SUMMARY
The mammary gland develops its adult form by a process referred to as branching morphogenesis. Many factors have been reported to affect this process. We have used cultured primary mammary epithelial organoids and mammary epithelial cell lines in three-dimensional collagen gels to elucidate which growth factors, matrix metalloproteinases (MMPs) and mammary morphogens interact in branching morphogenesis. Branching stimulated by stromal fibroblasts, epidermal growth factor, fibroblast growth factor 7, fibroblast growth factor 2 and hepatocyte growth factor was strongly reduced by inhibitors of MMPs, indicating the requirement of MMPs for three-dimensional growth involved in morphogenesis. Recombinant stromelysin 1/MMP3 alone was sufficient to drive branching in the absence of growth factors in the organoids. Plasmin also stimulated branching; however, plasmin-dependent branching was abolished by both inhibitors of plasmin and MMPs, suggesting that plasmin activates MMPs. To differentiate between signals for proliferation and morphogenesis, we used a cloned mammary epithelial cell line that lacks epimorphin, an essential mammary morphogen. Both epimorphin and MMPs were required for morphogenesis, but neither was required for epithelial cell proliferation. These results provide direct evidence for a crucial role of MMPs in branching in mammary epithelium and suggest that, in addition to epimorphin, MMP activity is a minimum requirement for branching morphogenesis in the mammary gland.
Keywords: Branching morphogenesis, Mammary gland, Stromelysin 1, Epimorphin, Plasminogen, Stromal/epithelial interactions, Mouse
INTRODUCTION
Branching morphogenesis is essential for development of several epithelial organs, including lung, kidney, salivary and mammary glands. Mammary gland morphogenesis and differentiation occur during postnatal development. At puberty, epithelial end buds proliferate and invade the mammary mesenchyme, establishing an extensive network of ductal branches. This is an intricate process that entails the coordination of several factors. These include the spatiotemporal control of the balance between cell proliferation and selective cell death, the adhesion of epithelial cells to the extracellular matrix (ECM), the regulated turnover of specific ECM components, cell-cell interactions, migration and differentiation (Gumbiner, 1992).
Mesenchymal-epithelial interactions are also crucial for ductal growth and morphogenesis. Several stromal growth factors have been implicated as autocrine and paracrine mediators of these processes in the mammary gland and other tissues. These include hepatocyte growth factor (HGF), which has been reported to stimulate branching of epithelial cells of different origin (Montesano et al., 1991), including the mammary gland (Niranjan et al., 1995; Yant et al., 1998), and epidermal growth factor (EGF; Coleman et al., 1988). Genetic ablation of the EGF receptor indicates that the EGF receptor-mediated signal transduction pathway is required for the branching process in vivo (Sebastian et al., 1998; Wiesen et al., 1999). Keratinocyte growth factor (KGF), fibroblast growth factor 7 (FGF7) and basic fibroblast growth factor 2 (bFGF2) also have been implicated in regulating mammary gland branching, owing to their spatiotemporal expression (Coleman-Krnacik and Rosen, 1994). However, transforming growth factor β (TGFβ), has been reported to both promote (Soriano et al., 1996) and inhibit (Silberstein and Daniel, 1987; Daniel et al., 1989) branching morphogenesis. Recent work from our laboratory has demonstrated that, while several growth factors can trigger epithelial branches to grow (and thus be visualized), epimorphin (syntaxin 2), another stromal-myoepithelial factor, is a required morphogen in the mammary gland (Hirai et al., 1998).
The expression pattern of type I and IV collagens and laminin also suggest an ECM involvement in mammary morphogenesis (Keely et al., 1995). Studies in transgenic mice expressing a chimeric dominant-negative β1 integrin suggest a requirement for β1 integrins for development and differentiation of the mammary epithelium (Faraldo et al., 1998). Furthermore, α2β1 and a3b1 integrin functions are required for branching in a human mammary cell culture system (Berdichevsky et al., 1994).
ECM and basement membrane (BM) structure and composition are modulated by the matrix metalloproteinases (MMPs; Lukashev and Werb, 1998). Most MMPs are secreted in a zymogen form, and are activated by proteolytic cleavage. The net MMP activity is determined by the rate of production and activation of the zymogen and by local concentration of tissue inhibitors of metalloproteinases (TIMPs). Growth factors and ECM molecules tightly control MMP expression. In turn, owing to their proteolytic activity, MMPs regulate ECM assembly, edit excess ECM components, remodel the microenvironment, and release bioactive ECM fragments and growth factors, all of which are implicated in morphogenesis of epithelial tissues (Basbaum and Werb, 1996; Werb, 1997).
Indirect evidence for involvement of MMPs in mammary gland morphogenesis has come from studies on the expression of stromelysin 1 (Str1/MMP3), during mammary gland development. Str1, which is normally expressed by mammary fibroblasts in vivo, is produced at elevated levels in the glands of virgin animals during ductal elongation, and later on during involution (Talhouk et al., 1992; Witty et al., 1995; Thomasset et al., 1998). The highest levels of Str1 are found around end buds and near branch points, where mammary epithelial cells display the highest mitotic activity (Korpsmeier, 1979; Witty et al., 1995; Thomasset et al., 1998). We have shown previously that targeted expression of an autoactivated Str1 mutant in mammary epithelium of transgenic mice increases branching morphogenesis, and leads to hyperplastic development of the breast, suggesting the possibility that this MMP may function as a morphogen in mammary gland development (Sympson et al., 1994; Witty et al., 1995).
To determine whether MMPs play a direct role in branching morphogenesis, we have used recombinant enzymes and specific proteinase inhibitors in primary epithelial organoids. To elucidate the minimum requirements for epithelial branching, we used a luminal epithelial cell line maintained under serum-free conditions. Our studies establish a crucial role for Str1, epimorphin and one of several growth factors in this process. As these factors are all produced by either stromal or myoepithelial cells in vivo, these studies further define the essential role of epithelial-mesenchymal interactions in luminal epithelial branching in the mammary gland.
MATERIALS AND METHODS
Preparation of primary mammary organoids
Primary epithelial organoids were prepared from 10-week-old, virgin CD-1 mice as previously described (Hirai et al., 1998). In brief, inguinal glands were removed aseptically, minced with scalpel blades and incubated with agitation (100 rpm) for 30 minutes at 37°C in 0.2% trypsin (Life Technologies) and 0.2% collagenase A (Boehringer Mannheim) in growth medium consisting of DMEM/F12 (1:1, Life Technologies), 5% fetal calf serum (FCS), 5 μg/ml insulin (Sigma) and 50 μg/ml gentamicin (Life Technologies). The resulting cell suspension was centrifuged at 80 g for 10 minutes. After discarding the supernatant containing fat tissue, the cell pellets were resuspended in growth medium supplemented with 1000 U DNase I, incubated for 2 minutes at ambient temperature, and washed once with growth medium. Separation of the single cell fraction (consisting mainly of fibroblasts) from the organoids was carried out by differential centrifugation in DMEM/F12. To eliminate most single cells, pellets were centrifuged 10 × 50 seconds each at 80 g. To culture fibroblasts, the supernatant fraction obtained from the first differential centrifugation was plated in growth medium in 10 cm dishes.
Preparation of SCp2 cell clusters
The functionally normal mouse mammary epithelial cell line, SCp2 (Desprez et al., 1993), was maintained in growth medium. SCp2 cell clusters were prepared as previously described (Hirai et al., 1998). In brief, 250 μl of 2% agarose in phosphate-buffered saline (PBS) was added to each well of a 24-well plate. After the agarose had gelled, 1.5 ml of growth medium was added to each well and incubated for 30 minutes at 37°C. This medium was then discarded and 5 × 104 cells, suspended in 500 μl of growth medium containing 1000 U DNase I (Sigma), were seeded on top of the agarose gel and incubated with gentle rotation (100 rpm) for 24 hours at 37°C. After incubation, cells formed smoothly rounded and well-packed clusters of 150–200 μm in diameter. The remaining single cells were removed by centrifugation for 30 seconds at 160 g.
Three-dimensional cultures
For three-dimensional cell cultures, primary organoids or SCp2 cell clusters were embedded in type I collagen gels as previously described (Hirai et al., 1998). In brief, acid-soluble collagen (7.5 volume of a 0.5% solution, Cellagen™ AC-5, ICN) was mixed gently on ice with 1 volume of 10× DMEM/F12, followed by 1 volume of 0.1 N NaOH. Two layers of collagen were poured into each well: a basal layer consisting only of collagen, and an upper layer that contained the cells. To allow gelation of the basal collagen layer, 100 μl of the collagen solution was poured into each well of a 48-well dish and incubated at 37°C for 15 minutes. The cell clusters or organoids were suspended in DMEM/F12 at a concentration of 2500–4000 clusters/organoids per ml, and 100 μl of the suspension was mixed with 5 ml of ice-cold collagen solution. This mixture (100 μl) was poured onto the gelled basal collagen layer, and placed immediately at 37°C. This yielded around 40–80 clusters/organoids per well. After gelation, 200 μl of chemically defined medium consisting of DMEM/F12 containing Nutridoma NS medium supplement (Boehringer Mannheim) and ITS (insulin/transferrin/selenium, Sigma) was added to each well. KGF, EGF, bFGF and HGF (all from Collaborative Research) were used at a final concentration of 50 ng/ml, and transforming growth factor β1 (TGFβ1, R&D Systems) was used at 20 ng/ml. All reagents were added to the cultures no later than 2 hours after the cells were embedded in collagen gels. Medium was replaced every 2–3 days.
For co-cultures, primary mammary fibroblasts were grown to confluence, trypsinized, and plated into 48 well dishes at a concentration of 20,000 cells per well in growth medium. After an overnight incubation, the medium was removed, cells were washed three times with chemically defined medium, and basal and cell-containing collagen gels were poured on top of the fibroblasts.
Proteinases and inhibitors
Leupeptin, pepstatin A, aprotinin and E64 (all from Sigma) were used at final concentrations of 1, 1, 1.5 and 10 μM, respectively. The hydroxamic acids, GM6001 (3-(N-hydroxycarbamoyl)-(2R)-isobutylpropionyl-L-tryptophan methylamide) and GM1210 ((N-tert-butyloxycarbonyl)-L-leucine-L-tryptophan methylamide), kind gifts from Dr R. Galardy (Glycomed, Alameda, CA), were dissolved at 100 mM in dimethylsulfoxide and used at a final concentration of 10 μM. GM6001 is a general inhibitor of all MMPs with Ki values of less than 100 nM (Grobelny et al., 1992; Gijbels et al., 1994). It has Ki values of 27 nM against Str1, 0.4 nM against skin fibroblast collagenase, 0.5 nM against gelatinase A, 0.2 nM against gelatinase B and 0.1 nM against neutrophil collagenase (Galardy et al., 1994). Recombinant human TIMP1 and TIMP2 were a kind gift from Joni Mott and Michael Banda (Lawrence Berkeley National Laboratory). They were used at a final concentration of 150 nM and 50 nM, respectively.
Recombinant human Str1 with the C-terminal hemopexin domain truncated was expressed and purified from the methyltrophic yeast Pichia pastoris (Lochter et al., 1999). Casein zymography indicated that the recombinant Str1 (rStr1) was proteolytically active. Before use in cell culture experiments, Str1 was dialyzed against DMEM/F12 (Life Technologies, Gaithersburg, MD). Subsequently, 1 mg/ml was activated by incubation with 1 mg/ml of trypsin (Life Technologies) for 1 hour at 37°C. Trypsin was subsequently inhibited with soybean trypsin inhibitor (Sigma) added at a final concentration of 10 μg/ml. A premixed solution of trypsin and SBTI was used as a control in the Str1 experiments.
Plasminogen (Sigma), plasmin (America Diagnostica) and uPA (Sigma) were used at a final concentration of 8.5 μg/ml, 10 μg/ml and 10 μg/ml, respectively.
Analysis of branching morphogenesis
The branching phenotype of organoids and cell clusters embedded in collagen gels was determined after cultivation for 4–6 days. A branching phenotype was defined as an organoid or SCp2 cell cluster having at least one process (branch) extending from its central body. The fact that the processes were branches and not individual cell spikes was confirmed by analyzing DAPI stained organoids under a fluorescent microscope. Quantification of branching was carried out by counting the percentage of branching clusters or organoids in each well or the number of branches per organoid. Experiments were carried out in duplicates or triplicates. Statistical significance was determined with One-way ANOVA, using the Graph Pad Instat program.
Zymography
Chemically defined culture medium conditioned by cells for 2 days was processed for casein and gelatin substrate gels as described previously (Talhouk et al., 1991; Lochter et al., 1997). In brief, conditioned medium concentrated 20 times using Centricon 10 filters (Amicon) was mixed with Laemmli sample buffer without reducing agents, incubated for 15 minutes at 37°C, and separated on 8.8% sodium dodecyl sulfate (SDS)-polyacrylamide slab gels containing 1 mg/ml of α-casein or gelatin (both from Sigma). After electrophoresis, gels were incubated for 30 minutes with 2.5% Triton X-100 and subsequently for 2 days at 37°C in 100 mM Tris-HCl, pH 7.4, containing 15 mM CaCl2. Gels were stained with Coomassie Blue R-250 and destained with water. Clear zones emerged against a blue background, indicating proteolytic activity. The metalloproteinase inhibitor, 1,10-phenanthroline (1 mM), was incubated with samples for 30 minutes at 37°C before addition of sample buffer (Unemori and Werb, 1986). This inhibitor was also included in all incubation steps after gel electrophoresis. To confirm which bands corresponded to the active forms of the MMPs, samples were treated for 1 hour with 1 mM APMA (p-aminophenylmercuric acetate; Sigma) before addition of sample buffer. The bands obtained were then compared with those of the samples not treated with APMA.
Organoid sections and immunofluorescence labeling
To analyze the organization of the mammary organoids, collagen gels containing organoids were fixed with 10% neutral buffered formalin (Sigma) for 30 minutes. Subsequently, they were washed twice for 20 minutes with 50 mM glycine in PBS followed by two 30 minutes washes with PBS. Gels were then incubated overnight at 4°C with 20% sucrose in PBS followed by incubation with 30% sucrose in PBS for 1 hour at ambient temperature. Finally, gels were incubated with OCT for 1 hour and then quick-frozen in a mixture of methanol and dry ice. Cryostat sections (5–10 μm thick) were cut and mounted onto glass slides. The samples were dried for 30 minutes and fixed for 10 minutes with 10% formalin or methanol/acetone (1:1). After rinsing three times with PBS containing 50 mM glycine, non-specific binding sites were blocked by incubation with PBS containing 15% FCS and 0.2% Tween 20 (blocking buffer) for 30 minutes at ambient temperature. Sections were then treated for 30 minutes with rabbit polyclonal antiserum against bovine keratins (1:10 dilution, Dako) and mouse monoclonal antibody against α-smooth muscle actin (1:200 dilution, Sigma) at ambient temperature. For detection of vimentin, sections were incubated with a mouse monoclonal antibody (1:200 dilution, Sigma) overnight at 4°C. For basement membrane staining, a rabbit polyclonal antibody against laminin (1:200 dilution, Sigma), and a rat monoclonal antibody against entactin (1:60 dilution, Upstate Biotechnologies) were used. After incubation with primary antibodies, slides were briefly washed five times with PBS and incubated with Texas Red-conjugated goat anti-mouse (1:100 dilution, Caltag), FITC-conjugated sheep anti-rabbit (1:100 dilution, Zymed) or Texas Red-conjugated goat anti-rat (1:100 dilution, Caltag) antibodies for 30 minutes at ambient temperature in blocking buffer. Slides were then washed five times with PBS and the nuclei were stained with DAPI (1:10,000 dilution in PBS, Sigma). Finally, they were mounted with Vectashield (Vector Laboratories).
To determine cell type distribution in the primary culture preparation, organoids were plated in glass chamber slides in medium containing 5% FCS and maintained in culture for 2 days. They were then washed twice with PBS, and fixed and stained as described above. The percentage of each cell type was determined by counting the number of cells positive for keratin, vimentin and α-smooth muscle actin in three randomly chosen areas of each slide. At least 200 cells were counted in each area. This experiment was carried out twice.
Proliferation assay and detection of apoptotic cells
Proliferation assays were carried out in 96-well plates with clustered SCp2 cells or primary organoids embedded in collagen gels. To embed the clusters/organoids, 50 μl of collagen was used for both the basal and the upper layers. The clusters/organoids were maintained for 6 days in 100 μl of chemically defined medium. To measure the cell number, 20 μl of Alamar Blue (Accumed International; Ahmed et al., 1994) were added to each well and incubated for 4 hours at 37°C. Absorbances were measured at 570 and 600 nm, and relative cell numbers were calculated as described by the manufacturer. Assays were carried out in quadruplicate.
Apoptosis was assessed by detection of FITC-labeled 3′OH DNA ends using an in situ apoptosis kit (Boehringer Mannheim) in 10 mm cryosections as previously described (Boudreau et al., 1996; Weaver et al., 1997).
Preparation of recombinant epimorphin
Soluble recombinant epimorphin was produced in Escherichia coli BL-21 and purified in the presence of 8 M urea as described previously (Oka and Hirai, 1996), with minor modifications (Hirai et al., 2001). Briefly, a deletion mutant missing the 3′ coiled-coil domain of epimorphin cDNA tagged with a 6× His sequence, called H12, was generated by PCR and inserted into the prokaryotic expression vector PET3a (Novagen). The recombinant protein was purified with Ni-NTA-agarose beads (Qiagen), and dialyzed against 150 mM NaCl for 1 hour, followed by 12 hours against PBS. It was then filter sterilized.
RT-PCR
For cDNA synthesis, 2.5 μg of total cellular RNA, prepared with TRIzol (Life Technologies) according to the manufacturer's instructions, and 20 units of reverse transcriptase (Boehringer Mannheim, Indianapolis, IN) was incubated at 37°C for three hours. Str1 was amplified with the 5′ primer (5′-GCT GCC ATT TCT AAT AAA GAG A-3′) and with the 3′ primer (5′-GCA CTT CCT TTC ACA AAG-3′) as described previously (Lochter et al., 1997). As a control for total RNA integrity, RT-PCR for actin was performed with the 5′ primer (5′- GCT GGT CGT CGA CAA CGG CT-3′) and the 3′ primer (5′-ATG ACC TGG CCG TCA GGC-3′). Absence of genomic DNA was verified by PCR for actin before cDNA synthesis. The resulting amplified fragments were analyzed on 1.5% ethidium bromide-stained agarose gels with an Eagle Eye II image analysis system (Stratagene, La Jolla, CA).
RESULTS
MMP activity is required for branching morphogenesis of primary mammary organoids
To study branching morphogenesis of primary mouse epithelial cells that were still in contact with each other and in a proper context, we isolated organoids from mammary glands of 10-week-old virgin mice as described in the Materials and Methods. Organoids were composed largely of epithelial and myoepithelial cells, along with a basement membrane, and a much smaller number of stromal cells. The preparation contained 64.9±2.1% keratin-positive and a-smooth muscle actin-negative luminal epithelial cells, 22.5±5.5% keratin positive and a-smooth muscle actin-positive myoepithelial cells, and 12.6±2.7% vimentin-positive fibroblasts. In cross-sections, many organoids were polar, with a central lumen surrounded by luminal epithelial and myoepithelial cells (Fig. 1A), and a basal, continuous basement membrane that contained laminin (Fig. 1B), entactin and collagen IV (data not shown). Thus, organoid cultures comprise several cell types, together with an endogenous BM, and should also contain some of the autocrine and paracrine factors present in the mammary tissue.
Fig. 1.
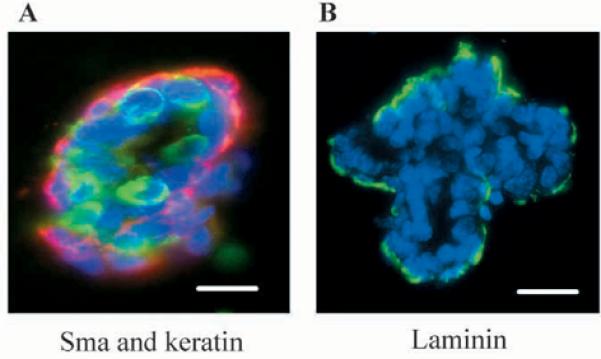
Primary organoids retain myoepithelial cells and basement membrane. (A) Detection of keratin-positive and α-smooth muscle actin-positive cells in primary mammary organoids at the time of preparation. Frozen sections of mammary organoids embedded in OCT were immunostained (A) simultaneously with mouse anti-α-smooth muscle actin to detect myoepithelial cells (red) and rabbit anti-keratin antibodies (green) as described in Materials and Methods. (B) Organoids were stained with rabbit anti-laminin antibodies (green). Scale bars: 50 μm. DAPI nuclear counterstain is in blue.
When mammary organoids were embedded in type I collagen gels, and incubated for 4 to 6 days in serum-free medium containing insulin as the only exogenous growth and survival factor, branching morphogenesis was low, with only 25–30% of organoids developing very short branches. EGF, HGF, bFGF and KGF all promoted branching in a dose-dependent manner (Fig. 2A,B). The organoids maintained their lumina even after several days in collagen, in both the presence and absence of growth factors (Fig. 2C).
Fig. 2.
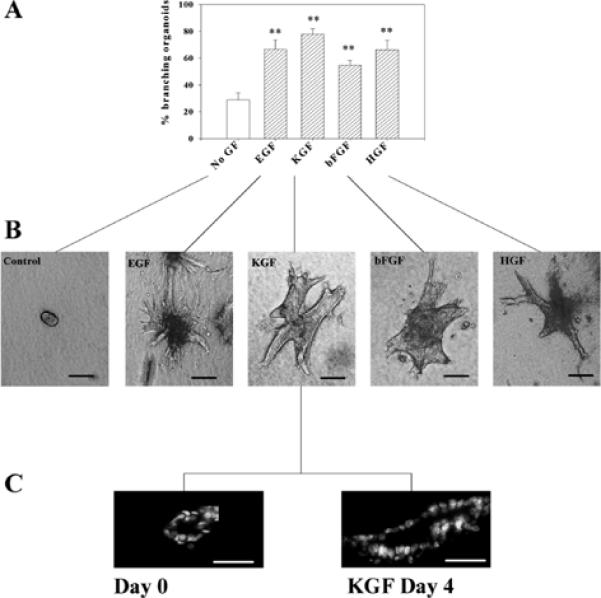
Growth factors induce branching morphogenesis in mammary organoids. Primary mammary organoids were embedded in collagen I gels and treated for 4 to 6 days with EGF, KGF, bFGF or HGF at a concentration of 50 ng/ml. (A) Quantification of the percentage of branching organoids for each of the treatments. The means±s.d. of three independent experiments are shown. The differences between the control group (no GF) and the growth factor-treated groups was statistically significant (**P≤0.01). (B) Appearance of control and growth factor-treated primary mammary organoids. (C) Cross sections of organoids showing the existence of a lumen at the day of preparation (day 0) and after 4 days of treatment with KGF. Scale bars: 200 μm in B; 100 μm in C.
Mammary organoids secreted latent and active isoforms of gelatinase A, latent gelatinase B and a high molecular weight gelatinase of 140 kDa demonstrable by substrate zymography (Fig. 3A). The gelatinolytic bands corresponding to gelatinase A and B, but not the 140 kDa gelatinase, were inhibited by the metalloproteinase inhibitor, 1,10 phenanthroline (not shown). Mammary organoids also secreted Str1, as determined by casein zymography (Fig. 3B) and urokinase plasminogen activator (uPA), as determined by casein-plasminogen zymography (Fig. 3C). Addition of growth factors did not change the activation status of any of the secreted proteinases.
Fig. 3.
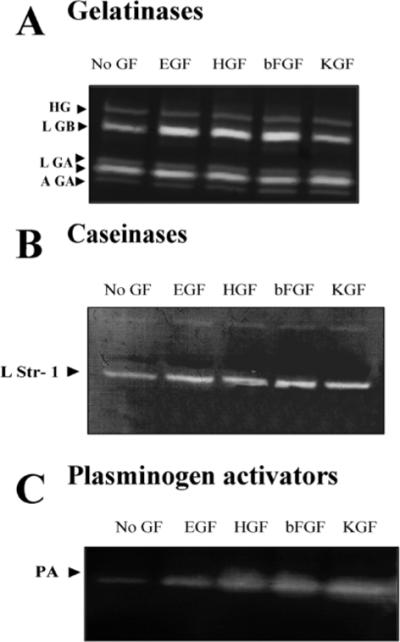
Growth factors increase the level but not the activation status of proteinases secreted by primary mammary organoids. Mammary organoids were treated for 4 to 6 days in collagen I gels with no growth factor (No GF) or with EGF, HGF, bFGF or KGF. Conditioned medium was collected, concentrated and run on substrate zymograms. (A) Gelatin zymogram. Latent gelatinase B (LGB), active and latent gelatinase A (AGA, LGA) and a high molecular weight gelatinase (HG) were detected. (B) Casein zymogram. A band corresponding to the molecular weight of latent stromelysin-1 (LStr1) was detected. (C) Plasminogen casein zymogram. A band corresponding to the molecular weight of uPA was detected. There were no differences in the activation status of the gelatinases, caseinase or plasminogen activator between growth factor treated organoids and untreated controls.
To determine whether proteinases were involved in branching morphogenesis, organoids were cultured in the presence of proteinase inhibitors. Inhibitors specific for cysteine proteinases (E64), serine proteinases (leupeptin and aprotinin) and aspartic proteinases (pepstatin) were without effect on branching morphogenesis of mammary organoids maintained in the presence or absence of growth factors. By contrast, treatment with the metalloproteinase inhibitor, GM6001, but not GM1210, an inactive structural homolog, dramatically reduced the ability of organoids to extend branches (Fig. 4A, parts a,b). Inhibition of branching by GM6001 occurred regardless of which growth factor was added to the culture medium. Furthermore, the background branching, which occurred in the absence of exogenous growth factors, was reduced further with GM6001 (Fig. 4A, part a). Inhibition of branching by GM6001 was completely reversible: organoids grown for 4 days in the presence of EGF and GM6001 began to branch after GM6001 was removed, and they were grown with EGF alone for an additional 6 days (not shown). To confirm that the effect seen with GM6001 was indeed due to a specific inhibition of MMP activity, we treated primary organoids in the presence of KGF with either recombinant TIMP1 or TIMP2. We found that both TIMPs inhibited branching morphogenesis to about the same extent as GM6001 (Fig. 4A, parts c,d). As with GM6001, the TIMP inhibition was reversible (not shown). These data indicate that MMPs are required for branching, and that the MMP inhibitors had not affected the viability of organoids.
Fig. 4.
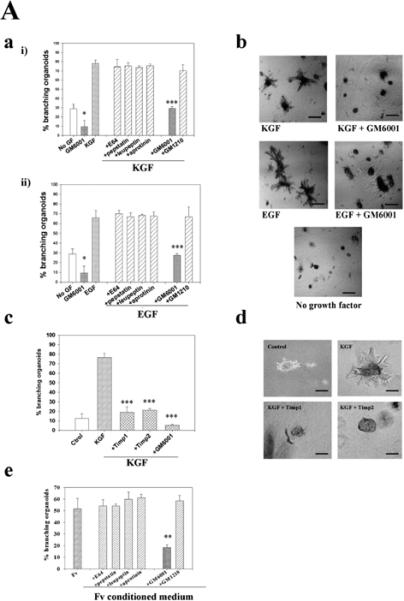
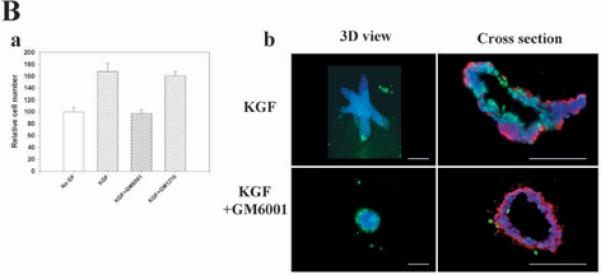
Proteinase inhibitors suppress branching morphogenesis induced by growth factors. (A, part a) Quantification of the percentage of branching organoids treated with (i) KGF or (ii) EGF in the presence of protease inhibitors E64, pepstatin, leupeptin, aprotinin, GM6001 and GM1210. Only the MMP inhibitor GM6001 had a statistically significant inhibitory effect on branching morphogenesis of both basal (*P≤0.05) and growth factor-treated organoids (***P≤0.001). The mean±s.d. of six samples in two independent experiments are shown. (A, part b) Primary organoids after 6 days of treatment with KGF and EGF with or without GM6001. Note the absence of branches in the organoids treated with the MMP inhibitor. Similar results were obtained with bFGF and HGF (not shown). (A, part c) Quantification of branching and (A, part d) appearance of the organoids treated with TIMP1 and TIMP2 in the presence of KGF. The mean±s.d. of six (TIMP1) and five (TIMP2) samples in two independent experiments are shown; (***P≤0.001). (A, part e) Quantification of the effect of proteinase inhibitors on branching morphogenesis induced by co-culturing the organoids with primary fibroblasts from virgin animals (Fv). Only GM6001 had a statistically significant inhibitory effect (**P≤0.01). The mean±s.d. of six samples in two independent experiments are shown. (B) GM6001 inhibits increase in cell number in organoids (B, part a), but does not alter apoptosis. Primary organoids were embedded in collagen I gels and treated for 6 days with KGF in the presence and absence of GM6001. (B, part a) Cell number was measured using Alamar Blue. The mean±s.d. of six samples in two independent experiments are shown. (B, part b) Whole organoids (3D) and cross sections are shown. Apoptotic nuclei were stained in green (FITC). Cross sections were also stained for α-smooth muscle actin-positive cells (red). There were no statistical differences in the number of apoptotic cells in control and treated groups. DAPI nuclear counterstain is in blue. Scale bars: 400 μm in A; 200 μm in B.
Mammary fibroblasts secrete HGF and other growth factors and also promote branching morphogenesis of mammary epithelia (reviewed by Cunha, 1994). We found that co-culture of organoids with mammary fibroblasts stimulated branching morphogenesis to about the same extent as individual growth factors (Fig. 4A, part e). This epithelial branching was also strongly inhibited by GM6001, but not by inhibitors of other classes of proteinases. These data suggest that the effects of mesenchymal cells on mammary branching, even if growth factor mediated, require MMP activity.
Branching morphogenesis requires both cell proliferation and invasion of the surrounding extracellular matrix. To test whether the MMP inhibitor was affecting cell proliferation in organoids cultured in collagen gels, we determined cell number in the presence of KGF and KGF plus GM6001 for 6 days. We found a decrease in cell number in those organoids treated with the MMP inhibitor (Fig. 4B, part a). This decrease could be due to an increase in cell death or a decrease in cell proliferation. We therefore stained organoids treated with KGF and KGF plus GM6001 for evidence of apoptosis, and found that there were low numbers of dying cells in both treated and untreated organoids (Fig. 4B, part b). In either case, organoids maintained a lumen and were positive for smooth muscle actin. Our results suggest that MMPs affect branching morphogenesis of primary organoids cultured in collagen I gels by affecting both cell invasion and proliferation. However, addition of GM6001 to isolated epithelial cells in collagen gels did not inhibit proliferation (see below), indicating that inhibition of cell proliferation is a secondary effect in the organoids.
Stromelysin 1 can induce branching in mammary organoids
The experiments using MMP inhibitors indicate that these proteinases are necessary for branching of primary organoids. If MMPs acted downstream of growth factors, then addition of MMPs, in the absence of growth factors, should be sufficient for branching. This indeed was the case: addition of activated recombinant Str1 (rStr1) to organoid cultures resulted in a dose-dependent increase in branching (Fig. 5A,C). The fact that the rStr1 was catalytically active was shown by its ability to activate endogenous gelatinase B (Fig. 5D). Expression and activation of gelatinase A was not affected (Fig. 5D). The effect of rStr1 on branching, however, was not due to activation of gelatinase B. Treatment of organoids with active recombinant gelatinase B did not induce branching. Nor was branching affected by active recombinant gelatinase A (data not shown). As expected, the effect of rStr1 on branching morphogenesis was inhibited with GM6001. These data suggest an important and possibly specific role for Str1 in branching morphogenesis.
Fig. 5.
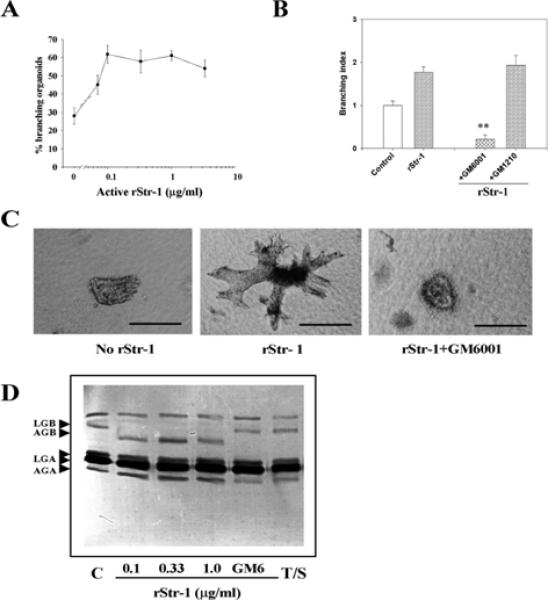
Recombinant stromelysin-1 induces branching morphogenesis of primary mammary organoids. (A) Dose response to rStr1. Mean±s.d. of three independent experiments is shown. (B) GM6001 inhibits rStr1-induced branching. rStr1 (1μg/ml) induced-branching morphogenesis compared with the control (**P<0.01). Control samples were treated with trypsin and soy bean trypsin inhibitor (SBTI), GM6001, (***P≤0.001). The means±s.d. of three independent experiments are shown. To calculate the branching index, control values were equated to 1. (C) Appearance of organoids in collagen I gels after 6 days with or without Str1 and GM6001 treatments. (D) Gelatin zymogram of conditioned media of organoids treated with buffer (Control, C), or different concentration of rStr1, with or without GM6001 (GM6) or trypsin+SBTI (T/S). Note the activation of gelatinase B in the presence of rStr1. This activation is inhibited in the presence of GM6001, as expected. Treatment with trypsin+SBTI (T/S) did not activate gelatinase B. Scale bars: 200 μm in C.
Plasmin is a positive regulator of branching morphogenesis
uPA is expressed at high levels in the mammary gland of the virgin mouse (Talhouk et al., 1992; Ossowski et al., 1979). To determine whether the plasminogen cascade has a physiologically relevant role in mammary gland branching, we added uPA, plasminogen or plasmin to primary organoid cultures. uPA had no effect on its own, but enhanced the branching effect induced by plasminogen slightly. Plasminogen and plasmin increased branching (Fig. 6A–C). The effect of plasmin was blocked by the serine proteinase inhibitor aprotinin (Fig. 6B), but not by aspartic and cysteine proteinase inhibitors pepstatin and E64 (data not shown).
Fig. 6.
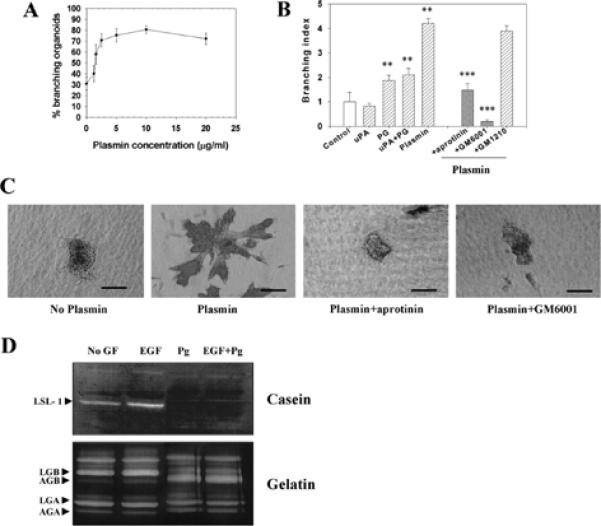
Plasmin induces branching morphogenesis of primary mammary organoids. (A) Dose response to increasing concentrations of plasmin. Mean±s.d. of three independent experiments is shown. (B) Effect of protease inhibitors on plasmin-induced branching morphogenesis. The difference with the control group (buffer) was statistically significant (**P≤ 0.01). Inhibition by both aprotinin and GM6001 were statistically significant (***P≤ 0.001). Mean±s.d. of three independent experiments is shown. To calculate the branching index, control values were taken as equal to 1. (C) Appearance of organoids treated for 6 days with no plasmin, or plasmin, with and without inhibitors. (D) Activation of MMPs by plasminogen. Casein zymogram (top panel) of conditioned media from organoids treated with buffer only (no GF), EGF, plasminogen (Pg) and EGF+plasminogen (EGF+Pg). When plasminogen was added to the treatment, the band corresponding to the latent form of Str1 disappeared. The bottom panel is a gelatin zymogram, using the same set of conditioned media as for the casein zymogram. Gelatinase B was activated in the presence of plasminogen. Scale bars: 150 μm in C.
Plasmin could act on branching either directly or indirectly by activating MMPs. Plasmin is known to activate Str1 and other MMPs (Nagase et al., 1990; Mazzieri et al., 1997), and its addition led to the disappearance of the band corresponding to the latent form of Str1, and the activation of gelatinase B, as shown by casein and gelatin zymography respectively (Fig. 6D). Furthermore we found that GM6001 inhibited the effect of plasmin on branching morphogenesis (Fig. 6B,C). This result implies that plasmin acts indirectly through MMP activation. These results suggest that plasminogen/ plasmin treatment of primary mammary organoids leads to branching morphogenesis through MMP activation.
Plasminogen/plasmin also synergized with growth factors in mammary organoid branching. The addition of plasminogen together with any of the four growth factors significantly increased both the percentage of branching organoids (Fig. 7A,B) and the number of branches per organoid (Fig. 7B). The same result was obtained using plasmin (data not shown). The fact that the number of branches increased when plasminogen/ plasmin were added together with the growth factors, implies that the number of branch initiation sites may be partly regulated by proteinase activity.
Fig. 7.
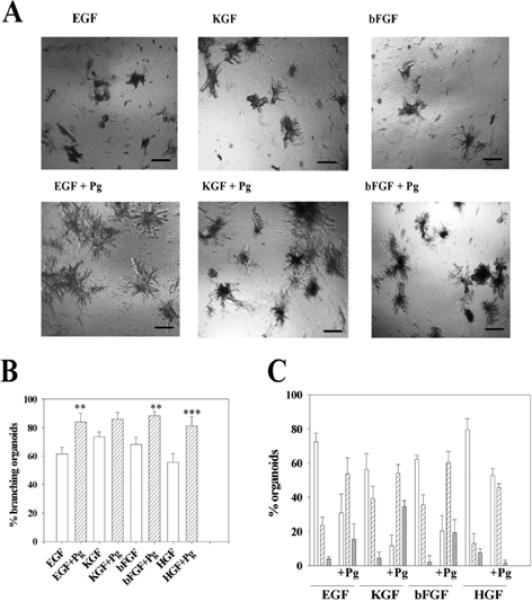
Plasminogen and plasmin synergize with growth factors. (A) Appearance of organoids treated with growth factors with or without added plasminogen (Pg). Note the increase in the size and number of branches of the organoids when plasminogen was added together with the growth factors. (B) Effect of plasminogen on the percentage of organoids with branches. Shown are the percentage of branching organoids in the presence of growth factors alone (white bars) and growth factors+Pg (hatched bars). The increase in the number of organoids with branches when plasminogen was added together with the growth factor, compared with the group without plasminogen was statistically significant in all cases except for the KGF group, (**, P≤ 0.01) and (***, P≤ 0.001). (C) Effect of plasminogen on the number of branches per organoid: one to five branches (white bars), six to ten branches (hatched bars) and more than ten branches (cross hatched bars). There were increased branches per organoid also for the KGF-treated cultures. The figures show the mean±s.d. of six wells in two independent experiments. Scale bars: 400 μm in A.
Epimorphin regulates branching morphogenesis in a MMP-dependent manner
The data presented thus far show that MMPs and/or growth factors are sufficient to promote branching morphogenesis in organoid cultures that contain luminal epithelial, myoepithelial and mesenchymal cells. However, myoepithelial and mesenchymal cells produce several factors including morphogens (Hirai et al., 1998) that may additionally affect the outcome of branching of the luminal cells in the organoids. To define the minimum requirements for branching of luminal epithelial cells, we next used SCp2 cells, a cloned mouse mammary epithelial cell line that can branch in three-dimensional type I collagen gels in the presence of epimorphin, a morphoregulatory molecule (Hirai et al., 1998).
We found that GM6001 inhibited epimorphin-induced branching of preclustered SCp2 cells embedded in collagen I gels (Fig. 8A,B). This result indicates that, as in the case of the primary mammary organoids, MMP activity is necessary for branching morphogenesis of mammary epithelial cell lines in the presence of epimorphin. This raises the question of whether epimorphin signaling modulates MMP activity. Treatment of SCp2 cells with epimorphin for 3 days led to a striking increase in the amounts of secreted gelatinase A, gelatinase B and Str1 as detected by casein and gelatin zymography (Fig. 8B). Str1 mRNA was also upregulated by epimorphin, as determined by RT-PCR (Fig. 8C). Taken together with the fact that GM6001 inhibited branching morphogenesis of SCp2 cells, these data suggest that MMPs are downstream of epimorphin. However, our results suggest that morphoregulatory signals induced by epimorphin are also required. In contrast to organoids, where epimorphin is provided by myoepithelial cells, Str1 added by itself or with growth factors was not sufficient to induce branching in the SCp2 cells (data not shown).
Fig. 8.
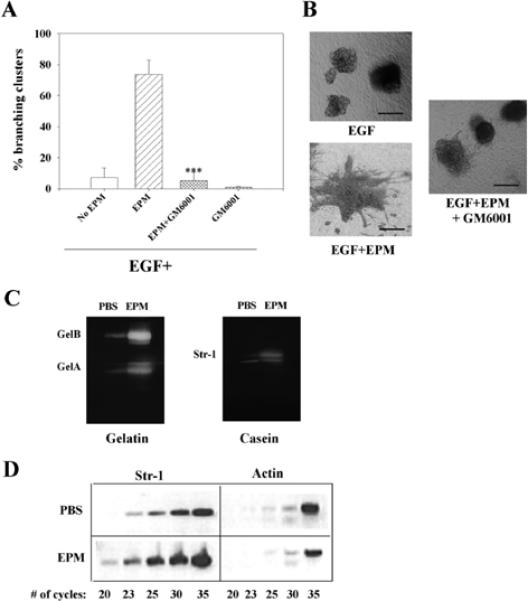
GM6001 inhibits epimorphin-induced branching morphogenesis in a mammary epithelial cell line. (A) Preclustered SCp2 cells were treated for 6 days in collagen I gels with EGF, EGF+epimorphin (EPM) with or without GM6001. GM6001 inhibited branching induced by epimorphin. This inhibition was statistically significant (***P≤0.001). Other proteinase inhibitors such as leupeptin, aprotinin, pepstatin and E64 did not show any inhibition of branching (not shown). The mean±s.d. of three independent experiments is shown. (B) Appearance of SCp2 cell clusters treated with EGF, EGF+EPM and EGF+EPM+GM6001. (C) Epimorphin upregulates gelatinases A and B and Str1 in SCp2 cells. SCp2 cells were treated for 48 hours with epimorphin or PBS as a control. Conditioned medium was collected, concentrated and separated on substrate gels. (D) Epimorphin regulates Str1 mRNA levels. RT-PCR analysis of Str1 expression in SCp2 cells treated with PBS or EPM for 48 hours. Twenty, 23, 25, 30 and 35 cycles of amplification were performed for detection of Str1 and actin. While the level of actin was not different statistically in the PBS and epimorphin-treated cultures (slightly higher in PBS in this experiment), Str1 levels were clearly higher in epimorphin-treated cultures. Scale bars: 200 μm in A.
Are MMPs required for growth or morphogenesis?
Proliferation is necessary to visualize branching morphogenesis (Hirai et al., 1998). Because in all the experiments described above, GM6001 led to an inhibition of branching, it was important to determine if inhibition by GM6001 was due to inhibition of proliferation of epithelial cells or whether it exerted its effect by inhibiting MMPs. SCp2 cell clusters embedded in collagen gels were treated with GM6001. In contrast to what we found with the primary organoids, there was no effect on cell number after 6 days of treatment with the inhibitor (Fig. 9). As a positive control for growth inhibition, we used TGFβ1, which inhibited mammary epithelial cell proliferation as expected (Fig. 9). Our results indicate that under the experimental conditions used, MMPs are not necessary for epithelial cell proliferation, but are necessary for branching morphogenesis.
Fig. 9.
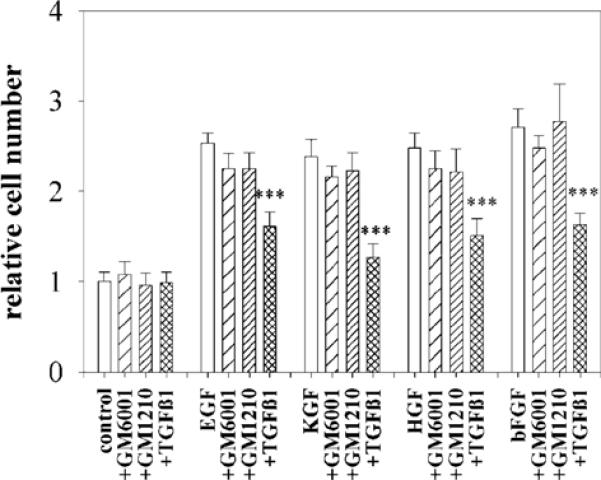
GM6001 does not inhibit SCp2 cell proliferation. SCp2 cells were embedded in collagen I gels and treated for six days with no growth factor, or with EGF, KGF, HGF, bFGF in the presence and absence of GM6001, GM1210 and TGFβ1. Quantification of cell number was carried out using Alamar Blue. The mean±s.d. of three independent experiments is shown. The data are normalized with values obtained for the control set to 1. Only TGFβ1 significantly reduced the cell number (***P≤0.001).
DISCUSSION
The goal of the present study was to delineate how the many different factors that have been postulated to be involved in mammary gland branching morphogenesis interact to bring about this complex morphogenetic end point. To determine the relationship between the various factors implicated in branching morphogenesis, we used two model systems: primary mammary epithelial organoids and SCp2 cells; a nontumorigenic and functionally normal mouse mammary epithelial cell line; both were cultured inside type I collagen gels. Our results show that MMP action is indispensable for mammary branching morphogenesis induced by both growth factors and epimorphin, that Str1 (MMP3), together with epimorphin, is sufficient to promote branching morphogenesis, and that plasmin, through activation of MMPs, is also a positive regulator of branching.
Polypeptide growth factors are expressed at elevated levels during ductal elongation in the mammary gland. In our assays EGF, HGF, KGF and bFGF increased branching of organoids without affecting activation of gelatinase A and gelatinase B, Str1 and uPA. Mechanisms of branching in other tissues may differ from the mammary gland. In embryonic lung rudiments, EGF and TGFα upregulate the expression of gelatinase A and gelatinase B; however, in this case, addition of growth factors promote growth, but inhibit branching morphogenesis (Ganser et al., 1991). In MDCK cells maintained in three-dimensional cultures, HGF increases uPA and uPAR, and also stimulates branching morphogenesis (Pepper et al., 1992). FGF10 has recently been identified as a mesenchyme-derived factor that plays a crucial role in patterning the early branching events in the mammalian lung and limb (Min et al., 1998). This growth factor is expressed in the mammary gland and promotes branching in culture (unpublished observation), but whether or not it is linked to proteinase activity still needs to be established. Thus, growth factors affect branching and proteinase expression in a tissue and cell type-specific manner in different systems; caution should be exercised when results from one cell type are applied to another.
Whereas inhibitors of serine, cysteine and aspartic proteinases did not affect morphogenesis induced by growth factors in primary organoids, the MMP inhibitors GM6001, TIMP1 and TIMP2, strongly impaired growth factor-induced branching. This suggests that MMPs are important modulators of this morphogenetic process in the mammary gland. Consistent with this notion, branching morphogenesis of primary mammary organoids was stimulated by an active form of recombinant Str1. These data, together with previous observations from our laboratory and others with transgenic mice expressing an auto-activated form of Str1 in mammary epithelium (Sympson et al., 1994; Witty et al., 1995), provide crucial evidence that Str1 has a selective role in mammary gland branching morphogenesis both in vivo and in culture. This is further supported by the fact that Str1 is produced at elevated levels during branching morphogenesis, compared with the resting, non-growing gland (Talhouk et al., 1991), and that its highest levels are found around end buds and near branch points (Witty et al., 1995). Moreover, Str1-null and TIMP1-overexpressing mice show less branching than their respective age- and estrous-matched littermates (M. Sternlicht, B. Wiseman, M. S., M. J. B. and Z. W., unpublished). Finally, analysis of mammary glands from mice with implanted pellets of TIMP1 show decreased ductal development (Fata et. al., 1999), supporting our results regarding the requirement of MMP activity during branching morphogenesis.
The pathway by which Str1 stimulates branching remains to be determined. We observed activation of gelatinase B by Str1 when the conditioned medium of organoids was analyzed. Recombinant gelatinase B, however, did not have any effect on its own when added to the cultures, suggesting that activation of gelatinase B does not directly mediate the effect of Str1 on branching. Gelatinase A also failed to affect branching. However, preliminary analysis of MMP-null mice indicates that gelatinase A does have a role in elongation of primary ducts, but not side branching (M. Sternlicht, B. Wiseman, M. S., M. J. B. and Z. W., unpublished). The correct spatial distribution or activation of these enzymes may be a factor in the branching process, and this may not be recapitulated by adding a recombinant protein to the culture medium.
MMPs may exert morphoregulatory activity by proteolysis of ECM components, resulting in a release of mechanical restraints. ECM cleavage could also release growth factors or other morphoregulatory molecules from the ECM or could unmask cryptic functional sites in laminin, for example. Laminin 1-derived peptides have been shown to enhance tumor cell invasion (Bresalier et al., 1995), and cleavage of laminin 5 by gelatinase A promotes locomotion of otherwise nonmotile mammary epithelial cells (Giannelli et al., 1997). Alternatively, MMPs may act through cleavage of cell surface molecules such as selectins and cell-cell adhesion molecules such as E-cadherin (Lochter et al., 1998), a process that may promote cell rearrangements and morphogenesis by loosening cell-cell contacts, or by triggering specific signal transduction pathways.
Analogous to Str1, uPA is developmentally regulated in the mouse mammary gland. Enzyme content and mRNA levels are high in the glands of virgin, early pregnant and involuting mice, and are low during late gestation and lactation (Busso et al., 1989). Nevertheless, when uPA was overexpressed in the mammary gland, there was no report of disruption of mammary morphogenesis, although the proteinase was secreted into the milk of the transgenic mice (Pittius et al., 1988; Hennighausen et al., 1991). Likewise, in the present study, uPA did not affect branching when added to the organoids, and growth factor-induced branching was not affected by serine proteinase inhibitors. However, we found that plasmin enhanced branching morphogenesis and activated latent MMPs in primary mammary organoids, and that plasmin-induced branching was inhibited by both serine and MMP inhibitors. These results point to a model in which branching morphogenesis is an MMP-dependent process and where Str1 activation is downstream from plasmin. This mechanism of MMP activation by plasmin has also been suggested to be involved in formation of aneurysms (Carmeliet et al., 1997). Previous culture studies using the NMuMG mammary cell line, also described an increase in uPA activity when cells were induced to branch by medium conditioned by fibroblasts; the branching process was inhibited by treatment with a serine proteinase inhibitor (Delannoy-Courdent et al., 1996; Delannoy-Courdent et al., 1998). Similar results were obtained using MDCK cells, which are derived from kidney (Pepper et al., 1992). Other investigators have shown that invasion of collagen matrices by MDCK cells is stimulated by MT-MMPs (Hotary et al., 2000). In our system, plasmin-induced branching morphogenesis was inhibited by a MMP inhibitor. It appears, therefore, that MMP activation by serine proteases may be a theme occurring in several cell types and biological processes.
Primary organoids are heterogeneous, and are composed of luminal epithelial and myoepithelial cells and fibroblasts. As such, they already contain epimorphin and basement membrane components such as laminin, entactin and type IV collagen (Hirai et al., 1998). Despite being a physiologically relevant model system for branching morphogenesis, it is difficult to determine which endogenous factors act on which cell types. To define the minimum requirements for branching morphogenesis in epithelial cells, we used a cloned luminal epithelial cell line. We have previously shown that branching morphogenesis of SCp2 cells in collagen I gels requires the presence of recombinant epimorphin (Hirai et al., 1998). We now show that epimorphin-induced branching is inhibited by GM6001, an MMP inhibitor, but not by serine, cysteine or aspartic proteinase inhibitors. Interestingly, epimorphin upregulated both gelatinases and Str1 in SCp2 cells. However, in contrast to primary mammary organoids, addition of recombinant Str1 to SCp2 cells did not induce branching, nor did it enhance the effect of epimorphin. This suggests that, while required for branching, MMPs are not by themselves sufficient to induce a full morphogenetic program in luminal epithelial cells, and that morphogenesis requires additional factors that are provided by myoepithelial cells and/or fibroblasts. Epimorphin is a prime candidate, because its addition to SCp2 cells was sufficient to induce branching in the absence of other cell types. Thus, epimorphin and MMP activity together constitute the minimum requirement for branching morphogenesis of mammary epithelial cells. The effect of epimorphin on morphogenesis is enhanced dramatically when growth factors are added (Hirai et al., 1998). Epimorphin and MMPs do not affect proliferation of mammary epithelial cells, and may thus be considered morphoregulatory factors. Growth factors provide the proliferative engine to manifest branching morphogenesis. The fact that we found MMP activity was required for primary mammary organoid proliferation but not for SCp2 cell proliferation suggests that, in the organoids, this may be a secondary effect. For example, the myoepithelial cells or the basement membrane may require remodeling to enable the release of active growth factors required for the proliferation of the epithelial cells within the organoids. However, it is also possible that growth within the organoids requires the degradation of the basement membrane and that this does not occur in the absence of MMP activity.
While epimorphin is expressed abundantly in and around the parenchyma in the mammary gland at all stages of development (Hirai et al., 1998), MMP and growth factor expression correlate well with tissue remodeling periods in the breast (Yang et al., 1995). As such, epimorphin could be viewed as a primary signal for branching (Hirai et al., 1998), but the actual timing of morphogenesis would occur through local regulation of MMP and growth factor expression. Growth factors, which must also be tightly regulated, would enable proliferation, which is essential for the development of new structures, but would not have a morphoregulatory role of their own, as discussed previously (Hirai et al., 1998). The finding in this paper that epimorphin can regulate MMP levels and activities raises the question of how and when the epithelial cells perceive epimorphin in the mammary gland during different stages of development. Thus, identification and regulation of the putative epimorphin receptors could help further define the temporal regulation in vivo.
Additional candidate molecules thought to regulate MMPs locally during morphogenesis are ECM constituents, many of which affect MMP expression and are precisely regulated during mammary development. We have previously shown that a reconstituted basement membrane (Matrigel) can regulate Str1 expression in mammary cell lines (Lochter et al., 1997). How the ECM molecules and their receptors are linked to MMP activity to bring about branching morphogenesis remains to be determined. Interestingly, the a2b1 integrin, which is necessary for mammary branching morphogenesis (Berdichevsky et al., 1994), can also mediate invasion of mammary carcinoma cells through basement membrane-coated filters by regulating the level of Str1 expression (Lochter et al., 1999). Our previous finding that Str1 can induce epithelial-to-mesenchymal conversion in mammary cells (Lochter et al., 1998) leads us to propose that such conversion may occur transiently at the tip of a growing branch allowing the normal epithelia to `invade' the surrounding fat pad. Although appealing, this possibility requires careful investigation.
A role for ECM remodeling in epithelial branching morphogenesis has long been hypothesized (Bernfield et al., 1984). It is only now that a link between matrix-degrading proteinase activity and morphogenesis has been established experimentally. In this study, we have used both a complex system of primary epithelial organoids and a simpler mammary epithelial cell line in collagen I gels to show that while MMP activity is an essential requirement for branching, specific morphogens and growth factors are also important players. Further studies using both culture models and in vivo studies are expected to broaden our understanding of how these factors interact in mammary gland development and to further delineate the signal transduction pathways involved.
Acknowledgments
We are grateful to R. Galardy for providing GM6001 and GM1210, to Joni Mott and Michael Banda for the recombinant TIMPs, to Lana Spivak, Jane Liaw and Davida Flattery for excellent technical assistance, to Philippe Pujuguet, Rick Schwarz and Derek Radisky for helpful discussions, and to Amy Ukena and Norene Jelliffe for expert administrative assistance. This work was supported by the US Department of Energy, Office of Biological and Environmental Research (contract DE-AC03-76SF00098) to M. J. B., the National Cancer Institute (CA 57621 to Z. W. and M. J. B., and CA 58207 to Z. W.) and by funds from the Human Frontiers Science Program (RG0051/1999M to Z. W.). Y. H. was supported by the Science and Technology Agency of Japan.
REFERENCES
- Ahmed SA, Gogal RM, Jr, Walsh JE. A new rapid and simple non radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H ]thymidine incorporation assay. J. Immunol. Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Basbaum CB, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr. Opin. Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- Berdichevsky F, Alford D, D' Souza B, Taylor-Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J. Cell Sci. 1994;107:3557–3568. doi: 10.1242/jcs.107.12.3557. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Banerjee SD, Koda JE, Rapraeger AC. Remodelling of the basement membrane: morphogenesis and maturation. Ciba Found. Symp. 1984;108:179–196. doi: 10.1002/9780470720899.ch12. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc. Nat. Acad. Sci. USA. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresalier RS, Schwartz B, Kim YS, Duh QY, Kleinman HK, Sullam PM. The laminin alpha 1 chain Ile-Lys-Val-Ala-Val (IKVAV) containing peptide promotes liver colonization by human colon cancer cells. Cancer Res. 1995;55:2476–2480. [PubMed] [Google Scholar]
- Busso N, Huarte J, Vassalli JD, Sappino AP, Belin D. Plasminogen activators in the mouse mammary gland. Decreased expression during lactation. J. Biol. Chem. 1989;264:7455–7457. [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genet. 1997;17:439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- Coleman S, Silberstein GB, Daniel CW. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev. Biol. 1988;127:304–315. doi: 10.1016/0012-1606(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Coleman-Krnacik S, Rosen JM. Differential temporal and spatial gene expression of fibroblast growth factor family members during mouse mammary gland development. Mol. Endocrinol. 1994;8:218–229. doi: 10.1210/mend.8.2.8170478. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74:1030–1044. doi: 10.1002/1097-0142(19940801)74:3+<1030::aid-cncr2820741510>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB, Van Horn K, Strickland P, Robinson S. TGF-beta 1-induced inhibition of mouse mammary ductal growth: developmental specificity and characterization. Dev. Biol. 1989;135:20–30. doi: 10.1016/0012-1606(89)90154-1. [DOI] [PubMed] [Google Scholar]
- Delannoy-Courdent A, Fauquette W, Dong-Le Bourhis XF, Boilly B, Vandenbunder B, Desbiens X. Expression of c-ets-1 and uPA genes is associated with mammary epithelial cell tubulogenesis or neoplastic scattering. Int. J. Dev. Biol. 1996;40:1097–1108. [PubMed] [Google Scholar]
- Delannoy-Courdent A, Mattot V, Fafeur V, Fauquette W, Pollet I, Calmels T, Vercamer C, Boilly B, Vandenbunder B, Desbiens X. The expression of an Ets1 transcription factor lacking its activation domain decreases uPA proteolytic activity and cell motility, and impairs normal tubulogenesis and cancerous scattering in mammary epithelial cells. J. Cell. Sci. 1998;111:1521–1534. doi: 10.1242/jcs.111.11.1521. [DOI] [PubMed] [Google Scholar]
- Desprez PY, Roskelley C, Campisis J, Bissell MJ. Isolation of functional cell lines from a mouse mammary epithelial cell strain: the importance of basement membrane and cell-cell interaction. Mol. Cell Differ. 1993;1:99–110. [Google Scholar]
- Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Perturbation of beta1-integrin function alters the development of murine mammary gland. EMBO J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Leco KJ, Moorehead RA, Martin DC, Khokha R. Timp-1 is important for epithelial proliferation and branching morphogenesis during mouse mammary development. Dev. Biol. 1999;211:238–254. doi: 10.1006/dbio.1999.9313. [DOI] [PubMed] [Google Scholar]
- Galardy RE, Cassabonne ME, Giese C, Gilbert JH, Lapierre F, Lopez H, Schaefer ME, Stack R, Sullivan M, Summers B, et al. Low molecular weight inhibitors in corneal ulceration. Ann. New York Acad. Sci. 1994;732:315–323. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- Ganser GL, Stricklin GP, Matrisian LM. EGF and TGF alpha influence in vitro lung development by the induction of matrix-degrading metalloproteinases. Int. J. Dev. Biol. 1991;35:453–461. [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Gijbels K, Galardy RE, Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J. Clin. Invest. 1994;94:2177–2182. doi: 10.1172/JCI117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Epithelial morphogenesis. Cell. 1992;69:385–387. doi: 10.1016/0092-8674(92)90440-n. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Westphal C, Sankaran L, Pittius CW. Regulation of expression of genes for milk proteins. Biotechnology. 1991;16:65–74. [PubMed] [Google Scholar]
- Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, Bissell MJ. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J. Cell Biol. 1998;140:159–169. doi: 10.1083/jcb.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Radisky D, Boudreau R, Simian M, Stevens M, Oka Y, Takebe K, Shinichiro N, Bissell MJ. Epimorphin mediates mammary luminal morphogenesis through control of C/EBPβ. J. Cell Biol. 2001;153:785–794. doi: 10.1083/jcb.153.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J. Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely PJ, Wu JE, Santoro SA. The spatial and temporal expression of the alpha 2 beta 1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation. 1995;59:1–13. doi: 10.1046/j.1432-0436.1995.5910001.x. [DOI] [PubMed] [Google Scholar]
- Korpsmeier KH. Proliferation kinetics in the mammary gland of the mouse during postnatal development. Anat. Anz. 1979;145:313–318. [PubMed] [Google Scholar]
- Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J. Biol. Chem. 1997;272:5007–5015. doi: 10.1074/jbc.272.8.5007. [DOI] [PubMed] [Google Scholar]
- Lochter A, Sternlicht MD, Werb Z, Bissell MJ. The significance of matrix metalloproteinases during early stages of tumor progression. Ann. New York Acad. Sci. 1998;23:180–93. doi: 10.1111/j.1749-6632.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- Lochter A, Navre M, Werb Z, Bissell MJ. α1 and α2 integrins mediate invasive activity of mouse mammary carcinoma cells through regulation of stromelysin-1 expression. Mol. Biol. Cell. 1999;10:271–282. doi: 10.1091/mbc.10.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev ME, Werb Z. ECM signaling: orchestrating cell behavior and misbehavior. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- Mazzieri R, Masiero L, Zanetta L, Monea S, Onisto M, Garbisa S, Mignatti P. Control of type IV collagenase activity by components of the urokinase- plasmin system: a regulatory mechanism with cell-bound reactants. EMBO J. 1997;16:2319–2332. doi: 10.1093/emboj/16.9.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Nagase H, Enghild JJ, Suzuki K, Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4- aminophenyl)mercuric acetate. Biochemistry. 1990;29:5783–5789. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- Niranjan B, Buluwela L, Yant J, Perusinghe N, Atherton A, Phippard D, Dale T, Gusterson B, Kamalati T. HGF/SF a potent cytokine for mammary growth, morphogenesis and development. Development. 1995;121:2897–908. doi: 10.1242/dev.121.9.2897. [DOI] [PubMed] [Google Scholar]
- Oka Y, Hirai Y. Inductive influences of epimorphin on endothelial cells in vitro. Exp. Cell. Res. 1996;222:189–198. doi: 10.1006/excr.1996.0024. [DOI] [PubMed] [Google Scholar]
- Ossowski L, Bieel D, Reich E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979;16:929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Matsumoto K, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor increases urokinase-type plasminogen activator (u-PA) and uPA receptor expression in Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 1992;267:20493–20496. [PubMed] [Google Scholar]
- Pittius CW, Sankaran L, Topper YJ, Hennighausen L. Comparison of the regulation of the whey acidic protein gene with that of a hybrid gene containing the whey acidic protein gene promoter in transgenic mice. Mol. Endocrinol. 1988;2:1027–1032. doi: 10.1210/mend-2-11-1027. [DOI] [PubMed] [Google Scholar]
- Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, Derynck R, Hom YK, Cunha GR, DiAugustine RP. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998;9:777–785. [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science. 1987;237:291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Soriano JV, Orci L, Montesano R. TGF-beta1 induces morphogenesis of branching cords by cloned mammary epithelial cells at subpicomolar concentrations. Biochem. Biophys. Res. Commun. 1996;220:879–885. doi: 10.1006/bbrc.1996.0499. [DOI] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Chin JR, Unemori EN, Werb Z, Bissell MJ. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991;112:439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J. Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, Werb Z, Bissell MJ. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am. J. Pathol. 1998;153:457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemori EN, Werb Z. Reorganization of polymerized actin: a possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J. Cell Biol. 1986;103:1021–1031. doi: 10.1083/jcb.103.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Wiesen JF, Young P, Werb Z, Cunha GR. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126:335–344. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol. Biol. Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Spitzer E, Meyer D, Sachs M, Niemann C, Hartmann G, Weidner KM, Birchmeier C, Birchmeier W. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J. Cell Biol. 1995;131:215–226. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant J, Buluwela L, Niranjan B, Gusterson B, Kamalati T. In vivo effects of hepatocyte growth factor/scatter factor on mouse mammary gland development. Exp. Cell Res. 1998;241:476–481. doi: 10.1006/excr.1998.4028. [DOI] [PubMed] [Google Scholar]


