Abstract
In eukaryotic organisms, gene expression requires an additional level of coordination that links transcriptional and posttranslational processes. Messenger RNAs have traditionally been viewed as passive molecules in the pathway from transcription to translation. However, it is now clear that RNA-binding proteins (RBPs) play an important role in cellular homeostasis by controlling gene expression at the posttranscriptional level. Here, we show that RBPs, as a class of proteins, show distinct gene expression dynamics compared to other protein coding genes in the eukaryote Sacchoromyces cerevisiae. We find that RBPs generally exhibit high protein stability, translational efficiency, and protein abundance but their encoding transcripts tend to have a low half-life. We show that RBPs are also most often posttranslationally modified, indicating their potential for regulation at the protein level to control diverse cellular processes. Further analysis of the RBP-RNA interaction network showed that the number of distinct targets bound by an RBP (connectivity) is strongly correlated with its protein stability, translational efficiency, and abundance. We also note that RBPs show less noise in their expression in a population of cells, with highly connected RBPs showing significantly lower noise. Our results indicate that highly connected RBPs are likely to be tightly regulated at the protein level as significant changes in their expression may bring about large-scale changes in global expression levels by affecting their targets. These observations might explain the molecular basis behind the cause of a number of disorders associated with misexpression or mutation in RBPs. Future studies uncovering the posttranscriptional networks in higher eukaryotes can help our understanding of the link between different levels of regulation and their role in pathological conditions.
Keywords: disease, posttranslational modifications, protein noise, regulation, systems biology
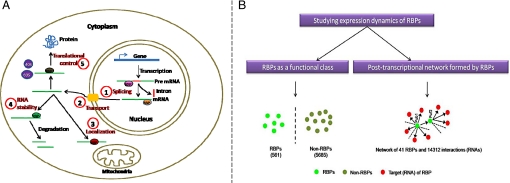
Gene expression is a highly regulated process and is controlled at several levels. In eukaryotes, control of gene expression first occurs at the level of transcription, where transcription factors regulate the synthesis of RNA of specific genes in response to different internal and external stimuli. On the other hand, at the protein level, several posttranslational modifications, such as phosphorylation by kinases and ubiquitin ligases, are known to spatially and temporally control the availability of functional protein products within the cell. However, a much less understood level of gene expression regulation, which occurs between these two layers, is due to the posttranscriptional control of RNAs. It is now increasingly known that this level is controlled by numerous factors with major players being the RNA-binding proteins (RBPs) (1–3) (see Fig. 1A). Therefore, intricate coordination of regulation from these three different layers is important for finely controlling the flow of genetic information from genes to proteins in different conditions. Indeed, changes in gene expression due to aberrations at any of these three levels have been shown to be responsible for the cause of a number of disorders (4–8).
Fig. 1.
(A) Schematic diagram showing the extensive role of RBPs in various posttranscriptional processes at different locations in eukaryotic cells. Circled number indicates the process in which RBPs are involved. RBPs are major players in splicing premRNA into mature mRNA in the nucleus, which are then exported into the cytoplasm by various other RBPs. In addition, RBPs are responsible for the localization of mRNAs to distinct subcellular compartments such as the mitochondria. In the cytoplasm, RBPs are also involved in governing the stability of transcripts by binding the substrate RNAs and in controlling the translation of mRNAs into corresponding protein products. For this reason, RBPs have been found to be key players either directly or indirectly responsible for the cause of several disorders due to changes in regulation they bring about at the posttranscriptional level (6, 7, 19). (B) In this study, we analyzed the expression dynamics of RBPs at two distinct levels. First, RBPs as a functional class, where we compared the properties of RBPs with the rest of the protein coding genes in the entire genome of Sacchoromyces cerevisiae. This involved comparison of 561 RBPs against 5,685 non-RBPs in the whole genome. Second, we studied the relationship between the RBP's connectivity, defined as the number of target mRNAs that are bound by a given RBP and their transcript and protein stability, transcript and protein expression, rate of translation, and expression noise.
Development of DNA microarray technology has made it possible to measure the expression of each annotated gene at the transcript level. Indeed, this technique has been the high-throughput approach of choice to efficiently characterize the transcriptomes of several model organisms. One common assumption in DNA microarray experiments is that the level of mRNA of a particular gene reflects the amount of protein and there is little regulation at the posttranscriptional level. Recent studies comparing the high-throughput data for mRNA and protein abundances indicate that there is a very weak correlation between the number of transcripts and protein products of a gene, challenging this notion (9, 10). This suggests that the regulation of gene expression at the posttranscriptional level is predominant. For instance, in the eukaryotic pathogen, Trypanosoma cruzi, it is well known that gene expression is primarily controlled at the posttranscriptional level through RNA-binding proteins (RBPs) (11). These studies suggest the extensive role of posttranscriptional regulation in controlling gene expression in eukaryotes (12, 13).
In eukaryotes, transcription and translation occur in different compartments. This allows for a plethora of options to control RNA at the posttranscriptional level, including their splicing, polyadenylation, transport, mRNA stability, localization, and translational control (2, 3). Although some early studies revealed the involvement of RBPs in the transport of mRNA from nucleus to the site of their translation, increasing evidence now suggests that RBPs regulate almost all of the posttranscriptional steps shown in Fig. 1A. For example, in humans, Nova protein is associated with splicing (14); PUF family proteins have been shown to play an important role during Caenorhabditis elegans oogenesis (15); Tap protein, like its yeast homolog Mex67, was reported as a bona fide mRNA nuclear export factor (16); Puf3p in yeast was shown to be responsible for localization of mitochondrial transcripts (17); and Pab1 was reported to regulate the initiation of translation (18). While the extensive role of RBPs in posttranscriptional control of cellular processes has been reviewed by several groups (1–3, 7), in yeast alone we found that RBPs are involved in multiple cellular processes and components (see Materials and Methods and SI Text S1). All these aspects highlight the importance of RBPs in regulating gene expression at the posttranscriptional level.
Due to their central role in controlling gene expression at the posttranscriptional level, alteration in expression or mutations in either RBPs or their RNA targets (i.e., the transcripts which physically associate with the RBP) have been reported to be the cause of several human diseases such as muscular atrophies, neurological disorders, and cancer (6, 7, 19, 20). In particular, disorders such as myotonic dystrophy (DM) and oculopharyngeal muscular dystrophy (OPMD) have been attributed to RNA's gain-of-function by CUG repeat expansion in the case of myotonic dystrophy protein kinase (DMPK) (19) and GCG repeat expansion in exon 1 of the RBP, PABPN1 in the case of OPMD (7), respectively. On the other hand, diseases like paraneoplastic opsoclonus-myoclonus ataxia (POMA) and spinal muscular atrophy (SMA) have been reported to be due to the RBPs loss of function (7), suggesting that mutations in either RBP or any of its interacting RNA target sequences can lead to extensive variations in their expression patterns and result in a number of diseases. In addition to the fitness defects that variations in RBPs can bring about in cells, it has been recently shown in yeast that RBPs form an important class of prionogenic proteins (21).
All these observations raise the questions: Are RBPs finely controlled in terms of their expression patterns and are there constraints on their expression patterns depending on the number of distinct RNA targets they control? To address this, in this study, we have analyzed the posttranscriptional network formed by RBPs in yeast, Saccharyomyces cerevisiae at two distinct levels (Fig. 1B). The first involved asking whether RBPs as a group show distinct dynamic properties in comparison to non-RBPs in the whole genome. The second composed of understanding the constraints placed on dynamic properties of RBPs in relation to the number of distinct transcripts controlled by them. Our analysis at the first level revealed that RBPs, as a functional class, are rapidly turned over (i.e., less stable) at the transcript level and are tightly controlled at the protein level. Analysis of the posttranscriptional network formed by RBPs indicated that highly connected RBPs are more abundant and ubiquitously present within the cell.
Results
RBPs Show High Abundance and Tight Regulation at the Protein Level.
To compare and understand the differences in the gene expression dynamics of RBPs with other protein coding genes in S. cerevisiae, we first compiled the set of RBPs and non-RBPs as described in Materials and Methods (Fig. 1B). This allowed us to define a set of 561 proteins in yeast as those that encode for RNA-binding proteins and the remaining 5,685 proteins (from the complete set of protein coding genes) as non-RNA-binding proteins. We also collected high-throughput data documenting various dynamic properties of messenger RNA transcripts and their translated protein products in yeast from different sources as described in Materials and Methods. These properties included the mRNA stability, mRNA copy number, ribosome occupancy, protein stability, and abundance. In addition to these attributes of mRNAs and proteins, we also obtained the data describing the cell-to-cell variation in protein expression in a genetically homogenous population of cells, typically referred to as protein expression noise.
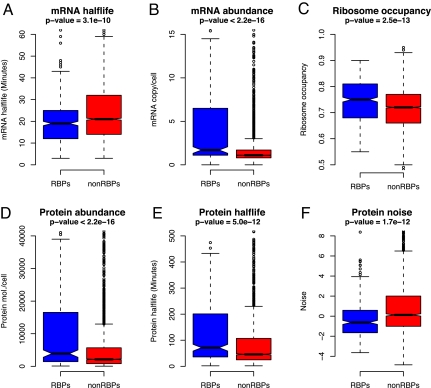
Messenger RNA half-life is a measure of transcript stability in the cell, whereas mRNA copy number reflects its abundance. We first asked whether RBPs as a functional class show a different tendency in comparison to non-RBPs in these properties. As a result of this analysis, we found that mRNAs encoding RBPs are significantly less stable (i.e., short half-life) at the transcript level compared to those genes that do not encode RBPs (P = 3.1 × 10−10, Wilcoxon test) (Fig. 2A). In yeast it has been shown that, in general, mRNAs of central physiological pathways have a longer half-life and mRNAs encoding regulatory and signaling proteins have a shorter half-life (22). In line with these observations, the observed lower half-life of RBPs in our analysis is consistent with their regulatory function and quick turn over at the transcript level. However, a comparison of the mRNA copy number of the two groups of genes, which is a proxy for mRNA abundance in the cell, indicated that RBPs are encoded by genes that exhibit much higher mRNA copy number (P < 2.2 × 10−16, Wilcoxon test) (Fig. 2B). Exclusion of translation and ribosome-associated genes, which form a significant fraction of the total repertoire of RBPs and are known to be highly expressed, did not change our results (SI Text S2). These observations suggest that RBPs tend to be less stable but more abundant at the transcript level, suggesting that abundance is a more prominent factor than their stability. Both mRNA half-life and mRNA abundance data indicate that RBP's expression at mRNA level is likely to be transient but whenever they are transcribed they are produced at high concentrations.
Fig. 2.
Comparing expression dynamics of RBPs with non-RBPs in the entire genome. Box plots showing the distribution of values for various regulatory properties for the two different groups of proteins (RBPs and non-RBPs) in S. cerevisiae. Blue and red bars correspond to RBP and non-RBP populations, respectively. Box plot identifies the middle 50% of the data, the median, and the extreme points. The entire set of data points is divided into quartiles and the interquartile range (IQR) is calculated as the difference between x0.75 and x0.25. The range of the 25% of the data points above (x0.75) and below (x0.25) the median (x0.50) is displayed as a filled box. The horizontal line and the notch represent the median and confidence intervals, respectively. Data points greater or <1.5 IQR represent outliers and are shown as dots. The horizontal line that is connected by dashed lines Above and Below the filled box (whiskers) represents the largest and smallest nonoutlier data points, respectively. (A) mRNA half-life, (B) mRNA copy number, (C) ribosome occupancy, (D) protein abundance, (E) protein half-life, and (F) protein noise. In each case, P-values shown correspond to the significance estimated on the basis of the Wilcoxon test comparing the RBP and non-RBP groups of proteins. RBPs were found to show significantly lower transcript stability, higher mRNA copy number, ribosome occupancy, protein stability, and abundance. However, protein noise, which reflects the extent of cell-to-cell variation in protein levels, was found to be significantly lower for RBPs compared to non-RBPs, suggesting that most RBPs are uniformly expressed across a homogenous population of cells. See SI Text S5 for a comparison of mean values of the groups for different properties.
Ribosome occupancy has been shown to be a measure of translational efficiency of mRNA. Higher ribosome occupancy relates to higher protein synthesis, and lower ribosome occupancy indicates low translation rate of mRNA. We next asked whether the ribosome occupancy i.e., rate of translation, of RBPs is higher than those for non-RBPs and whether their protein levels are higher within the cell. This analysis clearly revealed that RBPs have high ribosome occupancy (P = 2.5 × 10−13, Wilcoxon test) (Fig. 2C) and are also present in much higher concentrations (P < 2.2 × 10−16, Wilcoxon test) (Fig. 2D) with median abundances of RBPs being roughly double that observed for non-RBPs (3,895 versus 2,132 protein molecules/cell). These results indicate that RBPs are abundant and are translated rapidly, supporting the versatile nature of their involvement in multiple posttranscriptional control mechanisms at different cellular locations (SI Text S1). Exclusion of ribosome and translation-associated factors from RBPs to compare nonribosomal RBPs against non-RBPs indicated that ribosomal RBPs contribute significantly to the observed differences in the rate of translation and protein abundance of RBPs (SI Text S2). Comparing the protein concentrations of nonribosomal RBPs with non-RBPs indicated that the former are still significantly more abundant (P = 2.2 × 10−2).
Stability of a protein measured as its half-life can be considered as a proxy for the life time of a protein in a cell. Therefore, to understand the degradation rates of RBPs and to compare them against non-RBPs we analyzed their protein half-lives (see Materials and Methods). This analysis revealed that RBPs are significantly more stable than non-RBPs, with RBPs exhibiting a median half-life of 71 min as against non-RBPs with 46 min (P = 5 × 10−12, Wilcoxon test) (Fig. 2E). Repeating the analyses with nonribosomal RBPs showed a consistent trend despite their exclusion (P = 4.8 × 10−2) (SI Text S2). Our observations on the increased protein stability and concentration of the RBPs compared to other proteins in the cell suggests that RBPs, whose main functional role is in the processing and localization of their mRNA targets, might be required at multiple subcellular locations and be used throughout the cell cycle. This may likely warrant their higher abundance and stability at the protein level. It is important to note that although RBPs exhibit high protein stability, they also show low transcript stability, which indicates that most RBPs that are stable at the protein level, might be avoiding cellular crowding of their transcripts by quick turnover at the transcript level. Indeed, it has been shown in yeast that most RBPs autoregulate their own activity at the transcript level (23).
To understand how these properties vary with different processes in which RBPs are involved, we divided RBPs into four major categories: translation, transport, RNA localization, and processing using GO annotations and compared them with non-RBPs (SI Text S3). This analysis revealed that the general trends observed for different categories are similar to those seen for RBPs as a whole although certain categories comprised relatively few RBPs.
Several RBPs have been shown to be posttranslationally modified, which adds a layer of flexibility to their function. Many of these posttranslational modifications have been shown to modify their RNA-binding properties or their subcellular localization. Indeed, at least four types of posttranslational modifications namely phosphorylation, ubiquitination, methylation, and SUMOylation have been reported for RBPs (2). High stability of RBPs indicates the potential that posttranslational modifications can offer in the diversification of their function. In fact, analysis of the number of kinase substrates in RBP and non-RBP populations using the currently available protein phosphorylation map for yeast (24), suggests that some kinases not only target higher number of RBPs compared to non-RBPs (P = 2.7 × 10−2) but also more kinases are associated with RBPs (P < 2.2 × 10−16) (see SI Text S4).
Gene expression is a highly dynamic process and because of its dynamic nature there is a large variation in a protein's abundance among different cells in a population. This variation is termed as biological noise. Genes whose expression varies to a large extent show more noise and these are typically involved in stress response, amino acid biosynthesis, and heat shock. On the other hand, genes that show consistent expression during the cell cycle such as those involved in protein degradation and ribosomal proteins tend to show low noise (25). Here, we have explored this noise data, to address whether RBPs show significant difference from non-RBPs in terms of biological noise. As shown in Fig. 2F, RBPs were found to show significantly lower noise levels in comparison to non-RBPs (P = 1.7 × 10−12, Wilcoxon test). Reanalyzing the data by excluding ribosomal proteins still clearly indicated that RBPs exhibit much lower noise compared to other protein coding genes (P = 6.3 × 10−6, Wilcoxon test) (SI Text S2). This analysis unambiguously reveals that low noise is an inherent property of all RBPs and suggests that RBPs are tightly regulated at the protein level with little variation in their expression from cell to cell. An independent analysis to compare the dynamic properties of RBPs with all of the protein coding genes (including RBPs) and varying the test statistic used to calculate the significance, did not change our results. This suggests that the trends observed are generally robust and are independent of the statistical test used (SI Text S5).
The Number of Distinct Targets Bound by a RBP Is Correlated with Its Cellular Abundance.
RBPs are the key elements responsible for the posttranscriptional control of gene expression and when combined with their RNA targets, this information can be represented as a RBP-RNA network. Although, on a genomic scale, RBPs are believed to control diverse range of functions with some eukaryotic systems predominantly using posttranscriptional mechanisms for gene expression control (11, 13), large-scale elucidation of posttranscriptional networks is limited to few model organisms for a select set of RBPs. In yeast, few recent genomewide studies identified the targets for several RBPs using RIP-chip technology (23, 26). These studies revealed the important roles played by different families of RBPs and the structure of the posttranscriptional network formed by them. These high-throughput studies showed that the number of targets of a RBP can vary widely, from <10 to more than thousands. In this study we obtained this network, where nodes represent RBPs or their targets and links represent a distinct physical association between the RBP and the target RNA. We then systematically investigated the relationship between different dynamic properties of RBPs and the number of distinct RNA targets they control.
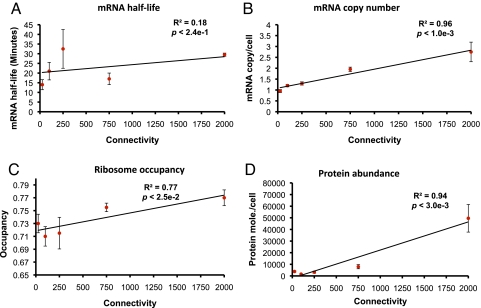
We first asked whether the number of targets of a RBP is correlated with its transcript stability by grouping the RBPs into different connectivity bins i.e., groups of RBPs comprising a number of distinct RNA targets (see Materials and Methods). As a result of this analysis, we found that there was a weak but positive correlation between them, suggesting that transcript turnover of RBPs may not be dependent on their number of targets (R2 = 0.18, P < 0.24) (Fig. 3A). On the other hand, a comparison of the mRNA copy number of a RBP and its number of targets revealed a strong positive correlation between them, suggesting that RBPs with a high number of targets are likely to be more highly expressed at the mRNA level (R2 = 0.96, P < 1 × 10−3) (Fig. 3B). For instance, PAB1 is a highly connected essential RBP that can bind to the poly(A) tail of an mRNA to regulate its translational initiation through its binding with eIF4G protein (18, 27). Indeed, it was reported to bind to 1,994 distinct RNA targets and was among the genes with very high mRNA copy number (7.1 mRNA copies/cell). These observations point to a direct link between the number of distinct targets of a RBP and its available number of copies of mRNA in the cell. To test the existence of a correlation between the connectivity and the rate of translation or the absolute protein abundance profile of RBPs, we further explored the relationship between them (Fig. 3 C and D). This comparison uncovered a more general link between translational efficiency of a RBP and its degree. For instance, Pub1p is another poly(A) binding protein (28) that binds to diverse sets of transcripts involved in ribosome biogenesis, cellular metabolism, and transport (29). This protein was reported to be localized to both nucleus and cytoplasm (30). Hence to be present at different locations and to bind to a large number of transcripts it has to be translated more often and should be present in a higher number of copies. Consistent with this, we find that its transcript exhibits high ribosome occupancy. Indeed, Hogan et al. (23) demonstrated that RNA targets of highly connected RBPs were enriched for multiple processes and subcellular localizations. These results clearly unveil the strong relationship between the concentration of a RBP and the number of distinct RNA targets bound by them, indicating that RBPs responsible for controlling a wide range of targets must occur in a higher number of copies at the protein level. It is important to note that although RBPs as a group of genes are significantly higher expressed at the transcript and protein levels compared to non-RBP population, relative abundance of the RBPs is correlated to the hierarchy of a RBP, defined as the number of distinct RNA targets. It is also noteworthy to mention that the RBPs analyzed for connectivity in this section did not comprise core ribosomal proteins, strengthening the generality of these observations.
Fig. 3.
Relationship between the number of targets of a RBP and its (A) transcript turn over, (B) estimated mRNA copy number per cell, (C) extent of ribosome occupancy, and (D) protein abundance. In each case, except for transcript stability, we found a strong correlation between the connectivity of a RBP and the regulatory property studied, suggesting that RBPs that regulate high numbers of targets are present at higher levels at the protein level. RBPs are divided into five bins, with approximately equal number of RBPs, based on their connectivity. Points correspond to the median values in the respective bins whereas the error bars show the normalized median deviation calculated as the ratio between the median absolute deviation (MAD) and the square-root of the number of values in the bin.
RBPs Bound to Many RNA Targets Are Less Frequently Degraded and Tightly Controlled at Protein Level.
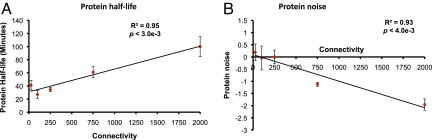
Although RBPs with a higher number of distinct targets are expressed at a higher level compared to those that control fewer targets, it is not evident whether their protein turnover rates would hold a similar trend. Therefore, to understand whether there is any dependence between the stability of a RBP and the number of transcripts it controls, we used a similar approach as above. This analysis clearly showed that RBPs that regulate many targets are highly stable at the protein level (R2 = 0.95, P < 3 × 10−3) (Fig. 4A). The link between protein stability and RBP's degree indicates that RBPs controlling several targets are less frequently degraded at the protein level and might be present throughout the cell cycle. Taken together, these observations raise the question: If highly connected RBPs are consistently expressed in large concentrations and are less frequently degraded, would their regulation be tightly controlled at the protein level? The fact that RBPs as a group show significantly lower noise in comparison to non-RBPs and that previous studies reported that regulatory proteins generally exhibit low noise (25) suggests that highly connected RBPs can be expected to show less noise in comparison to those that are poorly connected. Hence, we compared the connectivity of RBPs with their noise value. As shown in Fig. 4B, we found a strong correlation between the number of targets of a RBP and its protein noise. In particular, highly connected RBPs showed minimal variation in their protein expression across a population of cells (R2 = 0.93, P < 4 × 10−3). This suggests that RBPs controlling many targets are very tightly regulated with little cell-to-cell variation in their protein expression. These observations indicate that any significant change in their availability or regulation may result in an imbalance in cellular homeostasis as it may affect a vast number of transcripts. Indeed, a comparison of the number of essential genes in RBPs showed a twofold enrichment compared to the whole genome, suggesting their central role in maintaining cellular homeostasis (SI Text S6). These lines of evidence reveal that RBPs act as an important class of regulatory molecules in the cell whose expression is tightly controlled despite their occurrence in large cellular concentrations and in multiple subcellular locations.
Fig. 4.
Relationship between RBP's connectivity versus its (A) protein stability and (B) noise. RBPs controlling more numbers of targets showed an increasing tendency to be stable at the protein level and decreasing tendency in protein noise. RBPs are divided into five bins, with approximately equal number of RBPs, based on their connectivity. Points correspond to the median values whereas the error bars show the normalized median deviation calculated as the ratio between the median absolute deviation (MAD) and the square-root of the number of values in the bin.
Conclusion
RBPs form an important class of evolutionarily conserved proteins (31) and are known to be involved in a wide range of cellular processes. In addition to their functional roles in diverse processes, RBPs are also known to be implicated in a number of disorders due to their misexpression or mutations in the sequences that are used to recognize their cognate target RNAs. For instance, in humans, malfunctioning of RBPs like NOVA, which is a neuron-specific protein responsible for the alternative splicing of a subset of premRNAs, is known to be involved in the pathogenesis of the neurodegenerative syndrome POMA (14). In line with this and other observations on the impact of changes in the expression levels of RBPs being associated with diseases and fitness defects (6, 7), our analysis reveals that RBPs as a functional class show very little variation in their expression across cells, suggesting the importance in tightly controlling them. In addition, we also found that RBPs that regulate multiple transcripts show a significantly reduced noise, indicating that variations in the expression levels of these key posttranscriptional regulators can have significant impact on the functioning of the cell thereby leading to a disease phenotype.
Our analysis suggests that RBPs are generally less stable at the transcript level but exhibit higher stability and abundance at the protein level. Our results demonstrate that RBPs as a group follow the theoretically proposed time averaging effect on noise propagation (32), which suggests that if the protein has long half-life compared to its mRNA then it averages over the noisy fluctuations in the mRNA, decreasing the protein expression noise. These results also indicate that regulation of RBPs is predominantly controlled at the protein level through the use a number of posttranslational modifications (PTMs) like phosphorylation, arginine methylation, and sumoylation, which have been reported to occur in several well-studied RBPs (33–35). Indeed, a comparison of the number of phosphorylated targets in RBPs and non-RBPs unambiguously revealed the predominance of posttranslational control of RBPs. Therefore, it is possible to suggest that a wide variety of these PTMs might be responsible for their spatial and temporal regulation of transcripts in eukaryotic systems. It is possible to speculate from our observations that the low noise levels of RBPs together with extensive regulatory flexibility at the protein level might give them an advantage to control gene regulation at a finer level compared to transcriptional control by transcription factors. This might thereby provide a quick and extensive framework for controlling gene expression of a wide range of genes.
We also note that RBPs that are central to the cell are not only required in large quantities but are also found to be present for a longer time in the cell. All these observations suggest the importance of a posttranscriptional network of interactions in higher eukaryotes and raise several open questions in the regulation of gene expression beyond transcription. We believe that such questions could be addressed in the near future as more data from different levels of regulation become available (36–38).
Materials and Methods
Data on RNA-Binding Proteins in S. cerevisiae and Their Interactions.
The complete list of annotated RBPs and the data for well-studied RBPs in S. cerevisiae were obtained from Hogan et al. (23). The total number of annotated RBPs in yeast reported in this study was 561 and mRNA targets for 41 RBPs have been systematically identified on a whole genome scale by employing the RIP-chip technology. This approach essentially consists of two steps. The first involves generation of two RNA samples, isolation of RBP-bound mRNA by immunoprecipitation of messenger-ribonucleoproteins using affinity purification, and isolation of cellular RNA representing the whole set of transcripts in the cell. The second step involves hybridization of the two isolated RNA samples using dual-color microarrays and are analyzed for enriched transcripts to detect the bound targets of a RBP (39). A total of 14,312 interactions comprising 41 RBPs and 5,025 genes in the entire genome of S. cerevisiae, which forms a network of posttranscriptional interactions between RBPs and the target RNAs obtained using this approach, were used in this study (23).
Data for Comparative Analysis of Expression Dynamics.
To study the expression dynamics of RBPs in comparison to other protein coding genes in the genome and to analyze their relationship with the number of RNAs controlled by RBPs, we have used a variety of datasets. These include the transcript stability, mRNA copy number, ribosome occupancy, protein half-life, protein abundance, and protein noise. Transcript stability, which is measured as the RNA half-life of a transcript, was obtained from Wang et al. (22) and contained mRNA half-lives for 4,687 genes in the entire genome. A key parameter describing the translational status of a gene is the fraction of its transcripts engaged in translation, which is defined by the ribosome occupancy (40). Likewise, the number of mRNA copies of a gene can be best described by the parameter mRNA copy number per cell. Both these parameters for genes in S. cerevisiae were obtained from Arava et al. (40) where the authors used velocity sedimentation to separate mRNAs bound to ribosomes and quantified them using microarray analysis. mRNA copy number could be obtained for 5,643 genes whereas ribosome occupancy could be mapped for 5,700 genes, allowing us to study the extent of transcript abundance and translation rates of the genes and transcripts. Stability of a protein, which is an estimate of the duration it occurs within the cell is measured as the half-life of the protein. In yeast, protein half-lives have been estimated by Belle and coworkers for ≈3,750 proteins by inhibiting translation (41). In this study, we used these data by excluding proteins whose half-lives have been obtained by extrapolation. Protein abundance, which reveals the absolute number of protein molecules per cell, was obtained from Ghaemmaghami et al. (42). We could obtain abundance values for 3,868 proteins in the entire genome. Biological noise, which is typically defined as the variation in the expression of a protein between different cells in a homogenous population of cells, was obtained from Newman et al. (25). We could obtain noise data for 2,213 genes for cells grown on rich media. The authors in this study used two distinct measures for calculating protein noise, coefficient of variation (CV), which is the ratio of the standard deviation in the expression of a protein and its mean expression and distance from median (DM), which was calculated as the difference between the CV value of a protein and a running median of all CV values. In this study, we have used DM as a measure of protein noise as it was indicated to be a more robust measure compared to CV to understand protein-to-protein variations in noise levels (25). Since DM is the distance between the CV and median value of all CVs, negative values correspond to relatively less noise whereas positive values reflect higher levels of noise in the protein expression.
Comparison of the Regulatory Properties of RBPs with Other Protein Coding Genes.
To study whether RBPs show differences in dynamic properties when compared to other protein coding genes, we defined a non-RBP set of proteins. This set essentially comprised proteins in the whole genome after excluding the list of 561 RBPs defined above. To assess whether RBPs exhibit a different trend compared to non-RBPs for each of the properties studied, we used the Wilcoxon rank-sum test or the Mann–Whitney U test available in the R statistical package to calculate the significance. The Wilcoxon test enables the comparison of two samples to assess whether they come from the same distribution or not. Since this test is nonparametric and does not assume any inherent distribution of the samples it is ideal to compare different samples. Box plots were used to represent the distribution of values for each property. Independently, analysis of the mean values of a property for RBP and non-RBP sets of proteins was also carried out and P-values were estimated using the Welch t test, which gave similar results (SI Text S5). Because the RBP set comprised a number of ribosome-associated proteins we also excluded them from this list and repeated the analysis to test the robustness of the tendencies observed, in the absence of ribosomal proteins (SI Text S2).
Analysis of the Relationship Between the Number of Targets of a RBP and Their Dynamic Properties.
To understand the link between the number of targets of a RBP and their dynamic properties, RBPs were first grouped on the basis of their number of distinct RNA targets to which they were bound. This grouping was done in such a way that each bin of RBPs contained roughly an equal number of RBPs. This resulted in five different bins corresponding to varying degrees of RBPs, with some RBPs controlling as many as 2,000 mRNAs in the RBP-RNA network. To nullify the effect of outliers in each bin, median values were calculated for different dynamic properties and correlation was estimated between median values and connectivity of RBPs. P-values were calculated using the coefficient of correlation and the number of data points, on the basis of a linear fit.
Supporting Information.
For additional details relating to SI Text S1–S6, see Dataset S1, Figs. S1–S7, and Tables S1–S3. An analysis of enrichment of cell-cycle regulated genes, performed to address the concerns of a reviewer, is detailed in SI Text S7.
Supplementary Material
Acknowledgments.
We thank members of the Theoretical and Computational Biology group at MRC Laboratory of Molecular Biology (LMB) for their feedback and helpful discussions in the early stages of this work. We also thank S. De, B. Lang, Y. Kondo, and Y. Pilpel for providing helpful comments. This work was supported by MRC LMB (to N.M., S.C.J., and M.M.B.), by National Institute of Pharmaceutical Education and Research, Commonwealth split-site program (to N.M., N.R., and M.M.B.) and Cambridge Commonwealth Trust (to S.C.J.).
Supplementary URL: http://www.mrc-lmb.cam.ac.uk/genomes/sarath/RBP-dynamics
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906940106/DCSupplemental.
References
- 1.Mata J, Marguerat S, Bahler J. Post-transcriptional control of gene expression: A genome-wide perspective. Trends Biochem Sci. 2005;30(9):506–514. doi: 10.1016/j.tibs.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582(14):1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keene JD. RNA regulons: Coordination of post-transcriptional events. Nat Rev Genet. 2007;8(7):533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 4.Cookson W, Liang L, Abecasis G, Moffatt M, Lathrop M. Mapping complex disease traits with global gene expression. Nat Rev Genet. 2009;10(3):184–194. doi: 10.1038/nrg2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nica AC, Dermitzakis ET. Using gene expression to investigate the genetic basis of complex disorders. Hum Mol Genet. 2008;17(R2):R129–R134. doi: 10.1093/hmg/ddn285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136(4):777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukong KE, Chang KW, Khandjian EW, Richard S. RNA-binding proteins in human genetic disease. Trends Genet. 2008;24(8):416–425. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4(2):143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 9.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19(3):1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washburn MP, et al. Protein pathway and complex clustering of correlated mRNA and protein expression analyses in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2003;100(6):3107–3112. doi: 10.1073/pnas.0634629100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noe G, De Gaudenzi JG, Frasch AC. Functionally related transcripts have common RNA motifs for specific RNA-binding proteins in trypanosomes. BMC Mol Biol. 2008;9:107. doi: 10.1186/1471-2199-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell DA, Thomas S, Sturm NR. Transcription in kinetoplastid protozoa: Why be normal? Microbes Infect. 2003;5(13):1231–1240. doi: 10.1016/j.micinf.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Foth BJ, Zhang N, Mok S, Preiser PR, Bozdech Z. Quantitative protein expression profiling reveals extensive post-transcriptional regulation and post-translational modifications in schizont-stage malaria parasites. Genome Biol. 2008;9(12):R177. doi: 10.1186/gb-2008-9-12-r177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ule J, et al. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302(5648):1212–1215. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- 15.Lublin AL, Evans TC. The RNA-binding proteins PUF-5, PUF-6, and PUF-7 reveal multiple systems for maternal mRNA regulation during C. elegans oogenesis. Dev Biol. 2007;303(2):635–649. doi: 10.1016/j.ydbio.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Gruter P, et al. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell. 1998;1(5):649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 17.Saint-Georges Y, et al. Yeast mitochondrial biogenesis: A role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS ONE. 2008;3(6):e2293. doi: 10.1371/journal.pone.0002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler SH, Sachs AB. RNA recognition motif 2 of yeast Pab1p is required for its functional interaction with eukaryotic translation initiation factor 4G. Mol Cell Biol. 1998;18(1):51–57. doi: 10.1128/mcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musunuru K. Cell-specific RNA-binding proteins in human disease. Trends Cardiovasc Med. 2003;13(5):188–195. doi: 10.1016/s1050-1738(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 20.Kim MY, Hur J, Jeong S. Emerging roles of RNA and RNA-binding protein network in cancer cells. BMB Rep. 2009;42(3):125–130. doi: 10.5483/bmbrep.2009.42.3.125. [DOI] [PubMed] [Google Scholar]
- 21.Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137(1):146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, et al. Precision and functional specificity in mRNA decay. Proc Natl Acad Sci USA. 2002;99(9):5860–5865. doi: 10.1073/pnas.092538799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008;6(10):e255. doi: 10.1371/journal.pbio.0060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ptacek J, et al. Global analysis of protein phosphorylation in yeast. Nature. 2005;438(7068):679–684. doi: 10.1038/nature04187. [DOI] [PubMed] [Google Scholar]
- 25.Newman JR, et al. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature. 2006;441(7095):840–846. doi: 10.1038/nature04785. [DOI] [PubMed] [Google Scholar]
- 26.Gerber AP, Herschlag D, Brown PO. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol. 2004;2(3):E79. doi: 10.1371/journal.pbio.0020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachs AB, Davis RW, Kornberg RD. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol Cell Biol. 1987;7(9):3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matunis MJ, Matunis EL, Dreyfuss G. PUB1: A major yeast poly(A)+ RNA-binding protein. Mol Cell Biol. 1993;13(10):6114–6123. doi: 10.1128/mcb.13.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duttagupta R, et al. Global analysis of Pub1p targets reveals a coordinate control of gene expression through modulation of binding and stability. Mol Cell Biol. 2005;25(13):5499–5513. doi: 10.1128/MCB.25.13.5499-5513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson JT, Paddy MR, Swanson MS. PUB1 is a major nuclear and cytoplasmic polyadenylated RNA-binding protein in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13(10):6102–6113. doi: 10.1128/mcb.13.10.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anantharaman V, Koonin EV, Aravind L. Comparative genomics and evolution of proteins involved in RNA metabolism. Nucleic Acids Res. 2002;30(7):1427–1464. doi: 10.1093/nar/30.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulsson J. Summing up the noise in gene networks. Nature. 2004;427(6973):415–418. doi: 10.1038/nature02257. [DOI] [PubMed] [Google Scholar]
- 33.Schullery DS, et al. Regulated interaction of protein kinase Cdelta with the heterogeneous nuclear ribonucleoprotein K protein. J Biol Chem. 1999;274(21):15101–15109. doi: 10.1074/jbc.274.21.15101. [DOI] [PubMed] [Google Scholar]
- 34.Vassileva MT, Matunis MJ. SUMO modification of heterogeneous nuclear ribonucleoproteins. Mol Cell Biol. 2004;24(9):3623–3632. doi: 10.1128/MCB.24.9.3623-3632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu MC, et al. Arginine methyltransferase affects interactions and recruitment of mRNA processing and export factors. Genes Dev. 2004;18(16):2024–2035. doi: 10.1101/gad.1223204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halbeisen RE, Galgano A, Scherrer T, Gerber AP. Post-transcriptional gene regulation: From genome-wide studies to principles. Cell Mol Life Sci. 2008;65(5):798–813. doi: 10.1007/s00018-007-7447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lackner DH, et al. A network of multiple regulatory layers shapes gene expression in fission yeast. Mol Cell. 2007;26(1):145–155. doi: 10.1016/j.molcel.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hieronymus H, Silver PA. A systems view of mRNP biology. Genes Dev. 2004;18(23):2845–2860. doi: 10.1101/gad.1256904. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez M, Galy B, Hentze MW, Muckenthaler MU. Identification of target mRNAs of regulatory RNA-binding proteins using mRNP immunopurification and microarrays. Nat Protoc. 2007;2(8):2033–2042. doi: 10.1038/nprot.2007.293. [DOI] [PubMed] [Google Scholar]
- 40.Arava Y, et al. Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2003;100(7):3889–3894. doi: 10.1073/pnas.0635171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belle A, Tanay A, Bitincka L, Shamir R, O'Shea EK. Quantification of protein half-lives in the budding yeast proteome. Proc Natl Acad Sci USA. 2006;103(35):13004–13009. doi: 10.1073/pnas.0605420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425(6959):737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.