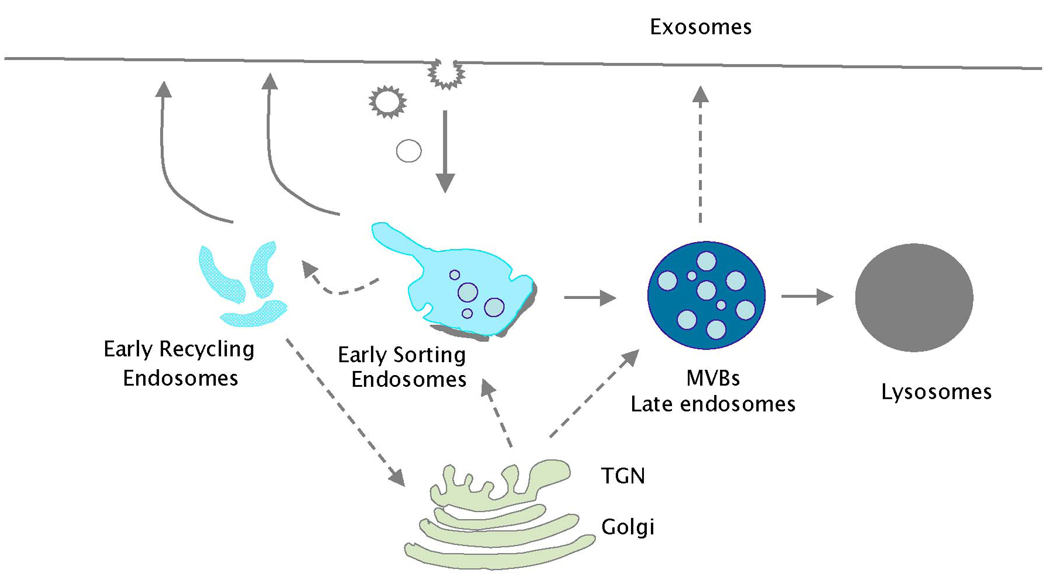
BOX 2. The endosomal system
The endosomal system consists of morphologically, compositionally and functionally distinct domains classified as early and late as they are sequentially accessed by tracers internalized by receptor-mediated or bulk flow endocytosis (see figure)1,2.
Early endosomes are subclassified into sorting and recycling endosomes based on differential kinetic access to recycling proteins, such as transferrin receptors, following internalization. Sorting endosomes consist of tubules continuous with closely apposed electron lucent vacuoles with few internal membranes and are accessed by endocytic cargo within 5–15 min119,120. The tubules transport cargo into and out of the vacuoles, including receptors that rapidly recycle to the plasma membrane. A proportion of recycling receptors accumulate in and recycle from perinuclear, tubulovesicular elements, apposed to the Golgi apparatus, called recycling endosomes2. The vacuolar domains of early endosomes are partially coated on the cytosolic face with an electron dense bilayered coat. This coat contains clathrin, but is distinct from clathrin coats on buds at the plasma membrane, TGN and tubular endosomes, and functions in sorting cargo between the limiting membrane and invaginating membrane destined to form intralumenal vesicles121,122.
As vacuolar endosomes mature, they accumulate within them internal vesicles enriched in integral membrane proteins destined for lysosomal degradation123,124. Such maturing endosomes are called multivesicular bodies (MVBs)45. MVBs that are largely filled with vesicles include mature late endosomes, defined by content of newly synthesized lysosomal enzymes and access to endocytic cargo by 30 min124. Most mature MVBs fuse with lysosomes to degrade their contents45. In some cell types, MVBs can also fuse with the plasma membrane and release their intralumenal vesicles, which are then called exosomes125.
This model is an overly simplified view of the endosomal system. Endosomal membranes are highly dynamic, and different endosomal domains vary in prevalence or content based on cell type, differentiation state, or response to extracellular signals. Specialized cell types like melanocytes subcompartmentalise the ubiquitous endosomal system to facilitate regulated cargo sorting and generate cell type-specific organelles.
MVB sorting of most well-studied cargo proteins, including MART1 in melanocytes34,46, requires ubiquitylation and interaction with an evolutionarily conserved machinery45. Indeed, endosomal sorting within melanocytes is regulated by the ubiquitin ligases NEDD4 and ITCH (which modify MART146), and possibly Mahogunin (MGRN1, which modifies the MVB sorting component TSG10147). By contrast, PMEL sorting to MVBs does not require ubiquitylation or components of the known MVB machinery34, but instead requires a lumenal sub-domain with homology to a polycystic kidney disease (PKD) repeat34,39. Because PMEL sorting to MVBs appears to be non-saturable upon ectopic expression in non-melanocytic cells34, the PKD domain likely facilitates interactions with a non-limiting component of the lumenal face of the invaginating membrane, such as a lipid head group.
Sorting to ILVs appears to be required for PMEL cleavage to Mα and Mβ34. This requirement might explain a delay in cleavage observed upon altering early endosomal membrane dynamics by overexpressing myosin 1b48, and might reflect either a conformational change that makes the PMEL cleavage site more available or induced proximity to a proprotein convertase on the ILVs. Whether the environment of the internal membranes provides additional support for fibril formation or for the segregation of fibrils from endocytic cargo remains to be tested.
HPS and sorting of melanosomal enzymes
Mature (stage III and IV) melanosomes are characterized by pigment deposits over the fibrils, and unlike stage II melanosomes, harbour melanogenic enzymes like tyrosinase and TYRP1. These proteins must be delivered to preformed premelanosomes such that melanin synthesis begins only after the complete formation of the fibrils on which melanin subsequently accumulates. This implies that melanogenic enzymes are delivered by pathways distinct from those used by premelanosome components such as PMEL. Several observations initially suggested that melanogenic enzymes could be transferred directly from the TGN to melanosomes by clathrin-coated vesicles13,49–51, a model supported by the finding that tyrosinase and TYRP1 accumulate in the pericentriolar "TGN" area in hypopigmented melanocytes that fail to synthesize glycosphingolipids52. However, the existence of a pericentriolar pool of early endosomes2, often bearing clathrin-coated buds, challenges some assumptions of the TGN-to-melanosome model. Recent studies, exploiting genetic deficiencies in humans and mouse models that affect pigmentation at late stages of melanogenesis, now support the notion that tyrosinase and TYRP1 are preferentially sorted to melanosomes from early endosomes.
Hermansky-Pudlak Syndrome (HPS) is a multisystem disorder characterized by partial albinism, excessive bleeding, and often lung fibrosis, sometimes accompanied by immunodeficiency and/or granulomatous colitis23. These systemic symptoms result from the malformation or malfunction of LRO, including melanosomes, platelet dense granules, and in some cases lung lamellar bodies and/or cytotoxic T-cell granules. HPS results from mutations in any of at least 8 genes; a similar disorder in mice, first identified based on coat colour dilution, results from mutations in at least 15 different genes, including the orthologues of the genes mutated in human HPS23,53. Although the HPS-associated genes are expressed in many cell types, they appear to be functionally essential for the generation of a restricted number of LROs within affected cell types. Most of the genes encode subunits of multi-subunit protein complexes (BOX 3). Some of the complexes, such as AP-3, are known to be involved in vesicular transport, whereas others, such as the biogenesis of lysosome-related organelle complex-1 (BLOC-1), BLOC-2 and BLOC-3, consist of subunits that lack common structural motifs or homology to proteins of known function (BOX 3). Biochemical analyses of these proteins and functional analyses of melanocytes from HPS models are shedding light on melanosomal enzyme trafficking and on the function of AP-3 and BLOCs in regulating early endosomal dynamics.
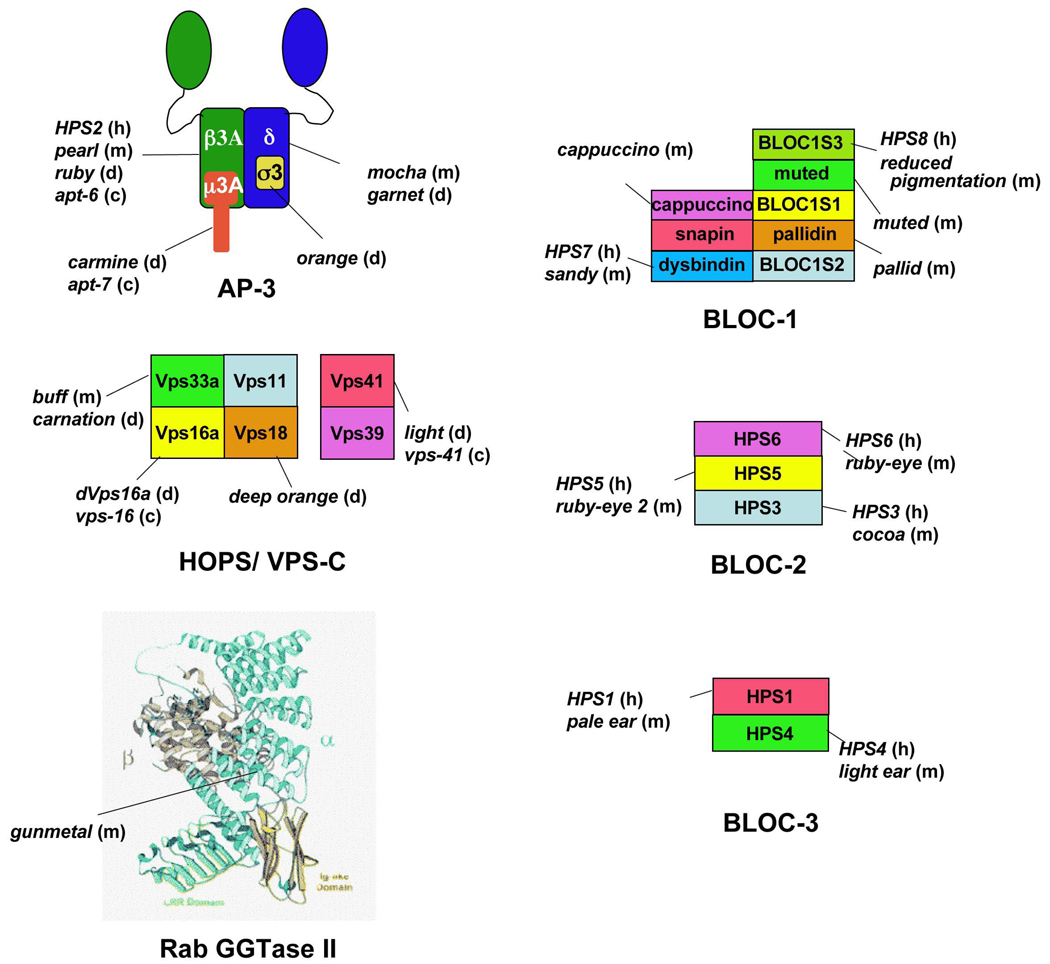
BOX 3. Gene products defective in Hermansky-Pudlak Syndrome
Hermansky-Pudlak Syndrome (HPS) results from mutations in any of at least eight genes in humans. Mutations in orthologues of the same eight genes or seven additional genes cause a similar disorder in mice23,53. Mutations in related genes in Drosophila melanogaster and Caenorhabditis elegans result in defective formation of eye pigment granules or gut granules, respectively53,55,73. Nearly all of these genes encode subunits of ubiquitously expressed protein complexes that are implicated in LRO biogenesis. Some of these complexes function in known protein sorting events. For example, Rab geranylgeranyl transferase (RGGTase II) catalyses the prenylation of Rab GTPases126, a modification required for Rab association with membranes. Its reduced activity in gunmetal mice titrates a subset of Rabs127,128 and blocks vesicle trafficking steps that require them. Similarly, AP-3 functions in lysosomal sorting56, and the homotypic protein sorting (HOPS) complex regulates SNARE interactions required for homotypic vacuole fusion in yeast129 and endosome dynamics in mammals87. By contrast, the subunits of the biogenesis of lysosome-related organelle complex-1 (BLOC-1), BLOC-2 and BLOC-3 lack common structural motifs or significant homology to proteins of known function. The figure illustrates the known subunits of the complexes, and human (h) HPS isoforms and/or mouse (m), D. melanogaster (d) and/or C. elegans (c) strains in which the subunits are defective or absent due to mutation. HOPS complex composition is based on the orthologous yeast complex, although mammalian HOPS likely possesses similar subunits; the composition of other complexes has been defined in mammals. The Rab GGTase II structure is reproduced with permission from ref. 130. Mutations in two other genes (the products of which are not depicted in the figure) cause HPS-like phenotypes in model organisms. The Slc7a11 gene, defective in the subtle gray (sut) mouse, encodes a cysteine/ glutamate exchanger required for pheomelanin formation in melanocytes. Genetic deficiency of RAB38, a tissue-specific member of the Rab superfamily, has been implicated in an HPS-like disorder in rats.
AP complexes in melanosome protein sorting
Mutations in subunits of the AP-3 complex have been identified in HPS type 2 patients, pearl and mocha mice, and D. melanogaster and C. elegans mutants with eye pigment and gut granule defects, respectively54,55. AP-3 is one of four heterotetrameric adaptor complexes that function in linking transmembrane cargo, through cytoplasmic sorting signals, to coated bud formation on post-Golgi membranes56. In melanocytes, AP-3 function has best been described with respect to its role in tyrosinase trafficking. The cytoplasmic domain of tyrosinase harbours a di-leucine-based sorting motif15,57 that binds to AP-3 in vitro and is required for sorting to lysosomes or neurosecretory granules in other cell types58–60. Importantly, a cohort of tyrosinase is mislocalized to endosomes in melanocytes from HPS type 2 patients and pearl mice15,61. As AP-3 coats are found primarily on buds on early endosomal tubules15,62 (FIG. 3A) that also contain tyrosinase in normal melanocytes15, AP-3 likely functions in tyrosinase sorting from endosomes. The mislocalized tyrosinase in AP-3-deficient cells accumulates primarily in early endosomes and in intralumenal vesicles of MVBs15, which suggests that AP-3 may have a function in vertebrate cells that is similar to its function in yeast63, to divert cargo from the MVB pathway.
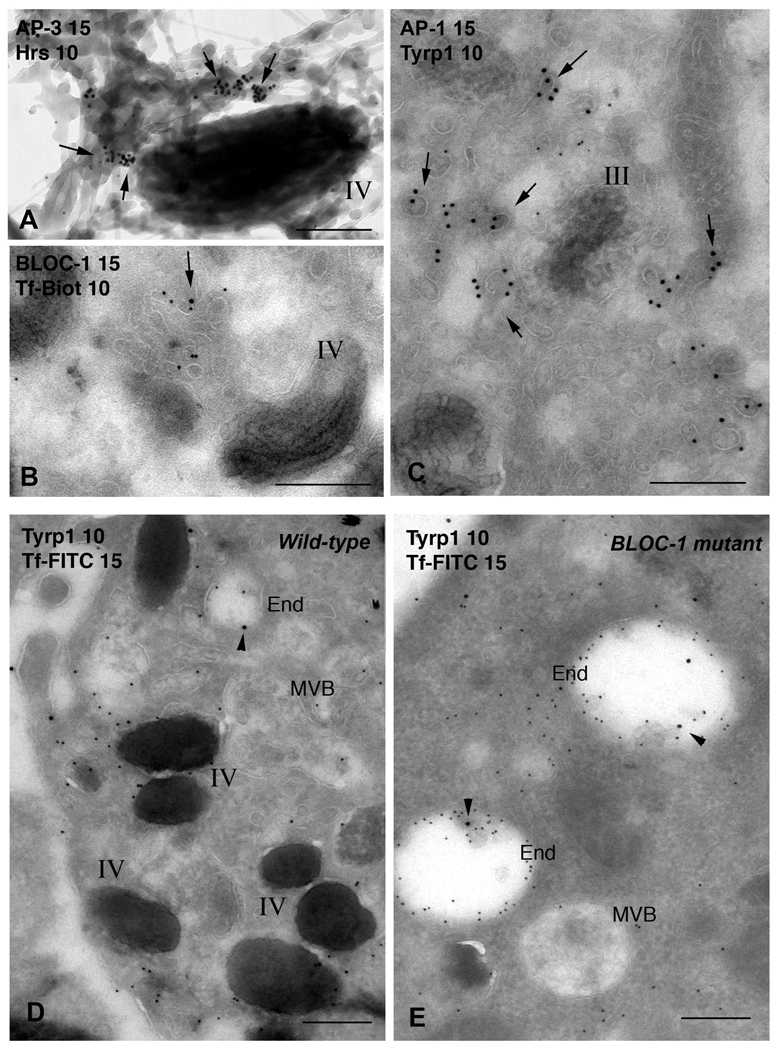
Figure 3. Endosomal protein complexes that regulate cargo sorting to melanosomes.
a–c. Adaptor proteins and BLOCs on endosomes. Whole mount preparations (a), and ultrathin cryosections (b and c) of MNT-1 human melanoma cells were double immunogold labeled using protein A conjugated to 10 or 15 nm gold particles as indicated. Prior to fixation, cells internalized transferrin conjugated either to horseradish peroxidase and treated with substrate to highlight the early endosomal network (a), or to biotin (Tf-biot) for immunogold labelling of early endosomes (b). Arrows, labelling for AP-3, AP-1 and BLOC-1. Note the predominant labelling for AP-3 and AP-1 near melanosomes and for BLOC-1 in Tf-biot filled tubules. D–E, TYRP1 is trapped in endosomes in BLOC-1 mutants. Early endosomes of immortalized melanocytes from BLOC-1-deficient muted mice that were untransfected (BLOC-1 mutant, E) or “rescued” with the missing Muted subunit (Wild-type, D) were loaded with Transferrin-FITC (Tf-FITC). Ultrathin cryosections of fixed cells were immunogold labeled with anti-TYRP1 and anti-FITC with 10 or 15 nm gold particles. In mutant cells, both TYRP1 and Tf-FITC accumulate in expanded vacuolar endosomes (End.). Rescued cells are normally pigmented and bear numerous mature melanosomes, many close to Tf/Tryp1-containing endosomes. Bars: 200 nm. Panels A, B, D and E are reproduced and modified with permission from refs. 15 (A), 69 (B) and 68 (D, E).
Although a cohort of tyrosinase is mislocalized to endosomes and pigmentation is attenuated in pearl and HPS type 2 melanocytes, these cells harbour pigmented mature melanosomes that are almost indistinguishable from the melanosomes in control melanocytes and retain a cohort of active tyrosinase15,64,65. This finding indicates that an additional pathway that is independent of AP-3 must deliver tyrosinase to melanosomes. Indeed, the tyrosinase cytoplasmic di-leucine-based sorting signal also binds in vitro to a second heterotetrameric adaptor complex, AP-115. Moreover, tyrosinase is found at steady state in distinct endosomal buds that contain either AP-3 or AP-1 in wild-type melanocytes, and the cohort within AP-1-containing buds increases in AP-3-deficient cells15. The presence of distinct AP-1- and AP-3-coated endosomal buds (FIG. 3A, C) is not unique to melanocytes, but is also found in other cell types62, in which AP-1 likely functions in recycling mannose 6-phosphate receptors to the TGN66 and other receptors to the cell surface67.
Whether AP-3 and AP-1 mediate redundant tyrosinase sorting pathways from endosomes to melanosomes is not clear, but a distinct role for AP-1 in melanosomal sorting is suggested by analysis of another mature melanosome cargo protein, TYRP1. TYRP1 localization to melanosomes is not grossly affected by AP-3 deficiency61,68. Moreover, TYRP1 harbours a di-leucine-based sorting signal21 that interacts with AP-1 but not AP-315, and colocalizes extensively with AP-1 (but not AP-3) in melanocytic cells13 (FIG. 3C). AP-1 is therefore likely to function in TYRP1 transport to melanosomes, and may “rescue” a cohort of tyrosinase that fails to be sorted by AP-3. How these two adaptors cooperate sequentially or concomitantly remains unknown.
Regulation of sorting by BLOCs
Cargo sorted by AP-3 is destined for late endosomes and lysosomes in several cell types; however, in pigment cells, tyrosinase and TYRP1 are destined for melanosomes, which indicates that additional effectors must participate in their sorting. Some of these effectors include BLOCs and other complexes that are defective in HPS.
BLOC-1 regulates sorting to melanosomes
BLOC-1 (BOX 3), a ~250 kDa protein complex, consists of eight-subunits; two subunits are encoded by genes that are mutated in human HPS, and these and three others are encoded by genes mutated in mouse HPS models53. BLOC-1 mutants are the most severely hypopigmented of mouse HPS models, correlating with a nearly complete loss of melanosomes from the RPE, choroid and skin23,53. Analyses of BLOC-1 mutant melanocytes relative to wild-type cells show that tyrosinase is only partially mislocalized but TYRP1 is grossly mislocalized68. TYRP1 accumulates in these mutant cells within early endosomal vacuoles (FIG. 3D, E) and at the cell surface, and undergoes dramatically enhanced endocytic flux due to a defect in exit from early endosomes towards melanosomes68. Because TYRP1 trafficking is differentially affected in AP-3 and BLOC-1 mutants, AP-3 and BLOC-1 define two distinct sorting pathways from early endosomes. Consistently, although both complexes localize at steady state to early endosomes, they populate distinct domains, with AP-3 enriched in buds and BLOC-1 components on tubular regions69 (FIG. 3A, B). What molecular function BLOC-1 serves on these domains is not clear, and whether BLOC-1, like AP-1 and AP-3, interacts directly with cargo remains to be established.
Given their distinct effects on intracellular cargo sorting in melanocytes, it is surprising that BLOC-1 and AP-3 in membrane fractions of several cell types can be co-immunoprecipitated69, and that both complexes are co-isolated on a sub-population of synaptic-like microvesicles70. Indeed, BLOC-1 and AP-3 may reciprocally regulate their association with membranes69, and consequently may regulate trafficking steps from the same organelle. However, to explain how tyrosinase and TYRP1 are destined for the same target organelle but differentially affected by deficiency of AP-3- and BLOC-1, these trafficking steps are likely to occur sequentially in space and time from contiguous but distinct early endosomal sub-domains. The accumulation of similar cargoes (e.g. CD63 and TYRP1) at the cell surface in cells that lack either AP-3 or BLOC-169,70, despite distinct intracellular fates68, most likely reflects indirect effects of endosomal perturbation, as documented in BLOC-1-deficient cells68.
BLOC-1 and BLOC-2 function in the same pathway
If BLOC-1 is required for cargo exit from early endosomes, how are cargo-bearing membranes directed toward melanosomes? A likely participant is BLOC-2. BLOC-2 consists of at least three subunits, HPS3, HPS5 and HPS671,72, which have all been found to be mutated in patients with HPS and mouse models23,53. Moreover, a mutation in the D. melanogaster orthologue of HPS5 results in eye pigment defects73. BLOC-2-deficient mice are more mildly hypopigmented than BLOC-1 mutants, and defects in melanosome architecture vary from dramatic in the RPE and choroid to less severe in hair bulb melanocytes65. The subcellular distribution of several melanosomal proteins is altered in melanocytes from patients with mutated HPS374,75. In BLOC-2-deficient mouse melanocytes, TYRP1 is more severely excluded from melanosomes than tyrosinase, and accumulates in a tubulovesicular endosomal compartment distinct from and apparently downstream of the endosomal vacuoles in BLOC-1-deficient cells68. Consistently, TYRP1 accumulates in endosome-like structures and is severely depleted due to lysosomal degradation in melanocytes from patients with mutated HPS576. These data suggest that BLOC-2 functions in the same pathway as BLOC-1, consistent with the physical and genetic interactions that have been observed between these two complexes69,77. However, BLOC-1 and BLOC-2 appear to function at different steps in this pathway, consistent with their localization to partially distinct endosomal sub-domains69. The phenotype of BLOC-1-deficient cells suggests a role for BLOC-1 in sorting and budding from endosomes. By contrast, the accumulation of vesicles75, some of which contain tyrosinase76, and the apparent mistargeting of TYRP1 for degradation68,69,76 in BLOC-2-deficient cells suggest a role for BLOC-2 in regulating directed targeting or fusion of endosome-derived membranes with maturing melanosomes.
HPS-associated proteins in other trafficking functions
HPS proteins as SNARE regulators?
How might BLOC-1 and BLOC-2 regulate these processes? Several endosomal SNAREs are highly upregulated in differentiated melanocytic cells78 and one of them, syntaxin 13, interacts directly in vitro with the BLOC-1 subunit, pallidin79,80. Moreover, syntaxin 13 is mislocalized to mature melanosomes in AP-3-deficient melanocytes68. The distribution of two other endosomal SNAREs, VAMP7-Ti and syntaxin 8, is altered in AP-3- or BLOC-1-deficient fibroblasts70. These data imply a role for BLOC-1 and AP-3 in defining SNARE dynamics.
Another likely SNARE regulator is the homotypic fusion and vacuole protein sorting (HOPS) complex. An amino-acid substitution in the HOPS subunit VPS33a underlies the HPS-like phenotype of buff mice, and mutations in additional subunits or associated proteins in D. melanogaster, zebrafish, or C. elegans cause severe defects in the formation of eye pigment granules81–85 and gut granules55. VPS33a itself is a member of the Sec1/Munc18 family of SNARE regulatory proteins86, and interacts directly with several endosomal SNAREs87. Sec1/Munc18 proteins appear to facilitate appropriate SNARE pairing88, and thus HOPS might regulate critical SNARE interactions required for endosome-to-melanosome trafficking.
A different function for BLOC-3?
Although most HPS-associated protein complexes clearly function in vesicular trafficking of melanogenic enzymes, the function of BLOC-3 is less clear. Mutations in the two known BLOC-3 subunits cause HPS types 1 and 4 — the most prevalent forms of HPS — and corresponding mouse models23,53. Melanocytes from patients with HPS type 1 display increased autophagy and accumulate melanosomes in compartments with features of autophagic vacuoles89. This finding suggests that the morphological disruption of melanosomes may be a consequence of a defect in a process other than trafficking of melanogenic enzymes. Indeed, in non-pigmented cells, BLOC-3 has been reported to regulate late endosome/lysosome motility and distribution90, which may have secondary consequences on endosomal membrane dynamics. HPS1 has been localized to the melanosomal membrane and to tubulovesicular membranes close to the Golgi91, but further studies using BLOC-3-deficient cells are needed to define whether and how BLOC-3 regulates melanosomal cargo.
Rabs in melanosome biogenesis
Rab GTPases are well known regulators of vesicular transport, and several have been implicated directly in membrane targeting to melanosomes. RAB32 and RAB38 are highly homologous Rab proteins that are each expressed in a limited set of tissues; melanocytes are among the few cell types that express both RAB32 and RAB3892. The Rab38 gene is mutated in ruby rats93, which suffer from an HPS-like syndrome, and in hypopigmented chocolate mice94; mutations in the D. melanogaster orthologue, lightoid, and its guanine nucleotide exchange factor, claret, cause eye pigment defects95. Chocolate mice and their derived melanocytes are only mildly hypopigmented. However, RNAi-mediated depletion of RAB32 in chocolate melanocytes severely impairs pigmentation and melanosome morphology, concomitant with missorting of tyrosinase and TYRP192. RAB38 localizes to tubulovesicular structures and to mature melanosomes, which implies a potential role in controlling trafficking or transport of vesicles that contain melanogenic enzymes to melanosomes. RAB32 presumably performs a function that is redundant in melanocytes but likely unique in cells that lack RAB38 expression. RAB7, a ubiquitous late endosomal Rab protein, has been implicated in TYRP1 protein trafficking based on TYRP1 redistribution upon siRNA depletion or dominant negative interference with RAB796. However, RAB7 also influences premelanosome motility97 and early endosome maturation98, so whether it functions directly in TYRP1 trafficking is not clear.
Functions of other melanosomal cargo
The defects in melanosome biogenesis that are observed in HPS and related models cannot be explained by effects on delivery of solely melanogenic enzymes. For example, because tyrosinase expression alone is sufficient to produce melanin in fibroblasts20, the severe hypopigmentation in BLOC-1-deficient melanocytes belies the cohort of tyrosinase delivered to melanosome-like compartments in these cells68. Indeed, additional melanocyte-specific constituents influence melanosome biogenesis (BOX 1). Analogous proteins likely function in the generation and maintenance of other LRO and perhaps all endosomal compartments.
Membrane transporters comprise a major class of proteins that regulate melanosome formation. Mutations in two transporters — OCA2/P and SLC45a2 (also known as AIM1 or MATP) — are respectively responsible for oculocutaneous albinism (OCA) types 2 and 4 in humans and corresponding mouse models99,100. In OCA2/P-deficient melanocytes, melanosome architecture is severely altered and tyrosinase and TYRP1 are mislocalized99,101, which suggests a role for OCA2/P in regulating delivery of enzymes to maturing melanosomes. OCA2/P is homologous to bacterial arsenate transporters99 and regulates sensitivity to arsenic toxicity102, which suggests that OCA2/P may function as an anion transporter, perhaps providing counterions for proton transport across melanosome membranes and intralumenal pH regulation99,103. OCA2/P-deficient melanocytes also have early secretory pathway defects104, but it is not clear whether these defects reflect OCA2 function in early secretory events or an indirect effect of cellular ion imbalance.
SLC45a2 has been identified in melanosome subcellular fractions17 and bears homology to sucrose/proton symporters in plants100, so it might regulate osmoregulation within melanosomes. SLC45a2-deficient melanocytes show abnormalities in melanogenic enzyme trafficking and loss of mature melanosomes105. A third putative membrane transporter, SLC24a5, was recently identified by positional cloning of the gene responsible for the golden mutation in zebrafish, which affects melanosome size, shape and number in the choroid106. SLC24a5 is a member of a family of potassium-dependent sodium/calcium exchangers, localizes to vesicular compartments in human melanocytes106, and copurifies with melanosomes17. Inhibitor and gene profiling analyses suggest that melanosomal sodium/hydrogen exchangers participate in pigmentation107, and additional solute transporters have been identified in melanosome subcellular fractions by proteomic analysis17.
Full melanosome function requires two other classes of protein. Genetic deficiency of OA1, a 7-transmembrane domain protein homologous to G-protein coupled receptors, is responsible for ocular albinism type I108. OA1-deficient RPE and choroidal melanocytes, and to a lesser extent skin melanocytes, have fewer, enlarged melanosomes relative to controls108. OA1 is localized to mature melanosomes and late endosomes and can signal through associated Giα proteins108,109, but neither the natural OA1 ligand nor how signalling might regulate melanosome size is known. A second unexpected contributor to melanosome biogenesis is presenilin, a component of the γ-secretase complex that is responsible for intramembrane cleavage of the Alzheimer’s precursor protein and Notch110. Selective ablation of presenilin expression or function in melanocytes results in hypopigmentation due to impaired transport of tyrosinase and perhaps other proteins to melanosomes, coupled with unusual proteolytic processing of tyrosinase, TYRP1 and DCT111. It is possible that presenilin regulates accessibility of membrane proteins that are required for melanogenic enzyme sorting to or fusion with melanosomes.
Conclusions and perspectives
The emerging picture (FIG. 4) suggests that melanosomes form by sequential, timed delivery of cargo from distinct endosomal domains, permitting progressive organelle maturation. The vacuolar domain of early endosomes provides cargo — including PMEL — required for the underlying melanosome structure, and is the likely source of the stage II melanosome limiting membrane. Distinct tubular domains of early endosomes then provide additional cargo — including melanogenic enzymes — that generate melanins and mediate organelle maturation through stages III and IV. The main challenges for the future will be to understand how these different targeting pathways are coordinated, how the molecular machinery functions in segregating these distinct domains, how tissue-specific membrane proteins shape the targeting pathways, and how ubiquitous protein complexes — like those deficient in HPS — are co-opted for the specific use of generating a distinct lineage of LRO.
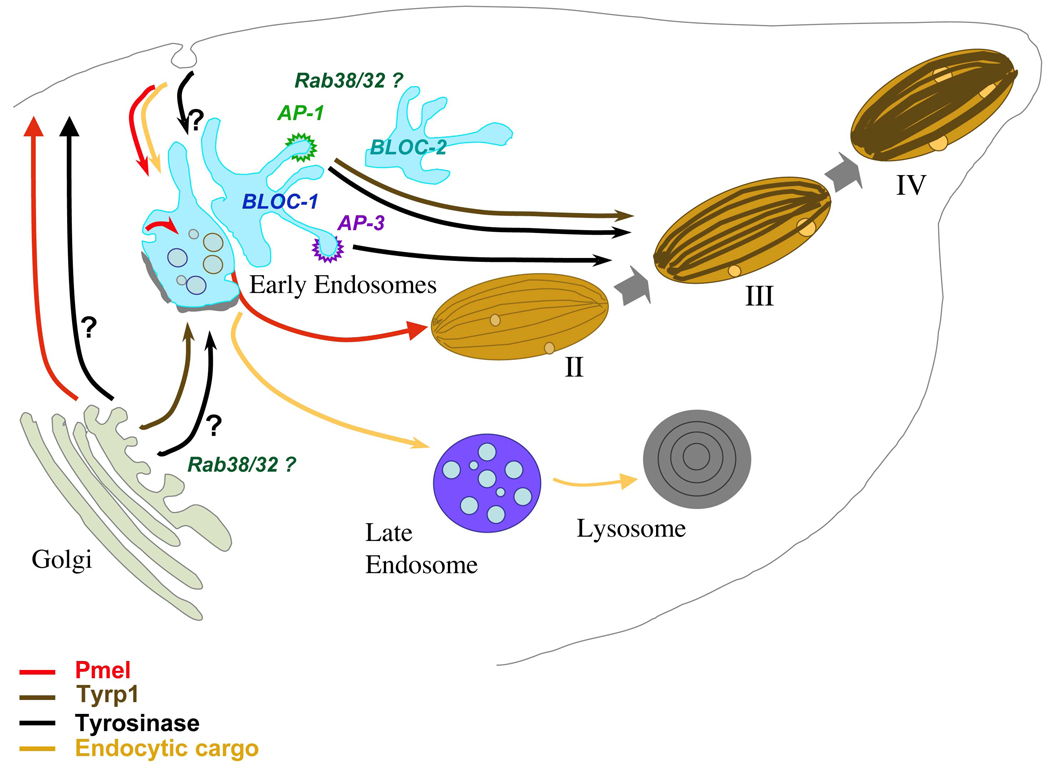
Figure 4. Model of melanosome maturation.
Schematic diagram of endosomal and melanosomal organelles of all melanocytic stages, and the biosynthetic transport pathways among them for three representative melanosome cargoes – PMEL, tyrosinase, and TYRP1. Divergence of endocytic cargo internalized by fluid phase and destined for lysosomes is also shown. Transport pathways for each cargo are indicated by solid or dashed lines as indicated in the legend. All melanosome cargoes derive from the Golgi and traverse vacuolar and/or tubular elements of early endosomes either directly or through the cell surface. From vacuolar elements, PMEL fibrils (green) segregate to stage II melanosomes and endocytic cargo segregates to late endosomes and eventually lysosomes. From tubuar elements of early endosomes, tyrosinase and TYRP1 are targeted to maturing melanosomes where they promote melanin deposition (orange). AP-3 coats initiate one endosome-to-melanosome pathway for a cohort of tyrosinase; BLOC-1, BLOC-2, and perhaps AP-1 regulate a second pathway traversed by a separate cohort of tyrosinase and by TYRP1. RAB32 and RAB38 might function at multiple sites along either or both of these pathways. The pathways taken by other melanosome components and the function of additional complexes defective in Hermansky-Pudlak syndrome within these pathways are not yet known.
One major unresolved question is how cargoes are segregated within stage I melanosomes for the conventional late endocytic pathway or stage II melanosomes – a process that does not occur in “non-specialized” cell types. Does Pmel trafficking, particularly ESCRT-independent sorting in MVBs, generate a “novel” endosomal intermediate within melanocytes distinct from late endosome precursors? Or do the fibrils themselves promote segregation from the intralumenal membranes from which they derive, separating the organelle into distinct domains – a fibrillar region and a multivesicular region – that eventually segregate toward two fates? Intriguingly, preliminary data suggest that as the PMEL-containing MVBs mature to stage II premelanosomes, they lose most of their internal membrane vesicles and either recruit or unmask effectors that render them competent to undergo further maturation to stage III and IV by docking and fusing with transport intermediates that carry melanogenic enzymes. These data might favour a model either in which the internal membranes segregate to a distinct domain or back-fuse to the limiting membrane, as occurs in antigen-processing compartments during dendritic-cell maturation112.
It is surprising that none of the HPS models analysed thus far interfere with either the segregation of stage II melanosomes from endosomes or grossly with fibril formation. This parallels the minimal effects of several HPS models on the formation of α granules in platelets — which, like stage II melanosomes, develop from MVBs113 — despite the complete loss of dense granules114. The underlying mechanisms behind these maturation events may be essential for normal endosomal maturation, such that mutations that affect them are not tolerated.
The lumenal domain-dependent mechanism of PMEL invagination onto internal membranes and the role of the internal membranes in regulating fibrillogenesis likely represent important ubiquitous events and must be better defined. Increasing evidence supports the role of MVBs and their associated lipids or derived exosomes in the formation of amyloid in Alzheimer’s and the prion diseases115–117. The fast rate and high efficiency of PMEL fibrillogenesis in vitro25 may provide a better mechanistic understanding of the role of such lipids in general processes of amyloidogenesis.
Another major unresolved question is how ubiquitous protein complexes, such as AP-3, the BLOCs, and HOPS, create distinct functional endosomal domains that function in tissue-specific sorting events. Perhaps each complex functions in concert with as-yet undefined tissue-specific factors, such as RAB32 or RAB38, to assemble specific macromolecular complexes at defined sites on endosomal membranes. Alternatively or additionally, altered expression levels of ubiquitous components might shift reaction equilibria in such a way as to generate novel pathways. For example, the increased expression of endosomal SNARE proteins in melanocytic cells78 might favour formation of novel trans-SNARE complexes or divert associated BLOCs or HOPS from “constitutive” pathways. Defining the SNARE distribution among melanosomal and endosomal membranes and the complexes they form in melanocytes will be critical for testing these hypotheses. The molecular functions of the BLOCs and HOPS complex must also be defined. How do they functionally interact with coats and SNAREs? How does BLOC-1 facilitate sorting of TYRP1 and other cargo toward melanosomes, and does it act in concert with adaptor complexes? To address these questions, it will be necessary to further define the subcompartmentalization of the endosomal system in these highly specialized cells and the protein-protein interactions that mediate it.
Finally, we need to learn more about the lumenal milieu of melanosomes and related organelles, and how the establishment of solute gradients regulates protein sorting. Transporters are becoming increasingly recognized as key regulators of membrane dynamics in the endocytic pathway (for example in cholesterol metabolism118). The characterization of melanosome membrane transporters and the availability of cell lines in which they fail to function provide an unprecedented opportunity to dissect how transporters modulate membrane dynamics via the lumenal environment of source and target compartments.
Our advances in melanosome biogenesis will allow us to better understand how other LRO form and the subcompartmentalization of the endosomal pathway in specific cell types. For example, endothelial cells use similar pathways to generate Weibel-Palade bodies, endosome-granule interactions may be required for maturation of cytolytic granules in T cells, and platelets likely use HPS-encoded protein complexes in similar ways to generate dense granules19. Thus, the dark organelle is clearly shedding light on the future of endosomal trafficking and the formation of specialized organelles.
Supplementary Material
Box 2.
Box 3: Figure.
ACKNOWLEDGEMENTS
We thank all the members of our laboratories for their contributions to many of the studies described here, including Figure 1 (Ilse Hurbain) and Figure 3 (Danièle Tenza). We also thank Esteban Dell’Angelica, Juan Bonifacino, Evelyne Coudrier, Willem Stoorvogel, Dan Cutler, Donata Rimoldi, M. Vittoria Schiaffino and Miguel Seabra for valuable discussions. We apologise to those authors whom we did not cite due to space limitations. This work was funded by grants from the National Institutes of Health (to MSM and GR), Institut Curie, CNRS, Fondation pour la Recherche Médicale, Institut National du Cancer, Cancéropole Ile de France (to GR).
Glossary Terms
- Lysosome-related organelles (LRO)
A class of tissue-specific intracellular organelles that share some features with lysosomes, such as acidic pH and lysosomal membrane protein and enzyme content.
- Eumelanins
The polymerized and cyclised products of successive oxidation reactions for which tyrosine is the initial substrate.
- Pheomelanins
The polymerized products of glutathionyl- or cysteinyl- adducts of tyrosine and its oxidation product, L-3,4-dihydroxyphenylalanine quinone.
- Dynein
A motor protein complex that regulates motility along microtubules, predominantly toward the minus end (toward the microtubule-organizing centre).
- Kinesins
A family of motor proteins that regulate microtubule-based motility, predominantly toward the plus end (away from the microtubule-organizing centre).
- Proprotein convertases
A small family of related proteinases that release mature peptides from many inactive proprotein precursors, including prohormones such as proinsulin.
- Weibel-Palade bodies
A lysosome-related organelle found in endothelial cells that is characterized by tubular fibrils of proteolytically processed von Willebrand factor.
- Amyloid
Fibrous structures that are composed of polymers of typically a single protein in a specific “cross β-sheet” pattern. Amyloid structures are associated with pathology in neurodegenerative diseases.
- Clathrin
A “coat” protein that forms patches on the plasma membrane, endosomes, or the TGN and is typically involved in budding of vesicles. Clathrin also plays structural roles in defining endosomal membrane domains.
- Multivesicular bodies (MVBs)
Endosomal domains that contain internal vesicles that are formed by invagination of the limiting membrane. Most mature MVBs correspond to late endosomes.
- Symporters
Ion transporters that move more than one type of ion across the same membrane at the same time.
- Rabs
Members of the largest sub-family within the superfamily of Ras small GTPases, each with its own function in membrane budding, fusion, or motility.
- SNAREs
Soluble N-ethylmaleimide-sensitive factor attachment protein receptors are membrane-associated proteins that are required for target membrane recognition and membrane fusion in the secretory and endocytic pathways.
- Ubiquitylation
The process by which the conserved 76-residue polypeptide, ubiquitin, is covalently conjugated to substrates.
- Ubiquitin ligases
Also known as E3 enzymes. Enzymes that bind to substrate proteins and are required directly or indirectly for the conjugation of ubiquitin.
Biographies
Graça Raposo is a Director of Research at the Centre National de la Recherche Scientifique (CNRS) in France. Since 1995, she has been a group leader in the Department of Cell Biology (UMR144-CNRS) at Institut Curie in Paris. She did her Ph.D. in Membrane Biology and Immunology at the University of Paris VII under the supervision of Lucio Benedetti. From 1990 to 1995 she was a post-doctoral fellow first at the Immunology Center in Marseille with Jean Davoust and then in the Department of Cell Biology, Utrecht University, The Netherlands, with Hans Geuze. Her studies focus on the modulations of the endocytic system in specialized cell types and in disease states such as viral and prion infections.
Michael Marks is an Associate Professor in the Department of Pathology & Laboratory Medicine at the University of Pennsylvania, USA, where he has been a faculty member since 1995. He did his Ph.D. with Peter Cresswell at Duke University, Durham, North Carolina, USA and two post-doctoral fellowships at the National Institutes of Health in Bethesda, Maryland, USA – first with Keiko Ozato and then with Juan Bonifacino. His laboratory focuses on post-Golgi protein sorting and organelle biogenesis.
REFERENCES
- 1.Gruenberg J. The endocytic pathway: a mosaic of domains. Nature Reviews, Mol. Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- 2.Maxfield FR, McGraw TE. Endocytic recycling. Nature Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 3.Spritz RA, Chiang PW, Oiso N, Alkhateeb A. Human and mouse disorders of pigmentation. Curr. Opin. Genet. Dev. 2003;13:284–289. doi: 10.1016/s0959-437x(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery G. Architecture of the optic chiasm and the mechanisms that sculpt its development. Physiol. Rev. 2001;81:1393–1414. doi: 10.1152/physrev.2001.81.4.1393. [DOI] [PubMed] [Google Scholar]
- 5.Wakamatsu K, Ito S. Advanced chemical methods in melanin determination. Pigment Cell Res. 2002;15:162–173. doi: 10.1034/j.1600-0749.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- 6.Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nature Rev. Mol. Cell Biol. 2001;2:738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- 7.Hearing VJ. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J. Dermatol. Sci. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Furumura M, et al. Characterization of genes modulated during pheomelanogenesis using differential display. Proc. Natl. Acad. Sci. USA. 1998;95:7374–7378. doi: 10.1073/pnas.95.13.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Futter CE. The molecular regulation of organelle transport in mammalian retinal pigment epithelial cells. Pigment Cell Res. 2006;19:104–111. doi: 10.1111/j.1600-0749.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Den Bossche K, Naeyaert J-M, Lambert J. The quest for the mechanism of melanin transfer. Traffic. 2006;7:769–778. doi: 10.1111/j.1600-0854.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 11.Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic. 2004;5:393–399. doi: 10.1111/j.1398-9219.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 12.Bennett DC, Lamoreux ML. The color loci of mice - a genetic century. Pigment Cell Res. 2003;16:333–344. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 13. Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 2001;152:809–823. doi: 10.1083/jcb.152.4.809. Showed that melanosomes are segregated from the endocytic pathway and are distinct from lysosomes, with a common precursor at the stage I melanosome/ vacuolar early endosome.
- 14.Kushimoto T, et al. A model for melanosome biogenesis based on the purification and analysis of early melanosomes. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10698–10703. doi: 10.1073/pnas.191184798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theos AC, et al. Functions of AP-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orlow SJ. Melanosomes are specialized members of the lysosomal lineage of organelles. J. Invest. Dermatol. 1995;105:3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- 17.Chi A, et al. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J. Proteome Res. 2006;5:3135–3144. doi: 10.1021/pr060363j. [DOI] [PubMed] [Google Scholar]
- 18.Blott EJ, Griffiths GM. Secretory lysosomes. Nature Rev. Mol. Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 19.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr. Opin. Cell Biol. 2007;19 doi: 10.1016/j.ceb.2007.05.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouchard B, Fuller BB, Vijayasaradhi S, Houghton AN. Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. J. Exp. Med. 1989;169:2029–2042. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vijayasaradhi S, Xu YQ, Bouchard B, Houghton AN. Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting of the human brown locus protein, gp75. J. Cell Biol. 1995;130:807–820. doi: 10.1083/jcb.130.4.807. Provided evidence for common signals for intracellular sorting of melanosomal and lysosomal proteins, and supported the notion that lysosomes and melanosomes share a common endosomal pathway of biogenesis. A related sorting signal in tyrosinase was later identified in refs. 58–60.
- 22.Berson JF, Harper D, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Biol. Cell. 2001;12:3451–3464. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei ML. Hermansky–Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 24.Theos AC, Truschel ST, Raposo G, Marks MS. The Silver locus product Pmel17/ gp100/ Silv/ ME20: Controversial in name and in function. Pigment Cell Res. 2005;18:322–336. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fowler DM, et al. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. Showed that PMEL17 cleavage products in vitro form fibrils with hallmarks of amyloid, and implicated amyloid structure in melanin polymerization.
- 26.Hoashi T, et al. MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes. J. Biol. Chem. 2005;280:14006–14016. doi: 10.1074/jbc.M413692200. [DOI] [PubMed] [Google Scholar]
- 27.Theos AC, et al. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell. 2006;17:3598–3612. doi: 10.1091/mbc.E06-01-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quevedo WC, Fleischmann RD, Dyckman J. Premature loss of melanocytes from hair follicles of light (Blt) and silver (si) mice. In: Seiji M, editor. Phenotypic expression in pigment cells. Tokyo: Tokyo Univ. Press; 1981. pp. 177–184. [Google Scholar]
- 29.Hamilton H. A study of the physiological properties of melanophores with special reference to their role in feather coloration. Anat. Rec. 1940;78:525–548. [Google Scholar]
- 30.Schonthaler HB, et al. A mutation in the silver gene leads to defects in melanosome biogenesis and alterations in the visual system in the zebrafish mutant fading vision. Dev. Biol. 2005;284:421–436. doi: 10.1016/j.ydbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Clark LA, Wahl JM, Rees CA, Murphy KE. Retrotransposon insertion in SILV is responsible for merle patterning of the domestic dog. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1376–1381. doi: 10.1073/pnas.0506940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunberg E, et al. A missense mutation in PMEL17 is associated with the Silver coat color in the horse. BMC Genet. 2006;7:46. doi: 10.1186/1471-2156-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berson JF, et al. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J. Cell Biol. 2003;161:521–533. doi: 10.1083/jcb.200302072. This paper, along with ref. 22, showed that PMEL17 is the main component of the intralumenal fibrils of stage II melanosomes and that the fibrils are generated by cleavage of PMEL17 in a post-Golgi compartment. These papers also highlight the role of MVBs as intermediates in the generation of stage II melanosomes.
- 34. Theos AC, et al. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. This paper was the first to define a lumenal sorting determinant for MVB sorting, using PMEL17 as a model. Along with ref. 39, it showed that this sorting and a separate lumenal determinant are required for formation of premelanosome fibrils.
- 35.Journet AM, Saffaripour S, Cramer EM, Tenza D, Wagner DD. von Willebrand factor storage requires intact prosequence cleavage site. Eur. J. Cell Biol. 1993;60:31–41. [PubMed] [Google Scholar]
- 36.Kelly JW, Balch WE. Amyloid as a natural product. J. Cell Biol. 2003;161:461–462. doi: 10.1083/jcb.200304074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler JC, Rochet JC, Lansbury PTJ. The N-terminal repeat domain of alpha-synuclein inhibits beta-sheet and amyloid fibril formation. Biochemistry. 2003;42:672–678. doi: 10.1021/bi020429y. [DOI] [PubMed] [Google Scholar]
- 38.Parham SN, Resende CG, Tuite MF. Oligopeptide repeats in the yeast protein Sup35p stabilize intermolecular prion interactions. EMBO J. 2001;20:2111–2119. doi: 10.1093/emboj/20.9.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoashi T, et al. The repeat domain of the melanosomal matrix protein Pmel17/gp100 is required for the formation of organellar fibers. J. Biol. Chem. 2006;281:21198–22208. doi: 10.1074/jbc.M601643200. [DOI] [PubMed] [Google Scholar]
- 40.Vischer UM, Wagner DD. von Willebrand factor proteolytic processing and multimerization precede the formation of Weibel-Palade bodies. Blood. 1994;83:3536–3544. [PubMed] [Google Scholar]
- 41.Basrur V, et al. Proteomic analysis of early melanosomes: identification of novel melanosomal proteins. J. Proteome Res. 2003;2:69–79. doi: 10.1021/pr025562r. [DOI] [PubMed] [Google Scholar]
- 42.Valencia JC, et al. Sorting of Pmel17 to melanosomes through the plasma membrane by AP1 and AP2: evidence for the polarized nature of melanocytes. J. Cell Sci. 2006;119:1080–1091. doi: 10.1242/jcs.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doray B, Lee I, Knisely J, Bu G, Kornfeld S. The γ/σ1 and α/σ2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell. 2007;18:1887–1896. doi: 10.1091/mbc.E07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valencia JC, et al. Sialylated core 1 O-glycans influence the sorting of Pmel17/gp100 and determine its capacity to form fibrils. J. Biol. Chem. 2007;282:11266–11280. doi: 10.1074/jbc.M608449200. [DOI] [PubMed] [Google Scholar]
- 45.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nature Rev. Mol. Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 46. Lévy F, et al. Ubiquitylation of a melanosomal protein by HECT-E3 ligases serves as sorting signal for lysosomal degradation. Mol. Biol. Cell. 2005;16:1777–1787. doi: 10.1091/mbc.E04-09-0803. Showed that unlike PMEL17, MART1 is sorted within MVBs by a classical ubiquitin-dependent signal and, together with ref. 26, that MART1 is required for proper melanosome biogenesis.
- 47.Kim BY, Olzmann JA, Barsh GS, Chin LS, Li L. Spongiform neurodegeneration-associated E3 ligase Mahogunin ubiquitylates TSG101 and regulates endosomal trafficking. Mol. Biol. Cell. 2007;18:1129–1142. doi: 10.1091/mbc.E06-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salas-Cortes L, et al. Myosin Ib modulates the morphology and the protein transport within multi-vesicular sorting endosomes. J. Cell Sci. 2005;118:4823–4832. doi: 10.1242/jcs.02607. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi T, et al. The Pmel 17/silver locus protein. Characterization and investigation of its melanogenic function. J. Biol. Chem. 1994;269:29198–29205. [PubMed] [Google Scholar]
- 50.Novikoff AB, Albala A, Biempica L. Ultrastructural and cytochemical observations on B-16 and Harding-Passey mouse melanomas. The origin of premelanosomes and compound melanosomes. J. Histochem. Cytochem. 1968;16:299–319. doi: 10.1177/16.5.299. [DOI] [PubMed] [Google Scholar]
- 51.Maul GG, Brumbaugh JA. On the possible function of coated vesicles in melanogenesis of the regenerating fowl feather. J. Cell Biol. 1971;48:41–48. doi: 10.1083/jcb.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sprong H, et al. Glycosphingolipids are required for sorting melanosomal proteins in the Golgi complex. J. Cell Biol. 2001;155:369–380. doi: 10.1083/jcb.200106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Pietro SM, Dell'Angelica EC. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 54.Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr. Opin. Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 55.Hermann GJ, et al. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol. Biol. Cell. 2005;16:3273–3288. doi: 10.1091/mbc.E05-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 57.Höning S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calvo PA, Frank DW, Bieler BM, Berson JF, Marks MS. A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J. Biol. Chem. 1999;274:12780–12789. doi: 10.1074/jbc.274.18.12780. [DOI] [PubMed] [Google Scholar]
- 59.Simmen T, Schmidt A, Hunziker W, Beermann F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. J. Cell Sci. 1999;112:45–53. doi: 10.1242/jcs.112.1.45. [DOI] [PubMed] [Google Scholar]
- 60.Blagoveshchenskaya AD, Hewitt EW, Cutler DF. Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells. Mol. Biol. Cell. 1999;10:3979–3990. doi: 10.1091/mbc.10.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huizing M, et al. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol. Biol. Cell. 2001;12:2075–2085. doi: 10.1091/mbc.12.7.2075. This paper, along with ref. 15, showed that the AP-3 complex, deficient in Hermansky-Pudlak syndrome type 2, regulates tyrosinase trafficking to melanosomes. Refs. 15 and 62 showed that AP-3 functions from early endosomes.
- 62.Peden AA, et al. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 2004;164:1065–1076. doi: 10.1083/jcb.200311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- 64.Huizing M, et al. Hermansky-Pudlak syndrome type 3 in Ashkenazi Jews and other non-Puerto Rican patients with hypopigmentation and platelet storage-pool deficiency. Am. J. Hum. Genet. 2001;69:1022–1032. doi: 10.1086/324168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nguyen T, et al. Melanosome morphologies in murine models of Hermansky-Pudlak syndrome reflect blocks in organelle development. J. Invest. Dermatol. 2002;119:1156–1164. doi: 10.1046/j.1523-1747.2002.19535.x. Provides a quantitative comparative analysis of the effects of different Hermansky-Pudlak syndrome mutations on melanosome morphology in epidermal melanocytes.
- 66.Meyer C, et al. µ1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deneka M, et al. Rabaptin-5α/rabaptin-4 serves as a linker between rab4 and γ1-adaptin in membrane recycling from endosomes. EMBO J. 2003;22:2645–2657. doi: 10.1093/emboj/cdg257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Setty SRG, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. Together with refs. 69 and 70, these papers define roles for BLOC-1 and BLOC-2 in cargo sorting from early endosomes. Refs. 69 and 70 suggest that BLOC-1 and AP-3 physically interact.
- 69.Di Pietro SM, et al. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol. Biol. Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salazar G, et al. BLOC-1 complex deficiency alters the targeting of Adaptor Protein complex-3 cargoes. Mol. Biol. Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gautam R, et al. The Hermansky-Pudlak syndrome 3 (cocoa) protein is a component of the biogenesis of lysosome-related organelles complex-2 (BLOC-2) J. Biol. Chem. 2004;279:12935–12942. doi: 10.1074/jbc.M311311200. [DOI] [PubMed] [Google Scholar]
- 72.Di Pietro SM, Falcon-Perez JM, Dell'Angelica EC. Characterization of BLOC-2, a complex containing the Hermansky-Pudlak syndrome proteins HPS3, HPS5 and HPS6. Traffic. 2004;5:276–283. doi: 10.1111/j.1600-0854.2004.0171.x. [DOI] [PubMed] [Google Scholar]
- 73.Falcón-Pérez JM, Romero-Calderón R, Brooks ES, Krantz DE, Dell'Angelica EC. The drosophila pigmentation gene pink (p) encodes a homologue of human Hermansky-Pudlak Syndrome 5 (HPS5) Traffic. 2007;8:154–168. doi: 10.1111/j.1600-0854.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- 74.Richmond B, et al. Melanocytes derived from patients with HermanskyPudlak Syndrome types 1, 2, and 3 have distinct defects in cargo trafficking. J. Invest. Dermatol. 2005;124:420–427. doi: 10.1111/j.0022-202X.2004.23585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boissy RE, et al. Melanocyte-specific proteins are aberrantly trafficked in melanocytes of Hermansky-Pudlak syndrome-type 3. Am. J. Pathol. 2005;166:231–240. doi: 10.1016/S0002-9440(10)62247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Helip-Wooley A, et al. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak Syndrome type-5. J. Invest. Dermatol. 2007;127:1471–1478. doi: 10.1038/sj.jid.5700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gautam R, et al. Interaction of Hermansky-Pudlak syndrome genes in the regulation of lysosome-related organelles. Traffic. 2006;7:779–792. doi: 10.1111/j.1600-0854.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 78.Wade N, et al. Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, Syntaxin 6, vesicle-associated membrane protein (VAMP)8, and VAMP7 in B16 melanoma cells. J. Biol. Chem. 2001;276:19820–19827. doi: 10.1074/jbc.M010838200. [DOI] [PubMed] [Google Scholar]
- 79. Huang L, Kuo YM, Gitschier J. The pallid gene encodes a novel, syntaxin 13-interacting protein involved in platelet storage pool deficiency. Nature Genet. 1999;23:329–332. doi: 10.1038/15507. Refs 79 and 80 show that an endosomal SNARE protein, syntaxin 13, interacts with the Hermansky-Pudlak syndrome complex BLOC-1, and were the first papers to provide evidence for a role of these complexes in endosomal transport. Ref. 78 further implies a functional role for syntaxin 13 and other endosomal SNAREs in regulating melanosome biogenesis.
- 80.Moriyama K, Bonifacino JS. Pallidin is a component of a multi-protein complex involved in the biogenesis of lysosome-related organelles. Traffic. 2002;3:666–677. doi: 10.1034/j.1600-0854.2002.30908.x. [DOI] [PubMed] [Google Scholar]
- 81.Yu J-F, Fukamachi S, Mitani H, Hori H, Kanamori A. Reduced expression of vps11 causes less pigmentation in medaka, Oryzias latipes. Pigment Cell Res. 2006;19:628–634. doi: 10.1111/j.1600-0749.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 82.Maldonado E, Hernandez F, Lozano C, Castro ME, Navarro RE. The zebrafish mutant vps18 as a model for vesicle-traffic related hypopigmentation diseases. Pigment Cell Res. 2006;19:315–326. doi: 10.1111/j.1600-0749.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 83.Pulipparacharuvil S, et al. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 2005;118:3663–3673. doi: 10.1242/jcs.02502. [DOI] [PubMed] [Google Scholar]
- 84.Warner TS, et al. The light gene of Drosophila melanogaster encodes a homologue of VPS41, a yeast gene involved in cellular-protein trafficking. Genome. 1998;41:236–243. [PubMed] [Google Scholar]
- 85.Sevrioukov EA, He JP, Moghrabi N, Sunio A, Kramer H. A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol. Cell. 1999;4:479–486. doi: 10.1016/s1097-2765(00)80199-9. [DOI] [PubMed] [Google Scholar]
- 86.Gerst JE. SNARE regulators: matchmakers and matchbreakers. Biochim. Biophys. Acta. 2003;1641:99–110. doi: 10.1016/s0167-4889(03)00096-x. [DOI] [PubMed] [Google Scholar]
- 87.Richardson SC, Winistorfer SC, Poupon V, Luzio JP, Piper RC. Mammalian late vacuole protein sorting orthologues participate in early endosomal fusion and interact with the cytoskeleton. Mol. Biol. Cell. 2004;15:1197–1210. doi: 10.1091/mbc.E03-06-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 89.Smith JW, Koshoffer A, Morris RE, Boissy RE. Membranous complexes characteristic of melanocytes derived from patients with Hermansky-Pudlak syndrome type 1 are macroautophagosomal entities of the lysosomal compartment. Pigment Cell Res. 2005;18:417–426. doi: 10.1111/j.1600-0749.2005.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falcón-Pérez JM, Nazarian R, Sabatti C, Dell'Angelica EC. Distribution and dynamics of Lamp1-containing endocytic organelles in fibroblasts deficient in BLOC-3. J. Cell Sci. 2005;118:5243–5255. doi: 10.1242/jcs.02633. [DOI] [PubMed] [Google Scholar]
- 91.Oh J, Liu ZX, Feng GH, Raposo G, Spritz RA. The Hermansky-Pudlak syndrome (HPS) protein is part of a high molecular weight complex involved in biogenesis of early melanosomes. Hum. Mol. Genet. 2000;9:375–385. doi: 10.1093/hmg/9.3.375. [DOI] [PubMed] [Google Scholar]
- 92. Wasmeier C, et al. Rab38 an Rab32 control early post-Golgi trafficking of melanogenic enzymes. J. Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. Following ref. 94, which showed that RAB38 functions in melanosome biogenesis, this paper shows that RAB32 and RAB38 function in a redundant manner to control trafficking of tyrosinase and TYRP1 and thereby melanosome formation.
- 93.Oiso N, Riddle SR, Serikawa T, Kuramoto T, Spritz RA. The rat Ruby (R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm. Genome. 2004;15:307–314. doi: 10.1007/s00335-004-2337-9. [DOI] [PubMed] [Google Scholar]
- 94.Loftus SK, et al. Mutation of melanosome protein RAB38 in chocolate mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4471–4476. doi: 10.1073/pnas.072087599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma J, Plesken H, Treisman JE, Edelman-Novemsky I, Ren M. Lightoid and Claret: a rab GTPase and its putative guanine nucleotide exchange factor in biogenesis of Drosophila eye pigment granules. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11652–11657. doi: 10.1073/pnas.0401926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirosaki K, Yamashita T, Wada I, Jin HY, Jimbow K. Tyrosinase and tyrosinase-related protein 1 require Rab7 for their intracellular transport. J. Invest. Dermatol. 2002;119:475–480. doi: 10.1046/j.1523-1747.2002.01832.x. [DOI] [PubMed] [Google Scholar]
- 97.Jordens I, et al. Rab7 and Rab27a control two motor protein activities involved in melanosomal transport. Pigment Cell Res. 2006;19:412–423. doi: 10.1111/j.1600-0749.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- 98.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 99.Brilliant MH. The mouse p (pink-eyed dilution) and human P genes, oculocutaneous albinism type 2 (OCA2), and melanosomal pH. Pigment Cell Res. 2001;14:86–93. doi: 10.1034/j.1600-0749.2001.140203.x. [DOI] [PubMed] [Google Scholar]
- 100.Newton JM, et al. Mutations in the human orthologue of the mouse underwhite gene (uw) underlie a new form of oculocutaneous albinism, OCA4. Am. J. Hum. Genet. 2001;69:981–988. doi: 10.1086/324340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Manga P, Boissy RE, Pifko-Hirst S, Zhou BK, Orlow SJ. Mislocalization of melanosomal proteins in melanocytes from mice with oculocutaneous albinism type 2. Exp. Eye Res. 2001;72:695–710. doi: 10.1006/exer.2001.1006. [DOI] [PubMed] [Google Scholar]
- 102.Staleva L, Manga P, Orlow SJ. Pink-eyed dilution protein modulates arsenic sensitivity and intracellular glutathione metabolism. Mol. Biol. Cell. 2002;13:4206–4220. doi: 10.1091/mbc.E02-05-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Manga P, Orlow SJ. Inverse correlation between pink-eyed dilution protein expression and induction of melanogenesis by bafilomycin A1. Pigment Cell Res. 2001;14:362–367. doi: 10.1034/j.1600-0749.2001.140508.x. [DOI] [PubMed] [Google Scholar]
- 104.Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol. Biol. Cell. 2002;13:1953–1964. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Costin G-E, Valencia JC, Vieira WD, Lamoreux ML, Hearing VJ. Tyrosinase processing and intracellular trafficking is disrupted in mouse primary melanocytes carrying the underwhite (uw) mutation. A model for oculocutaneous albinism (OCA) type 4. J. Cell Sci. 2003;116:3203–3212. doi: 10.1242/jcs.00598. [DOI] [PubMed] [Google Scholar]
- 106. Lamason RL, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. Identified a putative cation exchanger as responsible for the golden mutation in zebrafish, and then showed that allelic variation in the human orthologue correlates with variation in skin color.
- 107.Smith DR, Spaulding DT, Glenn HM, Fuller BB. The relationship between Na(+)/H(+) exchanger expression and tyrosinase activity in human melanocytes. Exp. Cell Res. 2004;298:521–534. doi: 10.1016/j.yexcr.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 108.Schiaffino MV, Tacchetti C. The ocular albinism type 1 (OA1) protein and the evidence for an intracellular signal transduction system involved in melanosome biogenesis. Pigment Cell Res. 2005;18:227–233. doi: 10.1111/j.1600-0749.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 109. Innamorati G, Piccirillo R, Bagnato P, Palmisano I, Schiaffino MV. The melanosomal/lysosomal protein OA1 has properties of a G protein-coupled receptor. Pigment Cell Res. 2006;19:125–135. doi: 10.1111/j.1600-0749.2006.00292.x. Using a cell transfection system, this paper showed that OA1 stimulates Giα signalling, which suggests that it functions as a classical G-protein coupled receptor.
- 110.Parks AL, Curtis D. Presenilin diversifies its portfolio. Trends Genet. 2007;23:140–150. doi: 10.1016/j.tig.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 111. Wang R, Tang P, Wang P, Boissy RE, Zheng H. Regulation of tyrosinase trafficking and processing by presenilins: partial loss of function by familial Alzheimer's disease mutation. Proc. Natl. Acad. Sci. U.S.A. 2006;103:353–358. doi: 10.1073/pnas.0509822102. By analysing melanocytes from targeted gene knockouts in mice, this paper makes the provocative suggestion that presenilins, which are part of an integral membrane protease complex, regulate the trafficking of tyrosinase and other proteins to mature melanosomes.
- 112.Kleijmeer M, et al. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J. Cell Biol. 2001;155:53–64. doi: 10.1083/jcb.200103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heijnen HF, et al. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91:2313–2325. [PubMed] [Google Scholar]
- 114.Huizing M, Parkes JM, Helip-Wooley A, White JG, Gahl WA. Platelet alpha granules in BLOC-2 and BLOC-3 subtypes of Hermansky-Pudlak syndrome. Platelets. 2007;18:150–157. doi: 10.1080/13576500600936039. [DOI] [PubMed] [Google Scholar]
- 115.Rajendran L, et al. Alzheimer's disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Février B, et al. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 2004;101 doi: 10.1073/pnas.0308413101. 9683-9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maxfield FR, Menon AK. Intracellular sterol transport and distribution. Curr. Opin. Cell Biol. 2006;18:379–385. doi: 10.1016/j.ceb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 119.Geuze HJ, Slot JW, Strous GJ, Lodish HF, Schwartz AL. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- 120.Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J. Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sachse M, Urbe S, Oorschot V, Strous GJ, Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr. Opin. Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 123.Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL. Late endosomes derive from early endosomes by maturation. Cell. 1991;65:417–427. doi: 10.1016/0092-8674(91)90459-c. [DOI] [PubMed] [Google Scholar]
- 124.Hopkins CR, Gibson A, Shipman M, Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990;346:335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- 125.Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 126.Leung KF, Baron R, Seabra MC. Thematic review series: lipid posttranslational modifications. geranylgeranylation of Rab GTPases. J. Lipid Res. 2006;47:467–475. doi: 10.1194/jlr.R500017-JLR200. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Q, et al. Cell-specific abnormal prenylation of Rab proteins in platelets and melanocytes of the gunmetal mouse. Br. J. Haematol. 2002;117:414–423. doi: 10.1046/j.1365-2141.2002.03444.x. [DOI] [PubMed] [Google Scholar]
- 128.Detter JC, et al. Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proc. Natl. Acad. Sci. USA. 2000;97:4144–4149. doi: 10.1073/pnas.080517697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sato TK, Rehling P, Peterson MR, Emr SD. Class C vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell. 2000;6:661–671. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 130.Zhang H, Seabra MC, Deisenhofer J. Crystal structure of Rab geranylgeranyltransferase at 2.0 Å resolution. Structure. 2000;8:241–251. doi: 10.1016/s0969-2126(00)00102-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.