Abstract
The neurotensin-1 (NT1) receptor has been implicated in mediating a number of important neurotensin effects. We have found that PD149163, a selective, brain penetrating, NT1 receptor agonist, produces a number of therapeutic-like preclinical effects after peripheral administration including pro-cognitive, antipsychotic and anxiolytic effects. In this study, we investigated PD149163's effect on food intake and thermal regulation, two physiological processes thought to be mediated by NT1 receptor.
Brown Norway rats and leptin-deficient mice (ob/ob) mice were administered subcutaneous PD149163 (0, 0.1, 0.25, or 1 mg/kg) for ten consecutive days. Weight and 24-hour food intake were measured in mice and rats and core body temperature was also measured in rats.
PD149163 significantly decreased food intake in rats and ob/ob mice and no tolerance was demonstrated to this effect over the course of the study. PD149163-treated animals exhibited weight loss compared to saline-treated animals. PD149163 produced hypothermia as expected but this effect did show tolerance over the course of the study, unlike feeding. The results suggest that NT1 receptor agonists are candidates for treatment of obesity and that somewhat different mechanisms are involved in NT1-induced feeding regulation and temperature regulation.
Introduction
Neurotensin (NT) is a 13 amino acid peptide present in the periphery and central nervous system (CNS). In the CNS, NT acts as a neurotransmitter and modulates several neurotransmitter systems such as dopamine, acetylcholine, glutamate and serotonin (St-Gelais et al., 2006).
Three NT receptors have thus far been cloned. NT1 and NT2 receptors are GCPR-coupled receptors, whereas NT3, the least studied receptor, is a non GCPR receptor similar to the protein sortilin (Pelaprat, 2006). NT administered into the brain of rodents decreases food intake, core body temperature, pain response, locomotor activity, enhances memory, sensorimotor gating (prepulse inhibition of startle reflex) and inhibits the effects of dopamine agonists such as amphetamine (St-Gelais et al., 2006). NT1 receptor has been the receptor most strongly implicated in these effects. As a result of these observed effects, there has been much interest in the possibility of targeting NT1 receptors as a strategy to develop novel treatments for several CNS disorders, most notably schizophrenia (Boules et al., 2005). To this end efforts have been directed at developing NT1 agonists that penetrate the brain after peripheral administration, since NT is not capable of this. One approach that has led to development of several distinct brain-penetrating NT analogs has been chemical modification of the c-terminal hexapeptide of NT, NT(8-13), which is the smallest fragment to retain full bioactivity of parent neuropeptide. PD149163, (Lys-(psiCH2NH)-Lys-Pro-Trp-Terleu-Leu-OEt) is one such example of this approach and receptor binding studies show it has affinity for the NT1 receptor (159 nM) but no detectable affinity for the NT2 receptor (Petrie et al., 2004), which distinguishes it from NT and other current NT analogs which have affinity for NT2 as well as NT1. Peripheral administration of PD149163 has been shown to produce robust positive effects in animal tests of antipsychosis (Feifel et al, 2008; Feifel et al, 2007; Feifel et. al, 2004; Feifel et al, 2003; Feifel et al, 1999), cognitive enhancement, (Azmi et al., 2006; Feifel et al., 2009), and anxiety (Shilling and Feifel, 2008) making it an attractive candidate for further drug development. The purpose of the current studies was to investigate the thermal and feeding effects of PD149163, which have not been previously characterized. Furthermore, we tested the effects of subchronic daily peripheral administration of PD149163 on temperature, feeding and body weight since the effects of peripheral administration of a NT1 receptor agonist daily during a subchronic time period have not been previously studied, and previous studies with PD149163 demonstrated that some of its acute effects, namely suppression of spontaneous locomotor activity, exhibits tolerance with daily subchronic dosing (Feifel et al., 2008), whereas other effects, namely the antipsychotic-like effects, do not (Feifel et al., 2008; Feifel et al., 2007).
Evidence exists for a mechanistic association between leptin, another anorexigenic peptide, and neurotensin. Therefore, in addition to testing PD149163 in normal rats, we tested it in ob/ob mice, a strain that is prone to develop obesity due to an engineered genetic knockout of leptin expression (Lin and Huang, 1997).
Methods
Thirty-two male Brown Norway rats (210-260 gms at testing, obtained from Harlan Laboratories, San Diego) and twenty leptin-deficient Ob/Ob mice (B6.V-Lep ob/J, 48-58 gms at testing, obtained from Jackson Labs) were tested. All animals were housed individually under a 12h:12h light:dark schedule (lights on at 7:00 AM). All testing occurred during the light phase of the animals' circadian illumination schedule.
In the first experiment, rats were randomly assigned to receive subcutaneous (s.c.) injections of saline or one of the following doses of PD149163: 0.1, 0.25, or 1 mg/kg (n=8). PD149163 was generously made available by the NIMH Chemical Synthesis and Drug Supply Program, and RTI International (Research Triangle Park, NC, USA). In a separate experiment performed following the experiment in rats, ob/ob mice were randomly assigned to receive either saline or 1 mg/kg PD149163 (n=10) administered intraperitoneally (i.p.). In both experiments, PD149163 was dissolved in saline (0.9% NaCl), and injected in a volume of 1.0 ml/kg. Each animal received the same dose daily for ten consecutive days.
Each rat and mouse was provided standard rat and mouse chow, respectively and water ad libitum throughout the experiments. Food consumption was measured for 24 hours after the first and the tenth injection. For the food intake measurement days about thirty grams of food were weighed and placed in each animal's hopper. After twenty four hours, the leftover chow in hopper was collected and weighed. Body weight was measured daily before each injection.
Core body temperature was also measured in rats on day 1 and day 10 of the experiment with a thermometer (Hi-Lo Temp Model 8200 (Malinckrodt), manufactured by Sensortek (now Physitemp Instruments)) using a rat rectal probe (Model Ret-2-Iso, Physitemp Instruments). Rectal probes were placed right after SC injections on day 1 and day 10 and readings were recorded 8 seconds after the probe was inserted. All procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Food intake and body temperature in rats were analyzed for day 1 and day 10 using separate one-way ANOVAs with Drug Treatment as a factor. This was followed with planned pair-wise comparison of each dose of PD149163 with saline for each treatment day that data was collected using Dunnett's test. In mice, food intake was compared using independent t-tests comparing saline-treated and PD149163-treated groups. In rats and mice body weight was analyzed using a repeated measures ANOVA with Treatment Day as a repeated measure and Treatment Group as between subject factor. Change in body weight from baseline to day 10 was compared in mice using independent t-tests and in rats using a one-way ANOVA with Treatment group as the factor, followed by pairwise comparisons of each drug dose with saline using Dunnett's test. Alpha was set at 0.05 for all tests.
Results
Temperature
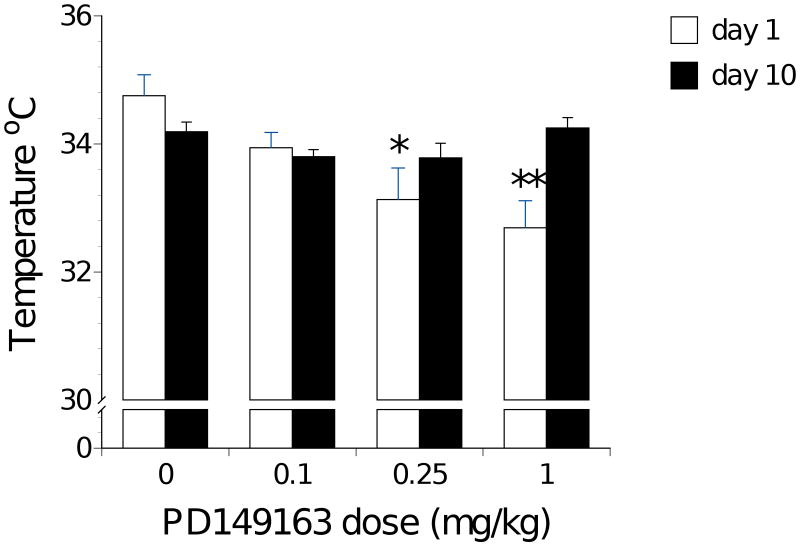
Figure 1 illustrates core body temperature data for rats. ANOVA performed on day 1 temperature data revealed a main effect of Drug Dose [F(3,31)=5.72, p<0.01], and as illustrated in figure 1, the medium (p <0.05) and high (p< 0.01) doses of PD149163 significantly lowered body temperature of rats compared to saline on the first treatment day. There was no significant effect of any Drug Dose on the body temperature on the tenth day of drug treatment.
Figure 1.
Rat core body temperature (± S.E.M.) recorded immediately after the first and tenth consecutive subcutaneous injection of PD149163. Significantly less (p<0.05) than saline represented by *.
Food Intake
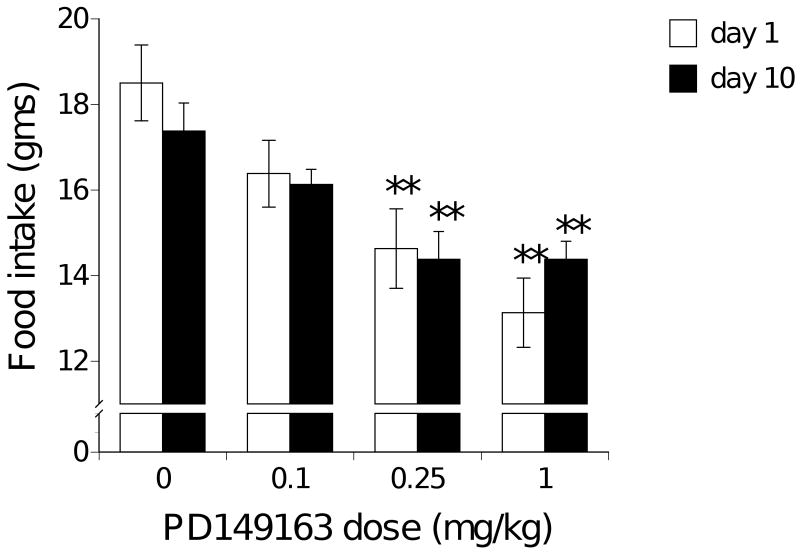
As figure 2 illustrates, the mid and high dose of PD149163 significantly reduced food intake in rats for the 24-hour period after the first [F(3)=7.380, p<0.01] and after the tenth consecutive injection (F(3)=7.434, p<0.01) compared to saline.
Figure 2.
Average food intake (± S.E.M.) during the 24-hour period after the first and tenth consecutive daily subcutaneous injection of PD149163 in rats. Significantly less (p<0.01) than saline represented by **.
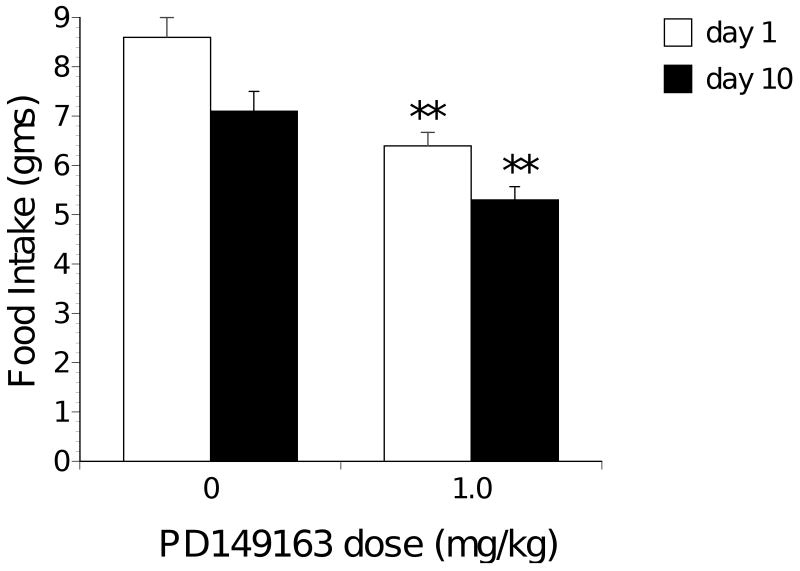
As Figure 3 illustrates, 1 mg/kg PD149163 also significantly reduced food intake compared to saline in ob/ob mice for the 24-hour periods following the first injections [t(18)=2.24, p<0.05] and the tenth consecutive injections [t(18)= 3.92, p<0.01]. T Food intake in both saline-treated and PD149163-treated ob/ob mice decreased from the first day to the tenth day but this was only significant in the saline-treated mice (t(9)= 4.39, p<0.01).
Figure 3.
Average food intake (± S.E.M.) during the 24-hour period after the first and tenth consecutive daily subcutaneous injection of PD149163 in ob/ob mice. Significantly less (p<0.01) than saline represented by **.
Body Weight
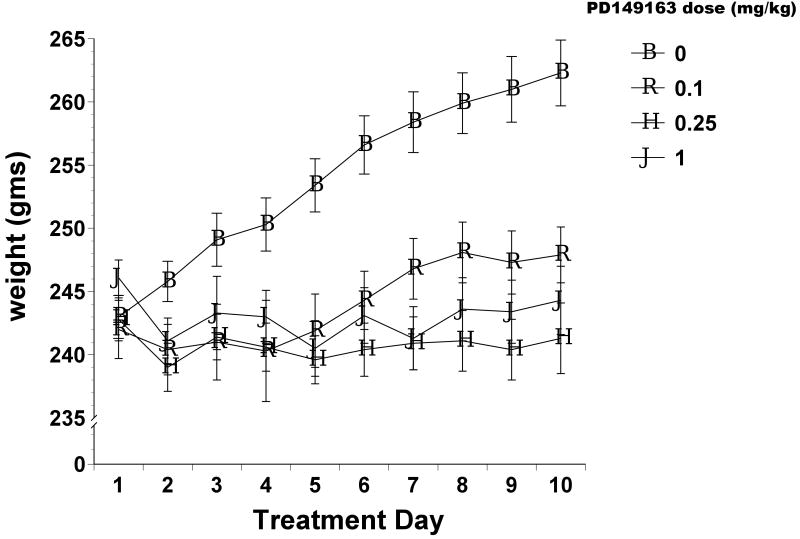
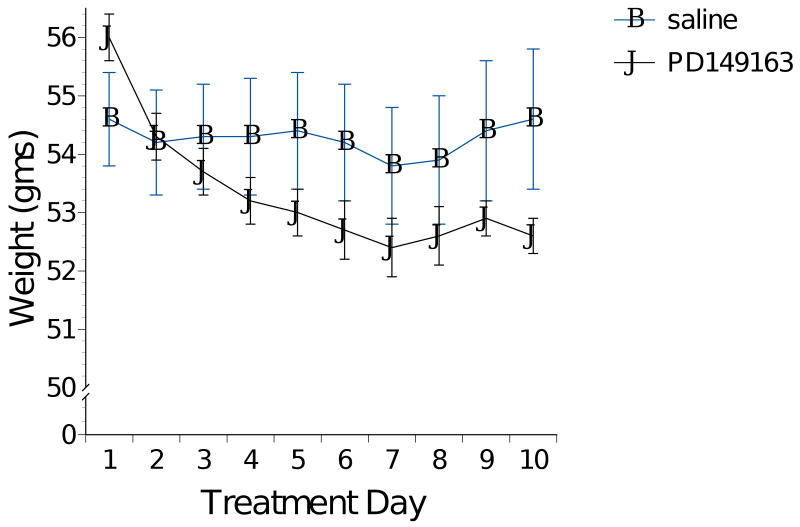
As figure 4 illustrates, all three doses of PD149163 significantly reduced weight gain in Brown Norway rats compared to saline. ANOVA revealed a significant main effect of Drug Dose [F(3)=7.395, p<0.01] and of Treatment Day [F(9)=31.847, p<0.001] as well as a significant Treatment Day X Drug Dose interaction [F(27)=14.421, p<0.001]. The weight difference between saline-treated and PD149163-treated rats became more pronounced across the 10-day experiment and this was due to consistent daily weight increase by saline-treated but not PD149163-treated rats.
Figure 4.
Average body weight (± S.E.M.) in Brown Norway rats. PD149163 was administered s.c. for 10 consecutive days.
As figure 5 illustrates, PD149163 also significantly reduced weight gain in ob/ob mice compared to saline. There was a significant effect of Day [F(9,162)=12.16, p<0.001] and significant Day X Treatment Group interaction [F(9,162)=45.00, p<0.001] but no effect of Treatment Group. The weight difference between saline treated and PD149163 treated rats became more pronounced across the 10-day experiment and in this case it was due to a progressive decrease in body weight in PD149163-treated mice but not saline-treated mice. The weight change between day 10 and baseline in PD149163 treated mice was significantly greater than saline treated mice [t(18)=-5.29, p<0.001].
Figure 5.
Average body weight (± S.E.M.) in ob/ob mice. PD149163 (1 mg/kg) was administered i.p. for 10 consecutive days.
Discussion
PD149163's reduction of food intake after acute administration demonstrated in rats and mice is consistent with previous studies testing centrally and peripherally administered NT and NT analogs (Boules et al., 2000; Cooke et al., 2009; Hawkins, 1986; Levine et al., 1983; Luttinger et al., 1982). PD149163's reduction of food intake in the leptin-deficient ob/ob mice indicates that the feeding effect of PD149163, and presumably NT itself, cannot be fully mediated by modulation of endogenous leptin, a mechanism which has been proposed for its anorexogenic effect (Beck et al., 1998). It has also been proposed that leptin's inhibitory regulation on feeding is mediated by endogenous NT (Kim et al., 2008; Sahu et al., 2001) and by histamine-1 receptors (Ohinata et al., 2004) and these mechanisms remain viable.
Since PD149163 is a selective NT1 receptor agonist (Petrie et al, 2004), this study suggests that NT's effect on food intake is, at least in part mediated through the NT1 receptor, which is consistent with results from other studies showing NT1 deficient mice did not respond to NT-induced feeding suppression (Remaury et al., 2002).
The ten days of consecutive PD149163 administration represents the longest study of the feeding effects of NT or a NT analog to date. In both Brown Norway and ob/ob mice, PD149163's effect on food intake persisted over the 10 day test period suggesting that there is no tolerance to this effect over a sub-chronic time period. Hawkins (Hawkins, 1986) administered NT ICV for six consecutive days and demonstrated no evidence of tolerance to its anorexogenic effects. On the other hand, Cooke et al. (Cooke et al., 2009) found that NT administered peripherally produced an acute (1 hour) suppression of feeding that seemed to develop tolerance after 2 days. Boules et al. (Boules et al., 2000) administered NT69L, another brain-penetrating hexapeptide NT analog, multiple times in healthy Sprague Dawley rats and obese Zucker rats, and found it suppressed food intake and body weight, but an idiosyncratic, non-consecutive day dosing schedule (days 1,2,7,8,11 & 12) used in that study prevents drawing any conclusion regarding tolerance due to repeated daily doses.
PD149163, like NT and other NT analogs, suppresses spontaneous activity and this could be due to sedation or malaise of some kind. However, this suppression of spontaneous activity dissipates by the tenth day of daily peripheral administration (Feifel et al., 2008), thus the persistence of PD149163's reduction of food intake for ten days makes it unlikely that it is secondary to sedation or malaise that may possibly be induced by PD149163. However future studies should investigate this further, for example, by investigating whether there is any induced taste aversion or pica behavior after PDt149163 administration.
The decrease in food intake by PD149163 was reflected in lower body weight in PD149163-treated rats and mice compared to their saline-treated counterparts suggesting that at least for 10 days, there are no compensatory metabolic changes sufficient to offset the reduced food intake. Future studies should explore whether the change in food intake is accompanied by changes in energy expenditure. This could be addressed, for example by measuring oxygen consumption. In the rats, the weight loss was due to a lower rate of weight gain in PD149163-treated rats compared to saline-treated rats. This is likely due to our testing of young Brown Norway rats, still in their growth phase. However, in ob/ob mice the weight effect of PD149163 was weight loss over time compared to relatively stable weight displayed by saline-treated ob/ob mice. The effects of PD149163 in the mice suggests that it's effect on food intake and weight is manifest in normal animals as well as animals with physiologically abnormal weight regulation that predisposes to obesity. This result is consistent with the findings using NT69L in obese Zucker rats (Boules et al., 2000).
As expected PD149163 reduced core body temperature in rats, an established effect displayed by NT treatment and mediated by NT1 receptors (Bissette et al., 1976; Boules et al., 2003; Remaury et al., 2002). However, tolerance to PD149163's temperature effect was observed with repeated administration. This effect is consistent with previous studies showing tolerance to the thermal effects of NT69L (Boules et al., 2003) and in contrast to the lack of tolerance observed with PD149163's effect on food intake in our study. In previous studies, it has been shown that PD149163's suppression of spontaneous locomotor activity exhibits tolerance with repeated treatment (Feifel, 2008), whereas PD149163's antipsychotic-like effects do not (Feifel et al., 2008; Feifel et al., 2007). Taken together it suggests that, though these different behaviors may all be mediated by NT1 receptors, the specific neural mechanisms underlying them are dissociable.
Acknowledgments
This research was supported by NIMH grant MH080910 to DF.
Footnotes
Disclosures: DF is a consultant and holds stock options in Argolyn Biosciences, and Tenzia, biopharmaceutical companies developing neurotensin agonists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azmi N, Norman C, Spicer CH, Bennett GW. Effects of a neurotensin analogue (PD149163) and antagonist (SR142948A) on the scopolamine-induced deficits in a novel object discrimination task. Behav Pharmacol. 2006;17:357–362. doi: 10.1097/01.fbp.0000224382.63744.20. [DOI] [PubMed] [Google Scholar]
- Beck B, Stricker-Krongrad A, Richy S, Burlet C. Evidence that hypothalamic neurotensin signals leptin effects on feeding behavior in normal and fat-preferring rats. Biochemical and Biophysical Research Communications. 1998;252:634–638. doi: 10.1006/bbrc.1998.9712. [DOI] [PubMed] [Google Scholar]
- Bissette G, Nemeroff CB, Loosen PT, Prange AJ, Jr, Lipton MA. Hypothermia and intolerance to cold induced by intracisternal administration of the hypothalamic peptide neurotensin. Nature. 1976;262:607–609. doi: 10.1038/262607a0. [DOI] [PubMed] [Google Scholar]
- Boules M, Cusack B, Zhao L, Fauq A, McCormick DJ, Richelson E. A novel neurotensin peptide analog given extracranially decreases food intake and weight in rodents. Brain Res. 2000;865:35–44. doi: 10.1016/s0006-8993(00)02187-9. [DOI] [PubMed] [Google Scholar]
- Boules M, Fredrickson P, Richelson E. Neurotensin agonists as an alternative to antipsychotics. Expert Opin Investig Drugs. 2005;14:359–369. doi: 10.1517/13543784.14.4.359. [DOI] [PubMed] [Google Scholar]
- Boules M, McMahon B, Wang R, Warrington L, Stewart J, Yerbury S, Fauq A, McCormick D, Richelson E. Selective tolerance to the hypothermic and anticataleptic effects of a neurotensin analog that crosses the blood-brain barrier. Brain Res. 2003;987:39–48. doi: 10.1016/s0006-8993(03)03227-x. [DOI] [PubMed] [Google Scholar]
- Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG. Peripheral and Central Administration of Xenin and Neurotensin Suppress Food Intake in Rodents. Obesity. 2009 Silver Spring; doi: 10.1038/oby.2008.652. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Murray RJ, Tina Tran DN, Rullan MA, Shilling PD. The reversal of amphetamine-induced locomotor activation by a selective neurotensin-1 receptor agonist does not exhibit tolerance. Psychopharmacology (Berl) 2008;200:197–203. doi: 10.1007/s00213-008-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Priebe K, Shilling PD. The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res. 2007;81:278–286. doi: 10.1016/j.bbr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. A systemically administered neurotensin agonist blocks disruption of prepulse inhibition produced by a serotonin-2A agonist. Neuropsychopharmacology. 2003;28:651–653. doi: 10.1038/sj.npp.1300083. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology. 2004;29:731–738. doi: 10.1038/sj.npp.1300378. [DOI] [PubMed] [Google Scholar]
- Feifel D, Mexal S, Melendez G, Liu PY, Goldenberg JR, Shilling PD. The Brattleboro Rat Displays a Natural Deficit in Social Discrimination That Is Restored by Clozapine and A Neurotensin Analog. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. Journal of Pharmacology and Experimental Therapeutics. 1999;288:710–713. [PubMed] [Google Scholar]
- Hawkins MF. Central nervous system neurotensin and feeding. Physiology and Behavior. 1986;36:1–8. doi: 10.1016/0031-9384(86)90064-8. [DOI] [PubMed] [Google Scholar]
- Kim ER, Leckstrom A, Mizuno TM. Impaired anorectic effect of leptin in neurotensin receptor 1-deficient mice. Behav Brain Res. 2008;194:66–71. doi: 10.1016/j.bbr.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Levine AS, Kneip J, Grace M, Morley JE. Effect of centrally administered neurotensin on multiple feeding paradigms. Pharmacology, Biochemistry and Behavior. 1983;18:19–23. doi: 10.1016/0091-3057(83)90244-7. [DOI] [PubMed] [Google Scholar]
- Lin S, Huang XF. Fasting increases leptin receptor mRNA expression in lean but not obese (ob/ob) mouse brain. Neuroreport. 1997;8:3625–3629. doi: 10.1097/00001756-199711100-00040. [DOI] [PubMed] [Google Scholar]
- Luttinger D, King RA, Sheppard D, Strupp J, Nemeroff CB, Prange AJ., Jr The effect of neurotensin on food consumption in the rat. European Journal of Pharmacology. 1982;81:499–503. doi: 10.1016/0014-2999(82)90116-9. [DOI] [PubMed] [Google Scholar]
- Ohinata K, Shimano T, Yamauchi R, Sakurada S, Yanai K, Yoshikawa M. The anorectic effect of neurotensin is mediated via a histamine H1 receptor in mice. Peptides. 2004;25:2135–2138. doi: 10.1016/j.peptides.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Pelaprat D. Interactions between neurotensin receptors and G proteins. Peptides. 2006;27:2476–2487. doi: 10.1016/j.peptides.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Petrie KA, Bubser M, Casey CD, Davis MD, Roth BL, Deutch AY. The neurotensin agonist PD149163 increases Fos expression in the prefrontal cortex of the rat. Neuropsychopharmacology. 2004;29:1878–1888. doi: 10.1038/sj.npp.1300494. [DOI] [PubMed] [Google Scholar]
- Remaury A, Vita N, Gendreau S, Jung M, Arnone M, Poncelet M, Culouscou JM, Le Fur G, Soubrie P, Caput D, Shire D, Kopf M, Ferrara P. Targeted inactivation of the neurotensin type 1 receptor reveals its role in body temperature control and feeding behavior but not in analgesia. Brain Res. 2002;953:63–72. doi: 10.1016/s0006-8993(02)03271-7. [DOI] [PubMed] [Google Scholar]
- Sahu A, Carraway RE, Wang YP. Evidence that neurotensin mediates the central effect of leptin on food intake in rat. Brain Res. 2001;888:343–347. doi: 10.1016/s0006-8993(00)03107-3. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Feifel D. The neurotensin-1 receptor agonist PD149163 blocks fear-potentiated startle. Pharmacol Biochem Behav. 2008;90:748–752. doi: 10.1016/j.pbb.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Gelais F, Jomphe C, Trudeau LE. The role of neurotensin in central nervous system pathophysiology: what is the evidence? J Psychiatry Neurosci. 2006;31:229–245. [PMC free article] [PubMed] [Google Scholar]