Abstract
The matrix metalloproteinases (MMPs) play a key role in normal and pathological angiogenesis by mediating extracellular matrix degradation and/or controlling the biological activity of growth factors, chemokines, and/or cytokines. Specific functions of individual MMPs as anti- or proangiogenic mediators remain to be elucidated. In the present study, we assessed the impact of single or combined MMP deficiencies in in vivo and in vitro models of angiogenesis (malignant keratinocyte transplantation and the aortic ring assay, respectively). MMP-9 was predominantly expressed by neutrophils in tumor transplants, whereas MMP-2 and MMP-3 were stromal. Neither the single deficiency of MMP-2, MMP-3, or MMP-9, nor the combined absence of MMP-9 and MMP-3 did impair tumor invasion and vascularization in vivo. However, there was a striking cooperative effect in double MMP-2:MMP-9-deficient mice as demonstrated by the absence of tumor vascularization and invasion. In contrast, the combined lack of MMP-2 and MMP-9 did not impair the in vitro capillary outgrowth from aortic rings. These results point to the importance of a cross talk between several host cells for the in vivo tumor promoting and angiogenic effects of MMP-2 and MMP-9. Our data demonstrate for the first time in an experimental model that MMP-2 and MMP-9 cooperate in promoting the in vivo invasive and angiogenic phenotype of malignant keratinocytes.
Keywords: angiogenesis, tumor invasion, proteolysis, gelatinases, stromal MMP
Matrix metalloproteinases (MMPs) are a family of structurally related zinc- and calcium–dependent endopeptidases that can degrade extracellular matrix (ECM) components (1, 2). MMP-mediated proteolysis is involved in various physiological and pathological processes such as embryonic development, involution, wound healing, inflammatory diseases, cancer invasion, and metastasis (3).
Among the MMPs, MMP-2 (gelatinase A), and MMP-9 (gelatinase B) are especially important in collagen degradation, through digestion of denatured collagen (gelatin) generated by the cleavage of fibrillar collagen triple helix by collagenases (MMP-1, MMP-8, MMP-13 and membrane type-1 MMP (MMP-14)). Both MMP-2 and MMP-9 are also active against native type IV and V collagens, elastin, aggrecan, and fibronectin (4, 5). Growth factors such as vascular endothelial growth factor (VEGF) that are sequestered in the extracellular matrix can become bioactive by MMP-9-mediated proteolysis (6). A number of nonmatrix MMP substrates such as cytokines, chemokines, and growth factors (7–9) have also been identified. The activity of MMPs is tightly regulated at multiple levels including transcription, secretion, proenzyme activation, and inhibition by tissue inhibitors of MMPs (TIMPs) (1, 2). MMP-2 and MMP-9 differ fundamentally in terms of transcriptional regulation (10, 11) and activation process. Like many other MMPs, MMP-9 can be activated by several proteases, including plasmin and MMP-3. The activation of MMP-2 is a more complex and specific cell-associated process involving a multiprotein cluster composed of MMP-14, TIMP-2, and pro-MMP-2 (12, 13).
Because of the lack of selective MMP inhibitors, the mechanisms of action and the individual roles of MMP-2 and MMP-9 have yet to be clearly defined. However, recent generation of gene-deficient mice provides a way to overcome this problem. Studies using MMP-deficient mice have brought new insights into the roles of MMP-2 and MMP-9 during physiological and pathological angiogenesis. MMP-9-deficient mice exhibit a specific embryo phenotype characterized by a delayed vascularization and ossification of the growth plate in long bones (14), as well as by decreased breeding efficiency (15). The importance of MMP-9 in tumor angiogenesis has been described using transgenic mouse models of skin cancer (16) and of RIP1-Tag2 insulinoma (6), as well as xenografts of human ovarian carcinoma (17). The reduced carcinogenesis in MMP-9-deficient mice can be restored by transplantation of bone marrow or spleen from mice expressing MMP-9 (16, 17). In addition, experimental metastasis of murine tumor cells is reduced in MMP-9-deficient mice (18). Although tumor angiogenesis and growth is reduced in MMP-2-deficient mice compared with wild-type mice (19), MMP-2 is not required for angiogenesis in the RIP1-Tag2 model (6). Taken together, these observations support the hypothesis that MMP-2 and MMP-9 play crucial roles in tumor progression.
No compensation by MMP-2 in MMP-9 KO mice or MMP-9 in MMP-2 KO mice is detectable by gelatin SDS-PAGE zymography of tissue extracts and cell cultures (20). This suggests that although MMP-2 and MMP-9 enzymatic activities may have similar functions in vitro, these MMPs may have distinct activities and regulation in vivo (20). In addition, opposite roles of MMP-2 and MMP-9 for the progression of antibody-induced arthritis have been reported (21).
To define the role of these MMPs in cancer growth and vascularization more precisely, we transplanted malignant keratinocytes into MMP-2−/−, MMP-9−/− and double MMP-2−/−: MMP-9−/− mice. We also investigated the role of MMP-3, a putative activator of MMP-9, by using this model in MMP-3−/− and double MMP-3−/− MMP-9−/− mice. MMP-9 was also mapped by using transgenic mouse line harboring the LacZ reporter gene driven by MMP-9 promoter (11).
The importance of both gelatinases is demonstrated by the complete inhibition of tumor vascularization and growth in double MMP-2−/− MMP-9−/− deficient mice.
MATERIALS AND METHODS
Genetically modified mice
Homozygous 7700 ExIn-LacZ mice (MMP-9/LacZ mice), which contain 7.7 kb of the 5′-flanking region and the first intron and exon of MMP-9 gene linked to a β-galactosidase gene were generated as described previously (11). For MMP-9-deficient mice (MMP-9−/−) (14), the progeny of heterozygous breeding pairs of mice was used and genotyped by RT-PCR (22). The MMP-2−/− and MMP-3−/− mice were those generated by Itoh et al. (19) and Mudgett et al. (23), respectively. MMP-2−/− or MMP-3−/− was interbred with MMP-9−/− to obtain the double MMP-2−/ − MMP-9−/− or MMP-3−/−:MMP-9−/− mice.
Transplantation assay in mice
Malignant murine keratinocytes (PDVA cells) (24) were routinely grown in modified Eagle’s minimal essential medium containing a fourfold concentration of amino acids and vitamins (Gibco Laboratories, Grant Island, NY), 10% fetal calf serum (Gibco) and antibiotics in a humidified incubator at 37°C, 5% CO2. Cells were plated on collagen gel (4 mg/mL of type I collagen isolated from rat tail tendons) inserted in Teflon rings (Renner GmbH, Dannstadt, Germany) and maintained in culture for 1 day before transplantation into 6- to 8-week-old mice or their corresponding wild type as described previously (25, 26). Briefly, the cell-coated collagen gels were covered with a silicone transplantation chamber (Renner, Germany) and implanted in toto onto the dorsal muscle fascia of mice. One week (for MMP9/LacZ mice) or three weeks (for all mice genotypes) after grafting, transplants were resected, embedded in Tissue Tek (Miles Laboratories Inc., Naperville, IL) and frozen in liquid nitrogen for cryostat sectioning. Each experimental group contained at least 6 animals.
Immunohistochemistry and β-galactosidase staining
Cryostat sections (6–7 μm thick) of MMP-9/LacZ transplants were fixed in 4% paraformaldehyde for 10 min, washed in Tris 50 mM-Triton X-100 0.1%, pH 7.6 buffer and incubated with rabbit polyclonal MMP-9 antibody (kindly provided by P. Carmeliet, KUL, Leuven, Belgium, diluted 1/350). Sections were incubated with horseradish peroxidase (HRP)-conjugated swine anti-rabbit IgG (Dako, Denmark, diluted 1/50) and color developed with 3-amino-9-ethyl carbazole (AEC+, Dako) or 3,3′diaminobenzidine (DAB+, Dako), before counterstaining with hematoxilin, safranin, or ethyl green. Expression of the LacZ transgene was determined in 2% paraformaldehyde–0.2% glutaraldehyde fixed serial sections (15 to 30 min), washed 3 times in PBS and stained with buffered 5-bromo-4-chloro-3-indolyl-β-galactopyronoside (X-Gal) solution as described previously (11).
For immunostaining of transplants, cryostat sections were fixed in acetone at −20°C and in 80% methanol at 4°C. When double immunofluorescent labeling was performed, sections were incubated for 1 h at room temperature with the primary antibodies: anti-type IV collagen Ab (rabbit polyclonal Ab; diluted 1/100), anti-keratin Ab (Sigma-Aldrich; guinea pig polyclonal Ab; diluted 1/20), anti-neutrophil Ab (Serotec; rat anti-mouse; diluted 1/200) and anti-αSMA Ab (Sigma-Aldrich; monoclonal anti-α-smooth muscle actin FITC conjugate; diluted 1/75). After washes in phosphate-buffered saline (PBS), fluorescein-isothiocyanate (FITC), appropriate Oregon green or Texas red-conjugated secondary antibodies were applied for 30 min: swine anti-rabbit (Dakopat; diluted 1/40) goat anti-rat (Molecular Probes, Eugene, OR; diluted 1/40) and mouse anti-guinea pig (Sigma-Aldrich, St. Louis, MO; diluted 1/40). After 3 washes in PBS, coverslips were mounted with Aqua Polymount (Polysciences, Warrington, PA) and specific labeling was observed using an inverted microscope equipped with epifluorescence optics.
Scoring of tumor vascularization
Tumor vascularization was scored on 5 sections in the middle of each transplant as described previously (26, 27): 0: vessels undetected in the collagen gel; +: vessels infiltrating the collagen gel without reaching the malignant epithelial layer; ++: blood vessels in close apposition to the epithelial layer, and +++: blood vessels intermingled with invasive epithelial tumor sprouts.
In situ gelatin zymography
In situ zymography was performed by incubating cryosections (7 μm) with 40 μg/ml fluorescein-conjugated gelatin (Molecular Probes, Eugene, OR) in 50 mM Tris-HCl pH 7.5, 10 mM CaCl2, 150 mM NaCl and 0.05 Brij-35 (Calbiochem, CA, USA) for 24 h at 37°C. Sections were washed 3 times with PBS and mounted with Vectashield (Vector Laboratories, Inc., Burlingame, CA). Gelatinase activity was visualized using fluorescent microscopy. Cells were counterstained using bisbenzimide (Calbiochem; fluorochromo-3HCl, 5 H2O; dilution 1/100). The MMP nature of gelatinolytic activity was assessed by incorporating a synthetic MMP inhibitor (R028-2653) (1 μm) in gelatin.
Aorta ring assay and cell outgrowth quantification
Angiogenesis was studied in vitro by culturing aorta rings from transgenic or deficient mice and their corresponding wild type in three-dimensional collagen gels. Mouse aortic rings were prepared, embedded in a collagen gel, and cultured during 9 days in MCDB131 medium supplemented with 5% of autologous serum according to the procedure described previously (28, 29). The endothelial nature of spreading cells was verified by incubating the aortic rings with fluorescent acetylated low density lipoprotein (Dil-Ac-LD), which is selectively taken up by endothelial cells (28, 29).
To quantify cell outgrowth, cell density was determined as a function of the distance to the aortic ring. For this, image analysis was performed on a PC using the software Aphelion 3.2 from Adsis. Images were first digitized in 760 × 570 pixel with 256 gray levels and filtered in order to make the background uniform. They were then binarized automatically with the threshold that maximizes the global average contrast of edges. Density distribution of spreading cells was determined using the methodology described previously (30, 31). Quantification was performed on two independent assays, by using at least triplicate culture per condition. The following statistical parameters were calculated (30): (1) the mean distribution (Dm) and standard deviation; (2) the mode (d) and (3) the maximal distance of migration of spreading cells (Lm). To compare the different distributions, the ANOVA was performed, and results were considered significantly different when the P value was less than 0.05.
RESULTS
MMP-9 promoter of host cells is activated in vivo in tumor transplants
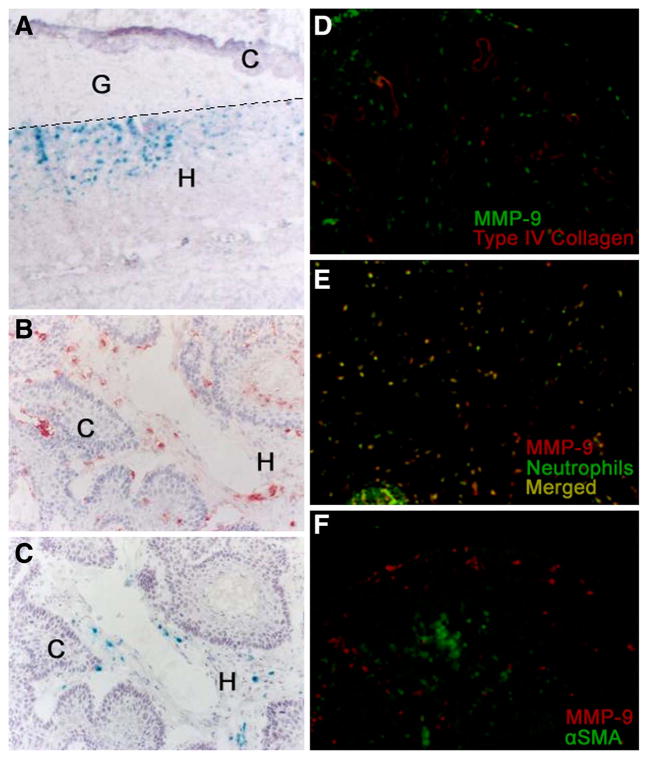
Malignant murine keratinocytes (PDVA cells) on collagen gels were implanted onto the dorsal muscle fascia of adult homozygous MMP-9/LacZ mice. In response to angiogenic stimuli produced by tumor cells, new blood vessels invaded the collagen gel and reached the malignant epithelial layer. Thereafter, malignant keratinocytes formed tumor sprouts that invaded the host tissue. Host-derived cells that infiltrated the collagen gel within the first 10 days expressed MMP-9 at the migration front (Fig. 1A). Three weeks after transplantation, when invasive malignant keratinocytes were intermingled with host cells, the latter displayed MMP-9 promoter activity as assessed by β-galactosidase staining, and produced endogenous MMP-9 protein as shown by immunostaining (Fig. 1B). In our model MMP-9 was not associated with vascular structures visualized by immunostaining with anti-type IV collagen antibody (Fig. 1D). MMP-9 was produced by neutrophils (Fig. 1E), but not by other stromal host cells such as α-smooth muscle actin-positive cells (Fig. 1F). However, it should be noted that not all cells producing MMP-9 (Fig. 1B) exhibited MMP-9 promoter activity (Fig. 1C). This apparent discrepancy between MMP-9 immunostaining and β-galactosidase activity might be related to the fact that immunolabeling localizes the proenzyme, while β-galactosidase staining reflects only transient promoter activation. In addition, the fate of β-galactosidase mRNA and/or protein might be different from that of endogenous MMP-9, and in the case of neutrophils, MMP-9 gene could be expressed in one site and the protein stored in intracellular granules during cell migration to the inflammatory site. Neither MMP-9 mRNA, nor protein was detected in PDVA cells cultured in vitro (data not shown).
Figure 1. In vivo invasive behavior of malignant PDVA cells.
PDVA cells were implanted into MMP-9/LacZ mice for one (A) or three (B, C) weeks. Immunohistochemistry (B, D, E, F) and β-galactosidase staining (A, C) were performed as described in Materials and Methods. Double immunostainings of tumor transplants resected from wild-type mice after three weeks of transplantation (D–F) reveal that MMP-9 does not colocalize with vascular structures stained with an antitype-IV collagen antibody (D), MMP-9 is produced by neutrophils (E) but not by α-smooth muscle actin (α-SMA) positive cells (F). The transplant areas analyzed in panels (D–F) correspond to the tumor part, where tumor cells are intermingled with host cells. C: carcinoma cells; G: collagen gel; H: host tissue. The dotted line delineates the limit of the collagen gel. Original magnification: (A) 40×, (B, C) 100×, (D–F) 1000×.
Deficiency of MMP-9 and/or MMP-3 does not impair in vivo tumor invasion and vascularization
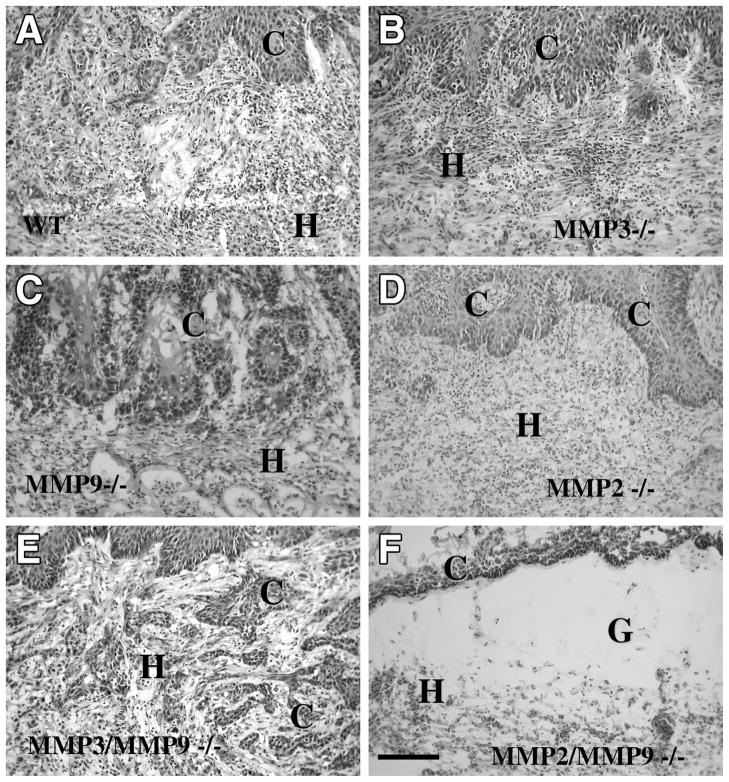
Wild-type (n=6) and MMP-9-deficient mice (n=6) were transplanted for three weeks with malignant PDVA keratinocytes. Neither tumor invasion (Fig. 2C) nor vascularization (Fig. 3 C) was affected by the single MMP-9 deficiency. Invading tumor sprouts surrounded by a rich capillary network were observed in both MMP-9-deficient and their corresponding wild-type mice (Fig. 3A, C). Tumor vascularization developed to a similar degree in both genotypes. Indeed, the numbers of tumors scored ++ (n=4) or scored +++ (n=2) were similar in MMP-9+/+ and MMP-9−/− mice.
Figure 2. Invasive behavior of malignant mouse keratinocytes (PDVA cells) in vivo, three weeks after transplantation.
Tumor cells invade the host tissue of wild-type (A), MMP-3−/− (B), MMP-9−/− (C), MMP-2−/− (D), and MMP-3−/−; MMP-9−/− (E), but not MMP-2−/−; MMP-9−/− mice (F). Sections were stained with hematoxylin eosin. C: carcinoma cells; G: collagen gel: H: host tissue. Original magnification: 200×.
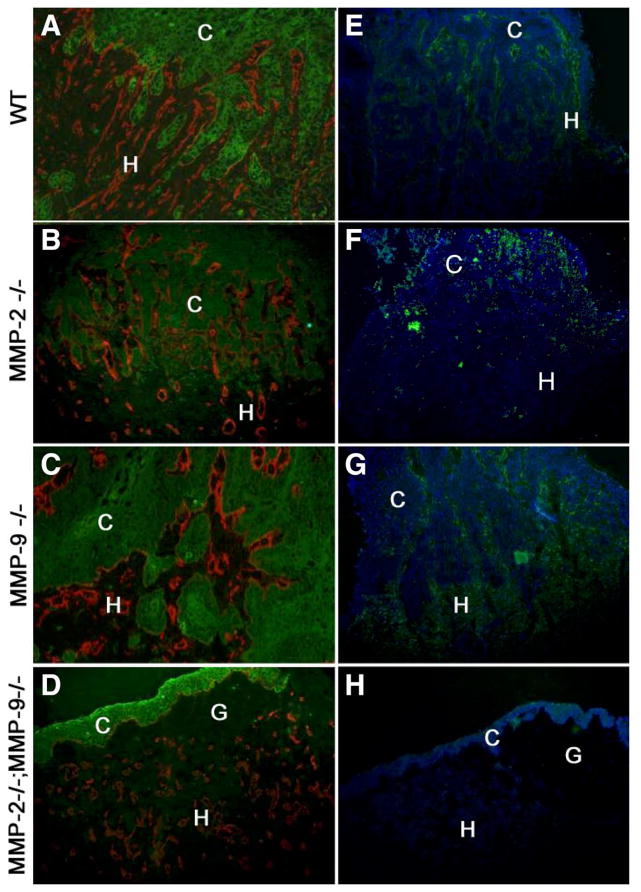
Figure 3. Invasive and angiogenic properties of malignant mouse PDVA keratinocytes in vivo.
Cells were transplanted for three weeks into (A, E) wild-type, (B, F) MMP-2−/−, (C, G) MMP-9−/−, or (D, H) MMP-2−/−; MMP-9−/− mice. (A–D) Malignant cells were stained with antikeratin Ab (green) and vessels with anti-type-IV collagen (red). Collagen type-IV collagen labeling was codistributed with immunostaining of endothelial cells recognized by anti-mouse platelet endothelial cell adhesion molecule (data not shown). (E–H) In situ zymography-detecting gelatinolytic activity (green), cells were counterstained with bisbenzimide (blue). C: carcinoma cells; G: collagen gel: H: host tissue. Original magnification: (A–D) 200×, (E–H) 100×.
Because MMP-9 can be activated by MMP-3, we evaluated the impact of the lack of an MMP acting upstream in the MMP activation cascade, by transplanting malignant cells into MMP-3−/− (n=6), MMP-3+/+ (n=6), double MMP3−/−:MMP-9−/− mice (n=7) and their corresponding wild-type (n=7) mice (Fig. 2B, E). The invasive and angiogenic phenotypes of malignant keratinocytes were preserved in these single- or double-MMP-deficient mice. Tumors were scored ++ (n=3 and n=4 in MMP-3+/+ and MMP-3−/− mice, respectively) and scored +++ (n=3 and n=2 in MMP-3+/+ and MMP-3−/− mice, respectively). Similar vascularization was also observed in double MMP-3−/−:MMP-9−/− mice (n=4 scored ++ and n=3 scored +++) and in their control mice (n=3 scored ++ and n=4 scored +++)
A combined deficiency of MMP-2 and MMP-9 impairs tumor invasion and vascularization
Tumor cells transplanted into MMP-2−/− mice (n=9) recruited angiogenesis (n=5 scored ++ and n=4 scored +++) and infiltrated into host tissue to similar extents as observed in wild-type mice (n=8) (n=4 scored ++ and n=4 scored +++) (Fig. 2D). In sharp contrast, when malignant cells were transplanted into double MMP-2−/−:MMP-9−/− mice (n=6), host-derived blood vessels were unable to migrate toward the tumor cell layer and remained confined beneath the collagen gel (all tumors were scored 0).
Even though the host alone was MMP-deficient, the tumor cells failed to invade the collagen gel and remained as an irregular stratified epithelium on top of the matrix (Fig. 2F, Fig. 3D). In situ zymography revealed that the gelatinolytic activity was confined to the host tissue and no activity could be detected in tumor layers (Fig. 3). This activity was completely absent in double MMP-2:MMP-9 deficient mice (Fig. 3H), strongly reduced in MMP-2−/− mice (Fig. 3F), but still evident in MMP-9−/− mice (Fig. 3). Of interest is the finding that gelatinolytic activity was differently distributed in each gelatinase deficient mice: largely distributed in the stroma of MMP-9−/− mice and much more dispersed in MMP-2−/− mice, in keeping with stromal expression of MMP-2 and inflammatory cell (neutrophil) expression of MMP-9.
Deficiency of MMPs does not impair the angiogenesis in vitro
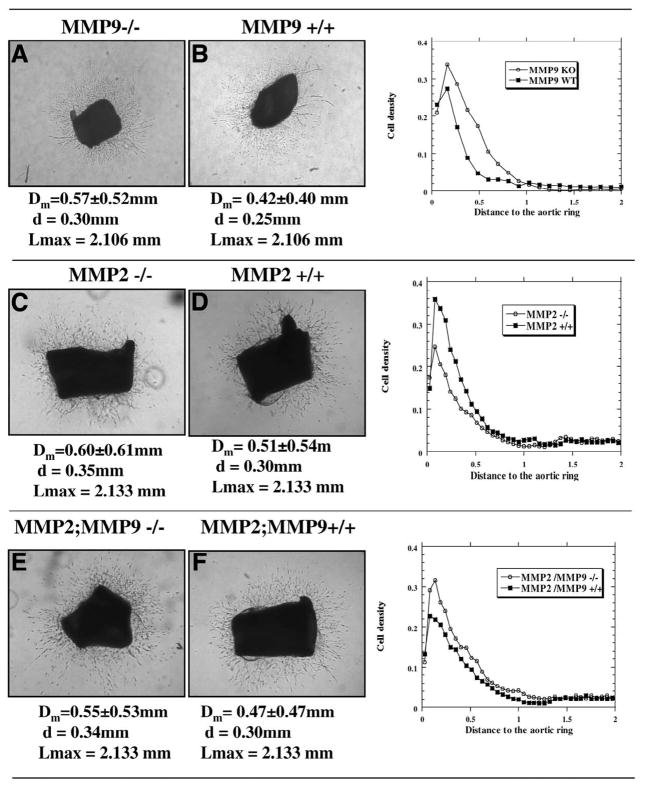
To determine whether the impact of MMP deficiency in angiogenesis was a direct effect on vascular cells or was indirect through the stromal and inflammatory cells present in the growing tumor mass, we next evaluated in vitro angiogenesis by using the aortic ring assay. We compared aortic explants resected from the MMP-deficient mice and their corresponding wild-type mice that were cultured in collagen gels. As early as day 4, small microvessels spread out from the aortic rings and isolated fibroblast-like cells migrated into the gel from explants from all the transgenic mice. At day 6, neoangiogenesis was maximal and similar vascular networks were observed in MMP-deficient and wild-type mice (Fig. 4). Interestingly, the double MMP-2:MMP-9 deficiency did not affect vessel outgrowth which was similar to that observed with wild-type mice. Expression of MMP-9 by vascular cells was assessed by using aortic rings from MMP-9/LacZ mice. Under the culture conditions, we observed β-galactosidase positive staining in microvessels spreading out and identified the activity of MMP-9 promoter in endothelial cells (data not shown). This clearly indicates that MMP-9 is expressed during the course of endothelial cells sprouting.
Figure 4. Microvascular outgrowth from mouse aortic rings after 6 days of culture.
A–D) Phase contrast microscopy showing capillary like structures spreading out from aortic rings obtained from (A) MMP-2+/+, (B) MMP-2−/−, (C) MMP-9+/+, (D) MMP-9−/−, (E) MMP-2+/+; MPP-9+/+ and (F) MMP-2−/−; MPP-9−/− mice. Statistical parameters of quantification based on image analysis (mean values ± SD) are indicated: Dm, mean distribution; d, mode; and Lmax, maximal length. The graphs illustrate the distribution of spreading cells around the aortic rings. Original magnification: (A, B, E, F) 50×, (C, D) 25×. Quantification was performed in triplicate cultures from 2 independent assays.
Objective quantification of cell spreading was performed by computer-assisted image analysis. The distribution of spreading cells around the aortic ring was characterized by determining the statistical parameters described in Materials and Methods: mean distribution (Dm), mode (d) and maximal length (Lmax). None of these different parameters was affected by gelatinase deficiency (P>0.05) (Fig. 4). However, comparison of the in vitro aortic ring assay and the in vivo malignant keratinocyte angiogenic phenotype clearly indicates that MMP-2 and MMP-9 are not absolute prerequisites for collagen penetration by activated endothelial cells per se.
DISCUSSION
In the present study, we evaluated the contribution of three host MMPs (MMP-2, MMP-3, and MMP-9) to the invasion and vascularization of malignant keratinocyte tumors transplanted into syngeneic MMP-deficient mice. The angiogenic and invasive phenotype of malignant cells was not affected by the single deficiency of host MMP-2, MMP-3, or MMP-9, or the combined deficiency of MMP-3 and MMP-9. Thus, although MMP-3 has a skin wound healing phenotype (32) and can activate MMP-9, acting upstream in the cascade of MMP activation (2), it has no significant contribution in this tumor model. However, we provide evidence that both MMP-2 and MMP-9 contribute to tumor invasion and vascularization. Tumor invasion and angiogenesis were impaired by the combined deficiency in both gelatinases.
In other models, the single deficiency of one of the gelatinase gene demonstrated a partial role of either MMP-9 or MMP-2 in the angiogenic switch (16, 17, 19, 22, 33). We have recently reported the synergistic contribution of MMP-2 and MMP-9 to choroidal neoangiogenesis induced by a laser burn (34). While both incidence and severity of choroidal angiogenesis were partially affected in single MMP-deficient mice, they were strongly attenuated in double-deficient mice.
A direct role for MMP-9 in the regulation of angiogenesis, at least via a modulation of vascular endothelial growth factor (VEGF) availability, has been reported in previous studies (6, 16, 17). The important contribution of MMP-9 in cancer progression has been further supported by the correlation of a low level of active MMP-9 and a suppression of spontaneous tumor growth in thrombospondin-deficient mice (35, 36). However, MMP-9 also suppresses tumor angiogenesis through production of angiostatic fragments of basement membrane collagens (36, 37).
We detected MMP-9 promoter activity in vivo in the host stroma, when we transplanted MMP-9/LacZ mice with malignant keratinocytes, as demonstrated by β-galactosidase staining. However double immunostaining showed that neutrophils are the main cellular source of MMP-9 in our model. This is consistent with previous studies of inflammatory processes such as asthma (38, 39). The inflammatory cell origin of MMP-9 in experimental tumor models has already been reported. Indeed, MMP-9 has been shown to be expressed by mast cells and neutrophils in a mouse model of skin carcinogenesis (16, 40) and by macrophages in breast and ovarian tumors (17, 41). Our finding supports the importance of neutrophils as the inflammatory cell origin of MMP-9 in an experimental cancer model.
Although MMP-9 promotor activity was detected in microvessels outgrowth from aortic rings issued from MMP-9/LacZ transgenic mice in vitro (data not shown), the single deficiency of MMP-9 did not affect microvessel sprouting. This observation highlights the importance of MMP-9 produced by inflammatory host cells.
Similarly to MMP-9 deficiency, the lack of MMP-2 did not affect tumor progression. Therefore, genetic ablation might underestimate the respective importance of MMP-2 or MMP-9 because of some compensatory response by the other gelatinase or other protease(s). In situ gelatin zymography revealed a partial and a strong reduction of gelatinolytic activity in MMP-9 or MMP-2 deficient mice, respectively, suggesting a lack of compensation of one gelatinase by the other. Although MMP-2 and MMP-9 are endowed with similar enzymatic activities in vitro, these MMPs may have distinct activities in vivo against nonmatrix substrates. For instance, MMP-9 is unable to cleave the MMP-2 cleavage site of monocyte chemoattractant protein-3 (8) and fibroblast growth factor receptor 1 (9), whereas MMP-2 is much poorer at cleaving type IV collagen α3 chain to generate tumstatin than MMP-9 (36).
The requirement of both MMP-2 and MMP-9 for successful tumor invasion and vascularization might reflect the necessity of specific interactions between stromal cells producing MMP-2 and inflammatory cells secreting MMP-9. The intact outgrowth of microvessels from aortic rings of mice with a combined gelatinase deficiency demonstrates that endothelial cell migration in a pure collagen matrix can occur in the absence of gelatinases. The normal in vitro microvessel outgrowth in combined deficient mice could be due to the lack of involvement of a gelatinase substrate in this in vitro model. In sharp contrast, in vivo, endothelial cell activation and migration might require gelatinases produced by other host cells to generate angiogenic or chemoattractant factors. Such a contribution of the two gelatinases produced by different cell types has been recently reported in a model of aneurysm (42). For aneurysm formation, both the local mesenchymal cell expression and the macrophage expression of gelatinases were required. Furthermore, we have previously shown that choroidal neoangiogenesis was fully prevented in double MMP-2−/−:MMP-9−/− mice, whereas it was only partly impaired in each single deficient mice (34). In our experimental cancer model, although both enzymes are separately provided by different host cell types, the enzymatic activity of a single gelatinase is sufficient to circumvent the absence of the other one. As assessed by in situ zymography, in both single MMP-2- and MMP-9-deficient mice, a gelatinolytic activity was detected in the host tissue, while it was completely absent in double-deficient mice. Therefore, a low level of gelatinolytic activity in the host tissue is likely to be sufficient to remodel the extracellular matrix or to activate growth factors or cytokines/chemokines leading to an appropriate microenvironment both for vessel sprouting and tumor invasion. Altogether, these studies underline the complexity of the dialogues occurring between inflammatory cells and mesenchymal cells which are likely to differ from a pathological condition to another.
In mice with combined deficiency of both MMP-2 and MMP-9 genes, pathological angiogenesis such as tumor angiogenesis and choroidal neovascularization (34) is significantly impaired. In sharp contrast, these mice develop normally suggesting that physiological angiogenesis was unaffected. This is the case for the effects of the MMP-9 generated antiangiogenic fragment, tumstatin, which only suppresses pathological angiogenesis, not physiological angiogenesis in wound healing or development (36). These observations highlight the difference between physiological and pathological angiogenic processes and are consistent with the differential effect of plasminogen activator inhibitor (PAI-1) deficiency in physiological and pathological angiogenesis (27, 43–46).
MMP-9-deficient mice exhibit an abnormal pattern of skeletal growth plate vascularization and ossification 3 weeks after birth. However, a skeletal of normal appearance is found in adult animals, suggesting that compensating mechanisms occur. This transient phenotype evokes a possible temporal regulation of MMP-9 and/or compensating mechanisms in adult life. In support of this hypothesis, autoimmune experimental models have shown a phenotype in MMP-9-deficient mice only in the early stage of life (47, 48).
Our data emphasize the fact that lack of requirement is not synonymous with lack of involvement in a specific process. Therefore, it is necessary to be cautious with conclusions as to function from single gene ablation of an MMP. When two enzymes have overlapping activities an apparently normal gross phenotype may be manifested, even though there may be significant molecular differences. Because of putative redundancy, it is crucial to determine when and which of the MMPs are the most important for tumor progression and metastasis dissemination. Indeed, first therapeutic approaches using broad spectrum MMP inhibitors have led to disappointing results (2, 16). It is likely that broad spectrum inhibitors might repress some beneficial effects such as, for instance, the generation of anti-angiogenic factors (e.g., angiostatin, endostatin, and tumstatin) (1) or the inactivation or activation of chemokines (49). Our study suggests that the specific and combined inhibition of MMP-2 and MMP-9 may be a promising strategy for anticancer treatment.
Acknowledgments
We thank I. Dasoul, P. Gavitelli, F. Olivier and G. Roland for their excellent technical assistance and J.S. Mudgett for providing us with the MMP-3 deficient mice. This work was supported by grants from the Communauté Française de Belgique (Actions de Recherches Concertées), the European Union (FP5 nº QLK3-CT2002-02136 and FP6 n° LSHC-CT-2003-503297), the Fonds de la Recherche Scientifique Médicale (FNRS), the Fonds National de la Recherche Scientifique (FNRS, Belgium), the Fédération Belge Contre le Cancer, the Fonds spéciaux de la Recherche (University of Liège), the Centre Anticancéreux près l’Université de Liège, the FB Assurances, the Fondation Léon Frédéricq (University of Liège), the D.G.T.R.E. from the “Région Wallonne,” the Interuniversity Attraction Poles Program - Belgian Science Policy (Brussels, Belgium), the National Cancer Institute USA CA072006 (to Z.W.). V.M., L.R., K.B. and M.J. are recipients of a grant from FNRS-Télévie. C. Munaut is a Research Associate from the FNRS (Belgium).
References
- 1.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 2.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 3.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shipley JM, Doyle GAR, Fliszar CJ, Ye QZ, Johnson LL, Shapiro SD, Welgus HG, Senior RM. The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. J Biol Chem. 1996;271:4335–4343. doi: 10.1074/jbc.271.8.4335. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen Q, Murphy G, Hughes CE, Mort JS, Roughley PJ. Matrix metalloproteinases cleave at 2 distinct sites on human cartilage link protein. Biochem J. 1993;295:595–598. doi: 10.1042/bj2950595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito A, Mukaiyama A, Itoh Y, Nagase H, Thogersen I, Enghild JJ, Sasaguri Y, Mori Y. Degradation of interleukin 1 beta by matrix metalloproteinases. J Biol Chem. 1996;271:14,657–14,660. doi: 10.1074/jbc.271.25.14657. [DOI] [PubMed] [Google Scholar]
- 8.McQuibban GA, Gong JH, Tam EM, McCulloch CAG, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 9.Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci USA. 1996;93:7069–7074. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998;290:S12–S23. doi: 10.1007/pl00007449. [DOI] [PubMed] [Google Scholar]
- 11.Munaut C, Salonurmi T, Kontusaari S, Reponen P, Morita T, Foidart JM, Tryggvason K. Murine matrix metalloproteinase 9 gene - 5′-upstream region contains cis-acting elements for expression in osteoclasts and migrating keratinocytes in transgenic mice. J Biol Chem. 1999;274:5588–5596. doi: 10.1074/jbc.274.9.5588. [DOI] [PubMed] [Google Scholar]
- 12.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 13.Sounni NE, Janssen M, Foidart JM, Noel A. Membrane type-1 matrix metalloproteinase and TIMP-2 in tumor angiogenesis. Matrix Biol. 2003;22:55–61. doi: 10.1016/s0945-053x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 14.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois B, Arnold B, Opdenakker G. Gelatinase B deficiency impairs reproduction. J Clin Invest. 2000;106:627–628. doi: 10.1172/JCI10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, Fidler IJ. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 18.Itoh T, Tanioka M, Matsuda H, Nishimoto H, Yoshioka T, Suzuki R, Uehira M. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin Exp Metastasis. 1999;17:177–181. doi: 10.1023/a:1006603723759. [DOI] [PubMed] [Google Scholar]
- 19.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 20.Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration arterial remodeling and geometrical. Circ Res. 2002;91:852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 21.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002;169:2643–2647. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- 22.Lambert V, Munaut C, Jost M, Noel A, Werb Z, Foidart JM, Rakic JM. Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am J Pathol. 2002;161:1247–1253. doi: 10.1016/S0002-9440(10)64401-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudgett JS, Hutchinson NI, Chartrain NA, Forsyth AJ, McDonnell J, Singer II, Bayne EK, Flanagan J, Kawka D, Shen CF, et al. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998;41:110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Fusenig N, Am SM, Boukamp P, Worst PK. Characteristics of chemically transformed mouse epidermal cells in vitro and in vivo. Bull Cancer. 1978;65:271–279. [PubMed] [Google Scholar]
- 25.Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 26.Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, Lund LR, Frandsen TL, Brunner N, Dano K, et al. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin: Implications for antiangiogenic strategies. J Cell Biol. 2001;152:777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajou K, Devy L, Masson V, Albert V, Frankenne F, Noel A, Foidart JM. Role of plasminogen activator inhibitor type 1 in tumour angiogenesis. Therapie. 2001;56:465–472. [PubMed] [Google Scholar]
- 28.Devy L, Blacher S, Grignet-Debrus C, Bajou K, Masson R, Gerard RD, Gils A, Carmeliet G, Carmeliet P, Declerck PJ, et al. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J. 2002;16:147–154. doi: 10.1096/fj.01-0552com. [DOI] [PubMed] [Google Scholar]
- 29.Masson V, Devy L, Grignet-Debrus C, Berndt S, Bajou K, Blacher S, Roland G, Chang Y, Fong T, Carmeliet P, et al. Mouse aortic ring assay: A new approach of the molecular genetics of angiogenesis. Biol Proced Online. 2002;4:24–31. doi: 10.1251/bpo30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blacher S, Devy L, Burbridge MF, Roland G, Tucker G, Noel A, Foidart JM. Improved quantification of angiogenesis in the rat aortic ring assay. Angiogenesis. 2001;4:133–142. doi: 10.1023/a:1012251229631. [DOI] [PubMed] [Google Scholar]
- 31.Blacher S, Devy L, Noel A, Foidart JM. Quantification of angiogenesis on the rat aortic ring assay. Image Anal Stereol. 2003;22:43–48. doi: 10.1023/a:1012251229631. [DOI] [PubMed] [Google Scholar]
- 32.Bullard KM, Lund L, Mudgett JS, Mellin TN, Murphy B, Ronan J, Werb Z, Banda MJ. Impaired wound contraction in stromelysin-1deficient mice. Ann Surg. 1999;230:260–265. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002;110:1105–1111. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambert V, Wielockx B, Munaut C, Galopin C, Jost M, Itoh T, Werb Z, Baker A, Libert C, Krell HW, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003;17:2290–2292. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA. 2001;98:12,485–12,490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang CQ, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha 3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alpha V beta 3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortega N, Werb Z. New functional roles for noncollagenous domains of basement membrane collagens. J Cell Sci. 2002;115:4201–4214. doi: 10.1242/jcs.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cataldo D, Tournoy KG, Vermalen K, Munaut C, Foidart JM, Louis R, Noel A, Pauwels RA. Matrix metalloproteinase-9 deficiency impairs cellular infiltration and hyper-responsiveness during allergen-induced airway inflammation. Am J Pathol. 2002;161:491–498. doi: 10.1016/S0002-9440(10)64205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cundall M, Sun YC, Miranda C, Trudeau JB, Barnes S, Wenzel SE. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucorticoids. J Allergy Clin Immunol. 2003;112:1064–1071. doi: 10.1016/j.jaci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 40.van Kempen LCL, Rhee JS, Dehne K, Lee J, Edwards DR, Coussens LM. Epithelial carcinogenesis: dynamic interplay between neoplastic cells and their microenvironment. Differentiation. 2002;70:610–623. doi: 10.1046/j.1432-0436.2002.700914.x. [DOI] [PubMed] [Google Scholar]
- 41.Man AK, Young LJT, Tynan JA, Lesperance J, Egeblad M, Werb Z, Hauser CA, Muller WJ, Cardiff RD, Oshima RG. Ets2-dependent stromal regulation of mouse mammary tumors. Mol Cell Biol. 2003;23:8614–8625. doi: 10.1128/MCB.23.23.8614-8625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longo GM, Xiong WF, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambert V, Munaut C, Noel A, Frankenne F, Bajou K, Gerard R, Carmeliet P, Defresne MP, Foidart JM, Rakic JM. Influence of plasminogen activator inhibitor type 1 on choroidal neovascularization. FASEB J. 2001;15:1021–1027. doi: 10.1096/fj.00-0393com. [DOI] [PubMed] [Google Scholar]
- 44.Rakic JM, Maillard C, Jost M, Bajou K, Masson V, Devy L, Lambert V, Foidart JM, Noel A. Role of plasminogen activator-plasmin system in tumor angiogenesis. Cell Mol Life Sci. 2003;60:463–473. doi: 10.1007/s000180300039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rakic JM, Lambert V, Munaut C, Bajou K, Peyrollier K, Alvarez-Gonzalez ML, Carmeliet P, Foidart JM, Noel A. Mice without uPA, tPA, or plasminogen genes are resistant to experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:1732–1739. doi: 10.1167/iovs.02-0809. [DOI] [PubMed] [Google Scholar]
- 46.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, Devos R, Vandenoord JJ, Collen D, Mulligan RC. Physiological consequences of loss of plasminogen-activator gene-function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 47.Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van den Oord J, Sciot R, Meinhardt T, Hammerling G, Opdenakker G, et al. Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest. 1999;104:1507–1515. doi: 10.1172/JCI6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, Shipley JM, Vu TH, Zhou XY, Diaz LA, Werb Z, Senior RM. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188:475–482. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, Lopez-Otin C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]