Abstract
Background: Sarcopenia is thought to be accompanied by increased muscle fat infiltration. However, no longitudinal studies have examined concomitant changes in muscle mass, strength, or fat infiltration in older adults.
Objective: We present longitudinal data on age-related changes in leg composition, strength, and muscle quality (MQ) in ambulatory, well-functioning men and women. We hypothesized that muscle cross-sectional area (CSA) and strength would decrease and muscular fat infiltration would increase over 5 y.
Design: Midthigh muscle, subcutaneous fat (SF), and intermuscular fat (IMF) CSAs and isokinetic leg muscle torque (MT) and MQ (MT/quadriceps CSA) were examined over 5 y in the Health, Aging, and Body Composition study cohort (n = 1678).
Results: Men experienced a 16.1% loss of MT, whereas women experienced a 13.4% loss. Adjusted annualized decreases in MT were 2–5 times greater than the loss of muscle CSA in those who lost weight and in those who remained weight-stable. Weight gain did not prevent the loss of MT, despite a small increase in muscle CSA. Only those who gained weight had an increase in SF (P < 0.001), whereas those who lost weight also lost SF (P < 0.001). There was an age-related increase in IMF in men and women (P < 0.001), and IMF increased in those who lost weight, gained weight, or remained weight-stable (all P < 0.001).
Conclusions: Loss of leg MT in older adults is greater than muscle CSA loss, which suggests a decrease in MQ. Additionally, aging is associated with an increase in IMF regardless of changes in weight or SF.
INTRODUCTION
Loss of muscle mass and strength is associated with age (1, 2). Cross-sectional studies indicate that the age-related loss in strength is associated with poor function (3, 4). Because muscle strength appears to be a critical component in maintaining physical function, mobility, and vitality in old age, it is essential to identify factors that contribute to the loss of strength as people age. Moreover, the association between the composition or the quality of skeletal muscle and the decline in strength in older people is poorly understood. Emerging evidence suggests that fat accumulation within skeletal muscle is associated with muscle weakness (5), poor function (3), and increased risk of incident mobility limitations (3). We reported previously that lower attenuation values of muscle on computed tomography (CT), a marker of greater muscle fat content (5) are associated with lower muscle strength and muscle quality (MQ) (ie, lower strength per muscle size) (6).
Cross-sectional studies have reported that muscle mass and strength are highly correlated, which has led to the common belief that loss of strength is a direct result of loss of muscle mass. A recent report from the Health, Aging and Body Composition (Health ABC) Study cohort showed that the loss of muscle mass measured by dual-energy X-ray absorptiometry (DXA) did predict loss of strength. However, the loss of strength was surprisingly much greater than the loss of muscle mass, which suggests the loss of MQ (4). Because CT can provide more detailed and precise estimates of muscle mass and adipose tissue content than DXA, as well as additional information on fatty infiltration of skeletal muscle, it is important to further explore whether decreasing muscle mass and quality occur with age.
Fat infiltrates skeletal muscle under a host of conditions, including obesity, weight gain, and certain disease states (eg, muscular dystrophy and McArdle disease) (7–10). Data from in vitro cell culture studies indicate that this increase in adipose tissue within skeletal muscle may arise because of the pluripotent capacity of progenitor cells of myocytes, which can differentiate into other cell types, including adipocytes, in response to various stimuli (11), such as the denervation of muscle tissue that accompanies aging. A higher intermuscular fat (IMF) area has been associated with various aging-related conditions, including insulin resistance (5, 8, 12), and has been hypothesized to be related to the development of type 2 diabetes mellitus. Finally, IMF has been positively associated with age (13). However, that study was limited by its cross-sectional design; thus, it is not clear whether aging results in changes in IMF. More recently, we found an increase of ≈18% in IMF over 1 y in a control group (n = 20) of older (77 ± 1 y) adults (14), but no long-term aging studies have been conducted on IMF change.
In this study we report novel longitudinal data on age-related changes in muscle strength, quality, and muscle composition in a large cohort of aging men and women. We hypothesized that CT-measured muscle cross-sectional area (CSA) would decrease and muscular fat infiltration would increase over a 5-y period in older men and women. Moreover, we hypothesized that the loss of strength will be disproportionately greater than the loss of muscle mass in older men and women.
SUBJECTS AND METHODS
Participants
The Health ABC Study is a longitudinal, observational study of 3075 well-functioning men and women between the ages of 70 and 79 y at baseline. Participants were recruited in 1997–1998 from Memphis, TN, and Pittsburgh, PA. Fifty-two percent of the participants were women and 41% were black. Eligible participants had no self-reported difficulty walking 0.25 mile (402 m), climbing 10 steps, or performing activities of daily living; did not report using a walking aid; and were free of cancer under active treatment. The institutional review boards of the University of Pittsburgh and the University of Tennessee approved the protocol, and all participants gave informed consent for study participation.
Because these analyses required a 5-y follow-up clinic visit, there was considerable loss to follow-up (n = 983), mainly because of death (n = 378) or other reasons, such as missed visits, proxy reports, phone visits, or drop-outs/refused (n = 605). Of those (n = 2092) who had a clinic visit after 5 y, some had missing baseline CT data (n = 54), others had invalid 5-y CT data (n = 92) as determined by a different thigh positioning during the second scan compared with the first scan, or missing 5-y CT data (n = 265). Additionally, 3 other participants were excluded because of invalid baseline or follow-up knee extensor muscle torque (MT) data. Thus, longitudinal analyses were conducted on the remaining 1678 participants (54.6% of the total cohort; 813 men and 865 women) who had complete 5-y follow-up CT data.
Body composition
DXA (QDR 4500A; Hologic Inc, Waltham, MA) was used to measure total body composition. Methods and validation were previously reported (15). In validation of this model of DXA against a 4-compartment model of body composition, a slight overestimate of fat-free mass (FFM) was detected, which resulted in study-wide adoption of a correction factor. Height and body mass were measured while subjects were wearing a hospital gown and no shoes by using a stadiometer and a calibrated balance-beam scale.
Thigh cross-sectional areas
The CSAs of muscle and IMF of the right thigh were measured at baseline at the level of the midfemur by using CT as previously described (3). Briefly, a single 10-mm axial image of the right thigh was acquired at the midpoint of the distance between the medial edge of the greater trochanter and the intercondyloid fossa. IMF was partitioned from subcutaneous fat (SF) by drawing a line along the deep fascial plane surrounding the thigh muscle. The total area of nonfat and nonbone tissues within the fascial border was used as a quantification of muscle area (cm2).
Knee extensor torque
Average maximum isokinetic MT of the knee extensors (Newton meters, N-m), was measured at baseline and at year six by using a Kin-Com 125 AP Dynamometer (Chattanooga, TN) at 60°/s on the right leg. The mean of the 3 most replicable and satisfactory trials out of a limit of 6 attempts was used (16). An interrater reliability study (n = 60) showed no significant differences between testers and a within-participant CV of ≈11%. There was also a significant site difference; thus, a study site adjustment for machine models was done. When the MT of the right leg was not available or valid because of knee replacement or pain, the MT of the left leg was used in all analyses and was matched with the left leg thigh CSA for calculation of muscle and fat areas and MQ values. MQ was defined and calculated as MT/quadriceps muscle area (N-m/cm2) (16).
Potential confounders and effect modifiers
Potential confounders and effect modifiers of the association between body weight change group values and changes in thigh composition and muscle function were assessed and included race (black or white), age, baseline values, smoking, alcohol use, comorbidity, physical activity level, body mass index (BMI), diabetes (indicated by self-report or diabetes medication use), and interim hospitalization.
Comorbidity was examined by summing the total of 11 conditions at baseline, assessed by self-report and validated with medication review, and grouping those with 0, 1, 2, or ≥3 conditions. Physical activity was defined by using the caloric expenditure (17) in the past week at baseline and after 5 y for self-reported walking, climbing stairs, and chores via a questionnaire. Interim hospitalization was defined as an overnight stay (ie, >24 h) in an acute-care hospital. These events were determined every 6 mo by interview at the annual clinic visit or interim phone call and confirmed by review of medical records.
Data analysis
Descriptive statistics (mean ± SD) were used to describe demographic and key clinical characteristics of the study population at baseline by sex and race. Paired t tests were used to test for differences in the distribution of continuous variables within persons. Bivariate correlations and multiple regression models were done to examine how changes in body composition explain changes in MT. To examine the effect of body weight change and weight stability on midthigh composition characteristics and muscle function, the cohort was further stratified by body weight change. There was a modest 5-y gain in body mass (P < 0.001) in this cohort in men (1.8 ± 0.2%; range: −17.4 to 44.0%) and women (1.7 ± 0.2%, range: −22.6 to 29.8%). Those participants who gained ≥3% of their baseline body weight were classified as having gained weight, whereas those who lost ≥3% of their body weight were considered to have lost weight (18, 19). The remaining participants were considered to be weight-stable. The association of weight change group on midthigh composition characteristics and muscle function measures was analyzed by using analysis of covariance (ANCOVA) for 5-y changes while adjusting for potential confounders. Additionally, we tested for an interaction between race and body weight change group for all midthigh composition and strength variables. All change models were run with and without baseline values as a sensitivity analysis and were found to have stable estimates (20). Finally, annualized rates (%) of change were calculated from all 5-y change outcomes in men and women. This was done by first taking the raw change score divided by the baseline value. This quotient was then divided by 5 and expressed as a percentage. For all analyses, overall P values for trends are reported, but when specific comparisons were done among weight change groups, a Bonferroni correction for multiple comparisons was used and a corrected P value is reported. Analyses were conducted with SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
The characteristics of the 1678 participants from the Health ABC Study in the current analysis are shown in Table 1. Many of the baseline characteristics in the cohort differed significantly between races within sex. Black men had a higher FFM (P < 0.01) but a lower total fat mass and percentage body fat (P < 0.001) than white men. Black women had a greater percentage body fat (P < 0.01) and greater total body mass, BMI, FFM, and total fat mass (all P < 0.001).
TABLE 1.
Baseline characteristics of Health, Aging, and Body Composition (Health ABC) participants (n = 1678)1
| White men (n = 567)2 | Black men (n = 246)3 | All men (n =813) | White women (n = 525)4 | Black women (n = 340)5 | All women (n = 865) | |
| Age (y) | 73.6 ± 2.8 | 73.5 ± 2.8 | 73.6 ± 2.8 | 73.4 ± 2.7 | 73.1 ± 2.8 | 73.2 ± 2.9 |
| Height (cm) | 173.5 ± 6.3 | 172.6 ± 6.6 | 173.2 ± 6.4 | 159.7 ± 5.9 | 160.0 ± 6.3 | 159.8 ± 6.1 |
| Total body mass (kg) | 81.6 ± 12.2 | 81.2 ± 14.3 | 81.5 ± 12.9 | 65.8 ± 11.7 | 76.4 ± 14.96 | 69.9 ± 14.0 |
| BMI (kg/m2) | 27.1 ± 3.5 | 27.2 ± 4.3 | 27.1 ± 3.8 | 25.8 ± 4.3 | 29.9 ± 5.56 | 27.4 ± 5.2 |
| Fat-free mass (kg) | 57.6 ± 6.9 | 58.8 ± 7.96 | 57.9 ± 7.3 | 39.7 ± 5.2 | 44.9 ± 6.26 | 41.8 ± 6.2 |
| Total fat mass (kg) | 23.9 ± 6.7 | 22.5 ± 7.66 | 23.5 ± 7.0 | 26.1 ± 7.5 | 31.5 ± 9.66 | 28.2 ± 8.8 |
| Total fat (%) | 28.8 ± 4.6 | 27.1 ± 5.36 | 28.3 ± 4.9 | 39.0 ± 5.5 | 40.4 ± 5.77 | 39.6 ± 5.6 |
| Physical activity (kcal · kg−1 · wk−1) | 54.6 ± 56.5 | 63.5 ± 72.3 | 57.3 ± 61.8 | 42.6 ± 49.9 | 55.8 ± 75.27 | 47.8 ± 61.4 |
All values are means ± SDs.
n = 550 participants for fat-free mass and total percentage fat, and n = 564 participants for total fat mass.
n = 242 participants for fat-free mass and total percentage fat, and n = 245 participants for total fat mass.
n = 509 participants for fat-free mass and total percentage fat.
n = 334 participants for fat-free mass and total percentage fat.
Significantly different from whites within sex (independent-samples t test): 6P < 0.001, 7P < 0.01.
Baseline midthigh composition and muscle function and characteristics in men and women are shown by race in Table 2. Black men had a greater total thigh muscle area, SF area (P < 0.05), and IMF area than did white men (all P < 0.001). Black women had a greater total thigh muscle area, SF area, and IMF area than did white women (all P < 0.001), but had a higher MT and lower thigh muscle attenuation than did white women (both P < 0.001).
TABLE 2.
Baseline midthigh composition and muscle function of Health, Aging, and Body Composition (Health ABC) Study participants1
| White men (n = 567) | Black men (n = 246) | All men (n = 813) | White women (n = 525) | Black women (n = 340) | All women (n = 865) | |
| Total thigh muscle area (cm2) | 129.5 ± 19.2 | 141.5 ± 23.62 | 133.2 ± 21.3 | 85.5 ± 13.5 | 104.1 ± 17.02 | 92.9 ± 17.5 |
| Quadriceps muscle area (cm2) | 60.9 ± 9.2 | 65.9 ± 11.42 | 62.4 ± 10.2 | 39.8 ± 6.6 | 47.3 ± 8.32 | 42.8 ± 8.2 |
| Average maximal muscle torque (N-m) | 133.9 ± 34.3 | 141.0 ± 35.6 | 135.8 ± 35.2 | 79.0 ± 19.9 | 90.6 ± 21.42 | 83.4 ± 21.4 |
| Muscle quality (N-m/cm2)3 | 2.18 ± 0.42 | 2.14 ± 0.42 | 2.17 ± 0.42 | 1.97 ± 0.39 | 1.91 ± 0.38 | 1.95 ± 0.39 |
| Subcutaneous fat (cm2) | 46.5 ± 19.0 | 50.4 ± 21.74 | 47.6 ± 19.9 | 96.9 ± 38.1 | 121.6 ± 49.62 | 106.7 ± 44.6 |
| Intermuscular fat (cm2) | 9.3 ± 6.2 | 10.7 ± 6.62 | 9.8 ± 6.4 | 8.5 ± 4.1 | 12.7 ± 7.62 | 10.1 ± 6.1 |
| Average muscle attenuation (HU) | 37.5 ± 6.5 | 37.8 ± 6.2 | 37.6 ± 6.4 | 35.2 ± 6.6 | 32.7 ± 7.52 | 34.2 ± 7.1 |
All values are means ± SDs. N-m, Newton meters; HU, Hounsfield units.
Significantly different from whites within sex (independent-samples t test): 2P < 0.001, 4P < 0.05.
Muscle quality = torque/quadriceps muscle area.
The 5-y changes in midthigh muscle composition and muscle function variables by sex are shown in Table 3. In both men and women, total thigh muscle area, average MT, MQ (all P < 0.001), and SF (P = 0.02 in men) all decreased significantly. In marked contrast, there was a significant increase in IMF area in both men and women (both P < 0.001). No race differences within sex were observed for changes in the muscle composition or strength characteristics with age (data not shown).
TABLE 3.
Five-year changes in midthigh composition and muscle function of Health, Aging, and Body Composition (Health ABC) participants by sex group1
| Men (n =
813) |
Women (n =
865) |
|||||
| Change | Percentage change | P value2 | Change | Percentage change | P value2 | |
| Total thigh muscle area (cm2) | −6.8 ± 10.0 | −4.9 ± 7.4 | <0.001 | −3.2 ± 7.6 | −3.2 ± 7.9 | <0.001 |
| Average maximal muscle torque (N-m) | −24.5 ± 28.1 | −16.1 ± 20.6 | <0.001 | −12.7 ± 17.5 | −13.4 ± 23.0 | <0.001 |
| Muscle quality (N-m/cm2)3 | −0.32 ± 0.41 | −13.1 ± 20.4 | <0.001 | −0.26 ± 0.37 | −11.1 ± 23.8 | <0.001 |
| Subcutaneous fat (cm2) | −0.8 ± 9.1 | −1.5 ± 19.8 | 0.020 | −3.2 ± 16.6 | −2.1 ± 16.9 | <0.001 |
| Intermuscular fat (cm2) | 3.1 ± 3.1 | 48.5 ± 59.6 | <0.001 | 1.7 ± 3.0 | 29.0 ± 43.6 | <0.001 |
All values are means ± SDs. N-m, Newton meters.
Derived by using paired-samples t tests.
Muscle quality = torque/quadriceps muscle area.
To examine the potential associations between changes in MT and changes in various body- and muscle-composition variables, bivariate correlations were conducted and are presented in Table 4. The loss of MT was positively associated with losses of body weight (P < 0.01), body fat mass (women only, P < 0.001), and quadriceps muscle area (P < 0.001). In women, the decrease in SF was also associated with losses in MT (P = 0.004), but this was not observed in men. Changes in MT were not significantly related to changes in IMF in either men or women.
TABLE 4.
Bivariate correlations between 5-y changes in various body- and muscle-composition variables and 5-y changes in knee extensor average maximal muscle torque by sex
| Men |
Women |
|||
| Five-year change variable | r | P value | r | P value |
| ΔBody weight (kg) | 0.105 | 0.005 | 0.186 | <0.001 |
| ΔBody fat mass (kg) | 0.066 | 0.081 | 0.150 | <0.001 |
| ΔQuadriceps muscle area (cm2) | 0.240 | <0.001 | 0.284 | <0.001 |
| ΔSubcutaneous fat (cm2) | 0.028 | 0.454 | 0.106 | 0.004 |
| ΔIntermuscular fat (cm2) | −0.025 | 0.500 | 0.048 | 0.200 |
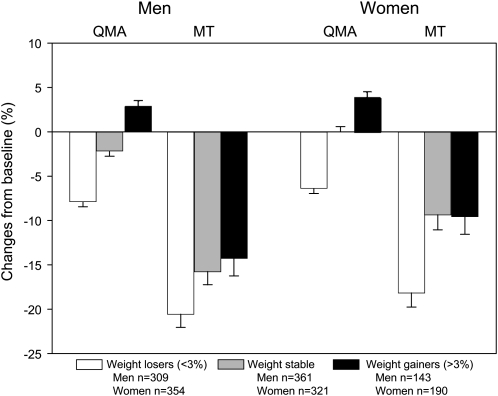
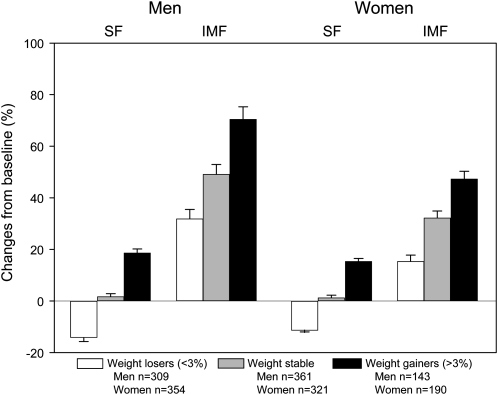
There were significant differences between weight-change groups for changes in quadriceps muscle area and MT (all P < 0.001), as shown in Figure 1. However, there was a greater proportionate decline in MT in each weight-loss group compared with changes in muscle area. In addition, the adjusted 5-y percentage changes in midthigh SF and IMF areas by weight-change group, stratified by sex, are shown in Figure 2. There were significant differences between weight-change groups for SF and IMF changes (all P < 0.001). Although IMF increased among all weight-change groups in men (35.5–74.6%) and women (16.8–50.0%), men and women who lost weight had a loss of SF (men = −14.1%; women = −11.3%).
FIGURE 1.
Adjusted least-squares mean (±SEM) 5-y percentage changes in quadriceps muscle area (QMA) and average muscle torque (MT) by weight change group and sex. There were significant within-group declines in MT in men and women in all weight groups (all P < 0.001) and changes in QMA (all P < 0.001), except for QMA changes in weight-stable women. There were significant differences between weight-change groups for QMA and MT changes (all P < 0.001). However, there was a greater percentage decline in MT in each weight-loss group than in changes in muscle area. Data were analyzed by using ANCOVA, with adjustment for baseline values, age, baseline drinking, smoking, baseline BMI, interim hospitalization, and baseline and change in physical activity level, baseline comorbidity, and diabetes.
FIGURE 2.
Adjusted least-squares mean (±SEM) 5-y percentage changes in midthigh subcutaneous fat (SF) and intermuscular fat (IMF) areas by weight-change group and sex. There were significant within-group changes in men and women for SF in the weight-loss and weight-gain groups (all P < 0.001). There were also significant within-group changes in men and women in all weight groups for IMF (all P < 0.001). There were significant differences between weight-change groups for SF and IMF changes (all P < 0.001). Data were analyzed by using ANCOVA, with adjustment for baseline values, age, baseline drinking, smoking, baseline BMI, interim hospitalization, and baseline and change in physical activity level, baseline comorbidity, and diabetes.
There was evidence of a race × body weight change group interaction in both men and women in the models (all P < 0.10) for change in thigh muscle area, SF, and IMF. Thus, an additional analysis was performed for these outcomes by race and body weight–change group within sex. Despite evidence of a race × body weight change interaction, there were similar patterns of adjusted annualized rates of thigh muscle area, IMF, and SF changes between blacks and whites within sex for all body weight change groups (data not shown).
The 5-y annualized rate of change (%) for these changes in muscle composition and function are presented in Table 5. There was a significant difference in the yearly rate of change in all thigh composition and function variables among weight-change groups (all P < 0.001), except for MQ. Moreover, there was a substantial difference between the rate of MT loss and change in muscle area in all groups, with an average 4-fold faster annual decline in MT. Thus, there was a consistent and similar loss of MQ in all weight-change groups within sex. It also appears that there may have been a sex difference with regard to increases in IMF with age by weight-change group. The annualized percentage increase in IMF across weight-change groups was ≈1.5–2.1 times those of women, although no evidence of a sex × weight-change group interaction was found. Because of the previously reported association between IMF and diabetes risk (12), we also analyzed the change in IMF stratified by the presence of diabetes within sex. There was a significantly greater increase in adjusted IMF in men with diabetes (n = 121) compared with men without diabetes (56.5 ± 5.4% compared with 44.6 ± 3.2%; P=0.037). However, there was no difference in the increase in IMF between diabetic (n = 94) and nondiabetic women (31.4 ± 4.3% compared with 28.7 ± 2.1%; P = 0.551).
TABLE 5.
Annualized rate (%) of midthigh composition and muscle function change during the 5-y follow-up time in Health, Aging, and Body Composition (Health ABC) participants by weight-change category1
| Men |
Women |
|||||||
| Weight loss2 (n = 309) | Weight-stable (n = 361) | Weight gain3 (n = 143) | P value for trend4 | Weight loss2 (n = 354) | Weight-stable (n = 321) | Weight gain3 (n = 190) | P value for trend4 | |
| Total thigh muscle area (cm2) | −1.9 ± 0.15 | −0.7 ± 0.15 | 0.2 ± 0.1 | 0.002 | −1.5 ± 0.15 | −0.3 ± 0.15 | 0.4 ± 0.15 | <0.001 |
| Quadriceps muscle area (cm2) | −1.6 ± 0.15 | −0.5 ± 0.15 | 0.6 ± 0.15 | <0.001 | −1.3 ± 0.15 | −0.03 ± 0.1 | 0.8 ± 0.15 | <0.001 |
| Average maximal muscle torque (N-m) | −4.1 ± 0.35 | −3.2 ± 0.35 | −2.9 ± 0.45 | 0.003 | −3.6 ± 0.35 | −1.9 ± 0.35 | −1.9 ± 0.45 | <0.001 |
| Muscle quality (N-m/cm2)6 | −2.9 ± 0.35 | −2.9 ± 0.35 | −3.0 ± 0.45 | 0.912 | −2.4 ± 0.35 | −1.9 ± 0.35 | −2.5 ± 0.45 | 0.184 |
| Subcutaneous fat (cm2) | −2.8 ± 0.25 | 0.4 ± 0.2 | 3.7 ± 0.35 | <0.001 | −2.3 ± 0.25 | 0.3 ± 0.2 | 3.1 ± 0.25 | <0.001 |
| Intermuscular fat (cm2) | 6.4 ± 0.75 | 9.9 ± 0.75 | 14.1 ± 1.05 | <0.001 | 3.1 ± 0.55 | 6.5 ± 0.55 | 9.5 ± 0.65 | <0.001 |
All values are least-squares means ± SEMs obtained by using ANCOVA, with adjustment for baseline values, age, race, baseline drinking, smoking, baseline BMI, interim hospitalization, diabetes, baseline and change in physical activity level, and baseline comorbidity. N-m, Newton meters.
Defined as a loss of ≤3% of body weight from baseline.
Defined as a gain of ≥3% of body weight from baseline.
P values represent differences between weight-change groups.
P < 0.001 for within-group change (paired-samples t tests).
Muscle quality = torque/quadriceps muscle area.
Finally, for all men and women, multiple linear regression analyses showed that higher lower baseline quadriceps muscle area, baseline MT, and greater loss of muscle area were associated with greater declines in MT with age. When these data were stratified by weight-change category, strength also declined in each weight-change category, but to a lesser extent in weight-stable men and women compared with those whose weight changed. Also, IMF showed a significant inverse relation as a predictor of MT decline only in men who remained weight-stable. No other potential predictors were found to predict the change in MT by weight-change category within sex.
DISCUSSION
The results of our investigation show for the first time that there is an age-related increase in fatty infiltration of midthigh skeletal muscle in men and women, as indicated by increases in IMF. Moreover, this fatty infiltration of skeletal muscle worsened over 5 y in both men and women regardless of weight changes and changes in subcutaneous thigh adipose tissue. We also found that the decreases in strength were 2–5 times greater than the loss of muscle size with aging. In addition, weight gain did not attenuate the loss of strength with aging, despite a small increase in midthigh muscle area. Together, these findings clearly indicate that progressive muscle weakness and increase in muscular fat infiltration with age occur regardless of changes in muscle mass or SF, implicating losses in MQ with aging. The present study is the first to clearly define a unique and consistent age-related increase in IMF.
The dramatically higher rates of strength loss relative to the loss of muscle mass in these older men and women is supported by our earlier report in this cohort that muscle strength declines at a more rapid rate than muscle mass as measured by DXA (4). These longitudinal studies are in apparent contrast with many earlier studies suggesting that a decline in muscle mass is strongly correlated with the losses of strength with aging (2, 21, 22). However, most of the evidence supporting the association between muscle mass and strength with aging is from cross-sectional studies, which can provide only estimates of change with age because they are composites of separate age groups that may have been affected by systematic differences in height, weight, physical activity, or other factors that might affect skeletal muscle. The few longitudinal studies indicate a more rapid decline in strength with aging than suggested by most cross-sectional studies. For example, Frontera et al (23) observed a 20–30% decline in strength of the knee and elbow extensors and flexors with an average ≈16% loss in the CSA of the quadriceps in older men over a 12-y span. Despite this difference, the change in CSA was an independent predictor of strength with aging. The findings in the present investigation extend these findings in a larger group of slightly older adults and also show that the decline in strength is relatively greater than the decline in muscle mass than has been previously reported. Moreover, our study indicates that the declines in muscle strength occur despite changes in body weight. It should be noted that the measure of MQ has not been consistent across studies, ie, different muscle groups have been examined, different methods for assessment of strength and/or muscle mass, making direct quantitative comparisons with younger subjects or longitudinal changes difficult. Our results agree, however, with previous cross-sectional studies (24, 25) suggesting significant age-related declines in MQ in older adults.
Another primary finding in our study was that there was an increase in muscle fat infiltration (IMF) regardless of body weight change or changes in the subcutaneous adipose tissue. This unexpected finding suggests that the gain in IMF is a remarkably consistent characteristic of aging even in those who lose weight. Although the increase in IMF appears to be influenced by weight change, with greater increases in IMF with weight gain, both muscle area and SF decline with weight loss. Although these results clearly indicate that the increase in IMF coincides with the progressive muscle weakness in aging, the increase in IMF was only significantly associated with the loss of strength in men who were weight-stable. This lack of direct association between IMF and loss of strength could have been due to the size of the IMF depot and the variability in MT and IMF changes. These novel findings regarding the age-associated gain in IMF complement prior studies on the role of IMF as an important contributor to adverse metabolic changes that accompany skeletal muscle loss. For instance, we (26) previously observed that, despite accounting for only ≈8% of total thigh fat, IMF, but not SF, is correlated with insulin resistance and the metabolic syndrome (26–28) and is proportionately greater in those with diabetes mellitus (27). In addition, Albu et al (12) found that, in both lean and obese women, higher IMF was associated with lower insulin sensitivity independent of race, weight, height, and skeletal muscle volume. Indeed, the increases in IMF with age were significantly greater in men with diabetes. Although this was not observed in women who had diabetes at baseline, the self-report of diabetes likely underestimated the effect of diabetes on IMF changes.
The direction of changes in IMF with age in the present study is somewhat analogous to an increased visceral adiposity, which is related to an increase in proinflammatory cytokines that negatively affect muscle function (29). However, whether IMF also plays an important role in this process remains unknown. Thus, it is unclear whether IMF is simply a marker of metabolic dysfunction of adjacent skeletal muscle or whether this fat depot plays a more active role in sarcopenia or muscle contractility.
Despite the novel findings of the current study, it had some limitations. First, although a considerable proportion of participants had follow-up data, the loss to follow-up participants could have biased our results. Although there were no differences in height, weight, BMI, lean mass, total fat mass, or percentage fat between the follow-up cohort and those not included in the analysis, participants included in this follow-up analysis were slightly younger (P < 0.001) at baseline. Moreover, participants in the follow-up cohort, compared with those without follow-up data, had a greater baseline MT (P < 0.001) and thigh muscle area (P < 0.001), but a lower thigh IMF area (P = 0.005). Second, this cohort began with relatively healthy, higher-functioning older adults in a somewhat narrow age range. Those with poor muscle function or greater fat infiltration may not have enrolled or could have been excluded because of other health problems. However, this is an ideal age range to examine the changes in muscle composition and function, with implications for designing prevention strategies to avoid body-composition changes with aging that may negatively affect muscle function. Third, although these data were stratified by weight-change group, the intentionality of weight change was not assessed. It is likely that some individuals lost or gained weight intentionally, whereas others could have experienced unintentional weight loss because of illness or other circumstances. However, the relatively small absolute difference in adjusted IMF change between weight groups was in the expected direction, with increased weight gain leading to greater increases in IMF. Finally, given the previously reported associated between IMF and diabetes risk (5, 8, 12), future studies should specifically address the relation between diabetes and age-related changes in muscle fat infiltration.
In summary, we found that, regardless of changes in muscle mass or body weight, older persons generally experience a progressive loss of strength and MQ. Furthermore, the accumulation of fat within skeletal muscle worsens with aging, even in weight-stable persons and those who lost body weight. These results show for the first time that a loss of strength with aging coincides with a gain in muscle fat infiltration. Although no associations were observed between the gain in muscle fat and loss of strength, additional studies are needed to determine whether these changes are mechanistically linked.
Acknowledgments
The authors' responsibilities were as follows—MJD, RB, and BHG: analysis and interpretation of data and preparation of manuscript; TBH and ABN: acquisition of subjects and data, study concept and design, interpretation of data, and preparation of manuscript; MV: interpretation of data and preparation of manuscript; SWP, MBC, PV-M, and TMM: interpretation of data and preparation of manuscript; and MN: acquisition of subjects and data, study concept and design, and preparation of manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Brooks SV, Faulkner JA. Skeletal-muscle weakness in old-age—underlying mechanisms. Med Sci Sports Exerc 1994;26:432–9 [PubMed] [Google Scholar]
- 2.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol 1991;71:644–50 [DOI] [PubMed] [Google Scholar]
- 3.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 2002;50:897–904 [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–64 [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 2000;89:104–10 [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol 2001;90:2157–65 [DOI] [PubMed] [Google Scholar]
- 7.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1991;54:509–15 [DOI] [PubMed] [Google Scholar]
- 8.Greco AV, Mingrone G, Giancaterini A, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes 2002;51:144–51 [DOI] [PubMed] [Google Scholar]
- 9.De Kerviler E, Leroy-Willig A, Duboc D, Eymard B, Syrota A. MR quantification of muscle fatty replacement in McArdle's disease. Magn Reson Imaging 1996;14:1137–41 [DOI] [PubMed] [Google Scholar]
- 10.Leroy-Willig A, Willig TN, Henry-Feugeas MC, et al. Body composition determined with MR in patients with Duchenne muscular dystrophy, spinal muscular atrophy, and normal subjects. Magn Reson Imaging 1997;15:737–44 [DOI] [PubMed] [Google Scholar]
- 11.Sinanan AC, Buxton PG, Lewis MP. Muscling in on stem cells. Biol Cell 2006;98:203–14 [DOI] [PubMed] [Google Scholar]
- 12.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 2005;82:1210–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkawa S, Odamaki M, Ikegaya N, Hibi I, Miyaji K, Kumagai H. Association of age with muscle mass, fat mass and fat distribution in non-diabetic haemodialysis patients. Nephrol Dial Transplant 2005;20:945–51 [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol 2008;105:1498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study—dual-energy x-ray absorptiometry and body composition working group. J Appl Physiol 1999;87:1513–20 [DOI] [PubMed] [Google Scholar]
- 16.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc 2003;51:323–30 [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–516 [DOI] [PubMed] [Google Scholar]
- 18.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr 2005;82:872–8 [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Kritchevsky SB, Tylavsky F, et al. Weight change, weight change intention, and the incidence of mobility limitation in well-functioning community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2005;60:1007–12 [DOI] [PubMed] [Google Scholar]
- 20.Yanez ND, III, Kronmal RA, Shemanski LR. The effects of measurement error in response variables and tests of association of explanatory variables in change models. Stat Med 1998;17:2597–606 [DOI] [PubMed] [Google Scholar]
- 21.Reed RL, Pearlmutter L, Yochum K, Meredith KE, Mooradian AD. The relationship between muscle mass and muscle strength in the elderly. J Am Geriatr Soc 1991;39:555–61 [DOI] [PubMed] [Google Scholar]
- 22.Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol 1990;45:M82–8 [DOI] [PubMed] [Google Scholar]
- 23.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 2000;88:1321–6 [DOI] [PubMed] [Google Scholar]
- 24.Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol 1997;83:1581–7 [DOI] [PubMed] [Google Scholar]
- 25.Lynch NA, Metter EJ, Lindle RS, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol 1999;86:188–94 [DOI] [PubMed] [Google Scholar]
- 26.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–92 [DOI] [PubMed] [Google Scholar]
- 27.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003;26:372–9 [DOI] [PubMed] [Google Scholar]
- 28.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005;165:777–83 [DOI] [PubMed] [Google Scholar]
- 29.Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol 2007;102:919–25 [DOI] [PMC free article] [PubMed] [Google Scholar]