Abstract
Patients with chronic ulcerative colitis are at increased risk of developing colorectal cancer. Though current hypotheses suggest that sporadic colorectal cancer is due to inability to control cancer stem cells, the cancer stem cell hypothesis has not yet been validated in colitis-associated cancer. Further, the identification of the colitis to cancer transition is challenging. We recently demonstrated that epithelial cells with the increased expression of aldehyde dehydrogenase (ALDH) in sporadic colon cancer correlate closely with tumor initiating ability. We sought to determine whether ALDH can be used as a marker to isolate tumor-initiating populations from patients with chronic ulcerative colitis. We utilized fluorescence-activated cell sorting to identify precursor colon cancer stem cells (pCCSC) from colitis patients and report both their transition to cancerous stem cells in xenografting studies as well as their ability to generate spheres in vitro. Similar to sporadic colon cancer, these colitis-derived tumors were capable of propagation as sphere cultures. However, unlike the origins of sporadic colon cancer, the primary colitic tissues did not express any histological evidence of dysplasia. To elucidate a potential mechanism for our findings, we compared the stroma of these different environments and determined that at least one paracrine factor is upregulated in the inflammatory and malignant stroma compared to resting, normal stroma. These data link colitis and cancer identifying potential tumor-initiating cells from colitic patients suggesting that sphere and/or xenograft formation will be useful to survey colitic patients at risk of developing cancer.
Keywords: Colitis, Colon Cancer, Aldehyde dehydrogenase, Colon sphere, Xenograft, tumor microenvironment
INTRODUCTION
The cancer stem cell hypothesis suggests that a very rare population of multipotent cells reside in tumors and retain limitless potential to regenerate (1). Colon cancer tumor initiating cells have been reported, using markers such as CD44 and CD133 (2–4). These markers had been used to detect tumor-initiating cells in other malignancies, (5–7) and led to significant enrichment of tumor-initiating cells. Further, use of these markers to identify colon cancer initiating cells has revealed their properties of self-renewal, multipotency, and chemoresistance. Another marker with functional significance is aldehyde dehydrogenase (ALDH1), a detoxifying metabolic enzyme often associated with stem and progenitor cell populations (8). Previous work by others demonstrated that ALDH1+ cells from breast tissue can be used as a predictor of clinical outcome in breast cancer patients (9). We recently showed that ALDH+ cells from colon cancer can be used as a marker for the tumor initiating subset (10) and may reflect a chemoresistant subset within CD44+/CD166+ tumor initiating colon cancer (11). In this current study, we selected ALDH+ cells from colitic patient samples to determine their potential for differentiation and their ability to generate tumors in mice.
To date, the cancer stem cell hypothesis holds true for established or frank malignancies but is virtually untested in cases of inflammation-associated cancers such as colitis-associated cancer. Inflammation contributes to the pathogenesis of a number of malignancies (12). Inflammation results in the release of significant numbers of bioreactive factors capable of inducing cell growth, differentiation, and potentially genetic mutations, as well as providing a supportive paracrine and stromal milieu for tumor development. Such a relationship is most poignantly demonstrated in the relationship between chronic ulcerative colitis and cancer, in which the incidence of malignant transformation is greatly increased (13). The genetic pathway via which benign colonic mucosa progresses into colon cancer is known as the adenoma to carcinoma sequence (14). Based on this knowledge, colonoscopy has been advocated to remove noninvasive, but potentially cancerous lesions known as polyps. This method of intervention has impacted the incidence of colon cancer, suggesting that early detection and prevention will decrease the incidence of colon cancer (15). However, for those patients with ulcerative colitis, surveillance colonoscopy seeking evidence of malignant transformation has proved challenging to interpret, while subsequent referral for surgical intervention may be controversial (16, 17). The accumulation of genetic alterations for colitis-associated cancers is believed to proceed via a pathway known as the inflammation-dysplasia-cancer progression (18). We, and others, have previously described methods to isolate CCSCs from patients with colorectal cancer (2–4). These methods utilized the surface markers ESAhigh, CD44+, CD166+ and CD133+ to enrich for cells that can form xenografts in mice, which recapitulate the phenotype of the original human tumor set as the functional definition of a CCSC.
The increased incidence of colon cancer in those patients with longstanding colitis may be due to cell-intrinsic factors including genetic and epigenetic phenomena. However, another explanation may be that cell-cell interactions from an inflammatory microenvironment promote oncogenesis. Current literature reveals that in some cases, the tumor microenvironment promotes this transformation (19–22). Furthermore, alterations in the stroma may precede those in the local epithelium (23). In the colon, the replacement of differentiated cells at the crypt surface is further augmented in both the inflammatory and the tumorigenic state (24–27). Both autocrine and paracrine microenvironmental signals contribute to maintenance of steady state, regeneration in the inflammatory state, and proliferation vs. apoptosis in malignancy.
In this study we used ALDH1 as a marker for colon tumor initiating cells, not only from colon cancer, but also from colitis. We show that rare epithelial cells from colitis can perpetuate xenograft growth in immunocompromised mice through an anaplastic stage and develop to cancer. In addition to multiple passages from very few cells, these colitis-initiated lesions may be grown in vitro as spheroid cultures, with a differentiation phenotype that strongly resembles that of spheroid cultures generated from colon cancer. Further, to interrogate the potential contribution of colitic and malignant stroma to transformation we examined the cytokine/chemokine profile from the normal colon, colitic, and colon cancer microenvironments. We reveal consistent upregulation of IL-6 and IL-8 in both the colitic and cancer microenvironments and demonstrate that inhibition of these signals results in significantly reduced tumor volume in xenograft experiments. In summary, we use stem cell based approaches to present the first documentation that benign colitic mucosa may be isolated and propagated as both xenografts and sphere cultures. Further, we document similarities between colitic and malignant colonic stroma.
MATERIALS AND METHODS
Immunohistochemistry
All tissues were retrieved under pathologic supervision with IRB approval at University of Michigan and the University of Florida. Normal colon tissues were retrieved from one of two sources: Either control tissue at least 10 cm away from a malignancy, or from patients presenting for resection of benign disorders such as slow-transit constipation. Routine immunohistochemistry/immunofluorescence for ALDH1 (1:100 BD Biosciences), CD44 (1:50 Clone G44-26, Bectin-Dickinson), IL8R (1:100 Abcam) and MUC2 (1:100 clone CCP58, Zymed) from paraffin embedded sections were completed. Either MOM (Vector labs for ALDH) or ARK (DAKOCytomation, for MUC2 or CD44) antigen retrieval methods were used in xenograft sections. Other antibodies include CD30 (1:10, Catalog #Z0751, DAKO), CDX2 (1:50 Abcam), CD45 (1:100, Catalog #M0701, DAKO), CAM 5.2 (1:1, Catalog #A0452, BD Biosciences), AE1/AE3 (1:100, Catalog #M4502, Millipore), CK19 (1:100 Abcam), CK7 (1:100, Catalog #M7018, DAKO), CD2 (1:100, Catalog #A0452, DAKO), CD3 (1:100, Catalog #M7254, DAKO), c-kit/CD117 (1:100, Catalog #A4502, DAKO) and S100 (1:250, Catalog Z0311, DAKO). The stromal isolates were stained with SMA (1:600 R&D Systems). For sphere studies, the spheres were concentrated into a 1% low melting point agarose plug prior to embedding in paraffin.
Patient subjects and xenograft assays
The University of Michigan and the University of Florida Institutional Animal Care and Use Committees approved the following protocols (UM 9510 and UF F090, respectively). For colon cancer tissue, cancer and matched normal tissues were retrieved from the operating room and maintained sterilely. For colitic tissues, both distal and proximal regions of the colon were implanted. Only those patients with ulcerative colitis were used. Patients with Crohn’s disease were not evaluated in this study. All tissue retrieval was completed under pathologic supervision. Tissues were minced and placed subcutaneously into the flanks of NOD-SCID mice. For cellular xenografts, the selected numbers of cells were pelleted post flow cytometry. The pellets were resuspended in Matrigel (BD Biosciences) such that the total volume for injection was not greater than 100 μl. The opposite flank received the control injection. For the antibody blocking experiments, commercially available pellets (Innovation Research of America, Sarasota, Florida) were embedded with either anti-IL8 or anti-IL6 (R&D Systems, 5 micrograms/day) or placebo (control) and implanted one day prior to placement of the xenografts. To account for variability, at least 8 mice/condition were initiated. Mice were monitored daily for tumor growth and measured at least weekly to establish tumor volume.
Flow cytometric sorting
The tissues were digested with collagenase. Antibodies were added at a concentration of 1:100 and maintained for 30 minutes on ice. The cell pellets were washed with HBSS/2%FBS, and in circumstances where a secondary antibody is required, the cells are resuspended in the appropriate secondary antibody. For identification of CD44 and ESA, the anti-CD44 allophycocyanin (APC, Pharmingen, Franklin Lakes, NJ) and anti-epithelial specific antigen (ESA)-FITC (Biomeda, Foster City, CA) were employed at a dilution of 1:40. Nonviable cells are eliminated by using the viability dye, DAPI, just prior to submission for flow cytometry. Murine cells were eliminated by staining with H2Kd (Southern biotech). Flow cytometry was performed on the FACS Aria (BD Immunocytometry Systems, Franklin Lakes, NJ). Side scatter and forward scatter profiles was utilized to eliminate cell doublets. Cells were routinely subjected to double sorting, and reanalyzed for purity, which is typically greater than 97%. The Aldefluor kit (Stem Cell Technologies, Vancouver, Canada) was used to identify the ALDH positive population. Controls included the viability stain, DAPI, the Aldefluor stained cells treated with inhibitor of the enzyme (DEAB), and staining with the secondary antibody alone.
Sphere culture
Flow cytometry was utilized to sort cells from xenografts either from colorectal oncogenic or colitic sources. Isolated cells were placed into a low attachment six well plate at a density of 2000–10,000 cells/well. These cells were suspended in a serum-free medium containing DMEM/F12 (Gibco), 10 nM progesterone (Sigma), glutamine, Gibco), 10 μg/ml insulin (Sigma), 13 μg/ml transferrin (Sigma), 15 nM sodium selenite (Sigma), 4 mg/ml BSA (Sigma), 50 μM putrescine (Sigma), and 15mM HEPES (Gibco), supplemented with 10 ng/ml FGF and 20 ng/mlEGF (Sigma).
Isolation and maintenance of primary stromal isolates
Tissues from colitic, colon cancer, and matched tissues were minced and digested with Collagenase type 1A (Sigma) for 30 minutes at 37°C. The resulting suspension was plated in serum containing media (DMEM 10% FBS, Gibco). For these experiments, successful isolates were employed in passages 4–9. Prior to use, observation of phenotype and staining with α smooth muscle actin was used to confirm these cells as fibroblasts For these experiments, successful isolates were employed in passages 4–9. For comparison, normal colon fibroblasts (CRL 1541) and colon cancer fibroblasts (CRL7213) were purchased from the American type culture collection (ATCC, Manassas, Virginia)
Cytokine array analysis
Conditioned media was harvested from stromal isolates. Conditioned media was harvested after plating of successful isolates at a density of 105 cells in a well of a 24-well tissue culture treated plate. After the cells had adhered, the media was removed and replaced with fresh media in the absence of serum. After 24 hours 37° C, 5% CO2, the overlying media was harvested for use in the cytokine/chemokine array. The Chemicon cytokine array (Human Cytokine Array III, Millipore) was conducted per manufacturer’s instructions.
Statistical Analysis
The incidence of ALDH expressing epithelium was enumerated in normal vs. colitic epithelium. In total, at least 5000 epithelia per disease type were counted. The data were analyzed as a two sample t-test with p < 0.05 taken as significant. For comparison of tumor latencies, growth rates, and size, ANOVA using Duncan’s multiple comparison procedure at the 0.05 significance level was determined.
RESULTS
ALDH+ epithelial cells from select colitis patients progress to adenocarcinomas in NOD-scid mice xenografts
The xenograft model is widely used for testing tumorigenicity of putative cancerous cells. Therefore, we employed xenografting of ESAhigh/ALDH+ primary tumor and colitic cells into NOD/SCID mice to determine tumor initiation and differentiation potential. In figure 1, we show staining for ALDH1 in normal (A), colitic, and malignant colon tissues and found increased ALDH1 immunoreactivity at the base of the crypt in a subset of colitic samples (B, and supplemental figure 1), and widespread expression throughout colitis-associated cancer tumor tissue (C). Quantification of epithelial cells in grossly normal and colitic tissues supported our observations that ALDH is overexpressed in colitic epithelium (D). In our studies, as few as 25 DAPIlow ESAhighALDHhigh cancer cells reproducibly generated serial xenografts with typical adenocarcinoma phenotypes (Figure 2A). To determine whether colitic tissue might bear the same tumorigenic potential as frank colon cancer, random tissue was isolated from patients presenting for resection of their colorectum due to chronic ulcerative colitis, with the majority of these patients presenting for surgery due to medically refractory disease (19/22; Supplemental Table 1). Classically, ulcerative colitis may be more severe distally in the colon, and less severe or relatively normal in the proximal colon. Therefore, portions of the proximal colon were retained as a matching control tissue. Proximal biopsies often revealed quiescent or nearly normal histology (data not shown). For every specimen retrieved, the pathologic evaluation based on histology of the flanking sections to the distal implanted tissues showed only ulcerative colitis without evidence of either dysplasia or adenocarcinoma (Supplemental Table 1). The histopathology of the flanking sections revealed inflammation ranging from quiescent disease to severe chronic colitis (Supplemental Table 1). Colitis samples were implanted into the subcutaneous flank of NOD-SCID murine hosts. and were observed for up to 5 months. In contrast to frank colorectal cancer, in which we now routinely engraft >75% (2) of the cancer tissue successfully, xenografts evolved from only the three of twenty-two colitic specimens (13.6%). No tumors were found in the contralateral flank containing the proximal normal colonic tissue implants from the same patients (not shown). Similar to the frank colon cancer xenografts, all three of the primary xenografts resulting from the colitic tissues could form secondary xenografts (Figure 2B) and were serially passaged in mice. Secondary xenografts were formed from both bulk primary xenograft cells and flow cytometric sorted for DAPIlowESAhighALDHhigh cells. ESA was included to ensure isolation of predominantly epithelial cells rather than any potential ALDHhigh hematopoietic cells in the colon/xenograft. As few as 50 DAPIlowESAhighALDHhigh cells were capable of tumorigenesis via serial passage of the xenograft. Further we were able to passage these tumors sequentially at least ten passages with continued enrichment for ALDHhigh cells.
Figure 1.
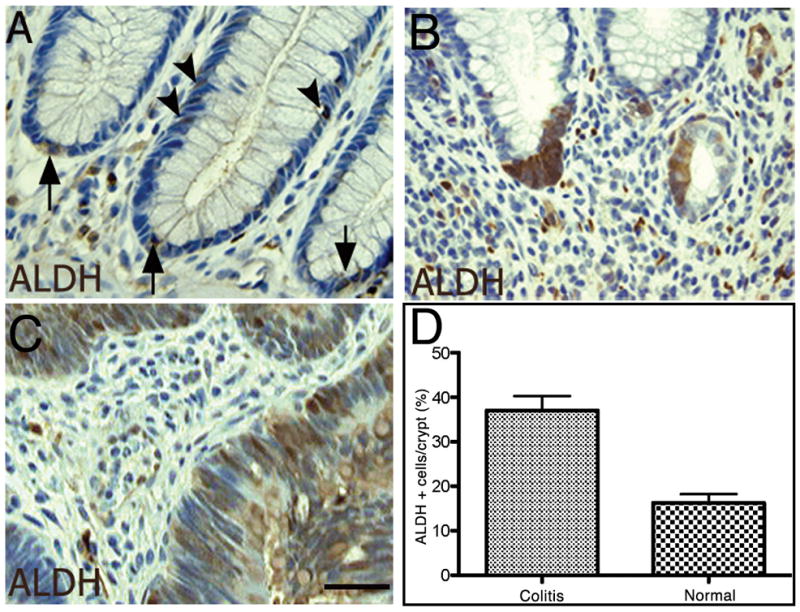
Immunohistochemistry of ALDH expression in normal colon, colitis, and colon cancer. Quantification of ALDH+ cells in epithelial tissue. Normal colonic epithelium reveals expression of ALDH at the cells at and near the base of the crypt (A, arrows) with several cells found further up the crypt (arrowheads). The colitic milieu demonstrated expansion of ALDH staining at the base of the crypt (B) compared to baseline normal mucosa. In colitis-associated cancer, the expression of ALDH is greatly expanded (C). ALDH-expressing cells in the epithelial portions of the primary tissue samples reveal an increase in immunoreactive ALDH-expressing cells in the colitic colon (see also Supplementary figure).
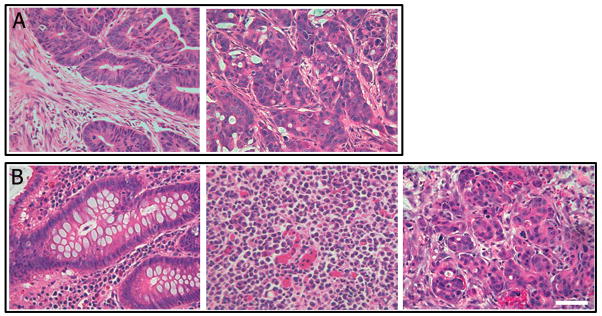
Figure 2. Generation of xenografts from colon cancer and colitis.
A portion of the colon was removed at operation and tissue was implanted into the flank of NOD-SCID mice. As few as 25 cells of either ESAhighCD44high or ESAhighALDHhigh were capable of reconstituting a tumor and histology strongly resembles the parent tumor (A; N=10). Colitic colon tissue (B, left panel) was implanted into the flank of NOD-SCID mice and observed for xenograft incorporation and growth. As few as 50 ESAhighALDHhigh cells were capable of reconstituting a tumor and H&E staining of tumor revealed anaplastic cells (B, middle panel; N=3). With serial passaging, xenografts from colitic tissues revealed an adenocarcinoma, with the epithelium forming glandlike structures (B, right panel; N=3). Scale Bar = 200 μm.
The colitic cells within the early passage anaplastic xenografts did not stain for a series of tissue specific markers including CDX2, c-kit, CD3, CD2, β-catenin, CD30, vimentin, CK20, cam52, and CD45 (data not shown). While CDX2 was employed to evaluate differentiated intestinal tissues, both CD3 and CD2 (pan T-cell and T-cell subset) markers were absent. Similarly, the CD45, or common leukocyte antigen, was absent. CD30, a marker of anaplastic lymphomas, was not seen. Staining for neither CK20, a marker of differentiated colorectal carcinomas, nor CAM 5.2, for low molecular weight cytokeratins, were visualized. Further evaluation included S100, evident in some melanomas and therefore included in the differential for anaplastic lesions, was absent. To evaluate the possibility that these tumors were variants of gastrointestinal stromal tumors, they were stained with the CD117 antibody (c-kit antibody), and they were negative for this marker. Instead, they displayed a classic anaplastic morphology of expanded, homogeneous cells (Figure 2B, middle). Continued xenograft passage of ESAhighALDHhigh selected cells resulted in progression of tumor morphology to a phenotype which was diagnostic of adenocarcinoma, with hallmark production of mucus and glandular formation (Figure 2B, right). In those colitic tissues with successful engraftment, the latency of tumor growth from bulk cell populations was similar to cancer counterparts with onset of tumor growth averaging six weeks. Enrichment for ESAhighALDHhigh selected cells resulted in palpable tumors in an average of two weeks. Once palpable, xenografts grew at similar rates regardless of source. Matched grossly normal tissues taken from the same patients did not engraft (N=22). This finding led us to categorize the original xenograft initiating cells isolated from colitis patients as precursor-Colon Cancer Stem Cells or pCCSC in order to distinguish them from the CCSC isolated from frank colon cancer.
Epithelial marker expression of primary tumors, xenografts, and spheres
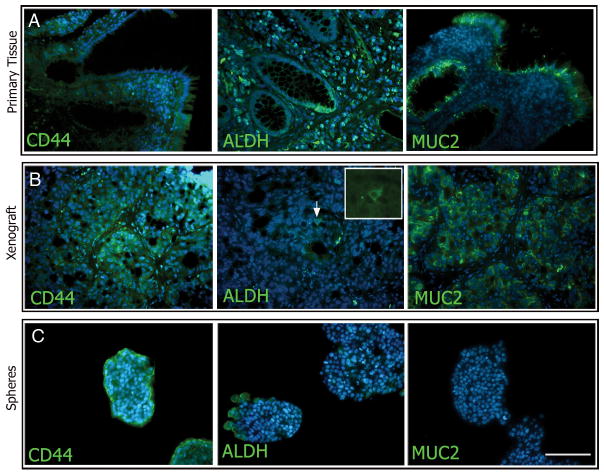
Findings by others have shown that neural stem cells can be characterized by their ability to form sphere shaped clusters, called neurospheres, in serum free medium (28, 29). Sphere formation has subsequently been used to isolate both normal and cancer stem/progenitor cells from a wide variety of tissues including the colon (4, 30, 31). We have established conditions that support the formation of colon spheres from both frank colon cancer and colitis, but not from normal colon. We used immunohistochemistry to delineate the phenotypes of the original tissues compared with both xenografted tissues and with the colon spheres (Figures 3 and 4). MUC2 is present in goblet cells during mucin formation, and is indicative of epithelial differentiation (32). We observed MUC2 staining in original tissues and in the xenografts from both colon cancer and serially passaged colitis sources with an adenocarcinoma histological profile (Figures 3 and 4A–B right panels). Expression of MUC2 was only visualized at the outer edges of the spheroid colonies generated from the frankly malignant source and not in the colitis spheres (Figure 3 and 4C, right panels), reflecting a more anaplastic nature. ALDH1 and CD44 immunoreactivities were also compared revealing dispersed cytological localization in both the primary tumors and resulting xenografts. CD44 expression was seen in primary tissues, xenografted tissues, and spheres from both origins (Figures 3 and 4A–C, left panels). However, though ALDH expression was seen in sections of all primary patient samples, the expression was much more restricted in successful xenografts and in the spheres from colitic origins (Figure 4B and C middle panels).
Figure 3. Marker analysis of primary tissue, xenografts, and spheres from malignant colon tissue.
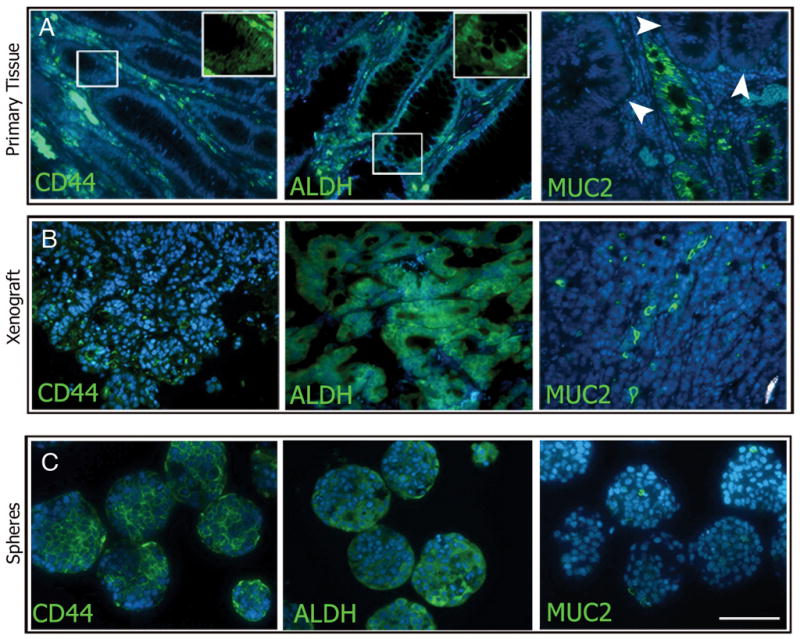
Expression of CD44 and ALDH was abundant in all tissue types examined (A–C left and middle panels, respectively). MUC2 expression in cancerous colon tissue was found in some crypts (A, right panel), while others showed no staining (arrowheads). MUC2 expression was relatively low and sporadic in the xenografts and spheres (B and C right panels, respectively). Scale bar = 200 μm.
Figure 4.
Marker analysis of primary tissue, xenografts, and spheres from colitic colons. Expression of CD44 was abundant in all tissue types examined (A–C, left panels). ALDH expression was expanded from the base of the crypts of colitic colons (A, middle panel), were found to be rare in xenografted tissue (B, middle panel), and was found in clusters in colitis-spheres (C, middle panel). MUC2 expression was abundant in the colitic and xenograft tissue (A and B, right panels) but was not observed in colitis-spheres (C, right panel). Scale bar = 200 μm.
The Stromal Microenvironment Changes in Colitis and Cancer
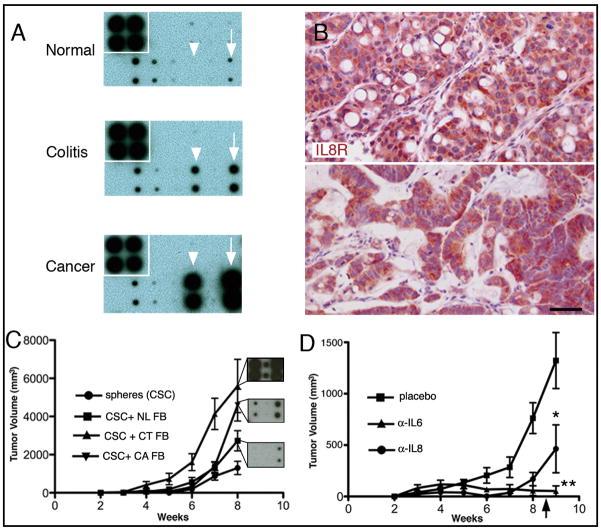
Recent findings suggest that alterations in normal stroma may support tumorigenesis, implying that the inflammatory environment found in some colon cancers and colitic tissues includes paracrine interactions that contribute to oncogenesis (18, 33). Indeed, cytokines including IL8 have been associated with inflammatory and oncologic processes in the colon (34). Therefore, we isolated colonic stromal cells (Supplemental Figure 2) from the colitic milieu and compared the cytokine profile of conditioned medium from the colitic fibroblasts to normal colon and malignant colon fibroblasts using cytokine array analysis. The expression of IL6 and IL8 was low to undetectable from the normal stromal cell medium (Figure 5A top), but was substantially increased from the medium taken from both the colitic (Figure 5A middle) and frankly malignant (Figure 5A bottom) stromal environments. In most cases the colitic stroma produced an intermediary amount of IL6 and IL8 compared to the baseline expression of normal colon stroma and high-level expression from colon cancer stroma. IL8 has at least one high affinity receptor, CXCR1, which we show is prominently expressed in malignant colon and colitic xenografts of ESAhighALDHhigh sorted cells (Figure 5B). We next evaluated whether colitis or cancer derived stroma enhance the ability of colon cancer spheres to form xenografts. We co-injected sphere-derived single cell suspensions alone or in the presence of normal colon/colon cancer/colitis associated fibroblasts and monitored the mice for tumor volumes and latencies. Those mice with both sphere-derived cells and cancer-associated fibroblasts developed tumors with a markedly decreased latency - mean time to palpable tumor <2 weeks with cancer/colitis fibroblasts versus ~6 weeks for spheres alone or with normal fibroblasts (Figure 5C) providing strong evidence that fibroblast-derived IL6 and/or IL8 plays a definitive role in tumorigenesis. Moreover, we show that colon cancer stem cells, when transplanted alone, result in longer tumor latency and smallest tumor volume in xenograft experiments (ANOVA, Duncan’s multiple comparison p < 0.05). Mixed model analysis for tumor growth rate in the colon cancer sphere with colitic fibroblasts revealed a growth rate which was significantly higher than that in the colon cancer spheres co-injected with either the colon cancer fibroblasts, normal colon fibroblasts, or spheres alone (p = 0.0006, < 0.0001, and < 0.0001, respectively). The tumor growth rate for the colon cancer spheres with colon cancer fibroblasts group was significantly higher that that in colon cancer spheres alone group (p = 0.0006). Injections consisting of fibroblasts alone failed to generate a xenograft, which was confirmed at necropsy (not shown). As shown in the insets (Figure 5C), for this experiment, the colitic fibroblasts secreted more IL6 and IL8 than the colon cancer fibroblast cell line.
Figure 5.
Cytokine array analysis of normal, colitic, and cancer stroma. Immunohistochemical expression of CXCR1 (IL8R). Tumorigenicity from cancer derived spheres in the presence/absence of stroma. Cytokine array analysis of conditioned media from normal (A, top), colitic (A, middle), and malignant (A, bottom) human colon. Cytokines IL6 (arrowhead), and IL8 (arrow) are relatively absent in normal conditions (N=4), with increases in colitic (N=3) and malignant conditions (N=3). IL8 receptor expression (CXCR1) in adenocarcinoma generated from colitic tissues reveals widespread expression (B, upper panel, N=2). IL8 receptor expression (CXCR1) in adenocarcinoma originating from human sporadic adenocarcinoma (B, lower panel, N=4). CXCR1 is also widely expressed in xenografts (passage 7) from sporadic colon cancer and resulting tumorigenic growth (B, lower panel). Co-injection of normal, colitic, or cancer-associated fibroblasts reveal a role for stromal elements in tumorigenesis and corresponding cytokine arrays demonstrate a likelihood that IL and/or IL8 play a role (C) p< 0.0001. Insets reveal relative cytokine/chemokine expression for IL6 (left) and IL8 (right). Pellets containing antibodies to IL8 or IL6 resulted in significantly reduced tumor sizes (D) and resulted in persistent inhibition of tumorigenesis until the source of antibodies was exhausted at ~ 8 weeks post-implant (arrow). *p < 0.03; **, p < 0.01; N > or = 4 per group. Scale bar = 100 μm.
To further determine a role for these cytokines in tumorigenesis, we implanted slow-release pellets containing either anti-IL8 or anti-IL6 antibodies adjacent to the xenografted cells. Both conditions resulted in significantly reduced tumor volume compared to the control group (Figure 5D). Moreover, palpable tumors increased in size after 8 weeks, the period at which the antibody supply in the pellets was exhausted. Together, these data indicate a direct role for these cytokines in tumor growth in vivo.
DISCUSSION
Colorectal cancer is the third most common cause of cancer and cancer associated mortality. The cancer stem cell paradigm suggests that a rare cell is responsible for the genesis, maintenance, recurrence and metastasis of cancer (35). For colorectal cancer, support for this paradigm has been reported using surface markers including CD44, CD133, and ALDH (2–4). Our initial examination employing both CD44 and CD133 revealed widespread expression in the normal crypt, often encompassing the entire lower third to half of the crypt(10, 36). With malignancy, the expression was usually expanded to all of the epithelium, particularly at the leading edge of tumors. We therefore sought alternative markers to assist with enrichment of tumorigenic cells.
We focused on ALDH for this study as its normal expression pattern was the most highly restricted being localized to only a few cells at the bottom of a crypt - the putative location of the normal colon stem cell (37, 38). Furthermore, enrichment with ALDH has rationale based on findings in blood, breast and brain cancer, in which ALDH bears a metabolic basis with its function as a detoxification enzyme (8, 9). Therefore, we reasoned that ALDH may provide the greatest degree of enrichment for colon stem/progenitor cell activity. Our studies reveal that a subset of colitic patients exhibit an expansion of ALDHhigh cells at the base of their crypts. Samples retrieved from resected colitic tissues can be assayed for pCCSC activity as defined by the ability to generate either anaplastic xenografts or in vitro colon spheres. ALDHhigh cells represent a highly enriched cell population for xenograft and sphere forming ability. Proliferative activity, whether due to regeneration or malignancy, may promote the function of this enzyme, thus explaining its increased expression in both colitis and colorectal cancer.
Though the pathogenic pathway for sporadic cancer is known as the adenoma to carcinoma pathway, (14) the parallel pathway for colitis is known as the inflammation to dysplasia to cancer pathway (18). The initial risk of neoplastic transformation is 0.5–1% per year in the first 7 years of disease, increasing thereafter at a rate of 1%/year to 18% by thirty years. This level of relative risk of colon cancer is 2.6–5.4-fold over the general population (13, 16). Our findings reveal an incidence of 14% of engraftment from colitic tissues, with further selection criteria for tumorigenic cells based on CCSC markers not significantly different from the rate of 18% incidence of transformation in patients with chronic colitis.
Current methods to survey the colitic colon for dysplasia are inexact for a number of reasons including lack of colonoscopic appearance for biopsy, and limited access to facilities to perform dye-enhancing techniques to increase the sensitivity of surveillance (39–41). These challenges often lead to clinicopathologic dispute over when surgical intervention is required (17, 42). Furthermore, surveillance colonoscopy does not diminish the risk of colorectal cancer, but instead may reveal malignancy prior to the development of symptoms or at an earlier stage.
We observed that xenografts from colitic patients initially have an anaplastic phenotype which can progress with serial in vivo passage to resemble classic adenocarcinoma of the colon. With consideration of the pathogenesis of colitis to cancer, this intermediate phenotype as seen in the early xenografts has implications for mechanistic dissection of malignant transformation. Therefore, our findings indicate that the in vivo transformation of colitis cells to adenocarcinoma cells may be a useful tool for providing earlier detection of colitis-associated cancer, while also providing insight into the molecular mechanisms involved in this often fatal transition.
Several other organ systems, including breast and brain, have reported both in vivo and in vitro approaches to examination of normal and cancer stem cells. We demonstrate successful perpetuation of pCCSC and CCSC through the development of colon spheres. The colon sphere in vitro culture system allows expansion of the number of pCCSC and CCSC from primary patient tissue. Previous studies which have demonstrated the utilization of colon cancer derived spheres have not shown this capacity in preneoplastic colitic lesions. As with these other studies, our colon cancer-derived spheres recapitulate human neoplasia upon xenoengraftment. Furthermore, our colitis-derived spheres also retain the ability to recapitulate human neoplasia upon xenoengraftment. The colon sphere assay will provide both a potential diagnostic tool for colitic propensity to cancer progression, and will also provide the ability to expand limited primary human samples in vitro for more detailed analysis.
The microenvironment in the colitic and oncogenic milieus with increased paracrine mediators such as IL6 and IL8 may play a dominant role in facilitating tumorigenesis with expansion of the pCCSCs and CCSCs. Moreover, the microenvironmental stroma has been identified as a key element in promoting oncogenesis in several models (19–22). Our findings suggest that several potential mediators, as assayed by examination of conditioned media from both cancer-derived and colitis-derived fibroblasts, are upregulated. In our studies, the levels of these cytokines revealed a hierarchy of expression and antibody-mediated blockage of these signals resulted in significantly reduced tumor volume. Additionally, the rapid increase in tumor volume following the 8 week time-point suggests that inhibition of these signals directly resulted in the absence of significant tumor growth. These mediators and the interactions between the stroma and epithelium with tumorigenic potential may serve as targets for both diagnostic and therapeutic intervention.
In this study we have established parallel and complementary assays that enrich for and expand precursor-cancer stem cells from a subset of colitis patients. We show that a minority of patients with colitis has expanded numbers of ALDH expressing crypt epithelial cells in their colitic tissues. Frank colon cancer had the most widespread expression of ALDH in the epithelial cells of the tumor. Selecting ALDH positive cells greatly enriched both sphere and xenograft formation from tissues with either colitis or colon cancer. Primary colitic xenografts formed anaplastic growths that progressed with subsequent xenograft passage into tumors indistinguishable from primary colon cancer xenografts. The strategies proposed within this report have implications for stem cells in clinical translation during cancer surveillance. Expansion of epithelial ALDH expression from biopsy specimens and/or the ability to form xenografts/colonospheres may serve as additional, persuasive evidence to mandate surgical intervention. However, clinical trial methods are necessary to rigorously define the correlation between these assays and outcomes on a broader scale.
Supplementary Material
Acknowledgments
We thank Marda Jorgensen and the immunohistochemistry core as part of CTAC, University of Florida, and to the flow cytometry cores at both the University of Florida and the University of Michigan. We are grateful for support from the Dr. Max Wicha and University of Michigan Cancer Center for the early studies and to Dr. Christophe Ginestier, Dr. Gabriella Dontu, and Dr. Bruce Boman for discussions and insights into the work. We appreciate Dr. Myron Chang, Division of Biostatistics, Department of Epidemiology and Health Policy Research for statistical review. We would also like to thank Dr. Sara Patterson for critical review of the manuscript and Dr. Brent Reynolds. JC is supported by NIH T32 DK 074367. EH was the recipient of the Sorkin Research Development award, and is currently supported by Department of Surgery Development Fund and the Evelyn F. and William L. McKnight Brain Institute of the University of Florida. EWS is supported by NIH R01s HL 070738 and HL 75258.
References
- 1.Kim CF, Dirks PB. Cancer and stem cell biology: how tightly intertwined? Cell Stem Cell. 2008;3:147–50. doi: 10.1016/j.stem.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 4.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–15. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–88. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–28. [PubMed] [Google Scholar]
- 7.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong L, Stojkovic M, Dimmick I, et al. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–51. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 9.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang EH, Hynes M, Zhang T, et al. Aldehyde Dehydrogenase 1 Is a Marker for Normal and Malignant Human Colonic Stem Cells (SC) and Tracks SC Overpopulation during Colon Tumorigenesis. Cancer Res. 2009:69. doi: 10.1158/0008-5472.CAN-08-4418. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dylla SJ, Beviglia L, Park IK, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71–4. doi: 10.1016/s0140-6736(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 14.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman D. Screening, surveillance, and prevention of colorectal cancer. Gastrointest Endosc Clin N Am. 2008;18:595–605. doi: 10.1016/j.giec.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–8. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 19.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–60. [PubMed] [Google Scholar]
- 20.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–85. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 21.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 22.Kuperwasser C, Chavarria T, Wu M, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moinfar F, Man YG, Arnould L, et al. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–6. [PubMed] [Google Scholar]
- 24.Boman BM, Fields JZ, Cavanaugh KL, Guetter A, Runquist OA. How dysregulated colonic crypt dynamics cause stem cell overpopulation and initiate colon cancer. Cancer Res. 2008;68:3304–13. doi: 10.1158/0008-5472.CAN-07-2061. [DOI] [PubMed] [Google Scholar]
- 25.Boman BM, Walters R, Fields JZ, et al. Colonic crypt changes during adenoma development in familial adenomatous polyposis: immunohistochemical evidence for expansion of the crypt base cell population. Am J Pathol. 2004;165:1489–98. doi: 10.1016/S0002-9440(10)63407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen R, Bronner MP, Crispin DA, Rabinovitch PS, Brentnall TA. Characterization of genomic instability in ulcerative colitis neoplasia leads to discovery of putative tumor suppressor regions. Cancer Genet Cytogenet. 2005;162:99–106. doi: 10.1016/j.cancergencyto.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Chen R, Rabinovitch PS, Crispin DA, et al. The initiation of colon cancer in a chronic inflammatory setting. Carcinogenesis. 2005;26:1513–9. doi: 10.1093/carcin/bgi106. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2:333–6. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 30.Todaro M, Alea MP, Di Stefano AB, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Todaro M, Perez Alea M, Scopelliti A, Medema JP, Stassi G. IL-4-mediated drug resistance in colon cancer stem cells. Cell Cycle. 2008;7:309–13. doi: 10.4161/cc.7.3.5389. [DOI] [PubMed] [Google Scholar]
- 32.Blache P, van de Wetering M, Duluc I, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 34.Rubie C, Frick VO, Pfeil S, et al. Correlation of IL-8 with induction, progression and metastatic potential of colorectal cancer. World J Gastroenterol. 2007;13:4996–5002. doi: 10.3748/wjg.v13.i37.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–90. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 36.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–31. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 37.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–20. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 38.Preston SL, Wong WM, Chan AO, et al. Bottom-up histogenesis of colorectal adenomas: origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer Res. 2003;63:3819–25. [PubMed] [Google Scholar]
- 39.Dekker E, van den Broek FJ, Reitsma JB, et al. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216–21. doi: 10.1055/s-2007-966214. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto T, Iwao Y, Igarashi M, et al. Endoscopic and chromoendoscopic atlas featuring dysplastic lesions in surveillance colonoscopy for patients with longstanding ulcerative colitis. Inflamm Bowel Dis. 2008;14:259–64. doi: 10.1002/ibd.20267. [DOI] [PubMed] [Google Scholar]
- 41.Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–68. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 42.Lim CH, Dixon MF, Vail A, et al. Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut. 2003;52:1127–32. doi: 10.1136/gut.52.8.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.