Abstract
The role of endogenous inducers of inflammation is poorly understood. To produce the proinflammatory master cytokine interleukin (IL)-1β, macrophages need double stimulation with ligands to both Toll-like receptors (TLRs) for IL-1β gene transcription and nucleotide-binding oligomerization domain-like receptors for activation of the inflammasome. It is particularly intriguing to define how this complex regulation is mediated in the absence of an infectious trigger. Biglycan, a ubiquitous leucine-rich repeat proteoglycan of the extracellular matrix, interacts with TLR2/4 on macrophages. The objective of this study was to define the role of biglycan in the synthesis and activation of IL-1β. Here we show that in macrophages, soluble biglycan induces the NLRP3/ASC inflammasome, activating caspase-1 and releasing mature IL-1β without the need for additional costimulatory factors. This is brought about by the interaction of biglycan with TLR2/4 and purinergic P2X4/P2X7 receptors, which induces receptor cooperativity. Furthermore, reactive oxygen species formation is involved in biglycan-mediated activation of the inflammasome. By signaling through TLR2/4, biglycan stimulates the expression of NLRP3 and pro-IL-1β mRNA. Both in a model of non-infectious inflammatory renal injury (unilateral ureteral obstruction) and in lipopolysaccharide-induced sepsis, biglycan-deficient mice displayed lower levels of active caspase-1 and mature IL-1β in the kidney, lung, and circulation. Our results provide evidence for direct activation of the NLRP3 inflammasome by biglycan and describe a fundamental paradigm of how tissue stress or injury is monitored by innate immune receptors detecting the release of the extracellular matrix components and turning such a signal into a robust inflammatory response.
IL-1β2 is a proinflammatory master cytokine produced by macrophages in response to inflammatory stimuli, such as LPS. The activity of IL-1β is regulated sequentially by synthesis of the 31-kDa precursor pro-IL-1β, intracellular proteolytic conversion into active IL-1β (17 kDa) by the cysteine protease caspase-1, also known as IL-1-converting enzyme (1, 2), and by secretion of IL-1β (3). The synthesis of pro-IL-1β is initiated by Toll-like receptor (TLR) agonists, whereas ATP stimulates cleavage and maturation of IL-1β (4, 5). Activation of caspase-1 requires the assembly and activity of a cytosolic multiprotein complex known as the inflammasome, consisting of nucleotide-binding oligomerization-like receptor family members (NLRs; NLRPs (NLR family, pyrin domain-containing 3), NAIP (NLR family, apoptosis inhibitory protein), and NLRC4 (NLR family caspase recruitment domain-containing 4)) (6), generating functional caspase-1 p20 and p10 subunits (1, 7, 8). TLRs and NLRs contain leucine-rich repeats (LRRs), which are used as ligand-sensing motifs (9, 10). NLRP3, the best characterized member of NLRs, recruits caspase-1 to the inflammasome via the adapter molecule ASC (apoptosis-associated specklike protein containing caspase activation and recruitment domain), thereby activating the inflammasome in response to toxins and ATP (11, 12). ASC is essential for activation of caspase-1 and secretion of mature IL-1β in response to various pathogen-associated molecular patterns (PAMPs) (12–15). Asbestos-, silica-, and ATP-mediated NLRP3 inflammasome activation is triggered by ROS (16, 17). Despite the great importance of endogenous regulators of the inflammasome, very little is known about how the complex regulation of IL-1β processing and release is mediated in the absence of an infectious trigger.
Macromolecules of the extracellular matrix (ECM) are commonly thought to function as purely structural components. However, there is growing evidence that the ECM exerts much more complex functions than being a mere scaffold for cells to attach to, including direct regulation of the inflammatory response reaction (18–25). Biglycan is a stationary component of the ECM and can be found in most tissues. It is a member of the family of small proteoglycans and has LRR motifs, similar to TLRs and NLRs (20, 26). However, when biglycan is released from the ECM during tissue injury or after secretion from activated macrophages, biglycan becomes available in its soluble form. Similar to PAMPs, soluble biglycan is an endogenous ligand for TLR4 and TLR2 in macrophages (27). The objective of this study was to define the role of biglycan in the regulation of IL-1β secretion by macrophages and its in vivo relevance, in order to better understand how tissue stress or injury is recognized and acted upon by the innate immune system.
Here we show that soluble biglycan organizes a multireceptor complex consisting of Toll-like and purinergic P2X receptors on the cell surface of macrophages. Thereby, biglycan regulates (i) the expression of NLRP3- and pro-IL-1β mRNA in a TLR2- and TLR4-dependent manner, (ii) the activation of the NLRP3/ASC inflammasome by interacting with purinergic receptors, and (iii) caspase-1 activation and the release of mature IL-1β. Importantly, both in a model of non-infectious inflammatory renal injury (unilateral ureteral obstruction) and in a prototypical innate immune process, such as LPS-induced sepsis, biglycan-deficient mice displayed lower levels of active caspase-1 and mature IL-1β, resulting in reduced infiltration of mononuclear cells and less damage to target organs. Thus, we propose that soluble biglycan acts as a critical danger and stress signal, which can activate the NLRP3 inflammasome, causing caspase-1-dependent processing and release of IL-1β.
The present study shows that by interacting with Toll-like and purinergic P2X receptors on the cell surface of macrophages, soluble biglycan activates the NLRP3 inflammasome and triggers a robust inflammatory response.
EXPERIMENTAL PROCEDURES
Mice
Male Bgn−/0 and Bgn+/0 mice (C57BL/6) (since the biglycan gene is located on the X chromosome, male animals are hemizygous, having only one allele) have been described previously (28). TLR2−/−, TLR4−/−, TLR2−/−/TLR4-M (TLR4-M mice with a TLR4 gene point mutation, C3H/HeJ) (29), ASC−/−, and P2X7R−/− mice were kindly provided by Dr. M. Freudenberg (Max Planck Institute for Immunology, Freiburg, Germany), Dr. M. Kirschfink (Technical University of Munich), Dr. V. M. Dixit (Genentech, San Francisco, CA), and Dr. B. Sperlágh (Hungarian Academy of Sciences, Budapest, Hungary), respectively. C57BL/6 and C3H/HeN (WT strains for TLR4-M) were purchased from Charles River Laboratories, and Casp-1tm1Sesh/LtJ was purchased from Jackson Laboratories. Mice were housed in a pathogen-free facility. Obstruction of the left ureter (unilateral ureteral obstruction (UUO)) (30) and sepsis (27) were performed in 2-month-old Bgn−/0 and Bgn+/0 male mice. The contralateral kidney served as control in UUO. Kidneys (n = 6 per group) were analyzed at day 3 (acute) and 21 (chronic) after induction of renal obstruction. Sepsis was induced by intraperitoneal injection of LPS in a dose of 200 μg/g body weight (Salmonella minnesota, trichloroacetic acid extraction (Sigma) or ultrapure S. minnesota (InvivoGen)). All animal work was done in accordance with the German Animal Protection Law and was approved by the Ethics Review Committee for laboratory animals of the District Government of Muenster and Darmstadt, Germany.
Purification of Human Biglycan
Expression of human biglycan in 293 HEK cells has been described previously (31). For purification of the native proteoglycan, containing two chondroitin/dermatan sulfate chains (31), the conditioned medium was supplemented with proteinase inhibitors (0.1 m ϵ-amino-n-caproic acid, 10 mm EDTA, 5 mm benzamidine, 10 mm N-ethylmaleimide, 1 mm phenylmethylsulfonyl fluoride) and passed over a DEAE-Trisacryl-M (Pall) column, followed by elution with 20 mm Tris/HCl, pH 7.4, containing 1 m NaCl. After concentrating the relevant fractions with Aquacide I, as instructed by the manufacturer (Calbiochem), the proteoglycans were dialyzed for 2 h against 20 mm Tris-HCl, pH 7.4, containing 150 mm NaCl and separated by high performance liquid chromatography (Prominence LC; Shimadzu) on a TSK-GEL-DEAE-5PW, 7.5-mm inner diameter × 7.5 cm, 10-μm column (Tosoh Bioscience) by a discontinuous binary NaCl gradient. The purity of the proteoglycans was verified by silver staining after SDS gel electrophoresis.
Cell Culture and Stimulation
Macrophages were harvested by peritoneal lavage 5 days after injection of thioglycollate and cultured in RPMI 1640 (Invitrogen) under serum-free conditions as described (27). When required, macrophages were preincubated for 1 h at 37 °C with Ac-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-CMK; 10 μm; Bachem), ATP oxidized sodium salt (oATP; 1 mm; Sigma), KN-62 (30 μm; Sigma), geldanamycin (200 nm; Axxora), IKK inhibitor III (10 μm; Merck), TNP-ATP (10 μm; Sigma), 6E-(bromomethylene)tetrahydro-3-(1-naphtalenyl-2H-pyran-2-one (bromoenol lactone (BEL); 20 μm; Cayman Chemical Co.), MEK1/2 inhibitor (U0126; 10 μm; New England Biolabs), p38 MAPK inhibitor (SB203580; 10 μm; Sigma), N-acetyl-l-cysteine (10 mm solution in RPMI 1640 adjusted to a pH of 7.4; Alexis), or diphenyleneiodonium chloride (0.5 μm; Sigma) and stimulated for 4 h with intact biglycan, biglycan protein core, or biglycan-derived glycosaminoglycan chains (all 4 μg/ml) (27). In some experiments, macrophages were subsequently pulsed for 30 min with ATP (5 mm; Sigma). For neutralization of biglycan activity, 4 μg/ml biglycan was incubated for 1 h at 37 °C with a biglycan-blocking antibody (IgG; 0.1–10 μg/ml) or as control with non-immune rabbit serum and subsequently added to thioglycollate-elicited macrophages for 4 h (27). To obtain biglycan protein core, the proteoglycan was digested with chondroitinase ABC (Seikagaku Corp.) and purified as described (32). Biglycan-derived glycosaminoglycan chains were obtained by β-elimination followed by dialysis against RPMI 1640 medium. For quantification, protein and hexuronic acid content in glycosaminoglycan chains and intact biglycan were measured (27). Alternatively to biglycan, macrophages were stimulated with ultrapure LPS from S. minnesota (2 ng/ml; InvivoGen), peptidoglycan from Staphylococcus aureus (5 μg/μl; Sigma), R837 (5 μg/ml; InvivoGen), chondroitin/dermatan sulfate (4 μg/μl; Medac), and human umbilical cord hyaluronan (25 μg/ml; Sigma) and pulsed for 30 min with ATP. Recombinant human TNFα, interferon-γ (IFNγ) and IL-1β (all 10 ng/ml) were from R&D Systems. Synthesis of hyaluronan was inhibited with 4-methylumbelliferone (2 mm; Sigma) for 24 h. Hyaluronan content in culture media from PMΦs was verified using the Hyaluronan DuoSet assay development kit (R&D Systems). NLRP3−/− and NLRP3+/+ bone marrow-derived macrophages (a kind gift from Dr. J. Tschopp, University of Lausanne, Switzerland) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 30% L929 supernatant for 5 days.
Northern Blot Analysis and Reverse Transcription-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen). Membranes were hybridized with 32P-labeled cDNA probes generated by reverse transcription-PCR (33): for IL-1β encompassing bp 257–790 (GenBankTM accession number M15131) with the primer pair 5′-CAGGCAGGCAGTATCACTCA-3′ and 5′-TACCAGTTGGGGAACTCTGC-3′; for NLRP1 encompassing bp 524–980 (GenBankTM accession number NM001004142) with the primer pair 5′-GAGAGCTGGCCCAGTATGAG-3′ and 5′-ACCCAGGGAACTTCACACAG-3′; for NLRP3 encompassing bp 1905–2365 (GenBankTM accession number AY355340) with the primer pair 5′-GCAGGAGGAAGACTTTGTGC-3′ and 5′-AGGAGATGTCGAAGCAGCAT-3′; for NLRC4 encompassing bp 1613–2098 (GenBankTM accession number NM001033367) with the primer pair 5′-GAGGTGAGCAAAGGGAACAG-3′ and 5′-TGCCTTGTCCTGTGACTCTG-3′; for ASC encompassing bp 22–573 (GenBankTM accession number AB059327) with the primer pair 5′-ATCCTGGACGCTCTTGAAAA-3′ and 5′-CTCCAGGTCCATCACCAAGT-3′; for caspase-1 encompassing bp 391–1163 (GenBankTM accession number BC008152) with the primer pair 5′-ACCCTCAAGTTTTGCCCTTT-3′ and 5′-TCAGCAGTGGGCATCTGTAG-3′; a mouse HPRT1 (hypoxanthine guanine phosphoribosyl transferase 1) probe encompassing bp 13–503 (GenBankTM accession number NM013556) with the primer pair 5′-AGTCCCAGCGTCGTGATTAG-3′ and 5′-AGAGGTCCTTTTCACCAGCA-3′. 18 S rRNA (GenBankTM accession number X00686; Applied Biosystems) was used as a housekeeping gene. Northern blots and reverse transcription-PCR were performed and quantified as described (33). Quantitative TaqMan PCRs were performed according to the manufacturer's instructions (Applied Biosystems). Amplification was detected by an increased fluorescent signal of 5-[(N-(30-diphenylphosphinyl-40-methoxycarbonyl) phenyl-carbonyl) aminoacetamido] fluorescein, using the AbiPrism 7500 sequence detection system (Applied Biosystems). Assay probes for NLRC4 (Mm01233151-m1), and glyceraldehyde-3-phosphate dehydrogenase (Mm03302249-g1) as well as the TaqMan Master Mix were purchased from Applied Biosystems.
Silencing of P2X4R and NLRC4 Gene Expression
P2X7R−/− (for P2X4R) and C57BL/6 (for NLRC4) PMΦs (1 × 106) were seeded in 6-well culture plates in antibiotic-free RPMI 1640 medium (Invitrogen) supplemented with 10% fetal calf serum. After 2 h, cells were washed, and only adherent cells were used for the experiments. P2X4R short hairpin RNA (shRNA) plasmid (sc42570SH), negative controls (control shRNA plasmid B, which encodes a scrambled shRNA sequence (sc108065), and control shRNA plasmid C, which is an alternate negative scrambled shRNA sequence control (sc108066)), transfection medium, and reagents were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and the protocol for transfection recommended by the manufacturer was followed. Protein P2X4R expression was verified by Western blots using an anti-P2X4R antibody (Alomone Laboratories). 24 h after transfection, cells were washed, serum-free RPMI 1640 medium was added, and cells were stimulated with biglycan (40 μg/ml) for 16 h. For silencing NLRC4 gene expression by small interfering RNA (siRNA), the siGENOME SMARTpool (M-055000-01, Dharmacon/Thermo Fisher Scientific) was used. siGENOME non-targeting siRNA 2 (D-001210-02-05; Dharmacon/Thermo Fisher Scientific) served as a negative control. Cells were transfected using the X-tremeGENE siRNA transfection reagent (Roche Applied Science), following instructions of the manufacturer. NLRC4 gene expression was verified by Quantitative TaqMan PCRs.
Immunoprecipitation, Western Blotting, and ELISA
Immunoprecipitations and Western and dot blots were performed and quantified as described (27, 32). The following antibodies were used: anti-mouse IL-1β (clone B122; BD Biosciences), anti-mouse caspase-1 (Millipore for immunoprecipitation and sc-514; Santa Cruz Biotechnology for Western blotting), anti-phosphorylated and -total Erk (Thr202/204), p38 (Thr180/Tyr182) MAPK (all from Cell Signaling Technology), anti-mouse TLR4 (BioCarta for immunoprecipitation) or rabbit polyclonal TLR4 antibody (H-80; Santa Cruz Biotechnology for Western blotting), anti-mouse TLR2 (Sanbio for immunoprecipitation; BioCarta for Western blotting), anti-P2X4R and anti-P2X7R (both from Alomone Laboratories), and anti-NLRP3 (D-12) and anti-β-tubulin (both from Santa Cruz Biotechnology). For coimmunoprecipitation of P2X7R and P2X4R with biglycan and TLR4 or TLR2, thioglycollate-elicited Bgn−/0 (3 × 106) macrophages were incubated with 8 μg of intact human biglycan for 2 h at 4 °C, followed by incubation with 1 mm 3,3′-dithiobis(sulfosuccinmidylpropionate) (Pierce) for cross-linking biglycan to binding proteins. Cells were harvested in radioimmune precipitation buffer, and a complex of normal rabbit serum bound to protein A-Sepharose (Sigma) was added for 6 h to remove nonspecifically precipitated proteins. Then 10 μg of rabbit anti-human biglycan (27) were added, and after 16 h, the immune complexes were precipitated with protein A-Sepharose. As control, a similar amount of protein A-Sepharose was incubated with the antibody but without cell lysate. Additional controls included homogenates of P2X7R−/−, TLR2−/−, and TLR4−/− macrophages, samples incubated without cross-linker and/or without biglycan and with non-immune rabbit serum (Sigma). After washing (3× lysis buffer, 2× phosphate-buffered saline), the material containing intact biglycan was divided into two aliquots. One aliquot was treated with chondroitinase ABC. Bound proteins were eluted by boiling in the respective sample buffer (27). IL-1β, TNFα, and IL-6 were measured in plasma and in culture media by mouse-specific ELISAs (R&D Systems). Results were normalized by protein content and cell number. All assays were performed in duplicate, and each experiment was carried out at least three times.
ATP Assay
The ATP content in supernatants from Bgn−/0 thioglycollate-elicited PMΦs incubated with biglycan (4 μg/ml) for 10 s to 240 min was quantified with the adenosine-5′-triphosphate bioluminescent assay kit (Sigma) in the TEKAN Infinite M200 luminometer (Crailsheim, Germany), using alamethicin (50 μg/ml; Sigma) as a positive control (34). The assay sensitivity was 10−9 to 10−1 mm of ATP.
Caspase-1 Activity Assay and ELISA for Mature IL-1β in Kidney Homogenates
Kidneys from Bgn−/0 and Bgn+/0 mice 3 days after UUO were homogenized and sonicated in lysis buffer containing 25 mm Na-HEPES, 2 mm dithiothreitol, 1 mm EDTA, 0.1% CHAPS, 10% sucrose, 1 mm phenylmethylsulfonyl fluoride, 1 μm pepstatin A, pH 7.2, for the measurement of caspase-1 activity. For the measurement of mature IL-1β buffer containing 137 mm NaCl, 20 mm Tris-Cl, pH 8.0, 5 mm EDTA, 10% glycerol, 1% Triton X-100 was used. Lysates were then centrifuged at 4 °C at 13,000 rpm for 15 min, and supernatants were snap-frozen and stored at −80 °C. Equal amounts of protein were used for each sample. The activity of caspase-1 was measured using the caspase-1 activity assay kit (R&D Systems) as instructed by the manufacturer and was expressed as the percentage of increase of activity in ligated in relation to contralateral control kidneys. IL-1β levels were measured by mouse-specific IL-1β ELISA (R&D Systems) and normalized to protein content. All assays were performed in duplicate for four animals in each group.
Morphology and Immunohistochemistry
Serial sections (3–5 μm) of formaldehyde-fixed and paraffin-embedded samples were stained with periodic acid-Schiff reaction and were further processed for immunohistochemical studies. Lung tissue sections were incubated with goat anti-mouse IL-1β (1:30; R&D Systems), followed by the alkaline phosphatase/anti-alkaline phosphatase technique (33) and Mayer's hematoxylin solution (Sigma) for counterstaining. Nonspecific staining was determined by the use of the secondary antibodies alone. To evaluate the number of infiltrating mononuclear cells in the interstitium of individual kidneys, 10 randomly selected non-overlapping fields of renal sections were examined under high power field magnification (×400) (Soft Imaging System; Olympus). Mean values of at least five kidneys per group were averaged. Tubulointerstitial damage was evaluated (tubular dilatation, epithelial cellular atrophy, and luminal cast formation with tubulointerstitial expansions) and scored on a scale of 0–4 (0, normal; 0.5, small focal area of the tubular injury; 1, involvement of >10% of the cortex; 2, involvement of up to 25% of the cortex; 3, involvement of up to 50–75% of the cortex; and 4, extensive damage involving >75% of the cortex), as described previously (35).
Other Methods
To ensure that all described effects of biglycan on macrophages were not attributable to contamination of the biglycan preparation, we carefully ruled out the presence of LPS, peptidoglycan, other proinflammatory factors, and TLR4 or TLR2 ligands, as shown previously (27). The endotoxin content was measured using the ENDOSAFE-PTS system, based on the Limulus amebocyte lysate test (Charles River Laboratories). The CytoTox non-radioactive assay kit (Promega) was used to detect l-lactate dehydrogenase. ROS formation was detected in peritoneal macrophages (PMΦs) incubated with the redox-sensitive probe dihydrodichlorofluorescein diacetate by using a microplate fluorimeter (Wallac, Victor; excitation 488 nm, emission 515 nm) (36). Protein concentrations were determined using the BCA protein assay reagent (Pierce).
Statistical Analysis
Data are given as means ± S.D. analyzed by the one-way analysis of variance, with Dunnett's significance correction test (SPSS software). Differences were considered significant at a p value of <0.05.
RESULTS
Biglycan Stimulates Maturation and Secretion of IL-1β in Macrophages without the Need for Additional Costimulatory Factors
Stimulation of Bgn−/0 thioglycollate-elicited PMΦs with intact biglycan in a time-dependent (up to 24 h) and dose-dependent (up to 80 μg/ml) manner resulted in enhanced secretion of mature IL-1β detected by ELISA (Fig. 1A and supplemental Fig. S1). Western blot analysis confirmed the presence of the mature 17-kDa form of IL-1β in culture supernatants from Bgn−/0 macrophages stimulated with biglycan (Fig. 1B). Similar results were obtained in resident peritoneal macrophages without stimulation with thioglycollate, further underlining the specificity of the response to biglycan (data not shown). Due to a limited number of resident peritoneal macrophages, in further experiments, only thioglycollate-elicited peritoneal macrophages were used.
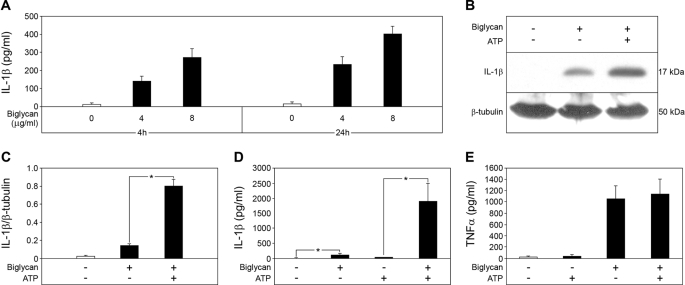
FIGURE 1.
Biglycan stimulates secretion of mature IL-1β without need for other costimulatory factors. A, ELISA for mature IL-1β in culture media from Bgn−/0 PMΦs non-stimulated and biglycan-stimulated (4 or 8 μg/ml) for 4 or 24 h. B, Western blot for mature 17-kDa IL-1β in PMΦ supernatants and cell lysates stimulated with biglycan (4 μg/ml) for 16 h without ATP or subsequently pulsed with ATP (5 mm) for 30 min versus non-stimulated controls, normalized to β-tubulin and quantified for three independent experiments (C). Shown are ELISA for mature IL-1β (D) and ELISA for TNFα (E) in media from Bgn−/0 PMΦs stimulated with biglycan for 4 h and then pulsed with ATP for 30 min. For A and C–E, data are means ± S.D. for at least n = 3. For C and D, *, p < 0.05.
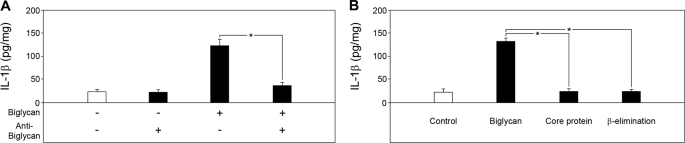
Surprisingly, biglycan-dependent secretion of mature IL-1β occurred without the costimulatory effect of ATP. However, when biglycan-stimulated Bgn−/0 macrophages were additionally pulsed with 5 mm ATP for 30 min, enhanced secretion of mature IL-1β was detected by Western blot (Fig. 1, B and C) and ELISA (Fig. 1D) in a dose-dependent manner (supplemental Fig. S1). Stimulation of IL-1β was specific for biglycan and was inhibited by biglycan-neutralizing antibodies (Fig. 2A). Neither the biglycan protein core nor biglycan-derived glycosaminoglycan (GAG) side chains (obtained by β-elimination) alone affected IL-1β-levels (Fig. 2B), suggesting that both core protein and GAG chains are necessary for the stimulation of macrophages.
FIGURE 2.
Effects of biglycan-blocking antibody and biglycan-derived protein core or glycosaminoglycan chains on the secretion of mature IL-1β from macrophages. A, ELISA of mature IL-1β in media from Bgn−/0 PMΦs stimulated for 4 h with biglycan (4 μg/ml) or without and after preincubation for 1 h with a biglycan-blocking antibody (10 μg/ml) indicated that biglycan is responsible for the enhanced secretion of mature IL-1β from PMΦs. B, ELISA of mature IL-1β in media from Bgn−/0 PMΦs stimulated for 4 h with intact biglycan (4 μg/ml), biglycan core protein, or biglycan-derived glycosaminoglycan chains (β-elimination). Data are means ± S.D. for at least n = 3. *, p < 0.05.
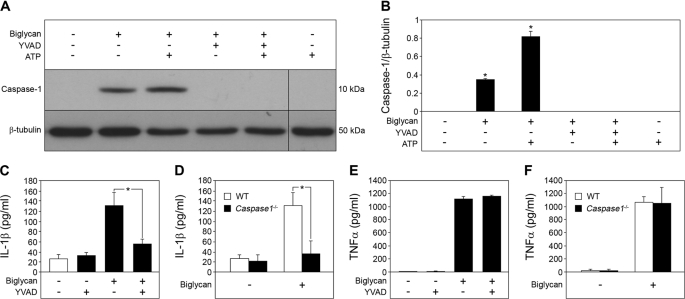
Since the cleavage of IL-1β into its mature 17-kDa form is processed by activated caspase-1 (1, 2), we also examined whether stimulation of macrophages with biglycan would lead to activation of caspase-1. In fact, the p10 active form of caspase-1 was detected by Western blot in macrophages incubated with biglycan alone for 16 h or additionally pulsed with 5 mm ATP for 30 min (Fig. 3, A and B). Furthermore, the caspase-1 inhibitor Ac-YVAD-CMK profoundly blocked biglycan-stimulated secretion of mature IL-1β (Fig. 3, A–C). Comparable inhibitory effects were observed in caspase-1−/− (Casp-1tm1Sesh) macrophages incubated with biglycan (Fig. 3D). The effects of biglycan were highly specific for IL-1β, since neither ATP (Fig. 1E) nor a functional lack of caspase-1 (inhibition (Fig. 3E) or deficiency (Fig. 3F)) had any influence on biglycan-mediated secretion of TNFα (27) from macrophages. As expected, PMΦs did not secrete mature IL-1β and showed signs of neither activation of caspase-1 nor release of endogenous ATP but overexpressed mRNA for pro-IL-1β and secreted TNFα and IL-6 in response to pure PAMP ligands (ultrapure LPS and peptidoglycan) to TLR2 and TLR4 (data not shown). Thus, biglycan regulates maturation and secretion of IL-1β in macrophages in a caspase-1-dependent manner even without the co-stimulatory effects of ATP.
FIGURE 3.
Biglycan mediates maturation of IL-1β in a caspase-1-dependent manner. A, immunoblot of whole cell lysates and media of Bgn−/0 PMΦs pretreated with caspase-1 inhibitor Ac-YVAD-CMK (YVAD; 10 μm) for 1 h, followed by stimulation with biglycan (4 μg/ml) for 16 h (without ATP or subsequently pulsed with 5 mm ATP for 30 min), analyzed with an antibody against caspase-1, normalized to β-tubulin, and quantified for n = 3 (B). C, ELISA for IL-1β in media from Bgn−/0 PMΦs pretreated with Ac-YVAD-CMK and stimulated with biglycan for 4 h versus non-stimulated PMΦs. D, ELISA for IL-1β in supernatants from WT and Casp1−/− PMΦs stimulated with biglycan for 4 h. E and F, ELISA for TNFα in supernatants from PMΦs stimulated as described in C and D, respectively. For B–F, data are means ± S.D. for at least n = 3. For B–D, *, p < 0.05.
Biglycan Requires the NLRP3 Inflammasome for IL-1β Maturation
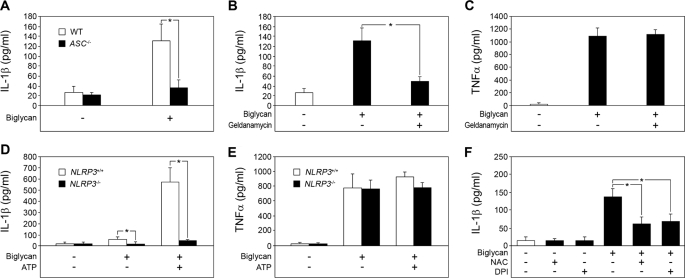
Activation of caspase-1 depends on procaspase recruitment into the inflammasome and requires the adapter protein ASC downstream of NLRs (7, 13, 37). To explore possible mechanisms of the biglycan-mediated activation of caspase-1, ASC−/− PMΦs were incubated with biglycan, and mature IL-1β was quantified by ELISA in supernatants of PMΦs. ASC deficiency completely abolished biglycan-dependent secretion of IL-1β (Fig. 4A), indicating the involvement of an ASC-dependent inflammasome in biglycan-mediated maturation and secretion of IL-1β. Next, we inhibited heat-shock protein 90 (HSP90), essential for the activity of various inflammasomes, using geldanamycin (38). Geldanamycin caused an 80% reduction in the level of mature IL-1β in supernatants from PMΦs stimulated with biglycan (Fig. 4B). In contrast to IL-1β, biglycan-induced secretion of TNFα was not affected by geldanamycin (Fig. 4C). NLRP3 interacts with ASC, can be activated by HSP90, and is a key player in caspase-1 activation in response to a variety of bacterial ligands, imidazoquinolines, double-stranded RNA, ATP, and various crystals (7, 12, 38–41). To demonstrate the potential requirement of NLRP3 in biglycan-triggered regulation of IL-1β, we used bone marrow-derived macrophages (BM-MΦs) from NLRP3+/+ and NLRP3−/− mice. In agreement with the data obtained in PMΦs, biglycan alone and in combination with ATP was capable of inducing secretion of mature IL-1β from NLRP3+/+ (Fig. 4D). However, higher concentrations of biglycan and longer periods of incubation were required for BM-MΦs (40 μg/ml, 16 h) to obtain comparable amounts of secreted mature IL-1β as for PMΦs (4 μg/ml, 4 h). Importantly, neither biglycan alone nor a combination of biglycan and ATP was capable of stimulating the secretion of IL-1β from NLRP3-deficient BM-MΦs (Fig. 4D). Conversely, TNFα was enhanced in supernatants from NLRP3−/− BM-MΦs stimulated with biglycan, showing that NLRP3 was necessary for biglycan-dependent regulation of IL-1β but not for stimulation of TNFα synthesis (Fig. 4E).
FIGURE 4.
Biglycan requires the NLRP3 inflammasome for IL-1β maturation. A, ELISA for mature IL-1β in culture media from WT and ASC−/− PMΦs stimulated with biglycan (4 μg/ml) for 4 h versus non-stimulated macrophages. B, ELISA for mature IL-1β; C, ELISA for TNFα in culture media from Bgn−/0 PMΦs pretreated with geldanamycin (200 nm) for 1 h followed by stimulation with biglycan (4 μg/ml) for 4 h versus non-stimulated macrophages. D, ELISA for mature IL-1β; E, ELISA for TNFα in supernatants from NLRP3+/+ and NLRP3−/− BM-MΦs stimulated with biglycan (40 μg/ml) for 16 h versus non-stimulated BM-MΦs. F, ELISA for IL-1β in culture media from Bgn−/0 PMΦs preincubated with N-acetyl-l-cysteine (NAC; 10 mm) or diphenyleneiodonium chloride (DPI; 0.5 mm) for 1 h and then stimulated with biglycan (4 mg/ml) for 4 h. For A–F, data are means ± S.D. for at least n = 3; *, p < 0.05.
Next, by silencing NLRC4 gene expression, we examined whether the NLRC4 inflammasome might be involved in biglycan-mediated maturation of IL-1β. Transfection of WT PMΦs with siRNA for 48 h significantly reduced NLRC4 gene expression in PMΦs transfected with NLRC4 siRNA but not in cells exposed to non-targeting siRNA, as verified by quantitative TaqMan PCRs (supplemental Fig. S2A). Subsequent stimulation of PMΦs with biglycan (4 μg/ml, 4 h) resulted in enhanced secretion of IL-1β without differences between control and PMΦs transfected either with NLRC4 or non-targeting siRNA (supplemental Fig. S2B), indicating that NLRC4 is not involved in biglycan-dependent IL-1β maturation. Thus, induction of the NLRP3 inflammasome appears to be essential for biglycan-dependent regulation of IL-1β.
Recent data have shown that activation of the NLRP3 inflammasome is triggered by ROS (16, 17). Therefore, we examined whether biglycan might induce ROS formation in PMΦs. In fact, enhanced ROS production was detected in supernatants from PMΦs after stimulation with biglycan (1.7 ± 0.2 times control, n = 3, p < 0.05, 1 h). Furthermore, N-acetyl-l-cysteine, an inhibitor of ROS, and diphenyleneiodonium, an inhibitor of NADPH oxidase (NADPH is the reduced form of nicotinamide adenine dinucleotide phosphate) and other flavin-containing oxidases, partially reduced biglycan-mediated secretion of IL-1β from PMΦs (Fig. 4F). This indicates that NADPH-mediated induction of ROS formation is involved in biglycan-mediated activation of the inflammasome.
Besides ROS, hyaluronan has also been shown to activate the NLRP3 inflammasome (42). To examine whether alterations in hyaluronan might be involved in biglycan-mediated IL-1β maturation, WT PMΦs were treated for 24 h with 2 mm 4-methylumbelliferone, a well known inhibitor of hyaluronan synthesis (43). Subsequently, biglycan (4 μg/ml) or TNFα plus IFNγ (10 ng/ml both) were added for 4 h. Incubation with 4-methylumbelliferone for 28 h significantly reduced hyaluronan content in culture media from PMΦs (supplemental Fig. S3A). Biglycan had no influence on hyaluronan synthesis. By contrast, TNFα plus IFNγ (positive control) enhanced the synthesis of hyaluronan (supplemental Fig. S3A). Inhibition of hyaluronan synthesis had no influence on biglycan-triggered secretion of mature IL-1β from PMΦs (supplemental Fig. S3B). Moreover, stimulation of PMΦs for 4 h with hyaluronan (1 or 25 μg/ml) was not sufficient to stimulate secretion of mature IL-1β (supplemental Fig. S3B). However, after 4 h, hyaluronan (25 μg/ml) markedly stimulated the secretion of TNFα from PMΦs (control PMΦs 41 ± 4 pg/ml versus hyaluronan-treated PMΦs 3280 ± 215 pg/ml; n = 3, p < 0.05, 4 h). In agreement with a previous report (42), increased secretion of mature IL-1β from PMΦs was triggered by hyaluronan after 18 h (control PMΦs 35 ± 3 pg/ml versus hyaluronan-treated PMΦs 184 ± 16 pg/ml; n = 3, p < 0.05). Thus, these data indicate that the effects of biglycan on the maturation of IL-1β are independent of those mediated by hyaluronan.
Biglycan-triggered Interaction of TLR2/4 and P2X4/P2X7 Receptors Leads to IL-1β Secretion in Macrophages
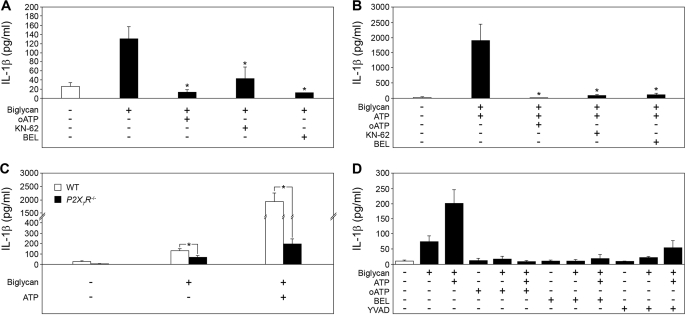
In a further step, we investigated how biglycan would stimulate secretion of IL-1β in the absence of other costimulatory factors. ATP, the best characterized costimulator required for IL-1β maturation and secretion, acts via P2X receptors, mainly the P2X7R, resulting in stimulation of Ca2+-independent phospholipase A2, which is required for caspase-1 activation (3, 44). To determine whether biglycan might use purinergic receptors, Bgn−/0 PMΦs were stimulated with biglycan in the presence of the P2X7R antagonists oATP and KN62 and of the Ca2+-independent phospholipase A2 inhibitor BEL with subsequent measurements of secreted mature IL-1β by ELISA (Fig. 5A). Biglycan-stimulated and ATP-pulsed PMΦs served as controls (Fig. 5B). In fact, oATP completely and KN62 partially inhibited biglycan-dependent secretion of mature IL-1β, indicating involvement of the P2X7R in this process (Fig. 5, A and B). Furthermore, BEL, known to block nigericin- or ATP-induced caspase-1 activation (45, 46), completely inhibited biglycan-mediated secretion of IL-1β (Fig. 5, A and B). To exclude the autocrine involvement of ATP in this process (47), we tested the release of lactate dehydrogenase after 4 h and the ATP content in supernatants from Bgn−/0 PMΦs incubated with biglycan for 10 s to 4 h, using the pore-forming peptide alamethicin as a positive control (34). There was no difference in lactate dehydrogenase levels between biglycan- and non-stimulated PMΦs (data not shown). Biglycan was not contaminated with ATP and did not affect the secretion of ATP (supplemental Fig. S4).
FIGURE 5.
Biglycan-mediated secretion of IL-1β involves the P2X7 receptor. A and B, ELISAs for IL-1β in media from Bgn−/0 PMΦs preincubated for 1 h with or without inhibitors: oATP (ATP periodate oxidized sodium salt, 1 mm), KN-62 (30 μm), BEL (20 μm) and stimulated with biglycan alone (4 μg/ml) for 4 h (A) or additionally pulsed with ATP (5 mm) for 30 min (B) versus non-stimulated PMΦs. C, mature IL-1β (ELISA) in media from P2X7R−/− versus WT (C57BL/6) PMΦs stimulated with biglycan (4 μg/ml) alone for 4 h or pulsed additionally with ATP (5 mm) for 30 min versus non-stimulated PMΦs. D, ELISA for mature IL-1β in media from P2X7R−/− PMΦs preincubated for 1 h in the presence or absence of inhibitors oATP, BEL, or YVAD (10 μm) and then stimulated for 4 h with biglycan (4 μg/ml) alone or additionally pulsed with ATP (5 mm) for 30 min versus non-stimulated PMΦs. For A–D, data are means ± S.D. for at least n = 3; *, p < 0.05.
To confirm that biglycan-mediated secretion of IL-1β involves P2X7R, WT and P2X7R−/− PMΦs were stimulated with biglycan alone or were additionally pulsed with ATP. As expected, the costimulatory effect of ATP was dramatically reduced in P2X7R−/− versus WT PMΦs (Fig. 5C). Interestingly, P2X7R deficiency only partially reduced biglycan-dependent secretion of IL-1β (Fig. 5C). However, each of the inhibitors of ATP-induced caspase-1 activation (oATP, BEL, and Ac-YVAD-CMK) completely blocked biglycan-induced secretion of IL-1β in P2X7R−/− PMΦs (Fig. 5D), suggesting additional involvement of other P2X receptors. These effects were specific for IL-1β, since oATP, KN62, BEL, and Ac-YVAD-CMK had no influence on biglycan-mediated secretion of TNFα (supplemental Fig. S5, A, C, and E) or IL-6 (supplemental Fig. S5, B, D, and F) from Bgn−/0 (Fig. supplemental Fig. S5, A–D) or P2X7R−/− (supplemental Fig. S5, E and F) macrophages.
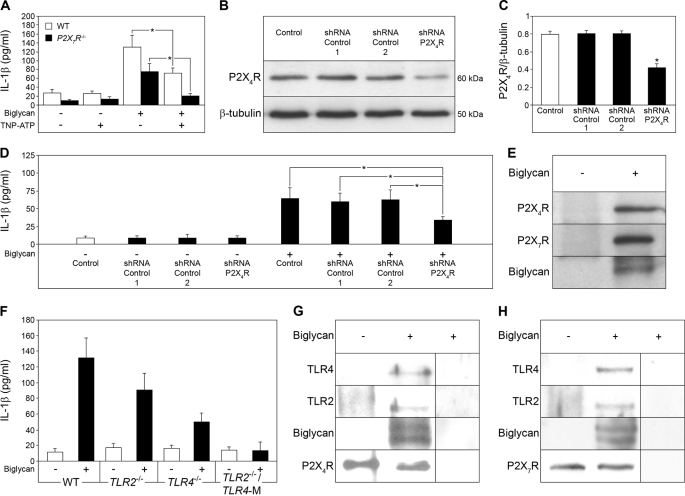
Among P2X receptors, P2X7R and P2X4R are constitutively expressed in primary mouse macrophages (48). Unlike P2X7R, which is ubiquitously expressed, P2X4R has a restricted pattern of expression and is markedly up-regulated due to activation of TLRs by LPS or other TLR ligands (49). To investigate whether P2X4R is involved in biglycan-mediated secretion of IL-1β, WT and P2X7R−/− PMΦs were incubated with biglycan in the presence of TNP-ATP, an unselective inhibitor of P2X4R. TNP-ATP resulted in a 41% inhibition in WT PMΦs, whereas biglycan-dependent secretion of IL-1β in P2X7R−/− PMΦs was completely abolished (Fig. 6A). TNP-ATP had no influence on biglycan-mediated secretion of TNFα (supplemental Fig. S6A) or IL-6 (supplemental Fig. S6B) from WT and P2X7R−/− PMΦs. Since a selective inhibitor of P2X4R is not available and TNP-ATP inhibits P2X1R, P2X2R, P2X3R, and other ATP-dependent proteins as well, a genetic approach based on shRNA-mediated knockdown of P2X4R was followed in P2X7R−/− PMΦs as proof of involvement of P2X4R in biglycan-induced secretion of IL-1β. Transfection of P2X7R−/− PMΦs with shRNA for 24 h resulted in a significant reduction of P2X4R protein verified by Western blot in PMΦs transfected with P2X4R shRNA but not in negative controls encoding two different scrambled shRNA sequences (Fig. 6, B and C). Subsequent stimulation of PMΦs with biglycan (40 μg/ml, 16 h) resulted in enhanced secretion of mature IL-1β in control P2X7R−/− PMΦs (Fig. 6D). Silencing of P2X4R abrogated biglycan-mediated IL-1β secretion (Fig. 6D) to a level correlating with the shRNA-mediated reduction of P2X4R protein (Fig. 6C). By contrast, TNFα (supplemental Fig. S6C) and IL-6 (supplemental Fig. S6D) were enhanced in supernatants from P2X7R−/− PMΦs transfected with P2X4R shRNA and stimulated with biglycan, demonstrating that P2X4R was necessary for biglycan-dependent regulation of IL-1β but not for stimulation of TNFα or IL-6 synthesis.
FIGURE 6.
Biglycan triggers the combined effects of TLR2/TLR4 and P2X7R/P2X4R on IL-1β secretion in macrophages. A, ELISA for mature IL-1β in media from WT or P2X7R−/− PMΦs pretreated with TNP-ATP (10 μm, 1 h) and stimulated with biglycan (4 μg/ml) for 4 h versus non-stimulated PMΦs. Shown are the Western blot for P2X4R (B), its quantification (C), and ELISA for mature IL-1β in P2X7R−/− PMΦs without transfection (control), transfected for 24 h with scrambled shRNA sequence (control 1 and control 2) or with P2X4R shRNA (D). The asterisk (C) indicates statistical differences between PMΦs transfected with P2X4R shRNA and controls. D, subsequently, PMΦs were incubated for the next 16 h under serum-free conditions in the absence or presence of biglycan (40 μg/ml). The asterisk indicates statistical difference between PMΦs transfected with P2X4R shRNA followed by biglycan stimulation and biglycan-stimulated controls. E, coimmunoprecipitation of biglycan with P2X4R and P2X7R. Lysates of WT PMΦs were immunoprecipitated with anti-biglycan antiserum and analyzed by immunoblot using anti-P2X4R, anti-P2X7R and anti-biglycan antibodies. F, ELISA for IL-1β in TLR4−/−, TLR2−/−, and TLR2−/−/TLR4-M PMΦs stimulated with biglycan (4 μg/ml, 4 h) versus non-stimulated PMΦs. Data are representative of at least three experiments. G and H, coimmunoprecipitation of P2X4R (G) and P2X7R (H) with TLR2/TLR4 in the presence of biglycan. Bgn−/0 PMΦs were incubated with or without biglycan, followed by immunoprecipitation with anti-P2X4R (G) or anti-P2X7R antibody (H) and analyzed by Western blot with anti-TLR4, anti-TLR2, anti-biglycan (G and H), anti-P2X4R (G), or anti-P2X7R antibodies (H). Non-immune rabbit serum instead of anti-P2X4R antibody (G) and homogenates of P2X7R−/− PMΦs (H) were used as controls. For A, C, D, and F, data are means ± S.D. for at least n = 3; *, p < 0.05.
Further proof for the involvement of P2X4R and P2X7R in the interaction with biglycan was provided by immunoprecipitation experiments. Incubation of WT PMΦs with intact biglycan in the presence of the 3,3′-dithiobis(sulfosuccinmidylpropionate) cross-linker and subsequent immunoprecipitation with anti-biglycan antiserum showed that biglycan coimmunoprecipitates with both P2X4R and P2X7R (Fig. 6E).
Since biglycan acts as a ligand of TLR4 and TLR2 (27), we also examined whether these receptors were important for biglycan-induced regulation of IL-1β in PMΦs. We found that biglycan-induced secretion of mature IL-1β was completely abrogated in TLR2−/−/TLR4-M and reduced in TLR2−/− or in TLR4−/− PMΦs (Fig. 6F). Taking into account that depletion of P2X4R and P2X7R led to inhibition of biglycan-induced secretion of IL-1β, we postulated that coactivation of TLR4 and/or TLR2 with P2X4R and/or P2X7R is obligatory for this process. Therefore, our next question was whether biglycan could serve as a linker between P2X receptors and TLRs. To test this hypothesis, Bgn−/0 PMΦs were incubated with and without biglycan, followed by immunoprecipitation using either anti-P2X4R-specific (Fig. 6G) or anti-P2X7R-specific (Fig. 6H) antiserum with subsequent detection of TLR4, TLR2, and biglycan by Western blotting. Coimmunoprecipitation of P2X receptors and TLRs occurred only in the presence of biglycan (Fig. 6, G and H). The non-immune rabbit serum (Fig. 6G) and P2X7R−/− PMΦs served as negative controls (Fig. 6H). Similar results were obtained using anti-TLR2 or anti-TLR4 antiserum for immunoprecipitation followed by detection of P2X4R and/or P2X7R and biglycan (data not shown). These data strongly suggest that biglycan serves as a mediator of IL-1β secretion by interacting with P2X4R/P2X7R and TLR4/TLR2, thereby activating these receptors and combining their effects via different signaling pathways.
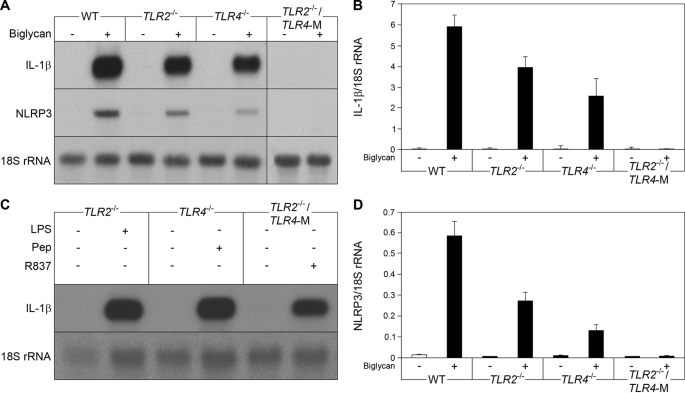
Biglycan Triggers IL-1β and NLRP3 mRNA Expression in Peritoneal Macrophages
Besides activation of the inflammasome, macrophages need TLR signaling for the induction of IL-1β gene transcription, as a second factor for the production of IL-1β (50). WT (C57BL/6) PMΦs were stimulated with intact biglycan for up to 24 h. Induction of IL-1β mRNA was observed already after 30 min (25.3 ± 1.6-fold control, n = 3, p < 0.05), reaching a maximum after 2 h (Fig. 7A), with a plateau at this level for up to 24 h. TNFα, a downstream mediator of biglycan (27), was not capable of increasing IL-1β mRNA within 30 min (data not shown). We therefore predicted that biglycan could have a direct effect on IL-1β expression in macrophages. To identify the receptor(s) involved, PMΦs from WT, TLR2−/−, TLR4−/−, and TLR2−/−/TLR4-M (TLR4-M, C3H/HeJ-mice are hyporesponsive to LPS due to a point mutation in the TLR4 gene) (29) were incubated with biglycan. Depletion of both TLR2 and TLR4 completely abolished biglycan-dependent stimulation of IL-1β mRNA, whereas the presence of one of the receptors was sufficient to trigger biglycan-mediated expression of IL-1β (Fig. 7, A and B). Next, we examined the influence of biglycan on mRNA expression of NLRP3, caspase-1, NLRC4, and ASC. Incubation of PMΦs with biglycan resulted in TLR2- and TLR4-dependent induction of NLRP3 mRNA expression (Fig. 7, A and D). This effect occurred within 30 min and could not be mimicked by TNFα or by IL-1β, two downstream effectors of biglycan (data not shown). By contrast, biglycan had no effect on the expression of caspase-1, ASC, and NLRC4 (data not shown), indicating that biglycan influences the inflammasome by regulating NLRP3 mRNA expression via TLR2 and TLR4. Agonists of TLR7 (R837), TLR2 (peptidoglycan), and TLR4 (LPS) were used as positive controls in TLR2−/−/TLR4-M, TLR4−/−, and TLR2−/− PMΦs, to show that these mice are deficient in the respective receptor but still able to express IL-1β mRNA (Fig. 7C). It is of note that NLRP3 mRNA was detected by Northern blots in unstimulated PMΦs as well. Since the mRNA levels of stimulated PMΦs were not in a linear range after such a long exposure, these data are not shown.
FIGURE 7.
Biglycan triggers IL-1β and NLRP3 mRNA expression in macrophages. A, Northern blots for IL-1β and NLRP3 mRNA in WT (C57BL/6), TLR2−/−, TLR4−/−, and TLR2−/−/TLR4-M PMΦs 2 h after stimulation with biglycan (4 μg/ml). B and D, quantification of IL-1β (B) and NLRP3 (D) mRNA levels normalized to 18 S rRNA. Data are means ± S.D.; all experiments were performed at least three times. C, expression of IL-1β mRNA in TLR-deficient PMΦs stimulated for 2 h with receptor agonists: TLR2−/− with LPS (2 ng/ml), TLR4−/− with peptidoglycan (5 μg/ml), and TLR2−/−/TLR4−/− with R837 (5 μg/ml). Data represent four independent experiments.
Subsequently, potential intracellular signaling cascades by which biglycan might stimulate IL-1β expression were explored. A combination of the MEK1/2 (U0126), p38 MAPK (SB203580), and IKK inhibitors (IKK inhibitor III) completely abolished the effects of biglycan on IL-1β mRNA expression in PMΦs (supplemental Fig. S7, A–D). Thus, biglycan stimulates IL-1β expression in a TLR2- and TLR4-dependent manner by signaling through NFκB, Erk, and p38 MAPK.
Collectively, in PMΦs in vitro interaction of biglycan with TLR2/4 serves as a trigger for IL-1β and NLRP3 gene transcription and for NLRP3-dependent caspase-1 activation due to receptor cooperativity with P2X4R/P2X7R.
Biglycan Deficiency Attenuates the Increase in Active Caspase-1 and Mature IL-1β in Non-infectious Inflammatory Renal Injury and in LPS-mediated Sepsis
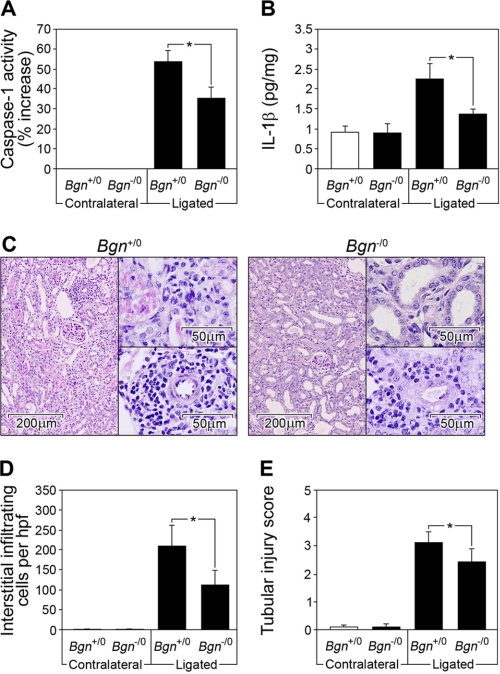
In a model of non-infectious inflammatory renal injury induced by the ligation of the left kidney (UUO), biglycan is markedly up-regulated in tubular epithelial cells prior to infiltration of macrophages (30). Therefore, UUO represents a good model to test whether biglycan might act in vivo as an endogenous inducer of inflammation via inflammasome-mediated induction of IL-β. Obstructed and contralateral Bgn−/0 and Bgn+/0 kidneys were harvested 3–21 days following UUO. Three days following ligation, active caspase-1 (Fig. 8A) and mature IL-1β (Fig. 8B) were enhanced in ligated versus contralateral kidneys from mice of both genotypes. Biglycan deficiency markedly attenuated the increase in active caspase-1 (Fig. 7A) and mature IL-1β (Fig. 8B) and resulted in lower numbers of infiltrating mononuclear cells in the renal interstitium (Fig. 8, C and D) and less damage to the kidney (Fig. 8, C and E). Thus, biglycan is involved in the regulation of caspase-1 activity and secretion of mature IL-1β in vivo in non-infectious inflammatory kidney disease.
FIGURE 8.
Biglycan deficiency attenuates the increase in active caspase-1 and mature IL-1β in non-infectious inflammatory renal injury (UUO). A, caspase-1 activity assay; B, ELISA for mature IL-1β in kidney homogenates from Bgn+/0 and Bgn−/0 mice 3 days following UUO. Equal amounts of protein were used for each sample. A, activity of caspase-1 was measured using the caspase-1 activity assay kit and was expressed as percentage of increase of caspase-1 activity in ligated (injured) to contralateral control kidneys. Caspase-1 activity in contralateral kidneys was taken as base line. B, levels of mature IL-1β in kidney homogenates were normalized to protein content. All assays (A and B) were performed in duplicate for four animals in each group. C, periodic acid-Schiff reaction staining of representative tissue sections from ligated kidneys from Bgn+/0 and Bgn−/0 mice 21 days following UUO. The lower insets show a magnified view of an area with infiltrating mononuclear cells. The upper insets show a magnified view of tubulointerstitial damage. Bars, magnification. D, the number of infiltrating mononuclear cells in the renal interstitium (10 randomly selected non-overlapping fields from renal sections of individual kidneys) was examined under high power field (hpf) magnification. E, tubulointerstitial damage was evaluated (tubular dilatation, epithelial cell atrophy, and luminal cast formation with tubulointerstitial expansion) and scored on a scale of 0–4. D and E, mean values of at least five kidneys per group were averaged. A, B, D, and E, data are means ± S.D. *, p < 0.05.
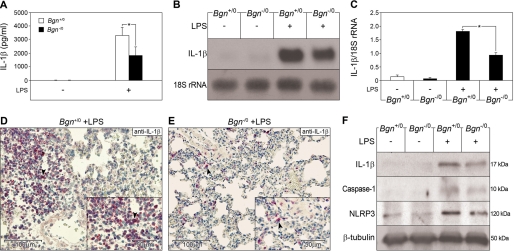
Based on in vitro findings that biglycan simultaneously interacts with P2X4R/P2X7R and TLR2/4, two receptors important for the recognition of both Gram-negative and Gram-positive pathogens, it would be conceivable that biglycan might potentiate PAMP-triggered inflammation by stimulating the NLRP3 inflammasome and by inducing synthesis of IL-1β via a second TLR not currently involved in pathogen-sensing. To test this assumption, a prototypical innate immune process, such as LPS-mediated sepsis, shown to be associated with enhanced expression of biglycan (27), was induced in Bgn−/0 and Bgn+/0 mice. Eight hours after injection of LPS, plasma IL-1β levels were elevated in all animals but were 2.1 ± 0.5-fold lower in Bgn−/0 than in Bgn+/0 animals (n = 6, p < 0.05) (Fig. 9A). In the lung, a major target organ in sepsis, biglycan deficiency resulted in attenuation of IL-1β mRNA expression (2.2 ± 0.5-fold lower than in Bgn+/0 mice, 8 h after LPS injection, Northern blots, n = 4, p < 0.05) (Fig. 9, B and C). IL-1β protein levels were also lower in septic lungs from Bgn−/0 compared with Bgn+/0 mice, as detected by immunostaining for pro- and mature IL-1β (Fig. 9, D and E) and Western blotting for mature IL-1β (Fig. 9F). The staining mainly corresponded to infiltrating cells (Fig. 9, D and E). Not only the levels of total IL-1β (Fig. 9E) but also those of the mature 17-kDa form were 2.6 ± 0.2-fold lower (n = 3, p < 0.05) in septic lungs from Bgn−/0 animals (Fig. 9F). This was associated with lower expression of NLRP3 (1.7 ± 0.2-fold lower, n = 3, p < 0.05) in septic lungs from Bgn−/0 mice (Fig. 8F). Importantly, the 10-kDa form of active caspase-1 was significantly reduced in septic Bgn−/0 lungs (1.9 ± 0.1-fold lower, n = 3, p < 0.05), clearly indicating the involvement of biglycan not only in the synthesis of pro-IL-1β but also in the activation of the inflammasome-dependent maturation of IL-1β. It is of note that both in PAMP-independent and -dependent in vivo models of inflammation, biglycan-deficient mice displayed lower levels of active caspase-1 and mature IL-1β, resulting in less damage to target organs. Thus, these in vivo results strongly support our in vitro findings that biglycan regulates both synthesis of pro-IL-1β and NLRP3-dependent activation of caspase-1 with subsequent IL-1β maturation.
FIGURE 9.
Biglycan deficiency attenuates the increase in pro-IL-1β expression, active caspase-1, and mature IL-1β in LPS-mediated sepsis. A, plasma levels of IL-1β in control mice without LPS (Bgn+/0 and Bgn−/0, ELISA, each group n = 6) and in septic Bgn+/0 and Bgn−/0 mice (each group n = 4). B, Northern blot for IL-1β mRNA in septic lungs of Bgn+/0 and Bgn−/0 mice normalized to 18 S rRNA and quantified for three experiments (C). D and E, immunostaining for (pro- and mature) IL-1β (marked by arrows) in septic lungs from Bgn+/0 (D) versus Bgn−/0 (E) mice. The lower right insets show a magnified view of cells expressing IL-1β. Bars, magnification. F, Western blots for mature IL-1β, active caspase-1 and NLRP3 normalized to β-tubulin in control and septic lungs from Bgn+/0 versus Bgn−/0. Quantification of F is included under “Results.” A–F, 8 h after LPS. Data are means ± S.D.; *, p < 0.05.
DISCUSSION
Here we show that the extracellular matrix component biglycan acts as an endogenous danger signal that activates the NLRP3 inflammasome by interacting with Toll-like and purinergic P2X receptors. By engaging these receptors, biglycan regulates the NLRP3 inflammasome in macrophages, stimulating the expression of NLRP3 and pro-IL-1β mRNA and activating the NLRP3/caspase-1 in a ROS- and HSP90-dependent manner, thereby driving the maturation and secretion of IL-1β without further need for other costimulatory factors. It is of note that both in a model of non-infectious inflammatory renal injury (UUO) and in a prototypical innate immune process, such as LPS-induced sepsis, biglycan-deficient mice displayed lower levels of active caspase-1 and mature IL-1β, resulting in less damage to target organs. In this report, we provide biochemical, pharmacological, and genetic evidence of direct activation of the NLRP3 inflammasome by biglycan, a matrix-derived molecule.
Contaminations are unlikely to have caused these effects. In contrast to many other endogenous ligands of innate immunity receptors (51), biglycan has not been prepared in a microbial system. Apart from the neutralizing effects of antibodies directed against biglycan, which strongly implicates biglycan as the active component, and in vivo relevance of biglycan/NLRP3/caspase-1 interaction in IL-1β maturation, contaminating products have been excluded by several assays (27). The final proof that activation of macrophages was in fact due to biglycan itself and not to potential other contaminants like heparan sulfate or hyaluronan (23) is based on the finding that only intact biglycan was capable of affecting macrophages, whereas neither the protein core nor the GAG side chains by themselves revealed any activity. At present, a few matrix molecules, such as fibronectin extra domain A (18), hyaluronan-derived oligosaccharides (19, 22), lumican (21), and biglycan (27), previously regarded only as docking sites for microbial pathogens, have been implicated to actively participate in the innate immune response as TLR agonists (18, 19). Our findings indicate that both TLRs and the NLRP3 inflammasome are activated by endogenous soluble biglycan and thereby might monitor tissue integrity and sense cellular stress under physiological and non-infection-driven pathological conditions, in addition to their obvious function in microbial defense (11). So far, only ATP (12), ROS (17, 52), amyloid-β (53), and crystals of monosodium urate (15) or calcium pyrophosphate dihydrate (15) and hyaluronan (42) have been described to trigger the NLRP3 inflammasome endogenously. Our findings are consistent with the recent observation that hyaluronan, another extracellular matrix molecule released upon injury, also activates the NLRP3 inflammasome by binding to CD44 with subsequent internalization and catabolism (42). We could show that effects of biglycan on IL-β maturation are independent of those mediated by hyaluronan. Furthermore, it appears that biglycan (4 μg/ml, 4 h) in comparison with hyaluronan (25 μg/ml, 18 h) stimulates the secretion of mature IL-1β from macrophages in a more rapid fashion. These findings suggest that the ECM contains various endogenous stimuli for IL-1β maturation and secretion, acting by different mechanisms and with different degrees of sufficiency, thereby modulating the inflammatory response reaction in terms of onset, intensity, and outcome (resolution or fibrosis).
Based on various pharmacological inhibitors and carefully proven by a genetic approach using NLRP3-, ASC-, caspase-1-, and TLR2/4-deficient mice, we were able to demonstrate in primary murine PMΦs and BM-MΦs that biglycan activates caspase-1 in a ROS/NLRP3/HSP90/ASC-dependent manner, thereby driving maturation and secretion of IL-1β, without the need for further costimulatory factors. In our experiments, we used mainly primary PMΦs, which displayed a better response to biglycan compared with macrophage-like cell lines or BM-MΦs. The majority of published data regarding the production of mature IL-1β in macrophages implicates the necessity of a double stimulation with ligands to TLRs to induce IL-1β gene transcription and with a second trigger, mainly ATP or muramyldipeptide, for activation of the inflammasome (50). Recent studies compared the response of human monocytes, macrophages, and dendritic cells to PAMP ligands of TLR2 and TLR4 (54) and showed that only monocytes are capable of releasing mature IL-1β without using additional exogenous costimulatory factors due to constitutively active caspase-1 and secretion of endogenous ATP (47, 54). In agreement with this report, in our hands, murine macrophages did not secrete mature IL-1β and showed no signs of activation of caspase-1 or release of endogenous ATP but overexpressed mRNA for pro-IL-1β in response to pure LPS (data not shown). Using highly sensitive methods, we did not detect biglycan-dependent secretion of ATP, which could have acted as a costimulatory factor. Therefore, in contrast to LPS, biglycan appears to be an autonomous trigger of IL-1β. Findings that the amounts of secreted IL-1β triggered by biglycan alone are much lower than those obtained by an additional pulse of 5 mm ATP have to be seen in light of the fact that physiological levels of extracellular ATP are only ∼10 nm and may maximally reach the micromolar range around living cells, creating an “ATP halo” (55). Therefore, the effects of ATP in the millimolar range used by us and others rather represent intracellular (3–10 mm) and not extracellular levels of ATP (55). Furthermore, the amount of mature IL-1β secreted from PMΦs stimulated by 4 μg/ml biglycan over 4 h was comparable with the response of macrophages to 25 μg/ml of hyaluronan over 18 h and much higher than the stimulation induced by large amounts of crude LPS (25 μg/ml, 18 h) (42), underlining the biological relevance of endogenous regulators in IL-1β maturation and secretion.
By binding to TLR2/4 and signaling through NFκB, Erk, and p38 MAPK, biglycan (i) stimulates the expression of NLRP3 mRNA and protein and (ii) increases the amount of pro-IL-1β, the major substrate of the inflammasome. Since various exogenous TLR ligands induce pro-IL-1β production via NFκB (56) and NLRP3 mRNA (39, 57), it is conceivable that biglycan as an endogenous ligand of TLR2/TLR4 (27) may exert similar effects. However, the somewhat surprising finding that besides binding to TLRs, biglycan independently interacts with purinergic P2X receptors, thereby inducing cumulative signaling capacities, suggests a novel mechanism of downstream activation of the NLRP3 inflammasome. Several mechanisms of NLRP3 inflammasome activation have been postulated up to now. Internalization with subsequent lysosomal damage appears to play a role in hyaluronan-, crystal-, and amyloid-β-dependent inflammasome activation (42, 53, 58). By contrast, ATP activates the inflammasome via the P2X7R with a subsequent efflux of K+ (3, 7, 16). The requirement of ROS or HSP90 in biglycan-dependent activation of the NLRP3 inflammasome, similar to monosodium urate, R837 (52), asbestos, silica (17), and ATP (16) as ROS mediators or to NLRP3-mediated gout-like inflammation involving HSP90 (38), indicates that HSP90 and ROS play a crucial role in the activation of the NLRP3 inflammasome independent of the initial trigger. The findings that biglycan triggers ROS formation fit well with previous observations that TLRs are involved in ROS formation via the NADPH oxidase enzyme complex (59).
Our genetic approach using TLR2/4- and P2X7R-deficient macrophages together with shRNA for P2X4R and further substantiated by biochemical methods indicates that the interaction of TLR2/4 with P2X7R/P2X4R occurs in the presence of biglycan. It is tempting to speculate that biglycan mediates clustering of TLR2/4 and purinergic P2X4/P2X7 receptors, inducing receptor cooperativity within these newly formed multireceptor complexes. Although several controls have been included, interpretation of the cross-linking experiments cannot totally rule out the possibility that biglycan might independently engage both receptors, being sufficient for activation of the inflammasome. The final proof for biglycan-triggered functional interaction of both signaling pathways at the proximal level by priming TLR2/4-deficient macrophages with a different TLR agonist is limited by the fact that, for example, R837 by itself is involved in activation of the inflammasome (41).
The core protein of biglycan is rife with leucine-rich repeats, which are considered to be involved in protein-protein interactions (26), and carries up to two GAG side chains. These complex structural features make biglycan particularly well endowed to serve as a cross-linker between different cell surface receptors (20). The requirement of intact biglycan for TLR/P2X receptor clustering underlines the intricacy of this interaction, in which besides CD14 and MD2 other molecules associated with TLRs and/or P2X receptors might also be involved. In fact, other proteins (e.g. β2 integrin) that might interact with biglycan have been identified to form signaling complexes with P2X7R (60). Thus, soluble biglycan, as an indicator of tissue injury, could be a pivotal danger signal, which is carefully monitored by the innate immune system.
Most importantly, the biological relevance of biglycan as a trigger of the NLRP3 inflammasome has been confirmed in vivo in PAMP-independent and -dependent mouse models of inflammation, sharing features of enhanced biglycan expression and extensive macrophage infiltration (27, 30). In both models, biglycan deficiency led to lower levels of active caspase-1 and mature IL-1β, resulting in less damage to target organs. It is tempting to speculate that biglycan, upon release from the ECM, acts as an autonomous trigger of the non-infectious inflammatory response due to interaction of TLR2/4 and P2X4/P2X7 receptors, followed by their alloyed signaling. On the other hand, in pathogen-mediated inflammation, biglycan might potentiate PAMP-triggered inflammation by stimulating the NLRP3 inflammasome and by inducing synthesis of IL-1β via a second TLR not involved in pathogen sensing. In this context, it would be conceivable that in LPS-induced sepsis, biglycan deficiency resulted in lower expression of IL-1β mRNA besides reduced capase-1 activity and mature IL-1β levels.
It is currently not understood how a stationary macromolecule of the ECM can turn into a soluble ligand triggering cellular responses. Biglycan undergoes strong interactions with collagen VI (61); however, in comparison with other LRR proteoglycans, biglycan has a relatively low affinity with soluble, monomolecular collagen I, the most abundant matrix component in mammalian organisms by far. No information exists on the affinity of biglycan with collagen I, which is incorporated into banded collagen fibrils. In addition, biglycan can form stable soluble dimers at least in vitro (62). It is conceivable, however, that monomeric biglycan interacts with ECM components, leading to sequestration of a potentially proinflammatory signal. As has been shown previously (27), stimulated macrophages step up their synthesis of biglycan. The newly synthesized proteoglycan may then displace biglycan weakly bound to the ECM by forming more stable soluble dimers, which, in turn, may become organizers of multireceptor complexes involving TLRs and P2XRs. Likewise, other matrix proteins may mobilize sequestered biglycan by heterodimer formation, although such heterodimers have not yet been reported. In the same context, it is intriguing that both TLRs and NLRs contain LRR motifs (9, 10) with the potential to interact in an analogous fashion with LRR proteins, including biglycan.
Taken together, our data suggest that soluble biglycan acts as a fundamental danger signal that signifies tissue injury and elicits a robust proinflammatory response by the innate immune system. By interacting with Toll-like and purinergic P2X receptors on the cell surface of macrophages, biglycan signals through these multireceptor complexes and activates the NLRP3 inflammasome in a ROS- and HSP90-dependent manner, thereby driving the caspase-1-mediated maturation and secretion of IL-1β. The observations described here implicate biglycan as a promising target for novel anti-inflammatory strategies. Mechanisms are conceivable whereby unsequestered (63) biglycan is trapped, most likely by other matrix components containing LRR motifs. In this way, receptor signaling of soluble biglycan might be prevented, thereby achieving tight control of its proinflammatory effects.
Supplementary Material
This work was supported by Deutsche Forschungsgemeinschaft SFB 815, Project A5 (to L. S.) and SCHA 1082/2-1 (to L. S. and H.-J. G.), the Excellence Cluster Cardiopulmonary System (to L. S. and O. E.), SFB 405, Project B10 (to H.-J. G.), Interdisciplinary Center of Clinical Research, Muenster, Grant Schae2/026/06 (to L. S., R. M. S., and P. B.), KliFo 118 (to O. E.), and Else Kröner-Fresenius-Stiftung (to L. S.). This work was also supported in part by the National Institutes of Health, NIDCR, Intramural Research Program (to M. F. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
- IL
- interleukin
- BEL
- bromoenol lactone
- BM-MΦ
- bone marrow-derived macrophage
- ECM
- extracellular matrix
- GAG
- glycosaminoglycan
- HSP90
- heat-shock protein 90
- IFNγ
- interferon-γ
- LRR
- leucine-rich repeat
- NLR
- nucleotide binding oligomerization domain-like receptor
- oATP
- ATP periodate oxidized sodium salt
- PAMPs
- pathogen-associated molecular patterns
- PMΦ
- peritoneal macrophage
- ROS
- reactive oxygen species
- TNP-ATP
- 2′3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate monolithium trisodium salt
- TLR
- Toll-like receptor
- UUO
- unilateral ureteral obstruction
- CMK
- chloromethylketone
- CHAPS
- 3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate
- LPS
- lipopolysaccharide
- WT
- wild type
- MAPK
- mitogen-activated protein kinase
- TNF
- tumor necrosis factor
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA
- ELISA
- enzyme-linked immunosorbent assay
- GAG
- glycosaminoglycan.
REFERENCES
- 1.Martinon F., Tschopp J. (2007) Cell Death Differ. 14, 10–22 [DOI] [PubMed] [Google Scholar]
- 2.Joshi V. D., Kalvakolanu D. V., Hebel J. R., Hasday J. D., Cross A. S. (2002) Infect. Immun. 70, 6896–6903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Virgilio F. (2007) Trends Pharmacol. Sci. 28, 465–472 [DOI] [PubMed] [Google Scholar]
- 4.Hogquist K. A., Nett M. A., Unanue E. R., Chaplin D. D. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 8485–8489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello C. A. (2005) J. Exp. Med. 201, 1355–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ting J. P., Lovering R. C., Alnemri E. S., Bertin J., Boss J. M., Davis B. K., Flavell R. A., Girardin S. E., Godzik A., Harton J. A., Hoffman H. M., Hugot J. P., Inohara N., Mackenzie A., Maltais L. J., Nunez G., Ogura Y., Otten L. A., Philpott D., Reed J. C., Reith W., Schreiber S., Steimle V., Ward P. A. (2008) Immunity 28, 285–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F., Gaide O., Pétrilli V., Mayor A., Tschopp J. (2007) Semin. Immunopathol. 29, 213–229 [DOI] [PubMed] [Google Scholar]
- 8.Fritz J. H., Ferrero R. L., Philpott D. J., Girardin S. E. (2006) Nat. Immunol. 7, 1250–1257 [DOI] [PubMed] [Google Scholar]
- 9.Gay N. J., Gangloff M. (2008) Handb. Exp. Pharmacol. 181–200 [DOI] [PubMed] [Google Scholar]
- 10.Inohara Chamaillard McDonald C., Nuñez G. (2005) Annu. Rev. Biochem. 74, 355–383 [DOI] [PubMed] [Google Scholar]
- 11.Pétrilli V., Dostert C., Muruve D. A., Tschopp J. (2007) Curr. Opin. Immunol. 19, 615–622 [DOI] [PubMed] [Google Scholar]
- 12.Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., Dixit V. M. (2006) Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 13.Mariathasan S., Newton K., Monack D. M., Vucic D., French D. M., Lee W. P., Roose-Girma M., Erickson S., Dixit V. M. (2004) Nature 430, 213–218 [DOI] [PubMed] [Google Scholar]
- 14.Kanneganti T. D., Body-Malapel M., Amer A., Park J. H., Whitfield J., Franchi L., Taraporewala Z. F., Miller D., Patton J. T., Inohara N., Núñez G. (2006) J. Biol. Chem. 281, 36560–36568 [DOI] [PubMed] [Google Scholar]
- 15.Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. (2006) Nature 440, 237–241 [DOI] [PubMed] [Google Scholar]
- 16.Cruz C. M., Rinna A., Forman H. J., Ventura A. L., Persechini P. M., Ojcius D. M. (2007) J. Biol. Chem. 282, 2871–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B. T., Tschopp J. (2008) Science 320, 674–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamura Y., Watari M., Jerud E. S., Young D. W., Ishizaka S. T., Rose J., Chow J. C., Strauss J. F., 3rd (2001) J. Biol. Chem. 276, 10229–10233 [DOI] [PubMed] [Google Scholar]
- 19.Termeer C., Benedix F., Sleeman J., Fieber C., Voith U., Ahrens T., Miyake K., Freudenberg M., Galanos C., Simon J. C. (2002) J. Exp. Med. 195, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaefer L., Iozzo R. V. (2008) J. Biol. Chem. 283, 21305–21309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu F., Vij N., Roberts L., Lopez-Briones S., Joyce S., Chakravarti S. (2007) J. Biol. Chem. 282, 26409–26417 [DOI] [PubMed] [Google Scholar]
- 22.Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 23.Jiang D., Liang J., Noble P. W. (2007) Annu. Rev. Cell Dev. Biol. 23, 435–461 [DOI] [PubMed] [Google Scholar]
- 24.Nathan C. (2002) Nature 420, 846–852 [DOI] [PubMed] [Google Scholar]
- 25.Park P. W., Pier G. B., Hinkes M. T., Bernfield M. (2001) Nature 411, 98–102 [DOI] [PubMed] [Google Scholar]
- 26.Iozzo R. V. (1999) J. Biol. Chem. 274, 18843–18846 [DOI] [PubMed] [Google Scholar]
- 27.Schaefer L., Babelova A., Kiss E., Hausser H. J., Baliova M., Krzyzankova M., Marsche G., Young M. F., Mihalik D., Götte M., Malle E., Schaefer R. M., Gröne H. J. (2005) J. Clin. Invest. 115, 2223–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu T., Bianco P., Fisher L. W., Longenecker G., Smith E., Goldstein S., Bonadio J., Boskey A., Heegaard A. M., Sommer B., Satomura K., Dominguez P., Zhao C., Kulkarni A. B., Robey P. G., Young M. F. (1998) Nat. Genet. 20, 78–82 [DOI] [PubMed] [Google Scholar]
- 29.Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 30.Schaefer L., Macakova K., Raslik I., Micegova M., Gröne H. J., Schönherr E., Robenek H., Echtermeyer F. G., Grässel S., Bruckner P., Schaefer R. M., Iozzo R. V., Kresse H. (2002) Am. J. Pathol. 160, 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kresse H., Seidler D. G., Muller M., Breuer E., Hausser H., Roughley P. J., Schonherr E. (2001) J. Biol. Chem. 276, 13411–13416 [DOI] [PubMed] [Google Scholar]
- 32.Schaefer L., Beck K. F., Raslik I., Walpen S., Mihalik D., Micegova M., Macakova K., Schonherr E., Seidler D. G., Varga G., Schaefer R. M., Kresse H., Pfeilschifter J. (2003) J. Biol. Chem. 278, 26227–26237 [DOI] [PubMed] [Google Scholar]
- 33.Schaefer L., Raslik I., Grone H. J., Schonherr E., Macakova K., Ugorcakova J., Budny S., Schaefer R. M., Kresse H. (2001) FASEB J. 15, 559–561 [DOI] [PubMed] [Google Scholar]
- 34.Mathew M. K., Balaram P. (1983) Mol. Cell. Biochem. 50, 47–64 [DOI] [PubMed] [Google Scholar]
- 35.Adams J., Kiss E., Arroyo A. B., Bonrouhi M., Sun Q., Li Z., Gretz N., Schnitger A., Zouboulis C. C., Wiesel M., Wagner J., Nelson P. J., Gröne H. J. (2005) Am. J. Pathol. 167, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plesková M., Beck K. F., Behrens M. H., Huwiler A., Fichtlscherer B., Wingerter O., Brandes R. P., Mülsch A., Pfeilschifter J. (2006) FASEB J. 20, 139–141 [DOI] [PubMed] [Google Scholar]
- 37.Martinon F. (2007) Eur. J. Immunol. 37, 3003–3006 [DOI] [PubMed] [Google Scholar]
- 38.Mayor A., Martinon F., De Smedt T., Pétrilli V., Tschopp J. (2007) Nat. Immunol. 8, 497–503 [DOI] [PubMed] [Google Scholar]
- 39.Sutterwala F. S., Ogura Y., Szczepanik M., Lara-Tejero M., Lichtenberger G. S., Grant E. P., Bertin J., Coyle A. J., Galán J. E., Askenase P. W., Flavell R. A. (2006) Immunity 24, 317–327 [DOI] [PubMed] [Google Scholar]
- 40.Lamkanfi M., Kanneganti T. D., Franchi L., Núñez G. (2007) J. Leukocyte Biol. 82, 220–225 [DOI] [PubMed] [Google Scholar]
- 41.Kanneganti T. D., Ozören N., Body-Malapel M., Amer A., Park J. H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Akira S., Núñez G. (2006) Nature 440, 233–236 [DOI] [PubMed] [Google Scholar]
- 42.Yamasaki K., Muto J., Taylor K. R., Cogen A. L., Audish D., Bertin J., Grant E. P., Coyle A. J., Misaghi A., Hoffman H. M., Gallo R. L. (2009) J. Biol. Chem. 284, 12762–12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vigetti D., Rizzi M., Viola M., Karousou E., Genasetti A., Clerici M., Bartolini B., Hascall V. C., De Luca G., Passi A. (2009) Glycobiology 19, 537–546 [DOI] [PubMed] [Google Scholar]
- 44.Andrei C., Margiocco P., Poggi A., Lotti L. V., Torrisi M. R., Rubartelli A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 9745–9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walev I., Klein J., Husmann M., Valeva A., Strauch S., Wirtz H., Weichel O., Bhakdi S. (2000) J. Immunol. 164, 5120–5124 [DOI] [PubMed] [Google Scholar]
- 46.Kahlenberg J. M., Dubyak G. R. (2004) J. Leukocyte Biol. 76, 676–684 [DOI] [PubMed] [Google Scholar]
- 47.Piccini A., Carta S., Tassi S., Lasiglié D., Fossati G., Rubartelli A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo C., Masin M., Qureshi O. S., Murrell-Lagnado R. D. (2007) Mol. Pharmacol. 72, 1447–1456 [DOI] [PubMed] [Google Scholar]
- 49.Raouf R., Chabot-Doré A. J., Ase A. R., Blais D., Séguéla P. (2007) Neuropharmacology 53, 496–504 [DOI] [PubMed] [Google Scholar]
- 50.Netea M. G., van de Veerdonk F. L., Kullberg B. J., Van der Meer J. W., Joosten L. A. (2008) Expert Opin. Biol. Ther. 8, 1867–1872 [DOI] [PubMed] [Google Scholar]
- 51.Tsan M. F., Gao B. (2004) J. Leukocyte Biol. 76, 514–519 [DOI] [PubMed] [Google Scholar]
- 52.Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. (2007) Cell Death Differ. 14, 1583–1589 [DOI] [PubMed] [Google Scholar]
- 53.Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. (2008) Nat. Immunol. 9, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Netea M. G., Nold-Petry C. A., Nold M. F., Joosten L. A., Opitz B., van der Meer J. H., van de Veerdonk F. L., Ferwerda G., Heinhuis B., Devesa I., Funk C. J., Mason R. J., Kullberg B. J., Rubartelli A., Van der Meer J. W., Dinarello C. A. (2009) Blood 113, 2324–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trautmann A. (2009) Sci. Signal. 2, pe6. [DOI] [PubMed] [Google Scholar]
- 56.Creagh E. M., O'Neill L. A. (2006) Trends Immunol. 27, 352–357 [DOI] [PubMed] [Google Scholar]
- 57.O'Connor W., Jr., Harton J. A., Zhu X., Linhoff M. W., Ting J. P. (2003) J. Immunol. 171, 6329–6333 [DOI] [PubMed] [Google Scholar]
- 58.Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008) Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogier-Denis E., Mkaddem S. B., Vandewalle A. (2008) Semin. Immunopathol. 30, 291–300 [DOI] [PubMed] [Google Scholar]
- 60.Kim M., Jiang L. H., Wilson H. L., North R. A., Surprenant A. (2001) EMBO J. 20, 6347–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiberg C., Heinegård D., Wenglén C., Timpl R., Mörgelin M. (2002) J. Biol. Chem. 277, 49120–49126 [DOI] [PubMed] [Google Scholar]
- 62.Scott P. G., Dodd C. M., Bergmann E. M., Sheehan J. K., Bishop P. N. (2006) J. Biol. Chem. 281, 13324–13332 [DOI] [PubMed] [Google Scholar]
- 63.Medzhitov R. (2008) Nature 454, 428–435 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.