Abstract
Agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) and endothelin −1 (ET-1) are suggested to be important links between placental ischemia and hypertension during preeclampsia. Activation of the angiotensin II type 1 receptor (AT1R) increases endothelial cell production of ET-1; however, the importance of ET-1 in response to AT1-AA mediated AT1 R activation during preeclampsia is unknown. Furthermore, the role of AT1-AA mediated increases in blood pressure during pregnancy remains unclear. The objective of this study was to test the hypothesis that AT1-AA, increased to levels observed in preeclamptic women and placental ischemic rats, increases mean arterial pressure (MAP) by activation of the ET-1 system. Chronic infusion of purified rat AT1-AA into normal pregnant (NP) rats for 7 days increased AT1-AA from 0.68 ± 0.5 to 10.88 ± 1.1 chronotropic units (P<0.001). The increased AT1-AA increased MAP from 99 ± 1 to 119 ± 2 mmHg (P<0.001). The hypertension was associated with significant increases in renal cortices (11-fold) and placental (4-fold) ET-1. To determine whether ET-1 mediates AT1-AA-induced hypertension, pregnant rats infused with AT1-AA and NP rats were treated with an ETA receptor antagonist. MAP was 100 ± 1 mmHg in AT1-AA + ETA antagonist treated rats versus 98 ± 2 mmHg in ETA antagonist treated rats. Collectively, these data support the hypothesis that one potential pathway whereby AT1-AAs increase blood pressure during pregnancy is by an ET-1 dependent mechanism.
Keywords: Preeclampsia, hypertension, kidney, placental, inflammation
Introduction
The initiating event in early onset preeclampsia is postulated to involve inadequate vascularization of the subplacental decidua with reduced placental perfusion that leads to hypertension during pregnancy by mechanisms not yet elucidated 1, 2. Recent studies have suggested that the production of agonistic autoantibodies to the angiotensin II (AngII) type I receptor (AT1-AA) may be an important link between placental ischemia and hypertension in preeclamptic women3-8. The AT1-AA induces signaling in vascular cells that are blocked by an AT1 receptor antagonist including activating protein-1, calcineurin and nuclear factor kappa B activation 3,4,7. Recent studies by Zhou et al. demonstrate that immunoglobulin isolated from preeclamptic women increases systolic blood pressure four days after retro-orbital injection into pregnant mice7,8. This hypertensive response was attenuated by administration of an AT1 receptor antagonist. While these findings suggest that AT1-AAs from preeclamptic women increases blood pressure in pregnant mice, possibly by activation of the AT1 receptor, it remains unclear by what mechanism purified AT1-AA mediates hypertension during pregnancy.
We recently reported that hypertension in response to reductions in uterine perfusion pressure in pregnant rats (RUPP) is associated with increased circulating levels of the AT1-AA9,10. Moreover, we found that the increased blood pressure response in RUPP pregnant rats decreased markedly by antagonism of the AT1 receptor. In addition we previously reported that the hypertension in RUPP rats is associated with significant increases in renal expression of preproendothelin and this blood pressure response is attenuated by administration of a selective ETA receptor antagonist. In contrast, ETA receptor blockade had no significant effect on blood pressure in the normal pregnant animal suggesting a role for ET-1 in mediating the hypertension in response to placental ischemia11 .
While our recent data implicate that AT1-AA and ET-1 are produced in response to placental ischemia and activation of the AT1R and ETA receptors contribute to hypertension in RUPP rats, it is unclear whether AT1-AAs have a direct effect to enhance ET-1 production or whether chronic AT1-AA increases arterial pressure during pregnancy via an ET dependent mechanism. Therefore, the overall goal of this study was to test the hypothesis that the AT1-AA stimulate ET-1 production and increases mean arterial pressure in pregnant rats by an ET-1 dependent mechanism. In order to achieve this goal we examined the effects of isolated, column purified active rat AT1-AA, on blood pressure and ET-1 production in pregnant rats. In addition, to determine a role for ET-1 activation in response to the purified rat AT1-AA, we compared the blood pressure effects of AT1-AA in pregnant rats in the presence of an ETA receptor antagonist.
Methods
Isolation and purification of rat AT1-AA
The female hAogen x male hRen (MDC, Berlin) transgenic rats (MDC, Berlin) were used as the source of rat AT1-AA. This model develops hypertension associated with the AT1-AA. On day 18 of gestation blood was collected and immunoglobulin was isolated from one ml of serum by specific anti-ratIgG column purification. AT1-AA was purified from rat IgG by epitope binding to the amino acid sequence corresponding to the second extracellular loop of the AT1 receptor covalently linked to Sepharose 4B CNBr-activated gel. Unbound IgG was washed away and bound IgG was eluted with 3 M potassium thiocyanate. AT1-AA activity was measured utilizing a bioassay that evaluates the beats/minute (bpm) of neonatal cardiomyocytes in culture3,12.
Protocol 1a: Effect of rat AT1-AA on MAP in pregnant rats
All studies were performed in timed pregnant Sprague Dawley rats purchased from Harlan Inc. (Indianapolis IN). Animals were housed in a temperature controlled room (23°C) with a 12:12 light: dark cycle. All experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
Twelve microliters/day of (1:50) purified rat AT1-AA fraction (collected as described above) diluted in saline was infused into pregnant rats for seven days. This protocol was adapted from a study by Dragun et al. that used similar fraction of isolated AT1-AA to demonstrate renal transplant rejection in rats. Purified rat AT1-AA 5,6,12,13 was infused intraperitoneally from day 12 to 19 of gestation via mini-osmotic pumps (model 2002, Alzet Scientific Corporation, Palo Alto, CA) into normal pregnant rats. Serum AT1-AA concentrations and activity was determined utilizing the procedure outlined above from 1 ml of serum collected on day 19 of gestation from normal pregnant control rats (n=14) and pregnant rats treated chronically with AT1-AA (n=16). A second group of normal pregnant rats were chronically treated with diluted control IgG (n=4) in order to compare the effects of the AT1-AA with an IgG molecule serving as a control to mediate hypertension during pregnancy. On day 18 of gestation, all rats were surgically instrumented with a carotid catheter for subsequent arterial pressure measurement. At day 19 of gestation arterial pressure was measured. Subsequently, a blood sample was collected, kidneys and placentas were harvested and litter size and pup weights were recorded while dams were under anesthesia using isoflurane (Webster, Sterling MA) delivered by an anesthesia apparatus (Vaporizer for Forane Anesthetic, Ohio Medical Products, Madison, WI).
Protocol 1b: Effect of AT1 receptor antagonist on AT1-AA induced hypertension
This protocol was performed in order to determine if AT1 receptor blockade attenuated hypertension induced by chronic AT1-AA. All groups of rats were treated with losartan (10mg/day), an AT1 receptor antagonist, in drinking water from day 14 to day 19 of gestation. Normal pregnant rats treated with losartan served as controls (NP+L, n=8). The experimental group included AT1-AA-induced hypertensive pregnant rats treated with losartan in drinking water (AT1-AA+L, n=9). Blood pressure was compared between NP+L and AT1-AA+L on day 19 of gestation.
Protocol 1c: Effect of ETA receptor antagonist on AT1-AA induced hypertension
This protocol was performed to determine if ETA receptor blockade attenuated AT1-AA-induced hypertension during pregnancy. Purified rat AT1-AA 5,6 10 was infused intraperitoneally from day 12 to 19 of gestation via mini-osmotic pumps (model 2002, Alzet Scientific Corporation, Palo Alto, CA) into normal pregnant rats (n=17) orally treated (drinking water) with the ETA receptor antagonist (ABT-627, 5mg/kg/day) from day 14 to day 19 of gestation. Normal pregnant rats treated with ETA receptor antagonist (n=11) alone served as controls. Blood pressure was compared between NP+ ETA and AT1-AA+ ETA on day 19 of gestation 9,11.
Measurement of arterial pressure in chronically instrumented conscious rats
Arterial pressure was determined in all groups of pregnant rats at day 19 of gestation as previously described (9,11,14). Briefly, on day 18 of gestation, pregnant rats were catheterized under anesthesia using isoflurane (Webster, Sterling MA) delivered by an anesthesia apparatus (Vaporizer for Forane Anesthetic, Ohio Medical Products, Madison, WI). A catheter of V-3 tubing (SCI, Lake Hayasu City, AZ) was inserted into the carotid artery for blood pressure monitoring. The catheter was tunneled to the back of the neck and exteriorized after implantation. On day 19 of gestation, pregnant rats were placed in individual restraining cages for arterial pressure measurements. Arterial pressure was monitored with a pressure transducer (Cobe III Transducer CDX Sema, Birmingham, AL) and was recorded continuously for a 2 hour period following a 1 hour stabilization.
Determination of Kidney and Placental Preproendothelin mRNA Levels
The cortex and medulla of kidneys were separated immediately after harvesting and quickly frozen in liquid nitrogen and stored at −80°C. Placentas were weighed and quickly frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted using the Totally RNA kit supplied by Ambion after the tissues were crushed in liquid nitrogen with a mortar and pestle. The isolation procedure was then performed as outlined in the instructions provided by the manufacturer. Real time PCR was utilized, as previously described, to determine tissue ppET-1.11,14,15.
Statistical analysis
Differences between control and AT1-AA infused rats were analyzed using an unpaired T test for blood pressure analysis and bioassay results. ANOVA with Tukey-Kramer multiple comparison test was used when comparing blood pressure analysis between control and experimental groups (groups treated with either losartan or ETA receptor antagonist). Data were considered statistically different at p values < 0.05. Statistical analysis of real time PCR results was calculated using the mean normalized cycle threshold (delta/delta CT) values and compared using ANOVA and Tukey-Kramer multiple comparison tests.
Results
Serum AT1-AA Levels and Mean Arterial Pressures in Control and Chronic AT1-AA treated Pregnant Rats
AT1-AA activity, determined via rat cardiomyocyte bioassay, increased from 0.68 ± 0.5 beats per minute (bpm) to 10.88 ± 1.1 bpm (P<0.001) with chronic AT1-AA (rat AT1-AA (1:50)) infusion into normal pregnant rats (Figure 1). Chronic infusion of purified rat AT1-AA into pregnant rats resulted in significant increases in arterial pressure, from 99 ± 1 mmHg in NP controls to 119 ± 2 mmHg (P<0.001) in AT1-AA treated pregnant rats (Figure 2). In contrast, chronic infusion of a control IgG into pregnant rats had no effect on MAP compared to normal pregnant rats (102 ± 2 vs 100 ± 2 mmHg).
Figure 1.
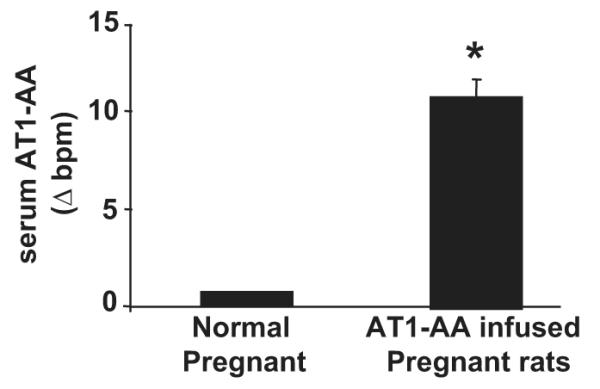
AT1-AA increase significantly in response to chronic infusion, indicated by increased beats per minute (bpm) of cultured rat neonatal cardiomyocyte assay. IgG was isolated from serum collected from NP and AT1-AA infused pregnant rats. The AT1-AA was column purified by epitope binding from the collection of IgG and activity tested by activation of AT1 receptors and increased chronotropic activity of cardiomyocytes in culture.
Figure 2.
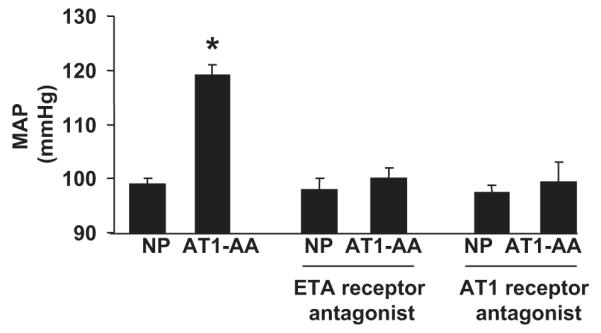
Changes in mean arterial pressure in normal pregnant (NP) rats in response to AT1-AA infusion. (* P<0.001 vs NP rats). Administration of either an ETA receptor antagonist or an AT1 receptor antagonist, losartan, abolished hypertension in response to AT1-AA in pregnant rats. All data are expressed as mean ±SEM.
Hypertension in response to AT1-AA is blocked by an AT1-receptor antagonist
MAP of pregnant AT1-AA treated rats that were administered AT1 receptor antagonist was not different than in pregnant rats chronically treated with AT1receptor antagonist alone (96 ± 2 mmHg vs 103 ± 3 mmHg) (Figure 2).
Preproendothelin mRNA levels in placenta and kidneys in control and AT1-AA treated pregnant rats
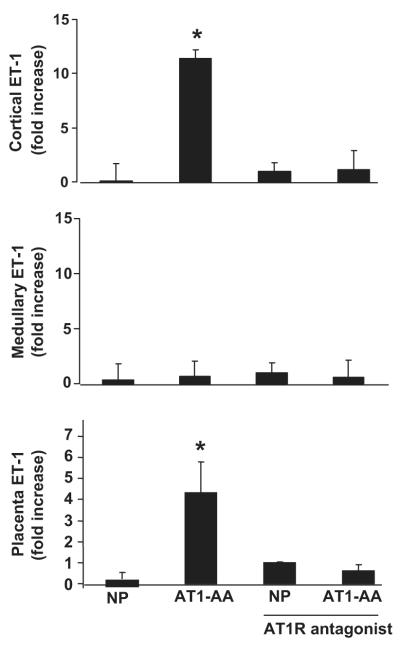
Real time PCR was used to measure preproendothelin in the placenta and the renal cortex and medulla. Preproendothelin mRNA levels in the renal cortices and placenta of the AT1-AA treated rats were significantly increased compared to normal pregnant rats (Figure 3). Conversely, medullary preproendothelin mRNA levels were not different in response to chronic AT1-AA excess compared to normal pregnant rats. In addition the increase in preproendothelin in response to AT1-AA induced hypertension was attenuated with administration of an AT1 receptor antagonist.
Figure 3.
Fold increase in tissue preproendothelin mRNA/B-actin determined by real time PCR. P values were <0.0073 in cortices (n=6) and < 0.02 in placenta (n=5). No difference was seen in medullary expression of ET-1 among the groups. AT1 receptor blockade significantly decreased preproendothelin in response to AT1-AA induced hypertension in renal cortices (n=6), and placenta (n=6).
Effect of ETA receptor antagonist on AT1-AA induced hypertension
The selective ETA receptor antagonist (ABT-627) was administered for 5 days to normal pregnant control rats and pregnant rats chronically treated with AT1-AA. Normal pregnant rats treated with ETA receptor antagonist alone served as controls. Administration of an ETA receptor antagonist attenuated AT1-AA induced hypertension (100 ± 1 mmHg in AT1-AA + ETA pregnant rats vs 98 ± 2 mmHg in ETA rats) (Figure 2).
Placental and pup weight in response to chronic AT1-AA
There was no difference in pup weight (2.3±0.04g vs 2.2±0.03g vs 2.3±0.03g vs 2.2±0.03g) or litter size (14 vs 13 vs 14 vs 14) between normal pregnant, AT1-AA, ETA receptor antagonist treated, or AT1-AA plus ETA receptor antagonist pregnant rats, respectively. Furthermore, placental weights were statistically unchanged (0.55±0.2 vs 0.54±0.2 vs 0.51±0.1 vs 0.48±2g) between normal pregnant, AT1-AA, ETA receptor antagonist treated, or AT1-AA plus ETA receptor antagonist pregnant rats, respectively.
Discussion
In this study we demonstrated that increasing levels of AT1-AA to levels observed in preeclamptic women and in placental ischemic rats, increases mean arterial pressure (MAP) in pregnant rats by activation of the endothelin system. We report that infusion of purified rat AT1-AA, isolated from serum collected from a pregnant transgenic rat cross overproducing components of the renin angiotensin system,12,13into pregnant rats from day 12 to day 19 of gestation, increased serum AT1-AA, blood pressure, and tissue levels of preproendothelin. Finally, we report that AT1-AA-induced hypertension in pregnant rats was attenuated by either oral administration of the AT1 receptor antagonist losartan or an ET type A receptor antagonist. In addition, the increase in endothelin transcript in response to AT1-AA induced hypertension was abolished by administration of an AT1 receptor antagonist.
Although a role for the AT1-AA to mediate hypertension during pregnancy has been suggested by other laboratories, potential mechanisms whereby the autoantibody increases pressure has remained undefined. In this study we demonstrate that administration of the AT1-AA to pregnant rats significantly increased ET-1 levels in renal cortices and placenta of pregnant rats (Figure 3). To confirm a role for the increase in ET-1 as a potential mechanism of AT1-AA-induced hypertension, pregnant rats and chronically AT1-AA treated pregnant rats were administered a selective ETA receptor antagonist in their drinking water. The hypertensive response to the AT1-AA in pregnant rats was attenuated with ETA blockade (Figure 2). These data indicate that the increased local production of ET-1 in response to chronic AT1-AA plays an important role in hypertension during pregnancy.
While AT1-AA are reportedly elevated in preeclamptic women5, 6, 10-20 , the importance of immune activation in mediating the cardiovascular and endothelial dysfunction in response to placental ischemia during pregnancy remains unclear. Although the AT1-AA has been implicated in endothelial dysfunction associated with preeclampsia the exact mechanism linking enhanced production of ET-1 to placental ischemia in pregnant rats or in preeclamptic women is unknown10,11,16-21. A recent study from our laboratory demonstrated that sera from pregnant rats exposed to chronic reduced uterine perfusion pressure (RUPP) increased ET-1 production by cultured endothelial cells and this response was attenuated by AT1 receptor antagonism21. This study suggested that the AT1-AA was a circulating component stimulated in response to placental ischemia. Further in vitro evidence included studies demonstrating that AT1-AAs induce signaling in vascular cells through activating protein-1, calcineurin and nuclear factor kappa B which were all blocked by AT1 receptor antagonism (3, 4, 7, 8). The signaling resulted in increased reactive oxygen species and sFlt-1 production, both of which have been implicated in preeclampsia (3,4,7). Finally, a recent study by Yang and colleagues demonstrated that human AT1-AA caused vascular constriction in isolated rat thoracic aortic rings, middle cerebral artery and coronary artery segments in a concentration-dependent fashion 22. The vasoconstrictive effect of AT1-AA was completely blocked by an AT1-receptor antagonist.
Recent studies from Zhou et al demonstrated that immunoglobulin isolated from preeclamptic women increases systolic pressure in pregnant mice. This increase in systolic pressure was associated with a reduction in placental size and fetal weight. This phenotype was ameliorated with co-injection of an AT1 receptor antagonist. These findings suggest a role for the AT1-AA component of the human IgG to mediate hypertension and intrauterine growth restriction in pregnant mice7, 8. These well designed and executed studies, however, did not determine the pathophysiological mechanisms associated with the hypertension. In this study we infused purified species specific AT1-AA into normal pregnant rats in order to test the role of the AT1-AA to induce hypertension during pregnancy. In addition, we confirm the presence of active rat AT1-AA seven days post-infusion by a previously established and validated bioassay utilized to determine increased chronotropic effects of the AT1-AA to activate AT1 receptors on cardiomyocytes in vitro .3-6,12 Finally, the findings reported in the present study are the first to demonstrate that one potential mechanism of the AT1-AA to elicit hypertensive effects is by ET-1 activation during pregnancy.
Data suggests that reduced placental perfusion in both humans and in animal models of preeclampsia is an important stimulus for AT1-AA production5-10, 21. Using the cardiomyocyte contraction assay, Walther et al. found that the AT1-AA was detectable between 18-22 weeks gestation in women with abnormal uterine perfusion 23. These women, when followed to term, fell into three groups; those who developed preeclampsia, those characterized by fetuses with intrauterine growth retardation, and those with a normal pregnancy outcome. AT1-AA was not observed in second trimester women with normal Doppler. At term, the AT1-AA was present in preeclamptic women, those exhibiting intrauterine growth retardation, and in healthy pregnant women with a history of abnormal uterine perfusion in the second trimester 23. The authors concluded that AT1-AA were present in patients with pathological uterine artery Doppler findings independent of preeclampsia suggesting that AT1-AA may not be the primary cause of preeclampsia. We recently reported that chronic reductions in uterine perfusion pressure in the pregnant rat increases arterial pressure, impairs endothelial function and is associated with intrauterine growth restriction and production of the AT1-AA9. Although reductions in placental perfusion is associated with AT1-AA production, we did not see intrauterine growth restriction or decreased pup viability with purified AT1-AA administration into pregnant rats, suggesting that this phenotype could result from a synergism between AT1-AA and other placental derived factors to elicit deleterious effects upon the fetus.
Perspectives
Although the data presented in this study demonstrate that hypertension in response to AT1-AA during pregnancy is mediated via an ET-1 dependent mechanism, there are a number of unanswered question in this field of investigation. While AT1-AA causes significant hypertension, the role of the AT1-AA in mediating impaired renal hemodynamics or proteinuria during pregnancy is unclear. Experiments inhibiting the generation of the AT1-AA in rats with placental ischemia will contribute to further defining the pathophysiological role of the autoantibody to mediate hypertension and renal dysfunction during pregnancy.
Furthermore, studies from our laboratories have shown that the AT1-AA is not produced in normal virgin rats indicating that the antigen to which the autoantibody is produced is predominantly expressed during pregnancy. The exact antigen to which the AT1-AA is produced remains to be determined. Thus, the immunological mechanisms that leads to AT1-AA production in response to reductions in uterine perfusion remains to be an important area of investigation
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by AHA SDG0835472N; NIH grants HL78147 and HL51971. RD is supported by the German Research Foundation (DFG 631/7-1).
Footnotes
There are no relationships to disclose
Reference List
- (1).Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- (2).Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- (3).Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- (4).Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–639. doi: 10.1161/01.CIR.0000058200.90059.B1. 1. [DOI] [PubMed] [Google Scholar]
- (5).Dechend R, Muller DN, Wallukat G, Homuth V, Krause M, Dudenhausen J, Luft FC. Activating auto-antibodies against the AT1 receptor in preeclampsia. Autoimmun Rev. 2005;4:61–65. doi: 10.1016/j.autrev.2004.07.002. [DOI] [PubMed] [Google Scholar]
- (6).Dechend R, Homuth V, Wallukat G, Muller DN, Krause M, Dudenhausen J, Haller H, Luft FC. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Investig. 2006;13:79–86. doi: 10.1016/j.jsgi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- (7).Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007;50:269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce preeclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).LaMarca BB, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Elevated agonistic autoantibodies to the angiotensin type 1 (AT1-AA) receptor in response to placental ischemia and TNF alpha in pregnant rats. Hypertension. 2008;52:7–11. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. 2008;51(4):982–988. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating TNF alpha-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- (12).Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- (13).Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schonemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. 10. [DOI] [PubMed] [Google Scholar]
- (14).LaMarca BB, Speed J, Fournier L, Babcock S, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: Effect of TNF alpha blockade. Hypertension. 2008;52:1–5. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).LaMarca BB, Chandler D, Grubbs L, Bain J, McMlemore G, Granger JP, Ryan MJ. Role of Sex Steroids in Modulating TNF alpha induced changes in Vascular Function and Blood Pressure. Am. J. Hypertens. 2007;20:1216–1221. doi: 10.1016/j.amjhyper.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82:1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- (17).Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- (18).Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H923–H924. doi: 10.1152/ajpheart.01278.2005. [DOI] [PubMed] [Google Scholar]
- (19).Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension. 1998;31:1065–1069. doi: 10.1161/01.hyp.31.5.1065. [DOI] [PubMed] [Google Scholar]
- (20).Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283:R29–R45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- (21).Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- (22).Yang X, Wang F, Chang H, Zhang S, Yang L, Wang X, Cheng X, Zhang M, Ma XL, Liu H. Autoantibody against AT1 receptor from preeclamptic patients induces vasoconstriction through angiotensin receptor activation. J Hypertens. 2008;26:1629–1635. doi: 10.1097/HJH.0b013e328304dbff. [DOI] [PubMed] [Google Scholar]
- (23).Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.