Abstract
Purpose
Host immune response to tumor may be an important prognostic factor for colon cancer patients. However, little is known on prognostic significance of histopathologic lymphoid reaction to tumor, independent of the number of lymph nodes examined and tumoral molecular alterations, including microsatellite instability (MSI) and the CpG island methylator phenotype (CIMP), both of which are associated with lymphocytic reaction and clinical outcome.
Experimental Design
Utilizing 843 colorectal cancer patients in two independent prospective cohorts, we examined patient prognosis in relation to 4 components of lymphocytic reaction (i.e., Crohn's-like reaction, peritumoral reaction, intratumoral periglandular reaction and tumor infiltrating lymphocytes, TIL) and an overall lymphocytic score (0-12). CIMP was determined using 8 markers including CACNA1G, CDKN2A (p16), CRABP1, IGF2, MLH1, NEUROG1, RUNX3 and SOCS1. Cox proportional hazard models computed hazard ratio (HR) for mortality, adjusted for covariates, including tumor stage, body mass index (BMI), the lymph node count, KRAS, BRAF, p53, cyclooxygenase-2 (COX-2, PTGS2), MSI, CIMP, LINE-1 methylation.
Results
Increasing overall lymphocytic reaction score including TIL was associated with a significant improvement in colorectal cancer-specific and overall survival (log-rank p<0.003). These findings remained significant (adjusted HR estimates, 0.49-0.71; Ptrend<0.009) in multivariate models that adjusted for covariates, including BMI, MSI, CIMP, LINE-1 hypomethylation, and COX-2. The beneficial effect of tumoral lymphocytic reaction was consistent across strata of clinical, pathologic and molecular characteristics.
Conclusions
Lymphocytic reactions to tumor were associated with improved prognosis among colorectal cancer patients, independent of the lymph node count and other clinical, pathologic and molecular characteristics.
Keywords: colon cancer, immune response, prognosis, molecular alterations, outcome
Introduction
Greater lymphocytic reaction to colorectal cancer observed by pathologic examination has been associated with longer patient survival (1-6). However, the true nature of this association and the exact mechanisms underlying it remains uncertain. Lymphocytic reaction may be an indicator of host immune response to tumor cells, leading to improved survival. Specific subsets of infiltrating lymphocytes (e.g., CD57+, CD8+, CD45RO+ or FOXP3+ cells) have been associated with improved clinical outcome in colorectal cancer (7-12). In addition, immune reaction to tumor may cause enlargement of lymph nodes, which may contribute to an increase in the number of recovered lymph nodes and, thereby, more accurate staging of colorectal cancer. In fact, lymphocytic reaction to colorectal cancer has been associated with an increase in the recovered node count (13), which has in turn consistently been associated with improved patient survival in colorectal cancer (13-18). Alternatively, lymphocytic reaction to tumor may reflect specific tumoral molecular alterations associated with indolent tumor behavior. Indeed, studies have shown that lymphocytic reaction to colorectal cancer is associated with microsatellite instability (MSI-high) (19-21) and the CpG island methylator phenotype (CIMP) (22). Studies have suggested that truncated peptides, which are produced by MSI and frameshift mutations, may be immunogenic and contribute to host immune response (23, 24). Because lymphocytic reaction, the lymph node count, MSI and CIMP have all been associated with prognosis (13-18, 25, 26), all of these variables can confound each other in survival analysis. To assess a prognostic role of lymphocytic reaction independent of the lymph node count and tumoral molecular features, it is necessary to examine the lymph node count and tumoral molecular features.
We therefore examined the prognostic significance of lymphocytic reaction to tumor in 843 stage I-IV colorectal cancer patients identified in two independent prospective cohort studies. Since we concurrently assessed the number of recovered lymph nodes as well as related molecular variables such as MSI, CIMP, BRAF mutation and LINE-1 hypomethylation, we could evaluate the effect of lymphocytic reaction to tumor, independent of these potential confounders.
Materials and Methods
Study Population
We utilized the databases of two independent prospective cohort studies; the Nurses' Health Study (N=121,701 women followed since 1976) and the Health Professionals Follow-up Study (N=51,529 men followed since 1986) (27). Every 2 years, participants were sent follow-up questionnaires to identify newly diagnosed cancer in themselves and their first-degree relatives. When a participant reported colorectal cancer, study physicians reviewed medical records, and recorded TNM stage, tumor location, and the number of positive and negative lymph nodes. We collected paraffin-embedded tissue blocks from hospitals where patients underwent tumor resections (27). We excluded cases preoperatively treated with radiation and/or chemotherapy. Based on availability of tissue specimens for pathologic analyses, we included a total of 843 stage I-IV colorectal cancer cases diagnosed up to 2003 (Table 1). Patients were observed until death or June 30, 2006, whichever came first. Ascertainment of deaths included reporting by the family or postal authorities. In addition, the names of persistent nonresponders were searched in the National Death Index. We identified >98% of deaths in the cohorts by these methods. The cause of death was assigned by physicians unaware of tumoral pathologic or molecular data. Written informed consent was obtained from all study subjects. This study was approved by the Human Subjects Committees at Brigham and Women's Hospital and the Harvard School of Public Health.
Table 1.
Clinical, pathologic and molecular features according to lymphocytic reactions to colorectal cancer
| Clinical or molecular feature | All cases | Overall lymphocytic reaction score | P value | ||
|---|---|---|---|---|---|
| 0-2 | 3-6 | 7-12 | |||
| Total N | 843 | 549 | 230 | 64 | |
| Sex | 0.32 | ||||
| Male (HPFS) | 361 (43%) | 240 (44%) | 90 (39%) | 31 (48%) | |
| Female (NHS) | 482 (57%) | 309 (56%) | 140 (61%) | 33 (52%) | |
| Mean age (years) ± SD | 66.3 ± 8.3 | 65.8 ± 8.4 | 66.8 ± 8.1 | 69.1 ± 7.8 | 0.002 |
| Body mass index (BMI) | 0.56 | ||||
| <30 kg/m2 | 663 (84%) | 425 (83%) | 185 (85%) | 53 (87%) | |
| ≥30 kg/m2 | 131 (16%) | 90 (17%) | 33 (15%) | 8 (13%) | |
| Family history of colorectal cancer in 1st degree relative(s) | 0.75 | ||||
| (-) | 637 (76%) | 411 (75%) | 178 (77%) | 48 (75%) | |
| (+) | 206 (24%) | 138 (25%) | 52 (23%) | 16 (25%) | |
| Year of diagnosis | 0.20 | ||||
| Prior to 1990 | 148 (18%) | 105 (19%) | 37 (16%) | 6 (9.4%) | |
| 1990 to 1999 | 598 (71%) | 381 (69%) | 164 (71%) | 53 (83%) | |
| 2000 to 2002 | 97 (12%) | 63 (11%) | 29 (13%) | 5 (7.8%) | |
| Tumor location | <0.0001 | ||||
| Proximal (cecum to transverse) | 381 (45%) | 206 (38%) | 131 (57%) | 44 (69%) | |
| Distal colon (splenic flexure to sigmoid) | 267 (32%) | 185 (34%) | 65 (28%) | 17 (27%) | |
| Rectum | 195 (23%) | 158 (29%) | 34 (15%) | 3 (4.7%) | |
| AJCC tumor stage | 0.002 | ||||
| I | 187 (22%) | 129 (24%) | 44 (19%) | 14 (22%) | |
| II | 242 (29%) | 139 (25%) | 76 (33%) | 27 (42%) | |
| III | 242 (29%) | 150 (27%) | 74 (32%) | 18 (28%) | |
| IV | 118 (14%) | 95 (17%) | 21 (9.1%) | 2 (3.1%) | |
| Unknown | 54 (6.4%) | 36 (6.6%) | 15 (6.5%) | 3 (4.7%) | |
| No. of lymph nodes examined | 0.0007 | ||||
| 0-3 | 80 (12%) | 62 (14%) | 14 (7.3%) | 4 (7.1%) | |
| 4-6 | 105 (15%) | 76 (17%) | 24 (13%) | 5 (8.9%) | |
| 7-12 | 245 (36%) | 162 (37%) | 67 (35%) | 16 (29%) | |
| ≥13 | 256 (37%) | 138 (32%) | 87 (45%) | 31 (55%) | |
| No. of negative lymph nodes | <0.0001 | ||||
| 0-3 | 131 (19%) | 99 (23%) | 27 (14%) | 5 (8.9%) | |
| 4-6 | 125 (18%) | 92 (21%) | 25 (13%) | 8 (14%) | |
| 7-12 | 223 (33%) | 138 (32%) | 70 (36%) | 15 (27%) | |
| ≥13 | 207 (30%) | 109 (25%) | 70 (36%) | 28 (50%) | |
| Tumor grade | <0.0001 | ||||
| Low | 755 (90%) | 515 (95%) | 200 (87%) | 40 (63%) | |
| High | 82 (9.8%) | 28 (5.2%) | 30 (13%) | 24 (37%) | |
| MSI | <0.0001 | ||||
| MSI-low/MSS | 702 (85%) | 503 (94%) | 169 (74%) | 30 (47%) | |
| MSI-high | 124 (15%) | 31 (5.8%) | 59 (26%) | 34 (53%) | |
| CIMP | <0.0001 | ||||
| CIMP-low/0 | 685 (84%) | 488 (93%) | 165 (73%) | 32 (50%) | |
| CIMP-high | 127 (16%) | 34 (6.5%) | 61 (27%) | 32 (50%) | |
| Mean LINE-1 methylation (%) ± SD | 61.2 ± 9.4 | 60.4 ± 9.5 | 62.3 ± 8.6 | 63.8 ± 10.7 | 0.001 |
| BRAF mutation | <0.0001 | ||||
| (-) | 699 (86%) | 479 (91%) | 178 (81%) | 42 (68%) | |
| (+) | 112 (14%) | 49 (9.3%) | 43 (19%) | 30 (32%) | |
| KRAS mutation | 0.11 | ||||
| (-) | 528 (63%) | 346 (64%) | 135 (59%) | 47 (73%) | |
| (+) | 304 (37%) | 195 (36%) | 92 (41%) | 17 (27%) | |
| p53 expression | <0.0001 | ||||
| (-) | 471 (57%) | 271 (50%) | 150 (66%) | 50 (78%) | |
| (+) | 358 (43%) | 267 (50%) | 77 (34%) | 14 (22%) | |
| COX-2 expression | 0.015 | ||||
| (-) | 136 (16%) | 74 (14%) | 50 (22%) | 12 (19%) | |
| (+) | 701 (84%) | 470 (86%) | 179 (78%) | 52 (81%) | |
(%) indicates the proportion of tumors with a specific clinical, pathologic or molecular feature in all patients or patients with a specific category of lymphocytic reactions.
Overall lymphocytic reaction score is the sum of scores for Crohn's-like reaction (0-3), peritumoral reaction (0-3), intratumoral periglandular reaction (0-3), and tumor infiltrating lymphocytes (TIL, 0-3).
CIMP, CpG island methylator phenotype; HPFS, Health Professionals Follow-up Study; LINE-1, long interspersed nucleotide element-1; MSI, microsatellite instability; MSS, microsatellite stable; NHS, Nurses' Health Study; SD, standard deviation.
Histopathologic Evaluations
Tissue sections from all colorectal cancer cases were examined by a pathologist (S.O.) unaware of other data. Of the 834 tumors, ≥3 tumor tissue blocks were available in 284 cases, 2 in 297 cases, and 1 in the remaining 262 cases. Tumor grade was categorized as high or low (≤50% vs. >50% glandular area). Four components of lymphocytic reactions [Crohn's-like lymphoid reaction, peritumoral lymphocytic reaction, intratumoral periglandular reaction, and tumor infiltrating lymphocytes (TIL)] were examined (Figure 1). Crohn's-like reaction was defined as transmural lymphoid reaction. Peritumoral lymphocytic reaction was defined as discrete lymphoid reactions surrounding tumor. Intratumoral periglandular reaction was defined as lymphocytic reaction in tumor stroma within tumor mass. TIL was defined as lymphocytes on top of cancer cells. For any given tumor, each of the 4 lymphocytic reaction components was scored as 0 (absent), 1+ (mild), 2+ (moderate) or 3+ (marked). The overall lymphocytic reaction score (0 to 12) was the sum of scores for the above 4 reaction components. Due to a skewed distribution of the lymphocytic scores (Supplemental Table 1), the majority of cases were in the group of scores 0-2. Most of them had score of 2. Thus, the reference group (i.e., low score) was set as those with scores 0-2, which provided the robust reference group. We divided non-low score cases into two groups because of a wide range of scores from 3 to 12. We set the cutoff between middle and high groups as ≤6 vs. ≥7, because it was in the middle of the total score of 12. A random selection of 398 cases were re-examined by a second pathologist (J.N.G.) unaware of other data, and the correlation on the lymphocytic scores between the two pathologists was good (Spearman correlation r = 0.65, p<0.0001; κ = 0.55 for score <3 vs. ≥3, p<0.0001). Kappa coefficients between the lymphocytic scores by the two pathologists for additional cutoffs were as follows: 0.49 for <2 vs. ≥2; 0.53 for <4 vs. ≥4; 0.46 for <5 vs. ≥5; 0.50 for <6 vs. ≥6; 0.49 for <7 vs. ≥7; 0.55 for <8 vs. ≥8; 0.57 for <9 vs. ≥9; 0.47 for <10 vs. ≥10.
Figure 1. Lymphocytic reaction to colorectal cancer.
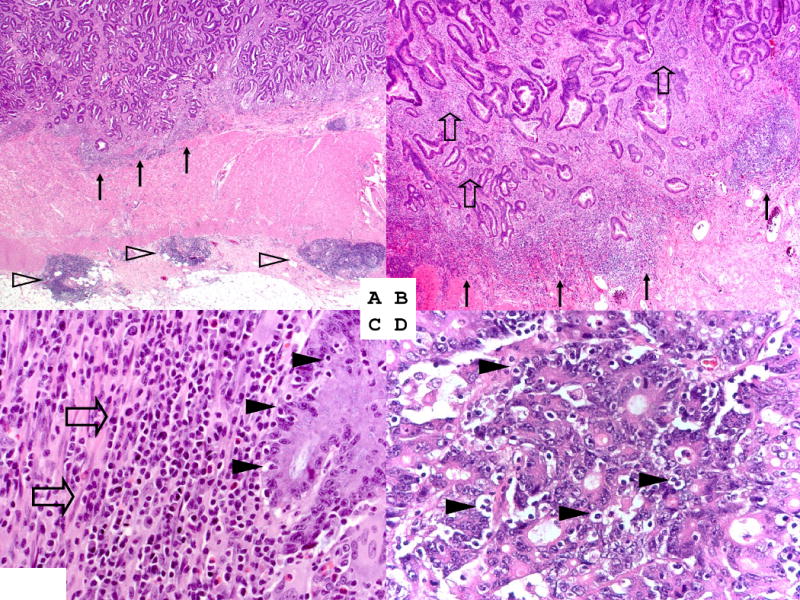
A. Crohn's-like reaction (open arrowheads) and peritumoral reaction (arrows) (original magnification ×20). B. Peritumoral reaction (arrows) and intratumoral periglandular reaction (open block arrows) (original magnification ×40). C. Intratumoral periglandular reaction (open block arrows) and tumor infiltrating lymphocytes (TIL; solid arrowheads) (original magnification ×400). D. TIL (solid arrowheads) (original magnification ×400).
Pyrosequencing of KRAS and BRAF, and Microsatellite Instability (MSI) Analysis
DNA from paraffin-embedded tissue was extracted, and PCR and Pyrosequencing targeted for KRAS codons 12 and 13 (28), and BRAF codon 600 (29) were performed. MSI status was determined using microsatellite markers, D2S123, D5S346, D17S250, BAT25, BAT26, BAT40, D18S55, D18S56, D18S67 and D18S487 (i.e., 10-marker panel) (30). MSI-high was defined as the presence of instability in ≥30% of the markers, MSI-low/microsatellite stability (MSS) as no or <30% unstable markers.
Real-Time PCR for CpG Island Methylation, and Pyrosequencing to Measure LINE-1 Methylation
Sodium bisulfite treatment on tumor DNA and subsequent real-time PCR (MethyLight) assays were validated and performed as previously described (31). We quantified promoter methylation in 8 CIMP-specific genes (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3 and SOCS1) (32-34). CIMP-high was defined as ≥6/8 methylated promoters using the 8-marker CIMP panel, CIMP-low/0 as 0 to 5 methylated promoters, according to the previously established criteria (33). In order to accurately quantify relatively high LINE-1 methylation levels, we utilized Pyrosequencing as previously described (35, 36).
Immunohistochemistry for p53 and COX-2
Tissue microarrays (TMAs) were constructed (37). p53 (38) and COX-2 (cyclooxygenase-2) immunohistochemistry was performed as previously described (27, 30). Appropriate positive and negative controls were included in each run of immunohistochemistry. All immunohistochemically-stained slides were interpreted by a pathologist (S.O.) unaware of other data. A random sample of 118 and 108 tumors were re-examined for p53 and COX-2, respectively, by a second observer (p53 by K.N., and COX-2 by R. Dehari, Kanagawa Cancer Center) unaware of other data, and the concordance between the two observers was substantial (κ=0.75, p<0.0001, for p53; and κ=0.62, p<0.0001, for COX-2).
Statistical Analysis
All analyses used SAS version 9.1 (SAS Institute, Cary, NC) and all p values were two-sided. The chi square test was used to assess an association between categorical variables. The analysis of variance (ANOVA) was performed to compare mean age and mean LINE-1 methylation level across the 3 lymphocytic categories. The κ coefficients were calculated to assess agreements between the two observers for lymphocytic reaction scores and immunohistochemical data.
In analysis of colorectal cancer-specific survival, death as a result of colorectal cancer was the primary end point and deaths as a result of other causes were censored. The Kaplan-Meier method was used to describe the distribution of survival time, and the log-rank test was performed. We used stage-matched (stratified) Cox proportional hazard models to calculate hazard ratio (HR) of death according to lymphocytic reaction, adjusted for age at diagnosis (continuous), sex, year of diagnosis (continuous), BMI (≥30 vs. <30 kg/m2), family history of colorectal cancer in any first degree relative (present vs. absent), tumor location (proximal vs. distal colon vs. rectum), tumor grade (high vs. low), negative lymph node count (ordinal categories; 0-3, 4-6, 7-12 and ≥13), MSI (high vs. low/MSS), CIMP (high vs. low/0), LINE-1 methylation (continuous), KRAS, BRAF, p53 and COX-2. Tumor stage (I, IIA, IIB, IIIA, IIIB, IIIC, IV, unknown) was used as a matching (stratifying) variable (using the “strata” option in the SAS “proc phreg” command) to minimize residual confounding; this fine tumor staging also included information on the number of positive lymph nodes. When we included all of the 4 lymphocytic reaction components (Crohn's-like reaction, peritumoral reaction, periglandular reaction and TIL) in multivariate Cox models, these 4 variables were collinear with each other. Spearman correlation coefficients among these 4 variables are shown in Supplemental Table 2. Thus, we analyzed each of the 4 components separately in Cox models. In the summary analysis, we used overall lymphocytic reaction score (0 to 12; the sum of scores for the 4 components). We classified tumors into 3 categories [overall reaction score 0-2 (low) vs. 3-6 (middle) vs. 7-12 (high)]. We examined the possibility of a non-linear relation between the lymphocytic reaction score and survival, non-parametrically with restricted cubic splines. In addition, we added squared term of lymphocytic score and/or cubic term of lymphocytic score in a multivariate model, and did not observe any significant improvement in model fitting (all p>0.5 by likelihood ratio tests). Nonetheless, our data presentations were mainly based on categorized groups according to the lymphocytic score, because categorized data were easily understandable. In the Cox regression analyses, the proportionality of hazards assumption was satisfied by evaluating time-dependent variables, which were the cross-product of the ordinal lymphocytic score variable and survival time (p=0.084 for colorectal cancer-specific survival; p=0.18 for overall survival). For cases with missing information on the negative lymph node count, we assign a separate (“missing”) indicator variable. For cases with missing information in other categorical covariates [including BMI (5.8% missing), tumor grade (0.7%), MSI (2.0%), CIMP (3.7%), KRAS (1.3%), BRAF (3.8%), p53 (1.7%) and COX-2 (0.7%)], we included those cases in a majority category, to minimize the number of variables in multivariate Cox models. We confirmed that excluding cases with missing information in any of the covariates did not substantially alter results (data not shown). An interaction was assessed by including the cross product of the ordinal lymphocytic score variable and another variable of interest in a multivariate Cox model, and p values for interaction were conservatively interpreted, considering multiple hypothesis testing.
Results
Lymphocytic reaction to colorectal cancer
We examined lymphocytic reaction under a light microscopy in 843 stage I-IV colorectal cancers identified in the two prospective cohort studies. We scored Crohn's-like reaction, peritumoral reaction, intratumoral periglandular reaction and tumor infiltrating lymphocytes (TIL) as: 0 (absent), 1+ (mild), 2+ (moderate) or 3+ (strong). Then, we calculated overall lymphocytic reaction score (0 to 12) as the sum of scores for the 4 components. As shown in Table 1, tumors with middle (score 3-6) to high (score 7-12) lymphocytic scores were associated with older age, proximal location, the larger negative node count, stage I-III, high tumor grade, MSI-high, CIMP-high, LINE-1 hypermethylation, BRAF mutation, and negative p53 (all p<0.003).
Overall lymphocytic reaction score and colorectal cancer survival
We examined patient survival according to the overall lymphocytic reaction score. There were a total of 373 deaths including 225 colorectal cancer specific deaths. Five-year colorectal cancer-specific survival was 73% among patients with a score of 0-2, 83% among patients with a score of 3-6, and 89% among patients with a score of 7-12 (log-rank p<0.0001), and five-year overall survival was 67 % among patients with a score of 0-2, 77% for a score of 3-6, and 88% for a score of 7-12 (log-rank p=0.002) (Figure 2A).
Figure 2. Unadjusted analysis of lymphocytic reaction and survival of stage I-IV colorectal cancer patients.
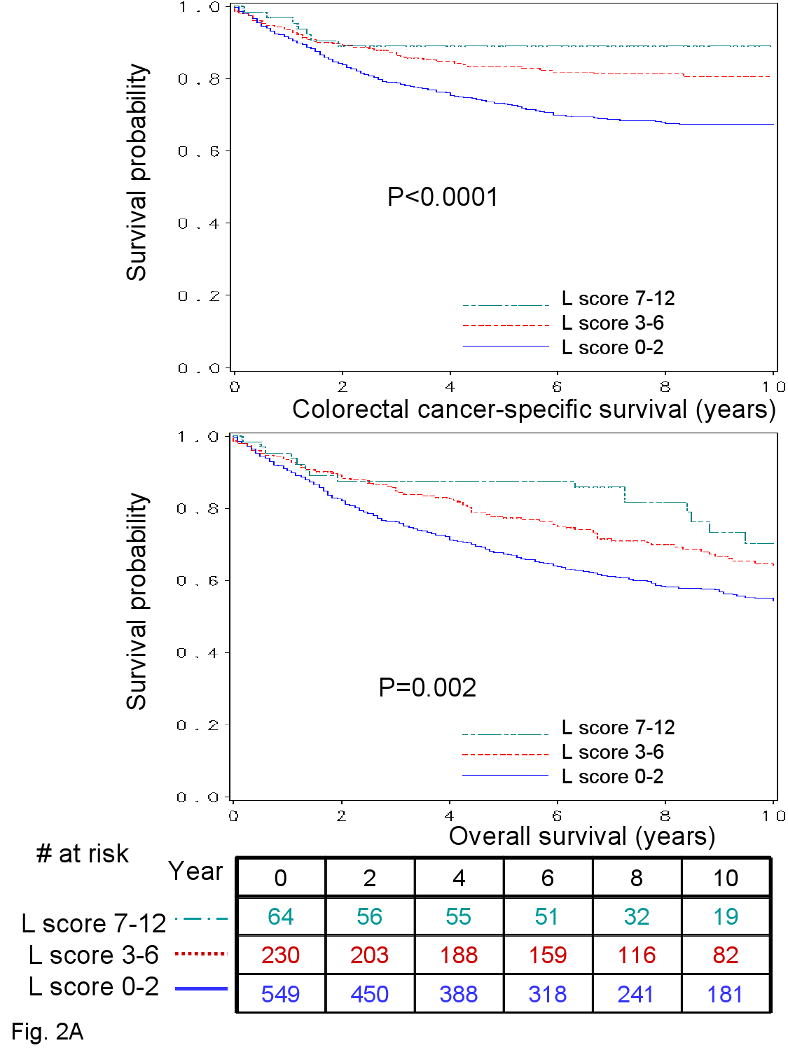
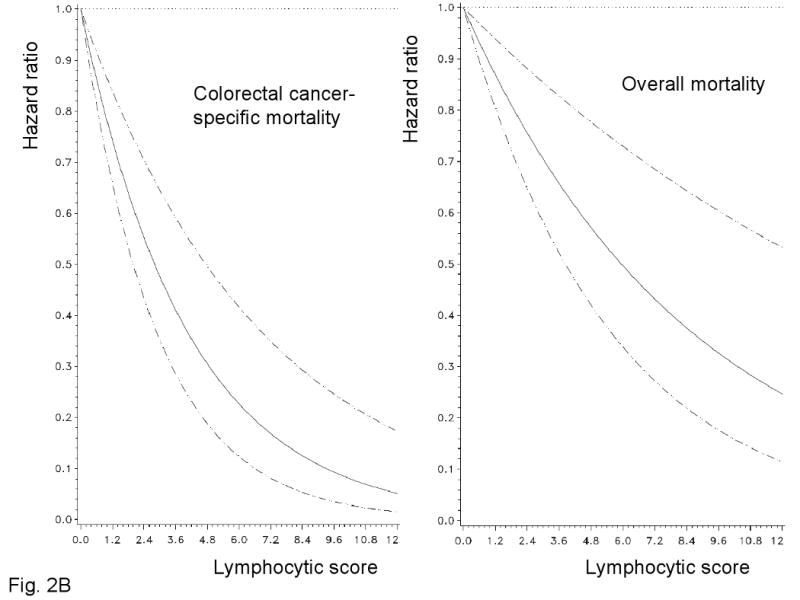
A. Kaplan-Meier curves for colon cancer-specific survival (upper panel) and overall survival (lower panel) with the numbers of at-risk individuals, according to the overall lymphocytic reaction score (L score). B. Smoothing spline plots of hazard ratio (HR) for colorectal cancer specific mortality (left panel) and overall mortality (right panel) according to lymphocytic reaction score (with score 0 as a referent). Hatched lines indicate 95% confidence interval.
We examined the possibility of a non-linear relation between the lymphocytic reaction score and survival, non-parametrically with restricted cubic splines (Figure 2B-C). This flexible method allowed us to examine the relation with survival without any categorization of the lymphocytic reaction score. Increasing lymphocytic reaction score was associated with a progressive decrease in mortality.
In multivariate Cox regression analyses, overall lymphocytic reaction score was associated with a significant improvement in colorectal cancer-specific and overall survival (Ptrend =0.008 and Ptrend=0.002, respectively) (Table 2). The attenuation of effect of lymphocytic reaction score on colorectal cancer-specific survival in the multivariate analysis was principally due to adjustment for tumor stage. A high lymphocytic reaction score was inversely associated with stage IV (Table 1). No other major confounders were identified.
Table 2.
Lymphocytic reaction score and survival of patients with stage I-IV colorectal cancer
| Overall lymphocytic reaction score* |
Total N | Colorectal cancer-specific survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths / person- years |
Univariate HR (95% CI) |
Stage- matched HR (95% CI) |
Multivariate HR (95% CI) |
Deaths / person- years |
Univariate HR (95% CI) |
Stage- matched HR (95% CI) |
Multivariate HR (95% CI) |
||
| 0-2 (low) | 549 (65%) | 175/4323 | 1 (referent) | 1 (referent) | 1 (referent) | 268/4323 | 1 (referent) | 1 (referent) | 1 (referent) |
| 3-6 (middle) | 230 (27%) | 43/1945 | 0.55 (0.39-0.76) | 0.64 (0.45-0.90) | 0.61 (0.42-0.89) | 88/1945 | 0.73 (0.57-0.93) | 0.80 (0.62-1.02) | 0.71 (0.54-0.93) |
| 7-12 (high) | 64 (7.6%) | 7/542 | 0.31 (0.15-0.66) | 0.60 (0.28-1.30) | 0.53 (0.23-1.23) | 17/542 | 0.50 (0.31-0.82) | 0.74 (0.45-1.23) | 0.49 (0.28-0.86) |
| P for trend | <0.0001 | 0.008 | 0.008 | 0.0004 | 0.050 | 0.002 | |||
The multivariate, stage-matched Cox regression model included age, year of diagnosis, sex, family history of colorectal cancer, tumor location, tumor grade, negative lymph node count, KRAS, BRAF, p53, LINE-1 methylation, microsatellite instability (MSI), the CpG island methylator phenotype (CIMP).
Overall lymphocytic reaction score is the sum of scores for Crohn's-like reaction (0-3), peritumoral reaction (0-3), intratumoral periglandular reaction (0-3), and tumor infiltrating lymphocytes (TIL, 0-3).
CI, confidence interval; HR, hazard ratio.
Each lymphocytic reaction component and colorectal cancer survival
We examined patient survival according to each of the 4 lymphocytic reaction components (Figure 3). All components of the lymphocytic reactions were associated with improved colorectal cancer-specific survival (long-rank p<0.014). We examined the prognostic significance of each component in stage-adjusted and multivariate Cox models (Table 3). Crohn's-like reaction, peritumoral reaction, and periglandular reaction appeared to be associated with long cancer-specific survival in multivariate analysis (Ptrend<0.053) (Table 3).
Figure 3.
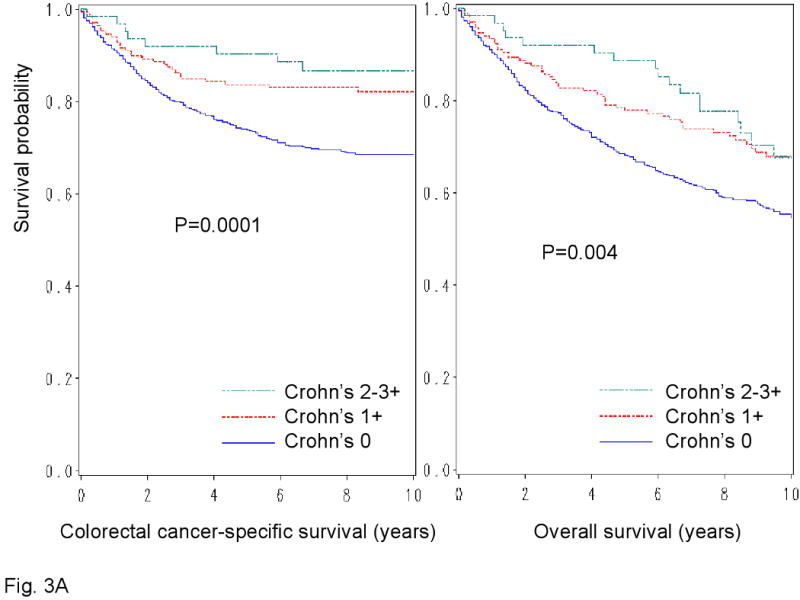
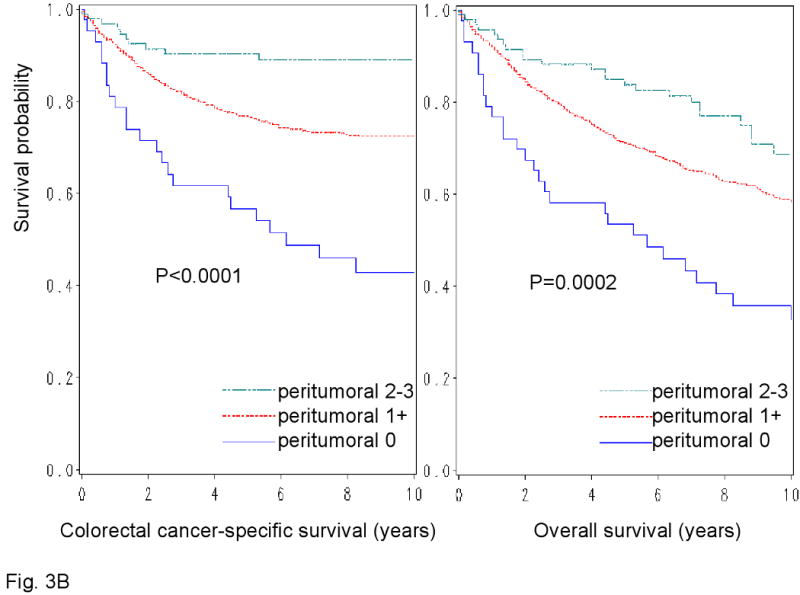
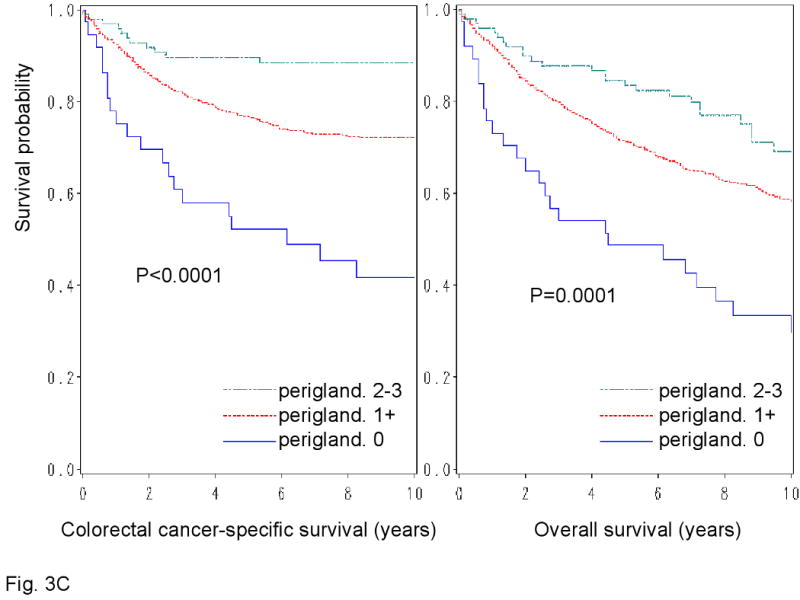
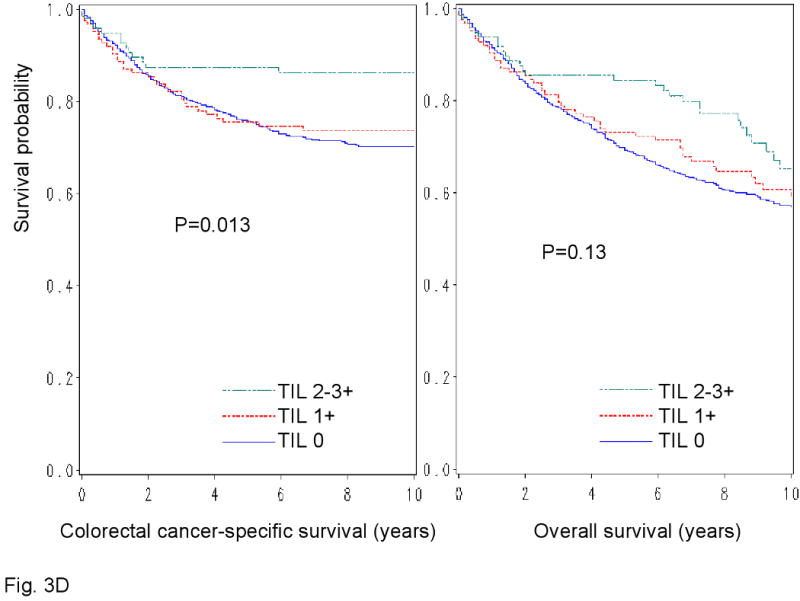
Kaplan-Meier survival curves for colon cancer-specific survival (left panel) and overall survival (right panel) according to each of the 4 components of lymphocytic reactions to stage I-IV colorectal cancer; Crohn's-like reaction (A), peritumoral reaction (B), intratumoral periglandular reaction (C) and TIL (tumor infiltrating lymphocytes) (D).
Table 3.
Lymphocytic reaction component and survival in stage I-IV colorectal cancer
| Lymphocytic reaction | Total N | Colorectal cancer-specific survival | Overall survival | ||||
|---|---|---|---|---|---|---|---|
| Deaths / person-years | Stage-matched HR (95% CI) |
Multivariate HR (95% CI) |
Deaths / person-years | Stage-matched HR (95% CI) |
Multivariate HR (95% CI) |
||
| Crohn's-like reaction* | |||||||
| 0 | 613 | 188/4778 | 1 (referent) | 1 (referent) | 292/4778 | 1 (referent) | 1 (referent) |
| 1+ | 168 | 29/1479 | 0.66 (0.42-0.98) | 0.77 (0.50-1.18) | 61/1479 | 0.77 (0.58-1.02) | 0.80 (0.59-1.09) |
| 2-3+ | 62 | 8/553 | 0.58 (0.28-1.20) | 0.53 (0.25-1.12) | 20/553 | 0.76 (0.48-1.21) | 0.51 (0.31-0.86) |
| P for trend | 0.020 | 0.052 | 0.059 | 0.007 | |||
| Peritumoral reaction* | |||||||
| 0 | 43 | 23/292 | 1 (referent) | 1 (referent) | 29/292 | 1 (referent) | 1 (referent) |
| 1+ | 707 | 192/5749 | 0.69 (0.44-1.09) | 0.72 (0.45-1.15) | 317/5749 | 0.78 (0.52-1.16) | 0.76 (0.50-1.16) |
| 2-3+ | 93 | 10/769 | 0.40 (0.19-0.87) | 0.38 (0.17-0.86) | 27/769 | 0.64 (0.37-1.13) | 0.48 (0.27-0.87) |
| P for trend | 0.017 | 0.019 | 0.12 | 0.014 | |||
| Intratumoral periglandular reaction* | |||||||
| 0 | 37 | 20/231 | 1 (referent) | 1 (referent) | 25/231 | 1 (referent) | 1 (referent) |
| 1+ | 709 | 194/5785 | 0.69 (0.43-1.12) | 0.65 (0.39-1.08) | 320/5785 | 0.71 (0.47-1.09) | 0.67 (0.43-1.04) |
| 2-3+ | 97 | 11/794 | 0.43 (0.20-0.93) | 0.37 (0.17-0.84) | 28/794 | 0.60 (0.34-1.05) | 0.42 (0.23-0.78) |
| P for trend | 0.028 | 0.016 | 0.093 | 0.005 | |||
| Tumor infiltrating lymphocytes (TIL)* | |||||||
| 0 | 624 | 180/5054 | 1 (referent) | 1 (referent) | 290/5054 | 1 (referent) | 1 (referent) |
| 1+ | 123 | 32/958 | 0.87 (0.59-1.27) | 0.81 (0.53-1.22) | 51/958 | 0.88 (0.65-1.19) | 0.77 (0.56-1.07) |
| 2-3+ | 96 | 13/798 | 0.80 (0.45-1.42) | 0.72 (0.37-1.40) | 32/798 | 0.99 (0.68-1.44) | 0.73 (0.47-1.13) |
| P for trend | 0.32 | 0.20 | 0.67 | 0.066 | |||
The multivariate, stage-matched Cox regression model included age, year of diagnosis, sex, family history of colorectal cancer, tumor location, tumor grade, negative lymph node count, KRAS, BRAF, p53, LINE-1 methylation, microsatellite instability (MSI), the CpG island methylator phenotype (CIMP), and each of the lymphocytic reaction components listed in the table.
The score represents reaction as follows: 0 (absent); 1+ (mild); 2+ (moderate); 3+ (strong).
CI, confidence interval; HR, hazard ratio.
Stratified analysis of lymphocytic reaction score and survival
We further examined whether the prognostic influence of the lymphocytic reaction score was modified by any of the other patient and tumoral features (Supplemental Figure). The prognostic effect of the lymphocytic reaction score was not significantly modified by any of the variables examined (all Pinteraction >0.30). Notably, the effect of the lymphocytic reaction score did not significantly differ across tumor stages (Pinteraction =0.32) or between the two independent cohort studies (Pinteraction =0.73).
Discussion
We examined the prognostic significance of lymphocytic reaction to tumor in a population of stage I-IV colorectal cancer patients who were concurrently assessed for other clinical and molecular predictors of patient outcome. We observed a significant relation between lymphocytic reaction and patient survival, independent of patient characteristics and other related molecular variables including the number of lymph nodes, p53, KRAS, BRAF, microsatellite instability (MSI), the CpG island methylator phenotype (CIMP) and LINE-1 hypomethylation. Although each of the 4 components of lymphocytic reactions [Crohn's-like reaction, peritumoral reaction, intratumoral periglandular reaction and tumor infiltrating lymphocytes (TIL)] appeared to predict longer survival of patients, the association with survival was most robust when overall lymphocytic score was used. Our results may support the role of the host immune reaction to tumor as an independent prognostic factor among colorectal cancer patients. The 4 components of lymphocytic reactions can be assessed upon routine histopathologic examination of resected colorectal cancer, and an evaluation of these features can be implemented in clinical practice. Our study also supports the use of immune cells as potential cancer treatment. The stimulation of immune response has a number of theoretical advantages over other forms of cancer therapy (1). Immune cells can direct to antigen-expressing tumor cells wherever they are located in the body, and can proliferate until all tumor cells are eradicated. Immunologic memory can be generated for surveillance against any tumor recurrence (1). Finally, targeting host immune cells may avoid the emergence of resistance mutations that are commonly observed during targeted treatment against molecules within cancer cells.
Examining prognostic and predictive factors is important in cancer research (39-45). Lymphocytic reaction to colorectal cancer has been associated with longer survival in colorectal cancer (2-6, 8). However, the mechanism underlying the survival advantage associated with lymphocytic reaction to tumor remains uncertain. Lymphocytic reaction may be an indicator of host immune response to tumor cells, leading to improved survival (7, 46). In addition, immune response may cause enlargement of lymph nodes, which may contribute to an increase in the recovered lymph node count and, thereby, more accurate staging of colorectal cancer. In fact, lymphocytic reaction to colorectal cancer have been associated with an increased lymph node count (13), and the lymph node count has consistently been associated with improved survival of colorectal cancer patients (13-18). Alternatively, lymphocytic reaction to tumor may reflect specific tumoral molecular alterations associated with indolent tumor behavior. Tumoral lymphocytic reaction has been associated with MSI-high (19, 47), which, in turn, is associated with longer patient survival (25). Recent studies have further shown that MSI-high in colorectal cancer is associated with the CpG island methylator phenotype (CIMP), BRAF mutation (33, 48), and high LINE-1 methylation level (35), and all of these factors (MSI, CIMP, BRAF mutation and LINE-1 methylation) have been independently related with survival of colon cancer patients (26, 36, 49). Therefore, In fact, numerous pathologic and molecular features (the lymph node count, MSI, CIMP, BRAF mutation, and LINE-1 methylation) could account for the beneficial effect of lymphocytic reaction to tumor. However, none of the previous studies of lymphocytic reaction and patient survival has comprehensively examined the aforementioned molecular features in colorectal cancer beyond MSI. In our analysis, the benefit associated with higher tumoral lymphocytic reaction remained significant after adjusting for these various pathologic and molecular features.
One unresolved question is whether subtyping of infiltrating lymphocytes provides any additional information beyond histopathologic evaluation of lymphocytic reaction patterns. Previous studies have shown that the presence, degree or localization of infiltrates by a specific subtype of lymphocytes (e.g., CD57+, CD8+, CD45RO+ or FOXP3+ lymphocytes) is associated with patient outcome in colorectal cancer (7-12). In addition, high tumoral expression of chemokine CXCL16 has been associated with TIL and good prognosis (50). However, none of these studies (7-12, 50) has comprehensively evaluated the distinct histopathologic patterns of lymphocytic infiltrates described in the current study. Moreover, none of the previous studies has examined potential confounding effect of the number of lymph nodes examined, and molecular features of colorectal cancer beyond MSI (i.e., CIMP, BRAF mutation and LINE-1 methylation). Thus, confounding effect by these variables (the lymph node count and tumoral molecular features) cannot be excluded in the previous studies (7-12, 50). Additional studies are necessary to clarify whether lymphocyte subtyping adds any additional or independent prognostic information beyond histopathologic evaluation of lymphocytic reaction patterns.
We calculated the lymphocytic reaction score using the four components, i.e., Crohn's-like reaction, peritumoral reaction, intratumoral periglandular reaction and TIL. TIL appeared to be less significantly associated with patient survival, than the other three components. Although using an overall score of the three components yielded similar results (data not shown), the significance and the magnitude of the effect were attenuated. Thus, in this study, we provide the data based on the four components. Nonetheless, utilization of the three components without TIL may represent a potentially reasonable alternative approach.
We confirmed the positive relation between lymphocytic reaction and the lymph node count, which has been previously reported (13). The recovered lymph node count has consistently been associated with longer survival of colorectal cancer patients (13-18). Our data provide evidence supporting that the recovered node count is influenced by host immune reaction to tumor. Nonetheless, the best effort should be made to recover and examine as many lymph nodes as possible for accurate staging. Additional studies are necessary to assess whether the beneficial prognostic effect of the lymph node count is independent of lymphocytic reaction to tumor.
There are limitations in this study. For example, data on cancer treatment were limited. Nonetheless, it is unlikely that chemotherapy use substantially differed according to lymphocytic reactions to tumor, since such data were not typically used for treatment decision making. In addition, beyond cause of mortality, data on cancer recurrences were not available in these cohorts. Nonetheless, given the median survival for metastatic colon cancer was approximately 10-12 months during much of the time period of this study, colorectal cancer-specific survival should be a reasonable surrogate for cancer-specific outcomes.
There are advantages in utilizing the database of the two independent prospective cohort studies, the Nurses' Health Study and Health Professionals Follow-up Study to examine prognostic significance of lymphocytic reaction and its interactions with tumoral and host factors. Anthropometric measurements, family history, other clinical information, pathologic and tumor staging data, and tumoral molecular features were prospectively collected, blinded to patient outcome. Cohort participants who developed cancer were treated at hospitals throughout the U.S., and thus more representative of colorectal cancers in the general U.S. population than patients in a single to several hospitals. There were no demographic difference between cases with tumor tissue analyzed and those without tumor tissue analyzed (27). Lymphocytic reaction to colorectal cancer was examined by the single study pathologist, and a subset of cases were re-examined by a second pathologist for the agreement study. Finally, our rich tumor database enabled us to simultaneously assess pathologic and tumoral molecular features and control for confounding by a number of tumoral molecular alterations. None of the previous studies on lymphocytic reactions and patient outcome has examined as many molecular variables as we did in this study.
In summary, our large cohort study suggests that lymphocytic reaction to tumor is associated with longer survival of colorectal cancer patients, independent of other clinical, pathologic and tumoral molecular characteristics. Our data suggest a possible role of host immune response as an independent prognostic factor in colorectal cancer patients. Future studies are needed to confirm these results as well as to elucidate exact mechanisms by which lymphocytic reaction to tumor affects clinical outcome in colorectal cancer.
Supplementary Material
Supplemental Figure. Lymphocytic reaction score and colorectal cancer-specific survival in various strata. Loge(adjusted HR) with 95% CI was calculated for middle-high lymphocytic reaction score (3-12) using a low score (0-2) as a referent.
CI, confidence interval; CIMP, CpG island methylator phenotype; COX-2, cyclooxygenase-2; BMI, body mass index; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; MSI, microsatellite instability; MSS, microsatellite stable; NHS, Nurses' Health Study.
Acknowledgments
We deeply thank the Nurses' Health Study and Health Professionals Follow-up Study cohort participants who have generously agreed to provide us with biological specimens and information through responses to questionnaires; hospitals and pathology departments throughout the U.S. for providing us with tumor tissue materials; and Frank Speizer, Walter Willett, Susan Hankinson, Meir Stampfer and many other staff members who have implemented and maintained the prospective cohort studies.
Funding: This work was supported by the U.S. National Institute of Health (P01 CA87969 to S. Hankinson, P01 CA55075 to W. Willett, P50 CA127003 to C.S.F., K07 CA122826 to S.O.), the Bennett Family Fund, and the Entertainment Industry Foundation National Colorectal Cancer Research Alliance. K.N. was supported by a fellowship grant from the Japan Society for Promotion of Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCI or NIH. Funding agencies did not have any role in the design of the study; the collection, analysis, or interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- COX-2
cyclooxygenase-2
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- MSI
microsatellite instability
- MSS
microsatellite stable
- NHS
Nurses' Health Study
- TIL
tumor infiltrating lymphocytes
Footnotes
Statement of Translational Relevance: Host immune response to tumor is an important prognostic factor in colorectal cancer. However, little is known on prognostic significance of lymphocytic reaction to tumor, independent of the number of lymph nodes and tumoral molecular alterations [including microsatellite instability (MSI) and the CpG island methylation phenotype (CIMP)]. We have utilized the database of 843 colorectal cancers in two independent cohort studies, with available clinical information, adequate follow-up, and important molecular events in colon cancers. To our knowledge, this is the first large study to demonstrate influence of lymphocytic reaction on clinical outcome independent of the lymph node count and molecular features including BRAF mutation, MSI, CIMP and LINE-1 hypomethylation, all of which are potential confounders. Lymphocytic reaction can be readily evaluated in routine pathology practice. Moreover, stimulating host immune response is a promising therapeutic strategy. Thus, our findings are relevant to practice in oncology.
References
- 1.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373:673–83. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- 3.Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84:981–7. doi: 10.1189/jlb.1107773. [DOI] [PubMed] [Google Scholar]
- 4.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–24. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Morris M, Platell C, Iacopetta B. Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res. 2008;14:1413–7. doi: 10.1158/1078-0432.CCR-07-1994. [DOI] [PubMed] [Google Scholar]
- 6.Graham DM, Appelman HD. Crohn's-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod Pathol. 1990;3:332–5. [PubMed] [Google Scholar]
- 7.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 8.Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–8. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Chiba T, Ohtani H, Mizoi T, et al. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–7. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 11.Zlobec I, Terracciano LM, Lugli A. Local Recurrence in Mismatch Repair-Proficient Colon Cancer Predicted by an Infiltrative Tumor Border and Lack of CD8+ Tumor-Infiltrating Lymphocytes. Clin Cancer Res. 2008;14:3792–7. doi: 10.1158/1078-0432.CCR-08-0048. [DOI] [PubMed] [Google Scholar]
- 12.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 13.George S, Primrose J, Talbot R, et al. Will Rogers revisited: prospective observational study of survival of 3592 patients with colorectal cancer according to number of nodes examined by pathologists. Br J Cancer. 2006;95:841–7. doi: 10.1038/sj.bjc.6603352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. 2002;26:179–89. doi: 10.1097/00000478-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–9. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 16.Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst. 2005;97:219–25. doi: 10.1093/jnci/dji020. [DOI] [PubMed] [Google Scholar]
- 17.Bui L, Rempel E, Reeson D, Simunovic M. Lymph node counts, rates of positive lymph nodes, and patient survival for colon cancer surgery in Ontario, Canada: a population-based study. J Surg Oncol. 2006;93:439–45. doi: 10.1002/jso.20499. [DOI] [PubMed] [Google Scholar]
- 18.Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–41. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 19.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527–35. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shia J, Ellis NA, Paty PB, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27:1407–17. doi: 10.1097/00000478-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins MA, Hayashi S, O'Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133:48–56. doi: 10.1053/j.gastro.2007.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogino S, Odze RD, kawasaki T, et al. Correlation of pathologic features with CpG island methylator phenotype (CIMP) by quantitative DNA methylation analysis in colorectal carcinoma. Am J Surg Pathol. 2006;30:1175–83. doi: 10.1097/01.pas.0000213266.84725.d0. [DOI] [PubMed] [Google Scholar]
- 23.Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–97. doi: 10.1053/j.gastro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Tougeron D, Fauquembergue E, Rouquette A, et al. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod Pathol. 2009 doi: 10.1038/modpathol.2009.80. [DOI] [PubMed] [Google Scholar]
- 25.Popat S, Hubner R, Houlston RS. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J Clin Oncol. 2005;23:609–18. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 26.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–6. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan AT, Ogino S, Fuchs CS. Aspirin and the Risk of Colorectal Cancer in Relation to the Expression of COX-2. New Engl J Med. 2007;356:2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 28.Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7:413–21. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8:582–8. doi: 10.2353/jmoldx.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogino S, Brahmandam M, Cantor M, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- 31.Ogino S, kawasaki T, Brahmandam M, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–17. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogino S, Cantor M, Kawasaki T, et al. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut. 2006;55:1000–6. doi: 10.1136/gut.2005.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9:305–14. doi: 10.2353/jmoldx.2007.060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 35.Ogino S, Kawasaki T, Nosho K, et al. LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG methylator phenotype in colorectal cancer. Int J Cancer. 2008;122:2767–73. doi: 10.1002/ijc.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogino S, Nosho K, Kirkner GJ, et al. A cohort study of tumoral LINE-1 hypomethylation and prognosis in colon cancer. J Natl Cancer Inst. 2008;100:1734–8. doi: 10.1093/jnci/djn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogino S, Brahmandam M, kawasaki T, Kirkner GJ, Loda M, Fuchs CS. Combined analysis of COX-2 and p53 expressions reveals synergistic inverse correlations with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Neoplasia. 2006;8:458–64. doi: 10.1593/neo.06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogino S, kawasaki T, Kirkner GJ, Yamaji T, Loda M, Fuchs CS. Loss of nuclear p27 (CDKN1B/KIP1) in colorectal cancer is correlated with microsatellite instability and CIMP. Mod Pathol. 2007;20:15–22. doi: 10.1038/modpathol.3800709. [DOI] [PubMed] [Google Scholar]
- 39.Cheng YW, Pincas H, Bacolod MD, et al. CpG island methylator phenotype associates with low-degree chromosomal abnormalities in colorectal cancer. Clin Cancer Res. 2008;14:6005–13. doi: 10.1158/1078-0432.CCR-08-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.French AJ, Sargent DJ, Burgart LJ, et al. Prognostic Significance of Defective Mismatch Repair and BRAF V600E in Patients with Colon Cancer. Clin Cancer Res. 2008;14:3408–15. doi: 10.1158/1078-0432.CCR-07-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginty F, Adak S, Can A, et al. The Relative Distribution of Membranous and Cytoplasmic Met Is a Prognostic Indicator in Stage I and II Colon Cancer. Clin Cancer Res. 2008;14:3814–22. doi: 10.1158/1078-0432.CCR-08-0180. [DOI] [PubMed] [Google Scholar]
- 42.Jorissen RN, Lipton L, Gibbs P, et al. DNA copy-number alterations underlie gene expression differences between microsatellite stable and unstable colorectal cancers. Clin Cancer Res. 2008;14:8061–9. doi: 10.1158/1078-0432.CCR-08-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suehiro Y, Wong CW, Chirieac LR, et al. Epigenetic-Genetic Interactions in the APC/WNT, RAS/RAF, and P53 Pathways in Colorectal Carcinoma. Clin Cancer Res. 2008;14:2560–9. doi: 10.1158/1078-0432.CCR-07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weichert W, Roske A, Niesporek S, et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res. 2008;14:1669–77. doi: 10.1158/1078-0432.CCR-07-0990. [DOI] [PubMed] [Google Scholar]
- 45.Zlobec I, Baker K, Terracciano LM, Lugli A. RHAMM, p21 combined phenotype identifies microsatellite instability-high colorectal cancers with a highly adverse prognosis. Clin Cancer Res. 2008;14:3798–806. doi: 10.1158/1078-0432.CCR-07-5103. [DOI] [PubMed] [Google Scholar]
- 46.Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol. 2006;24:3570–5. doi: 10.1200/JCO.2006.06.8866. [DOI] [PubMed] [Google Scholar]
- 47.Jass JR, Do KA, Simms LA, et al. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673–9. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samowitz W, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–45. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 49.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–9. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 50.Hojo S, Koizumi K, Tsuneyama K, et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67:4725–31. doi: 10.1158/0008-5472.CAN-06-3424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Lymphocytic reaction score and colorectal cancer-specific survival in various strata. Loge(adjusted HR) with 95% CI was calculated for middle-high lymphocytic reaction score (3-12) using a low score (0-2) as a referent.
CI, confidence interval; CIMP, CpG island methylator phenotype; COX-2, cyclooxygenase-2; BMI, body mass index; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; MSI, microsatellite instability; MSS, microsatellite stable; NHS, Nurses' Health Study.


