Abstract
Antagonists of anti-apoptotic Bcl-2 family members hold promise as cancer therapeutics. Apoptosis is triggered when a peptide containing a BH3 motif or a small molecule BH3 peptidomimetic, such as ABT 737, binds to the relevant Bcl-2 family members. ABT-737 is an antagonist of Bcl-2, Bcl-xL, and Bcl-w but not of Mcl-1. Here we describe new structures of mutant BH3 peptides bound to Bcl-xL and Mcl-1. These structures suggested a rationale for the failure of ABT-737 to bind Mcl-1, but a designed variant of ABT-737 failed to acquire binding affinity for Mcl-1. Rather, it was selective for Bcl-xL, a result attributable in part to significant backbone refolding and movements of helical segments in its ligand binding site. To date there are few reported crystal structures of organic ligands in complex with their pro-survival protein targets. Our structure of this new organic ligand provided insights into the structural transitions that occur within the BH3 binding groove, highlighting significant differences in the structural properties of members of the Bcl-2 pro-survival protein family. Such differences are likely to influence and be important in the quest for compounds capable of selectively antagonizing the different family members.
Apoptosis, or programmed cell death, is a fundamental cellular process required by all multicellular organisms for the elimination of redundant, damaged, or potentially dangerous cells (1). A consequence of dysregulated cell death is the survival of abnormal cells, which in some cases can proliferate uncontrollably and form tumors. Hence, strategies to activate cell death pathways may represent one avenue by which cancer cells can be killed. A major pathway to cell death is regulated by the Bcl-2 family of proteins that consists of both pro-apoptotic and pro-survival members (2). Those family members that promote cell death are divided into two subgroups; that is, the Bax/Bak molecules, which are the essential mediators of apoptosis, and the BH3-only proteins (such as Bim, Bad, Puma, Noxa, and several others), which are the initiators of the apoptotic cascade. Within the pro-survival faction there are five members including Bcl-2 itself, Bcl-xL, Bcl-w, Mcl-1, and A1. Overexpression of pro-survival proteins can confer a survival advantage to cancer cells. Critically, conventional anti-cancer therapies are often rendered ineffective by this overexpression and other up-stream defects, most prominently mutations in the tumor suppressor p53.
One strategy to kill cancer cells is to develop molecules that can mimic the BH3-only proteins (3). These proteins function by engaging the pro-survival proteins, although the downstream consequences of this interaction remain controversial (4). These interactions are mediated by a short sequence motif called the BH3 domain on the BH3-only protein. The structures of a number of BH3 domains in complex with pro-survival proteins have been solved, and all reveal that the BH3 sequence forms an amphipathic helix that inserts into a hydrophobic groove on the surface of the pro-survival proteins (5–10). These structures suggest that it might be possible to develop drugs based on small organic molecules that mimic natural BH3 ligands and activate the cell death pathways.
Although a number of “BH3-mimetic” molecules have now been described (11–21), many of these appear to kill cells in a non-mechanism-based manner (22). Only ABT-737, developed by Abbott Laboratories (15, 23), has been shown to be a bona fide BH3-mimetic (22, 24), binding in the same hydrophobic groove of Bcl-xL as do BH3 ligands (25). ABT-263, a molecule in the same chemical class as ABT-737, has shown efficacy as a single agent in cancer cell lines and animal models of cancer (26–28) and is currently in a phase I/II clinical trial in leukemia and lymphoma patients.
One of the important aspects of BH3 peptide interactions with pro-survival proteins is selectivity; some BH3 ligands only bind certain subsets of pro-survival proteins (e.g. Bad only binds Bcl-xL, Bcl-2, and Bcl-w, whereas Noxa only binds Mcl-1 and A1), but others such as Bim and Puma are more promiscuous and bind all pro-survival proteins tightly (29–31). This selectivity has important implications as it appears that a range of pro-survival proteins needs to be neutralized for cell death to proceed in some cell types (30, 32–34). Selectivity is also an important issue for drug potency. ABT-737 and ABT-263 share the same binding profile as Bad, and consequently, their efficacy seems to be restricted to cells/tumors in which Mcl-1 (to which they do not bind with high affinity) is inactivated or limiting (22, 28, 35–38). Hence, only a small subset of all tumors, in particular some hematological malignancies and small-cell lung cancers, appear to respond to ABT-737/ABT-263 when used as a single agent (15, 28). Therefore, to expand the repertoire of cancers that could be treated with BH3 mimetics, it is likely that the range of binding specificities of such molecules also needs to be expanded, with Mcl-1 being an obvious target candidate. Obatoclax (GX15-070), a small molecule developed by Gemin X Biotechnologies, has been reported to function in this way (14). However, this compound can kill cells deficient in both Bax and Bak, the essential mediators of apoptotic cell death (28); hence, its true mechanism of action is uncertain.
Peptide ligands can provide a template for small molecule drug design, particularly when a structure is available for it in complex with its target. For example, highly effective small molecule antagonists of the inhibitors of apoptosis proteins were designed using peptides as a starting point (39, 40). Antagonists of Bcl-xL based on derivatized terphenyl and terephthalamide scaffolds that apparently mimic the BH3 α-helix have also been reported (17, 18), although in this case no complexes with the protein target have yet been described.
Here we use mutagenesis data and structures of BH3 peptides in complex with pro-survival proteins to guide attempts to develop a BH3-mimetic that binds to Mcl-1. Although that goal was not achieved, our results demonstrate extreme conformational flexibility in the pro-survival protein Bcl-xL and yielded a novel Bcl-xL-selective antagonist. In contrast, Mcl-1 displays no backbone conformational flexibility when presented with the various ligands we describe.
EXPERIMENTAL PROCEDURES
Compounds
ABT-737 was supplied by Abbott Laboratories. The synthesis of W1191542 is described in the supplemental material.
Protein Expression and Purification/Peptides
Expression and purification of the loop-deleted form of human Bcl-xL (Δ27–82 ΔC24) and the human/mouse chimeric Mcl-1 (ΔN170 ΔC23) used for crystallization was performed as described previously (5, 41). Biacore assays were performed using human Bcl-2 ΔC22, Bcl-xL ΔC24, Bcl-w C29S/A128E ΔC29, and human/mouse Mcl-1 ΔN170 ΔC23 purified as described previously (5, 35). Human Bim BH3 peptides (26-mers) were synthesized and purified by reverse phase high performance liquid chromatography to >90% purity by Mimotopes. All peptides used for crystallization have a free amine at the N terminus and are either amidated (wild-type Bim and L12F) or have a free acid (L12Y) at the C terminus. The wild-type Bim BH3 sequence used here is 1DMRPEIWIAQELRRIGDEFNAYYARR26.
Crystallization
Crystals of Bcl-xL and Mcl-1 complexes with Bim BH3 peptides and with compounds were obtained by mixing the protein and ligand at a molar ratio of 1:1.3, then concentrating the sample to 10 mg/ml. Crystals were grown by the sitting drop method at room temperature under the following conditions: BimL12Y·Mcl-1 (0.15 m zinc acetate, 0.15 m imidazole, pH 5.75), W1191542·Bcl-xL (0.2 m calcium acetate, 10% (w/v) polyethylene glycol 8000, 0.1 m imidazole, pH 8.0), Bim·Bcl-xL (1 m sodium citrate, 0.1 m sodium cacodylate, pH 6.5), BimL12F·Bcl-xL (0.02 m zinc acetate, 2.5 m NaCl, 0.1 m imidazole, pH 8.0). Prior to flash-freezing in liquid N2, crystals were equilibrated into cryoprotectant consisting of reservoir solution plus increasing concentrations of ethylene glycol to a final concentration of 20% (v/v) for all complexes except BimL12Y·Mcl-1 where the final concentration was 25% (v/v).
Crystal Data Collection and Structure Refinement
X-ray data were collected at 100 K using an in-house RAXIS-IV++ detector with a micro-max007 rotating anode x-ray source or at the Australian synchrotron (42). All data were integrated and scaled with HKL2000 (43) (Table 1). Most structures were determined by molecular replacement with PHASER (44–46) using the following coordinates as search models: W1191542·Bcl-xL (PDB 3FDL (47)), Bim·Bcl-xL (PDB 2P1L (48)), BimL12F·Bcl-xL (PDB 2P1L). In each case the ligand and solvent was removed before calculation. For BimL12Y·Mcl-1 a rigid body refinement in Refmac5 was performed using the structure of Mcl-1 in which the Leu-12 residue was mutated to alanine (PDB 2NL9 (5)). Several rounds of building in COOT (49) and refinement in REFMAC5 (50) and PHENIX (51) led to the final models. The crystals of BimL12F·Bcl-xL were twinned, exhibiting a twinning fraction of 0.407, determined, and refined in PHENIX.
TABLE 1.
Crystallographic statistics for Mcl-1 and Bcl-xL protein complexes
Numbers in parenthesis are for the highest resolution shell.
| Data Set | BimL12Y·Mcl-1 | W1191542·Bcl-xL | Bim·Bcl-xL | BimL12F·Bcl-xL |
|---|---|---|---|---|
| PDB | 3IO9 | 3INQ | 3FDL | 3IO8 |
| Crystal parameter, space group | I222 | P212121 | C2 | P62 |
| Unit cell parameters | a = 52.5 Å | a = 62.6 Å | a = 74.11 Å | a = 65.7 Å |
| b = 74.1 Å | b = 68.2 Å | b = 35.68 Å | c = 170.1 Å | |
| c = 117.5 Å | c = 71.4 Å | c = 62.76 Å | ||
| β = 111.5 ° | ||||
| Data collection | ||||
| Wavelength (Å) | 1.5418 | 1.5418 | 1.5418 | 0.9566 |
| Resolution (Å) | 50.00-2.20 (2.28-2.20) | 50.00-2.00 (2.07-2.00) | 50.00-1.78 (1.84-1.78) | 50.00-2.30 (2.38-2.30) |
| Rmerge | 0.086 (0.378) | 0.086 (0.867) | 0.046 (0.163) | 0.147 (0.835) |
| I/σ(I) | 25.7 (6.0) | 31.4 (2.3) | 33.7 (11.1) | 15.7 (2.7) |
| Completeness (%) | 98.0 (99.1) | 99.9 (99.9) | 96.0 (94.9) | 99.8 (99.3) |
| Redundancy | 6.5 (5.4) | 6.8 (6.3) | 5.7 (5.8) | 7.4 (5.8) |
| No. of copies/asymmetric unit | 1 | 2 | 1 | 2 |
| Temperature | 100 K | 100 K | 100 K | 100 K |
| Refinement | ||||
| Resolution (Å) | 29.39-2.40 (2.46-2.40) | 31.63-2.00 (2.09-2.00) | 29.19-1.78 (1.82-1.78) | 47.30-2.30 (2.39-2.30) |
| No. of reflections Rwork | 8,615 (610) | 21,183 (2450) | 13,529 (946) | 18,425 (1,925) |
| No. of reflections Rfree | 474 (40) | 1,090 (126) | 728 (50) | 948 (111) |
| Rwork | 0.209 (0.175) | 0.194 (0.285) | 0.160 (0.239) | 0.185 (0.317) |
| Rfree | 0.251 (0.242) | 0.250 (0.364) | 0.208 (0.300) | 0.231 (0.319) |
| No. of atoms | ||||
| Protein | 1,146 | 2,272 | 1,163 | 2,319 |
| Ligand | 207 | 110 | 222 | 368 |
| Solvent | 70 | 182 | 209 | 32 |
| Root mean square deviations | ||||
| Bond lengths (Å) | 0.013 | 0.013 | 0.015 | 0.004 |
| Bond angles (°) | 1.4 | 1.4 | 1.5 | 0.76 |
| Chiral (Å3) | 0.096 | 0.095 | 0.265 | 0.055 |
Cytochrome c Release Assay
The ability of peptides or compounds to elicit cytochrome c release from digitonin-permeabilized cells was examined as described previously (37). Mitochondria-containing crude lysates from mcl-1−/− or bcl-x−/− mouse embryonic fibroblasts (MEFs)3 were incubated with 10 μm peptide or compound for 1 h at 30 °C before pelleting intact mitochondria. The supernatant was retained as the soluble fraction. The pellet fraction containing intact mitochondria was then lysed with buffer containing 1% (v/v) Triton X-100. The amount of cytochrome c in the soluble and pellet fractions was determined by Western blot using an anti-cytochrome c antibody (7H8 C12; BD Biosciences).
Cell Killing Assays
Short-term cell viability assays were measured using the CellTitre-Glo Luminescent Cell Viability Assay (Promega). Mouse embryonic fibroblasts (mcl-1−/− and bcl-x−/−) were plated at 3000 cells/well in a 96-well plate overnight. The media was then replaced with fresh media containing serial dilutions of W1191542, ABT-737, or solvent (DMSO), and the cells incubated for a further 24 h. CellTitre-Glo reagent was then added, and the luminescent signal was determined with a luminometer. Percentage viability was determined as the ratio of the signal in the presence of compound to the signal obtained for wells in which only DMSO was added.
For long term clonogenic survival assays, mcl-1−/− and bcl-x−/− MEFs were plated at 150 cells/well in 6-well plates then incubated for 6 days in the presence of serial dilutions of ABT-737, W1191542, or solvent (DMSO). Colonies were then stained with Coomassie Blue and scored. Results were expressed as the ratio of the number of colonies in the presence of compound to the number of colonies in the presence of DMSO only.
Binding Assays
Binding of compounds to pro-survival proteins was measured by surface plasmon resonance using a Biacore 3000 biosensor for solution competition assays as described previously (30). In these assays proteins were added at a final concentration of 10 nm and the compounds were diluted from 10 mm stock prepared in dimethyl sulfoxide. The running/dilution buffer was 10 mm Hepes, 150 mm NaCl, 3.4 mm EDTA, 0.005% (v/v) Tween 20, pH 7.2. Direct binding assays were performed using a Biacore S51 biosensor as previously described (52). The running buffer was 10 mm NaH2PO4, 40 mm Na2HPO4, 150 mm NaCl, 1.0 mm EDTA, 0.03% (v/v) Tween 20, 5% (v/v) DMSO, pH 7.4. Dilutions of each compound were injected at a flow rate of 90 μl/min over three spots (spot 1, immobilized with anti-GST antibody only; spot 2, coated with GST-Bcl-xL captured by the anti-GST antibody; spot r, free) at 25 °C. The association time and dissociation times were 90 and 240 s, respectively. Buffer blanks without or containing 4–6% (v/v) DMSO were injected periodically for double referencing and solvent correction. All sensorgrams were processed using double referencing; first, the response from the reference spot (spot 1) was subtracted from the binding response (spot 2) followed by solvent correction, then the response from an average of the buffer injections was subtracted. To obtain kinetic rate constants (ka and kd), corrected response data were then fitted to a one-to-one binding site model that included mass transport limitations. The equilibrium dissociation constant (KD) was determined from the ratio kd/ka.
RESULTS
Structure of BimL12Y·Mcl-1
BH3 domain peptides present four conserved hydrophobic residues (h1–h4) on one face of an amphipathic helix that bind into four pockets within the hydrophobic ligand binding groove on the pro-survival protein (5, 7, 10). In the 26-mer Bim BH3 peptides used here (1DMRPEIWIAQELRRIGDEFNAYYARR26), these four hydrophobic amino acids are Ile-8, Leu-12, Ile-15, and Phe-19 (shown in bold). The structure of Bcl-xL in complex with ABT-737 demonstrates that the chlorobiphenyl and thiophenyl groups of ABT-737 occupy pockets accommodating Leu-12 and Phe-19 in the Bim BH3 complex (25). In associated saturation mutagenesis studies we also showed that Mcl-1 is highly tolerant of any side-chain substitutions at the h4 residue of Bim (25), consistent with the structure of the Bim·Mcl-1 complex which shows that the h4 binding site on Mcl-1 is poorly formed and exposed to solvent compared with the corresponding region on Bcl-xL (5, 7). We, therefore, decided to focus on the h2 binding pocket on Mcl-1 as a potential site that restricts ABT-737 from binding to it tightly.
Our mutagenesis studies also showed that although substitution of the h2 leucine residue on Bim for tyrosine caused a significant reduction in binding affinity for Mcl-1 compared with all other pro-survival proteins where only a minor effect on binding was observed (i.e. 60-fold decrease in affinity for Mcl-1 compared with wild-type Bim versus a 3-fold decrease for Bcl-xL) (25), this mutant still bound Mcl-1 with ∼470 nm affinity. As a tyrosine side chain bears some resemblance to the terminal chlorophenyl group of ABT-737, we reasoned that a structure of the Bim BH3 domain with the L12Y mutation in complex with Mcl-1 could provide some insights into how ABT-737 could be modified to bind Mcl-1.
We determined the x-ray structure at 2.2 Å resolution of human/mouse chimeric Mcl-1 (5) with a 26-residue Bim BH3 peptide in which the h2 leucine residue was replaced with tyrosine (L12Y, see Table 1 for crystallographic statistics). The crystals grow under identical conditions and with similar cell dimensions to those of the wild-type peptide complex (5). The structure shows no significant differences in chain tracing between wild-type and mutant complexes. Only minor structural changes within the h2 binding pocket are observed, allowing the protein to accommodate the bulkier tyrosine residue which projects more deeply into this pocket than does the wild-type leucine residue at this position (Fig. 1a). This deep penetration of the h2 pocket is somewhat reminiscent of the way in which the chlorobiphenyl group on ABT-737 associates with the Bcl-xL h2 pocket. Notably though, the angle at which the tyrosine residue enters the Mcl-1 h2 pocket compared with the way in which the chlorobiphenyl of ABT-737 enters the corresponding region on Bcl-xL is different (Fig. 1b). Hence, although Mcl-1 can accommodate a bulky aromatic group on a peptidic ligand in the h2 binding pocket, it appears to do so differently to Bcl-xL, at least in the context of a small molecule ligand.
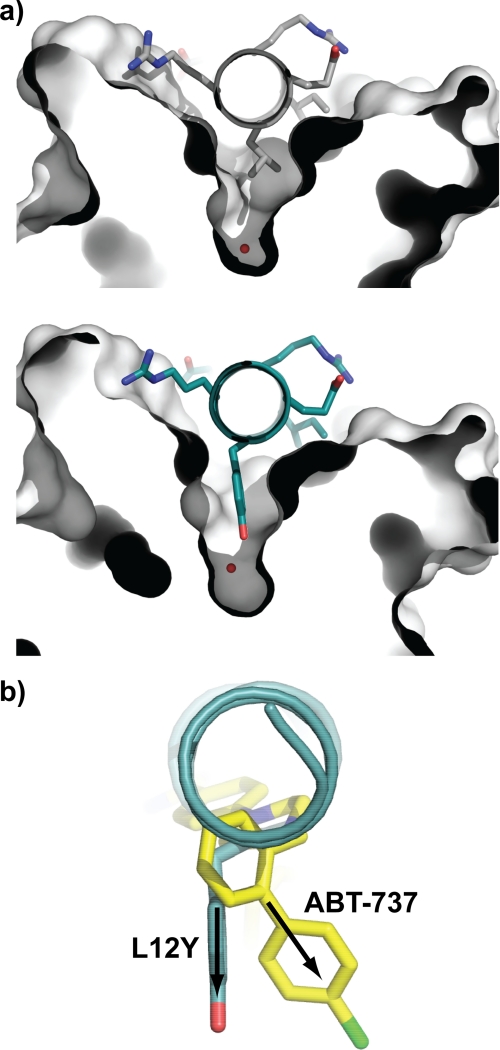
FIGURE 1.
Structure of BimL12Y·Mcl-1 complex. a, comparison of Leu-12 and L12Y binding pockets on wild-type (PDB 2NL9) and mutant Bim BH3 peptides bound to Mcl-1. The tyrosine at position 12 in the Bim mutant peptide (lower panel) penetrates the h2 binding pocket deeper than the wild-type leucine residue. A water molecule (indicated in red) is seen at the bottom of the pocket in both structures. b, overlay of Mcl-1 and Bcl-xL structures in their respective complexes with BimL12Y (teal) and ABT-737 (yellow, PDB 2YXJ), showing only the peptide and organic ligand. The tyrosine in BimL12Y penetrates the h2 pocket to approximately the same depth that the chlorobiphenyl group of ABT-737 penetrates the corresponding pocket of Bcl-xL; however, the angle of entry (arrows) is different.
Modifying ABT-737 to Mimic BimL12Y
The BimL12Y· Mcl-1 structure suggested a strategy by which ABT-737 could be modified to gain binding to Mcl-1. Switching the chlorophenyl group from the ortho to meta position more closely recapitulates the bond entry vector observed for the tyrosine residue in the Bim L12Y peptide complex with Mcl-1, potentially allowing it to engage the h2 binding pocket. We synthesized the compound (W1191542), although with a phenyl group in place of the chlorophenyl (Fig. 2a), as our mutagenesis studies showed that a phenylalanine substitution at Leu-12 in Bim BH3 was better tolerated in Mcl-1 (binding with 10 nm affinity) than the tyrosine substitution (25).
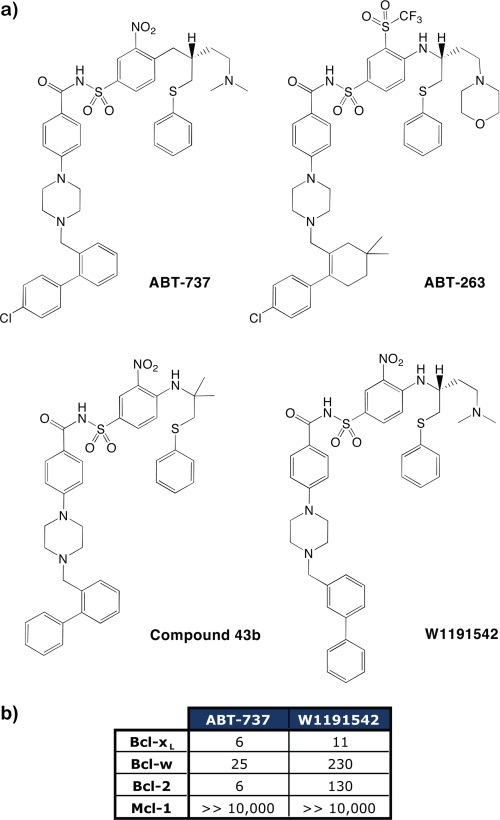
FIGURE 2.
A designed ABT-737 derivative, W1191542. a, chemical structures of ABT-737, ABT-263, Compound 43b (23) and W1191542. b, the IC50 values (in nm) determined by solution competition assays for either ABT-737 or W1191542 binding mammalian pro-survival proteins. ABT-737 binds tightly to Bcl-xL, Bcl-w, and Bcl-2, whereas W1191542 demonstrates a more selective binding profile for Bcl-xL.
To determine whether W1191542 had gained binding affinity for Mcl-1, we performed solution competition assays using a Biacore biosensor (Fig. 2b). No apparent increase in affinity for Mcl-1 was observed. However, this compound retained high affinity binding to Bcl-xL and indeed became significantly more selective for Bcl-xL over Bcl-2 and Bcl-w compared with ABT-737. Hence, a subtle alteration in the structure of ABT-737 significantly influenced its binding selectivity profile, although not in the way intended.
W1191542 Retains Biological Activity
Although our initial aim in modifying ABT-737 to bind Mcl-1 was not achieved, W1191542 was still of interest because of its selective binding profile for Bcl-xL. To test whether it retained biological activity, it was initially examined in an in vitro cytochrome c release assay using digitonin-permeabilized MEFs. As neutralization of both Mcl-1 and Bcl-xL is required for cell killing in MEFs (33), we initially used cells deficient in Mcl-1 (mcl-1−/− MEFs) to remove the Mcl-1 “brake” on Bak/Bax. In this assay W1191542 was able to cause cytochrome c release similar to both ABT-737 and the Bad BH3 domain peptide (Fig. 3a), both of which target a wider subset of pro-survival proteins (Bcl-xL, Bcl-2 and Bcl-w) with high affinity (15, 30). As a control, Bcl-xL-deficient MEFs were also examined, and no cytochrome c release was observed with W1191542, although release was observed with the Noxa BH3 domain peptide that specifically targets Mcl-1 (30) (Fig. 3a).
FIGURE 3.
Biological functioning of W1191542. a, W1191542 elicits cytochrome c release from digitonin-permeabilized mcl-1−/− MEFs, similar to ABT-737 and Bad BH3 domain peptide but not bcl-x−/− MEFs, evidenced by its translocation into the soluble fraction. WB, Western blot. b and c, W1191542 kills MEFs deficient in Mcl-1 in short-term (24 h) (b) viability and long term (6 days) clonogenic assays (c) but is significantly less efficacious than ABT-737. Crosses indicate the absence of colony formation. d, binding kinetics of ABT-737 and W1191542 to Bcl-xL. W1191542 binds Bcl-xL more rapidly but dissociates faster than ABT-737.
To test whether W1191542 could induce apoptosis in intact cells, we examined its ability to kill mcl-1−/− MEFs. Although significant activity in a 24-h assay was observed (EC50, 2 μm), it was several orders of magnitude weaker than ABT-737 (Fig. 3b). No killing of bcl-x−/− cells was observed (Fig. 3b), consistent with the binding data showing there was no significant affinity of this compound for Mcl-1. W1191542 was also examined for its ability to inhibit long term (6 days) clonogenic survival of mcl-1−/− MEFs. Again, although clonal suppression was observed with mcl-1−/− MEFs (but not bcl-x−/− MEFs), it was significantly weaker than ABT-737 (Fig. 3c). Hence, the killing potency of MEFs by small molecules appears to be significantly affected by the range of pro-survival proteins they target.
Finally, we examined W1191542 in a direct binding assay using a Biacore S51 instrument that enables the kinetics of small organic compounds binding to their targets to be determined. This approach eliminates some of the limitations of solution competition studies and expands the dynamic range of the assay. Interestingly, this assay provided a similar result to the competition assay, indicating that ABT-737 bound Bcl-xL ∼2-fold tighter than W1191542 (Fig. 3d and supplemental Fig. 2). However, the kinetics of the interactions are somewhat different; W1191542 binds Bcl-xL almost three times faster than ABT-737, but ABT-737 has a greater than 5-fold slower off-rate. The shortened half-life of the W1191542·Bcl-xL complex, 190 s versus 1000 s for the ABT-737 complex, seems a better indicator of its reduced potency than does its KD.
Structure of W1191542 Complexed to Bcl-xL
The binding kinetics of W1191542 and ABT-737 suggest that these compounds engage Bcl-xL differently despite the similarity in their chemical structures (Fig. 2a). We were also interested in how W1191542 would bind Bcl-xL after our modification to ABT-737; that is, whether the biphenyl moiety would now project directly down into the h2 pocket rather than toward the α3 helix. To help address both of these issues, we determined the crystal structure of W1191542 with Bcl-xL at 2.0 Å resolution using a loop deleted form of Bcl-xL (41, 47, 48). This variant forms a domain swapped dimer in which the α1 helix of each Bcl-xL molecule crosses into its neighbor. This domain exchange is distant from, and has no apparent effect on the ligand binding groove (47, 48). The advantage of using this form of Bcl-xL is that it allows crystals of Bcl-xL complexes to grow reproducibly within days instead of months, as previously reported (7, 53).
Two W1191542·Bcl-xL complexes are present in the asymmetric unit, each providing a different view of the interaction between the compound and its target. The major difference is in the biphenyl moiety (Fig. 4); in molecule B the biphenyl penetrates the h2 binding pocket, whereas in molecule A it is rotated around the methyl-biphenyl-piperazine C-N bond ∼45° relative to molecule B and projects out of the groove. There is also a discernable difference in the orientation of the α3 helix in molecule B resulting in a wider binding groove. The different crystal environments of molecules A and B are manifest as weak intermolecular contacts involving the ligand bound to molecule B. In molecule B helix α3 also participates in intermolecular crystal contacts. The two views provided by this structure may have captured the binding groove in transition from the closed to opened state upon ligand binding.
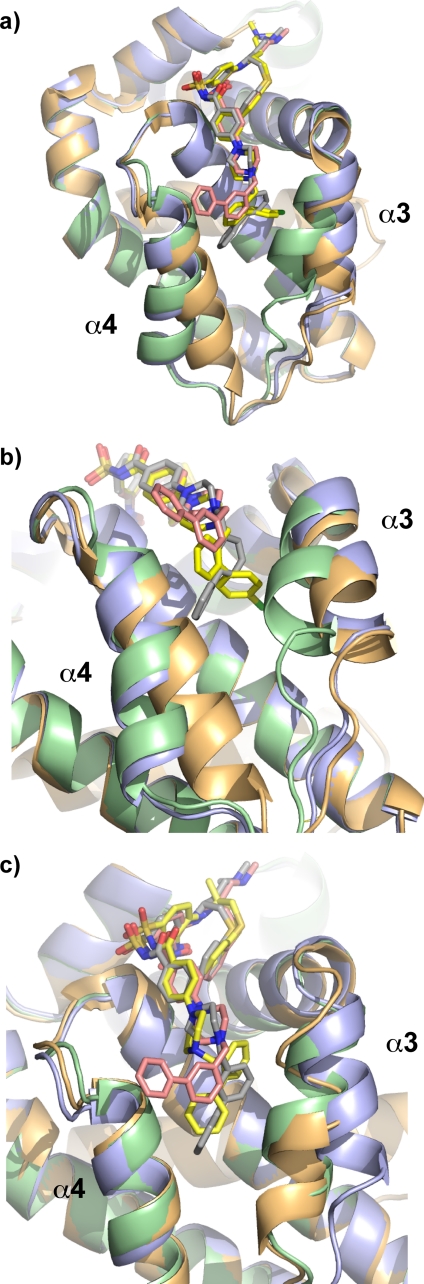
FIGURE 4.
Structure of the W1191542·Bcl-xL complex. a, overlay of structures of Bcl-xL in complex with ABT-737 (Bcl-xL, orange; ABT-737, yellow), W1191542 molecule A (Bcl-xL, green; W1191542/A, pink), or W1191542 molecule B (Bcl-xL, blue; W1191542/B, gray). Note the significant differences in the orientation of the α3 and α4 helices. b, close-up view of each compound in the region of the h2 pocket. The biphenyl of W1191542 molecule A projects out of the binding groove located between the α3 and α4 helices, whereas in W1191542 molecule B and ABT-737 it projects into the groove, although their orientations are different. c, overlay of the structures of Bcl-xL in complex with Compound 43b (Bcl-xL, orange; Compound 43b, yellow) (23), W1191542 molecule A (Bcl-xL, green; W1191542/A, pink), or W1191542 molecule B (Bcl-xL, blue; W1191542/B, gray). The space occupied by the biphenyl of Compound 43b overlays with that used by the biphenyl moiety in W1191542 molecule B.
When compared with the ABT-737·Bcl-xL structure, it is apparent that W1191542 binds similarly with the exception of the chlorobiphenyl moiety on ABT-737, which penetrates the h2 pocket differently from the biphenyl of both molecules in this new structure (Fig. 4, a and b). There are also significant differences in the orientation of the α3 and α4 helices; the α4 helix (orange) in the ABT-737 complex is different in both molecules of the W1191542 complex, whereas the α3 helix adopts a conformation similar to that seen in the molecule where the biphenyl group of W1191542 is buried (blue).
Structure of Wild-type Bim and BimL12F Complexed with BclxL
The structures of W1191542 and ABT-737 in complex with Bcl-xL revealed two distinct binding modes, especially in the region of the h2 pocket within the Bcl-xL hydrophobic groove. We, therefore, examined how a peptide ligand with an aromatic moiety at h2 would engage Bcl-xL as this could provide further insights into the structural plasticity of Bcl-2 family proteins.
By again using the loop-deleted form of Bcl-xL (47, 48), we were also able to crystallize and determine structures of both wild-type Bim and the BimL12F mutant in complex with Bcl-xL (Table 1). These two closely related Bim peptides crystallized in different conditions, suggesting differences in the structures, unlike with Mcl-1, where closely related Bim peptides (wild-type and L12Y) crystallized in very similar conditions and yielded similar structures. The wild-type complex is noticeably different from the previously published structure of the homologous murine complex (7) only at the C-terminal end of the peptide where the murine Bim BH3 extends in helical conformation for two full turns beyond the binding groove on Bcl-xL (although this feature is an artifact of the crystals of the murine complex and is not observed here). Crystals of the BimL12F·Bcl-xL complex are twinned and have two molecules in the asymmetric unit; chains A and B describe one peptide-protein complex (AB), and chains C and D describe the other (CD). Helix α3 is poorly resolved in both molecules, although it is somewhat better in the complex CD, which we describe here. Although the overall structure is similar to the wild-type Bim·Bcl-xL complex (Fig. 5a), substantial differences are apparent in helix α3. The side chain of the substituted phenylalanine residue in the h2 position of the peptide penetrates into its binding pocket in a manner very similar to that observed for the chlorobiphenyl group of ABT-737 (Fig. 5b) and unlike the biphenyl moiety of W1191542 (Fig. 5c).
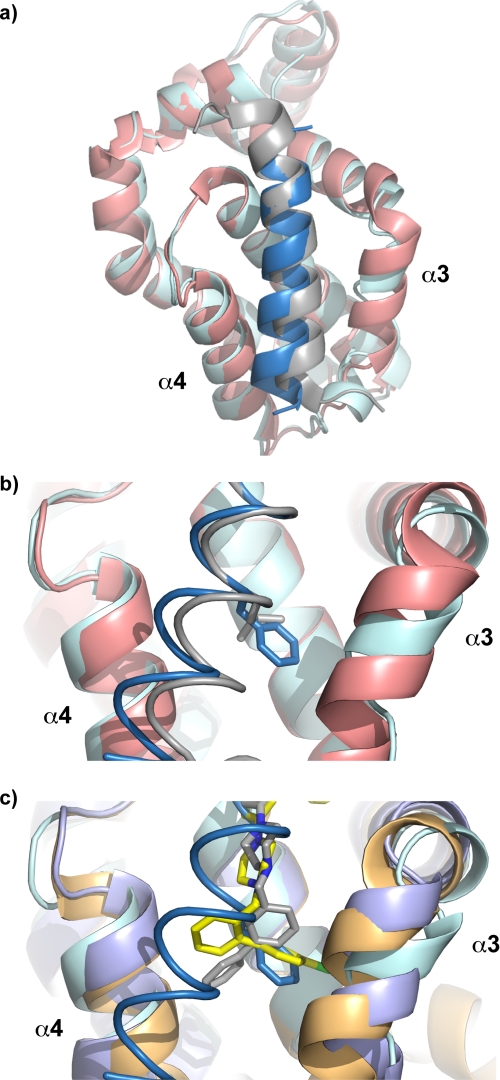
FIGURE 5.
Structure of the BimL12F·Bcl-xL complex. a, overlay of structures of Bcl-xL in complex with wild-type Bim BH3 (Bcl-xL, pink; Bim BH3, gray) or BimL12F (Bcl-xL, cyan; BimL12F, dark blue). b, close-up view in the region of the h2-binding pocket showing the relative orientation of the wild-type leucine residue in Bim BH3 (gray) or the phenylalanine residue at the same position in the mutated peptide (dark blue). c, overlay of the structures of Bcl-xL in complex with BimL12F (Bcl-xL, cyan; BimL12F, dark blue), ABT-737 (Bcl-xL, orange; ABT-737, yellow), or W1191542 molecule B (Bcl-xL, light blue; W1191542/A, gray). The phenylalanine side chain projects into the h2 binding pocket similarly to the chlorobiphenyl moiety of ABT-737.
DISCUSSION
A Novel Bcl-xL-selective Antagonist
The anti-tumor activity of ABT-737/ABT-263 in cell culture systems in in vivo models of cancer and, more recently in human patients, provides incentive to develop further anti-cancer therapeutics of the BH3-mimetic class. An obvious starting point for extending the range of tumors that could be treated with such molecules would be through varying the spectrum of pro-survival proteins they target. An Mcl-1-specific compound is likely to prove useful for cancers such as multiple myeloma, which are dependent on Mcl-1 for their survival (54, 55).
Guided by the structures of peptide complexes with Bcl-xL and Mcl-1 and of ABT-737 with Bcl-xL, we designed compound W1191542 to acquire binding affinity for Mcl-1. W1191542 shows no significant affinity for Mcl-1 but instead is selective for Bcl-xL over other pro-survival proteins. Another analogue of ABT-737 (A-385358) with a similar binding profile has been reported previously and shown to be significantly less potent than ABT-737 in both in vitro and in vivo assays (56). Although this compound was selective for Bcl-xL over Bcl-2, it also bound to Bcl-xL weaker than ABT-737, which may account for its reduced activity. However, W1191542 is several orders of magnitude less active than ABT-737 in cell-based assays despite binding Bcl-xL with only slightly lower affinity (2-fold). Reasons for this apparent discrepancy could include compound solubility and/or cell permeability, different binding kinetics of the compounds to Bcl-xL, or factors relating to loss of binding of W1191542 to Bcl-2 even though it has previously been shown that in MEFs only Bcl-xL and Mcl-1 need to be neutralized for Bak-mediated cell death (33).
Bcl-xL Plasticity
Structural studies of the murine pro-survival Bcl-2 family member A1 concluded that structural plasticity allowed the protein to bind diverse peptide ligands (10). In that case, however, no significant differences in the backbone trace of A1 in the different BH3 peptide complexes are discernable. In contrast, the structures we describe here for Bcl-xL display large differences in the folding and location of the chain segment connecting helices α2 and α4 and in the position of helix α4. Previous studies have shown that the Bcl-xL α2-α3 connection is folded differently in the complexes with Bak BH3 (9) and Beclin BH3 (48, 57) compared with the Bim BH3 complex. The structures of the BH3 binding grooves of Bcl-xL reported here in complex with Bim BH3 L12F and in molecule B of the W1191542 complex are both novel. The structure of the BH3 binding groove of molecule A of the W1191542 complex is similar to the Bcl-xL complex with Compound 43b (PDB 2O2N) (23).
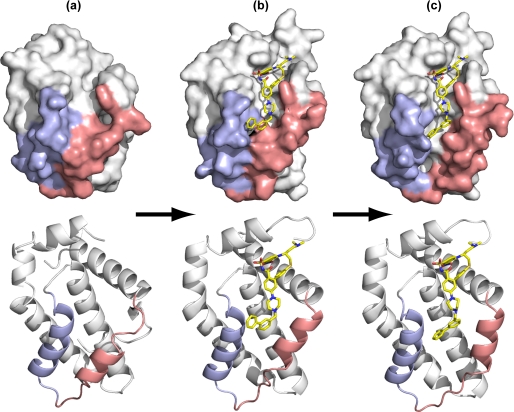
Especially interesting are the two different views of the W1191542·Bcl-xL complex in our crystal structure. When combined with the structure of unliganded Bcl-xL (58), one can speculate about the series of events that lead to the final complex (Fig. 6). For example, if W1191542 from molecule A is overlaid with the unliganded Bcl-xL, the portion of the molecule from the acyl sulfonamide to the thiophenyl moiety can engage the top half of the groove with minimal overlap. The biphenyl group in this complex projects out of the groove which is more closed in the region of the h2 pocket, notably by Leu-108. In molecule B of the W119542 complex, the groove is widened by the displacement of α3 and the accommodation of the biphenyl moiety in the h2 pocket. In both W119542 complex structures, α4 is unperturbed from its conformation in unliganded Bcl-xL. However, when ABT-737 binds, α4 is observed to lie closer to α3 than in the W119542 complex structures (see Fig. 4). Hence, molecules similar to ABT-737 may initially engage Bcl-xL in the h4 pocket, which is relatively “open” in the unliganded structure, and then progressively bind along the groove toward the h1 pocket with coordinated rearrangements in the α3 and α4 helices that open up the groove. It may be that BH3 peptides also bind Bcl-xL in this way. Notably the speculative structural transition presented in Fig. 6 encompasses the full range of structures observed to date from closed (PDB 1MAZ), partially open, and open (PDB 3INQ).
FIGURE 6.
Proposed structural transitions in the binding of W1151952 to Bcl-xL. a, the lower half of the binding groove (α3 helix, pink; α4 helix, blue) is occluded in unliganded Bcl-xL (PDB 1MAZ). b, upon engaging W1191542, there is a partial opening of the groove to accommodate the conformer observed in molecule A. c, when the biphenyl moiety penetrates the h2 pocket, as in molecule B, the groove is further widened.
There is not yet any evidence that the softness in the structure of Bcl-xL helix α3 is also a property of the Mcl-1 structure. There the BH3 binding groove is apparently more open in the unliganded state (5, 59). Furthermore, there is a striking difference in the accommodation of the Bim BH3 variants L12Y into Mcl-1 and L12F into Bcl-xL, the former causing only minor local perturbations in the Mcl-1 structure and the latter partially dissolving the α3 of Bcl-xL. Recently, Fu et al. (60) attempted to model a BimL12F variant into Bcl-xL. Our results showing the significant structural plasticity in Bcl-xL upon ligand binding (including with BimL12F) highlight the potential challenges involved in such modeling studies that will equally apply to modeling small molecule ligands.
Design Issues
Compound W1191542 did not acquire the capacity to bind to Mcl-1. Saturation mutagenesis of Leu-12 of Bim BH3 (25) showed a 10-fold or less loss of binding of L12Y to Bcl-2, Bcl-w, and Bcl-xL but a 60-fold loss of binding to Mcl-1. The structure of that mutant complexed to Mcl-1 and reported here suggests little capacity in Mcl-1 to reshape itself around the mutated residue. Thus, a plausible explanation for the failure of W1191542 to bind to Mcl-1 is that Mcl-1 is less forgiving of structural alterations in the h2 pocket. Abbott Laboratories have reported the structure of a complex of Bcl-xL with a molecule similar to ABT-737, Compound 43b (PDB 2O2N (23)) (Fig. 2a) but with the chlorobiphenyl moiety replaced with a biphenyl, as in W1191542 (although the biphenyl group is in the ortho position, not meta, as in W1191542). The space occupied by the biphenyl of Compound 43b overlays that utilized by the biphenyl of molecule B in the W1191542 complex (Fig. 4c). This is achieved through differences in the conformation of the piperazine ring and the torsion angles in the methylene connector to the biphenyl. Thus, we attribute the retention of binding of W119542 to Bcl-xL to flexibility of both the protein and the compound. These results highlight significant differences in the structural properties of members of the Bcl-2 pro-survival protein family, differences that are likely to be important in the quest for compounds capable of selectively antagonizing the different family members. In conclusion, we suggest that differential binding of the Bcl-2 family members to particular peptides or compounds might reflect their capacity to adopt a compatible conformation to the ligand and not merely amino acid sequence differences among the family members in the ligand binding groove.
Supplementary Material
Acknowledgments
Infrastructure support from National Health and Medical Research Council IRIISS Grant 361646 and the Victorian State Government OIS grant is gratefully acknowledged. Crystallization trials were performed at the Bio21 C3 Collaborative Crystallization Centre. Data for the BimL12F BH3 complex with Bcl-xL were collected on the PX1 beamline at the Australian Synchrotron, Victoria, Australia. We thank Abbott Laboratories for provision of ABT-737, David Huang, Andreas Strasser, Philippe Bouillet, Priscilla Kelly, and Mark van Delft for essential reagents, and Madelin Tan for assistance with crystallization.
This work was supported by Australian National Health and Medical Research Council Program fellowships (to W. D. F. and P. M. C.) and Grant 461221 (to P. M. C.) and Project Grant 575561 (to P. E. C.), Leukemia and Lymphoma Society Grant SCOR 7015-02, Cancer Council of Victoria Grant-in-aid 461239 (to W. D. F.) and a fellowship (to E. F. L.), Leukemia Foundation of Australia Phillip Desbrow post doctoral fellowship (to E. F. L.), and by the Australian Cancer Research Foundation (to P. M. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
The atomic coordinates and structure factors (codes 3INQ, 3IO9, and 3IO8) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1.Cory S., Huang D. C., Adams J. M. (2003) Oncogene 22, 8590–8607 [DOI] [PubMed] [Google Scholar]
- 2.Youle R. J., Strasser A. (2008) Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 3.Fesik S. W. (2005) Nat. Rev. Cancer 5, 876–885 [DOI] [PubMed] [Google Scholar]
- 4.Chipuk J. E., Green D. R. (2008) Trends Cell Biol. 18, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czabotar P. E., Lee E. F., van Delft M. F., Day C. L., Smith B. J., Huang D. C., Fairlie W. D., Hinds M. G., Colman P. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6217–6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day C. L., Smits C., Fan F. C., Lee E. F., Fairlie W. D., Hinds M. G. (2008) J. Mol. Biol. 380, 958–971 [DOI] [PubMed] [Google Scholar]
- 7.Liu X., Dai S., Zhu Y., Marrack P., Kappler J. W. (2003) Immunity 19, 341–352 [DOI] [PubMed] [Google Scholar]
- 8.Petros A. M., Nettesheim D. G., Wang Y., Olejniczak E. T., Meadows R. P., Mack J., Swift K., Matayoshi E. D., Zhang H., Thompson C. B., Fesik S. W. (2000) Protein Sci. 9, 2528–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sattler M., Liang H., Nettesheim D., Meadows R. P., Harlan J. E., Eberstadt M., Yoon H. S., Shuker S. B., Chang B. S., Minn A. J., Thompson C. B., Fesik S. W. (1997) Science 275, 983–986 [DOI] [PubMed] [Google Scholar]
- 10.Smits C., Czabotar P. E., Hinds M. G., Day C. L. (2008) Structure 16, 818–829 [DOI] [PubMed] [Google Scholar]
- 11.Degterev A., Lugovskoy A., Cardone M., Mulley B., Wagner G., Mitchison T., Yuan J. (2001) Nat. Cell Biol. 3, 173–182 [DOI] [PubMed] [Google Scholar]
- 12.Enyedy I. J., Ling Y., Nacro K., Tomita Y., Wu X., Cao Y., Guo R., Li B., Zhu X., Huang Y., Long Y. Q., Roller P. P., Yang D., Wang S. (2001) J. Med. Chem. 44, 4313–4324 [DOI] [PubMed] [Google Scholar]
- 13.Mohammad R. M., Goustin A. S., Aboukameel A., Chen B., Banerjee S., Wang G., Nikolovska-Coleska Z., Wang S., Al-Katib A. (2007) Clin. Cancer Res. 13, 2226–2235 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen M., Marcellus R. C., Roulston A., Watson M., Serfass L., Murthy Madiraju S. R., Goulet D., Viallet J., Bélec L., Billot X., Acoca S., Purisima E., Wiegmans A., Cluse L., Johnstone R. W., Beauparlant P., Shore G. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19512–19517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oltersdorf T., Elmore S. W., Shoemaker A. R., Armstrong R. C., Augeri D. J., Belli B. A., Bruncko M., Deckwerth T. L., Dinges J., Hajduk P. J., Joseph M. K., Kitada S., Korsmeyer S. J., Kunzer A. R., Letai A., Li C., Mitten M. J., Nettesheim D. G., Ng S., Nimmer P. M., O'Connor J. M., Oleksijew A., Petros A. M., Reed J. C., Shen W., Tahir S. K., Thompson C. B., Tomaselli K. J., Wang B., Wendt M. D., Zhang H., Fesik S. W., Rosenberg S. H. (2005) Nature 435, 677–681 [DOI] [PubMed] [Google Scholar]
- 16.Tzung S. P., Kim K. M., Basañez G., Giedt C. D., Simon J., Zimmerberg J., Zhang K. Y., Hockenbery D. M. (2001) Nat. Cell Biol. 3, 183–191 [DOI] [PubMed] [Google Scholar]
- 17.Yin H., Lee G. I., Sedey K. A., Kutzki O., Park H. S., Orner B. P., Ernst J. T., Wang H. G., Sebti S. M., Hamilton A. D. (2005) J. Am. Chem. Soc. 127, 10191–10196 [DOI] [PubMed] [Google Scholar]
- 18.Yin H., Lee G. I., Sedey K. A., Rodriguez J. M., Wang H. G., Sebti S. M., Hamilton A. D. (2005) J. Am. Chem. Soc. 127, 5463–5468 [DOI] [PubMed] [Google Scholar]
- 19.Lessene G., Czabotar P. E., Colman P. M. (2008) Nat. Rev. Drug Discov. 7, 989–1000 [DOI] [PubMed] [Google Scholar]
- 20.Prakesch M., Denisov A. Y., Naim M., Gehring K., Arya P. (2008) Bioorg. Med. Chem. 16, 7443–7449 [DOI] [PubMed] [Google Scholar]
- 21.Feng W. Y., Liu F. T., Patwari Y., Agrawal S. G., Newland A. C., Jia L. (2003) Br. J. Haematol. 121, 332–340 [DOI] [PubMed] [Google Scholar]
- 22.van Delft M. F., Wei A. H., Mason K. D., Vandenberg C. J., Chen L., Czabotar P. E., Willis S. N., Scott C. L., Day C. L., Cory S., Adams J. M., Roberts A. W., Huang D. C. (2006) Cancer Cell 10, 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruncko M., Oost T. K., Belli B. A., Ding H., Joseph M. K., Kunzer A., Martineau D., McClellan W. J., Mitten M., Ng S. C., Nimmer P. M., Oltersdorf T., Park C. M., Petros A. M., Shoemaker A. R., Song X., Wang X., Wendt M. D., Zhang H., Fesik S. W., Rosenberg S. H., Elmore S. W. (2007) J. Med. Chem. 50, 641–662 [DOI] [PubMed] [Google Scholar]
- 24.Vogler M., Weber K., Dinsdale D., Schmitz I., Schulze-Osthoff K., Dyer M. J., Cohen G. M. (2009) Cell Death Differ. 16, 1030–1039 [DOI] [PubMed] [Google Scholar]
- 25.Lee E. F., Czabotar P. E., Smith B. J., Deshayes K., Zobel K., Colman P. M., Fairlie W. D. (2007) Cell Death Differ. 14, 1711–1713 [DOI] [PubMed] [Google Scholar]
- 26.Ackler S., Xiao Y., Mitten M. J., Foster K., Oleksijew A., Refici M., Schlessinger S., Wang B., Chemburkar S. R., Bauch J., Tse C., Frost D. J., Fesik S. W., Rosenberg S. H., Elmore S. W., Shoemaker A. R. (2008) Mol. Cancer Ther. 7, 3265–3274 [DOI] [PubMed] [Google Scholar]
- 27.Shoemaker A. R., Mitten M. J., Adickes J., Ackler S., Refici M., Ferguson D., Oleksijew A., O'Connor J. M., Wang B., Frost D. J., Bauch J., Marsh K., Tahir S. K., Yang X., Tse C., Fesik S. W., Rosenberg S. H., Elmore S. W. (2008) Clin. Cancer Res. 14, 3268–3277 [DOI] [PubMed] [Google Scholar]
- 28.Tse C., Shoemaker A. R., Adickes J., Anderson M. G., Chen J., Jin S., Johnson E. F., Marsh K. C., Mitten M. J., Nimmer P., Roberts L., Tahir S. K., Xiao Y., Yang X., Zhang H., Fesik S., Rosenberg S. H., Elmore S. W. (2008) Cancer Res. 68, 3421–3428 [DOI] [PubMed] [Google Scholar]
- 29.Certo M., Del Gaizo Moore V., Nishino M., Wei G., Korsmeyer S., Armstrong S. A., Letai A. (2006) Cancer Cell 9, 351–365 [DOI] [PubMed] [Google Scholar]
- 30.Chen L., Willis S. N., Wei A., Smith B. J., Fletcher J. I., Hinds M. G., Colman P. M., Day C. L., Adams J. M., Huang D. C. (2005) Mol. Cell 17, 393–403 [DOI] [PubMed] [Google Scholar]
- 31.Kuwana T., Bouchier-Hayes L., Chipuk J. E., Bonzon C., Sullivan B. A., Green D. R., Newmeyer D. D. (2005) Mol. Cell 17, 525–535 [DOI] [PubMed] [Google Scholar]
- 32.Fletcher J. I., Huang D. C. (2008) Cell Cycle 7, 39–44 [DOI] [PubMed] [Google Scholar]
- 33.Willis S. N., Chen L., Dewson G., Wei A., Naik E., Fletcher J. I., Adams J. M., Huang D. C. (2005) Genes Dev. 19, 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willis S. N., Fletcher J. I., Kaufmann T., van Delft M. F., Chen L., Czabotar P. E., Ierino H., Lee E. F., Fairlie W. D., Bouillet P., Strasser A., Kluck R. M., Adams J. M., Huang D. C. (2007) Science 315, 856–859 [DOI] [PubMed] [Google Scholar]
- 35.Chen S., Dai Y., Harada H., Dent P., Grant S. (2007) Cancer Res. 67, 782–791 [DOI] [PubMed] [Google Scholar]
- 36.Konopleva M., Contractor R., Tsao T., Samudio I., Ruvolo P. P., Kitada S., Deng X., Zhai D., Shi Y. X., Sneed T., Verhaegen M., Soengas M., Ruvolo V. R., McQueen T., Schober W. D., Watt J. C., Jiffar T., Ling X., Marini F. C., Harris D., Dietrich M., Estrov Z., McCubrey J., May W. S., Reed J. C., Andreeff M. (2006) Cancer Cell 10, 375–388 [DOI] [PubMed] [Google Scholar]
- 37.Lee E. F., Czabotar P. E., van Delft M. F., Michalak E. M., Boyle M. J., Willis S. N., Puthalakath H., Bouillet P., Colman P. M., Huang D. C., Fairlie W. D. (2008) J. Cell Biol. 180, 341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin X., Morgan-Lappe S., Huang X., Li L., Zakula D. M., Vernetti L. A., Fesik S. W., Shen Y. (2007) Oncogene 26, 3972–3979 [DOI] [PubMed] [Google Scholar]
- 39.Varfolomeev E., Blankenship J. W., Wayson S. M., Fedorova A. V., Kayagaki N., Garg P., Zobel K., Dynek J. N., Elliott L. O., Wallweber H. J., Flygare J. A., Fairbrother W. J., Deshayes K., Dixit V. M., Vucic D. (2007) Cell 131, 669–681 [DOI] [PubMed] [Google Scholar]
- 40.Zobel K., Wang L., Varfolomeev E., Franklin M. C., Elliott L. O., Wallweber H. J., Okawa D. C., Flygare J. A., Vucic D., Fairbrother W. J., Deshayes K. (2006) ACS Chem. Biol. 1, 525–533 [DOI] [PubMed] [Google Scholar]
- 41.Kvansakul M., Yang H., Fairlie W. D., Czabotar P. E., Fischer S. F., Perugini M. A., Huang D. C., Colman P. M. (2008) Cell Death Differ 15, 1564–1571 [DOI] [PubMed] [Google Scholar]
- 42.McPhillips T. M., McPhillips S. E., Chiu H. J., Cohen A. E., Deacon A. M., Ellis P. J., Garman E., Gonzalez A., Sauter N. K., Phizackerley R. P., Soltis S. M., Kuhn P. (2002) J. Synchrotron Radiat. 9, 401–406 [DOI] [PubMed] [Google Scholar]
- 43.Otwinowski Z., Minor W. (1997) Methods in. Enzymology 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 44.McCoy A. J., Grosse-Kunstleve R. W., Storoni L. C., Read R. J. (2005) Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 [DOI] [PubMed] [Google Scholar]
- 45.Read R. J. (2001) Acta Crystallogr. D Biol. Crystallogr. 57, 1373–1382 [DOI] [PubMed] [Google Scholar]
- 46.Storoni L. C., McCoy A. J., Read R. J. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 432–438 [DOI] [PubMed] [Google Scholar]
- 47.Lee E. F., Sadowsky J. D., Smith B. J., Czabotar P. E., Peterson-Kaufman K. J., Colman P. M., Gellman S. H., Fairlie W. D. (2009) Angew. Chem. Int. Ed. Engl. 48, 4318–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oberstein A., Jeffrey P. D., Shi Y. (2007) J. Biol. Chem. 282, 13123–13132 [DOI] [PubMed] [Google Scholar]
- 49.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 50.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 51.Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., Terwilliger T. C. (2002) Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 [DOI] [PubMed] [Google Scholar]
- 52.Lee E. F., Chen L., Yang H., Colman P. M., Huang D. C., Fairlie W. D. (2008) Cell Death Differ 15, 1609–1618 [DOI] [PubMed] [Google Scholar]
- 53.Liu X., Zhu Y., Dai S., White J., Peyerl F., Kappler J. W., Marrack P. (2006) J. Exp. Med. 203, 2953–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wuillème-Toumi S., Robillard N., Gomez P., Moreau P., Le Gouill S., Avet-Loiseau H., Harousseau J. L., Amiot M., Bataille R. (2005) Leukemia 19, 1248–1252 [DOI] [PubMed] [Google Scholar]
- 55.Zhang B., Gojo I., Fenton R. G. (2002) Blood 99, 1885–1893 [DOI] [PubMed] [Google Scholar]
- 56.Shoemaker A. R., Oleksijew A., Bauch J., Belli B. A., Borre T., Bruncko M., Deckwirth T., Frost D. J., Jarvis K., Joseph M. K., Marsh K., McClellan W., Nellans H., Ng S., Nimmer P., O'Connor J. M., Oltersdorf T., Qing W., Shen W., Stavropoulos J., Tahir S. K., Wang B., Warner R., Zhang H., Fesik S. W., Rosenberg S. H., Elmore S. W. (2006) Cancer Res. 66, 8731–8739 [DOI] [PubMed] [Google Scholar]
- 57.Feng W., Huang S., Wu H., Zhang M. (2007) J. Mol. Biol. 372, 223–235 [DOI] [PubMed] [Google Scholar]
- 58.Muchmore S. W., Sattler M., Liang H., Meadows R. P., Harlan J. E., Yoon H. S., Nettesheim D., Chang B. S., Thompson C. B., Wong S. L., Ng S. L., Fesik S. W. (1996) Nature 381, 335–341 [DOI] [PubMed] [Google Scholar]
- 59.Day C. L., Chen L., Richardson S. J., Harrison P. J., Huang D. C., Hinds M. G. (2005) J. Biol. Chem. 280, 4738–4744 [DOI] [PubMed] [Google Scholar]
- 60.Fu X., Apgar J. R., Keating A. E. (2007) J. Mol. Biol. 371, 1099–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.