Abstract
Toll-like receptors (TLR) play a central role in the initiation of the innate immune response to pathogens. Upon recognition of molecular motifs specific for microbial molecules TLR mediate pro-inflammatory cytokine secretion and enhance antigen presentation; in B cells they further promote expansion, class switch recombination and immunoglobulin secretion. As a result of their adjuvant properties, TLR ligands have become an integral component of antimicrobial vaccines. In spite of this, little is known of the direct effects of TLR engagement on B-lymphocyte function. The scope of this review is to outline the differences in TLR expression and reactivity in murine and human B-cell subsets and to provide an overview of the currently available literature. We will further discuss the possible roles of TLR in regulating B-cell effector functions and shaping antibody-mediated defence against microbial pathogens in vivo.
Keywords: antibody responses, B cells, bacteria, Toll-like receptors, viruses
Introduction
Among the pattern recognition receptors Toll-like receptors (TLR) represent an important receptor family involved in the recognition of molecular structures specific for microbial pathogens [reviewed in refs 1–3). They can be grouped into two main categories: (i) cell surface receptors and (ii) receptors localized in the endosome. It is important to make this distinction because surface TLR bind molecules on the bacterial cell wall such as bacterial lipopeptides (TLR2) or lipopolysaccharide (LPS; TLR4), whereas endosomal TLR that are activated by microbial nucleic acids are less readily accessible. This difference in subcellular localization translates into distinct functions within the antimicrobial immune response.
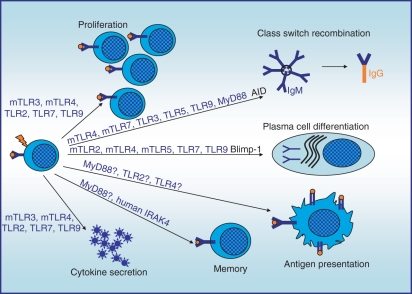
In B cells, TLR activation results in the up-regulation of activation markers, proliferation, cytokine secretion, terminal differentiation and, finally, immunoglobulin secretion. Figure 1 summarizes the main B-cell functions, and denotes the murine and human TLR involved. Engagement of TLR results in myeloid differentiation primary response protein 88 (MyD88) -dependent activation of nuclear factor-κB (NF-κB), mitogen-activated protein kinase and protein kinase B (PKB)/Akt signalling pathways,4–6 as well as Toll/interleukin-1 receptor (TIR)-domain-containing adaptor inducing interferon-β (TRIF)-mediated interferon-β (IFN-β) induction upon TLR3 or murine TLR4 activation.7,8 Yet, it is currently unknown whether the TLR2/4-associated adapter proteins Mal/TIRAP and TRAM play a role in B-cell activation.
Figure 1.
Role of Toll-like receptor (TLR) in B-cell functions. The graph depicts the main functions exerted by the B cell. The TLR that have been proven to impact these functions are indicated above the arrows. mTLR = murine TLR; hTLR = human TLR; TLR = murine and human TLR. Question marks (?) indicate that a function has not been addressed in B cells but may be postulated based on data obtained in other cellular systems or from clinical case studies.
Furthermore, because of the paucity of studies, little is known of the molecular events integrating TLR signalling into the classical B-cell receptor (BCR) -mediated signalling cascades leading to B-cell activation. Only a few groups have made an attempt to distinguish BCR-signalling from TLR-signalling; these studies indicate that there is significant overlap between these pathways.9–12 However, in murine B cells TLR signalling via MyD88 abolishes the requirement for BCR-mediated recruitment of Bruton’s tyrosine kinase (Btk) and phosphoinositide 3-kinase (PI3K) for Erk phsophorylation.9,12 What is more, there is an increasing body of evidence indicating that TLR play a major role in determining the degree of B-cell activation and the quality of B-cell effector functions. The scope of this review is therefore to provide an overview of the current literature addressing TLR expression and function in murine and human B cells, and to discuss the significance of the available findings.
Differences in B-cell expression of TLR in mouse and man
Several studies have addressed TLR expression patterns and their functions in different B-cell subsets. Expression of TLR on B lymphocytes varies depending on the mammalian species so the authors of this review have chosen to focus on the comparison of TLR expression and function in murine and human B cells. The differences are outlined in Table 1. The most prominent difference lies in TLR4 expression. Murine B cells constitutively express TLR4. Its ligand, LPS, stimulates B-cell proliferation, cytokine secretion and class switch recombination (CSR).13–15 In marked contrast, human B cells are irresponsive to LPS, which is reflected by the absence of TLR4 expression.16,17 Similarly, murine B cells are strongly responsive to stimulation with TLR2-active lipopeptides,18 but human B cells need to be sensitized for TLR2 ligands by cross-linking of the BCR with anti-immunoglobulin or protein A from Staphylococcus aureus.19,20 In both immune systems TLR2 is expressed in association with its co-receptors TLR1 and TLR6, whereas CD36 expression has only been described in murine B cells,21 and expression of the putative co-receptor TLR10 is limited to human B cells.22
Table 1.
B-cell expression and function of Toll-like receptor (TLR) in human and murine B cells
| Ligand | Expression in human B cell | Function in human B cell | Expression in murine B cell | Function in murine B cell | |
|---|---|---|---|---|---|
| Surface TLR | Ref. 17,23,24 | Ref. 25–27 | |||
| TLR1 | +++ | co-receptor for TLR2 | + | co-receptor for TLR2 | |
| TLR2 | lipopeptides | ++ | proliferation (with BCR stimulus) Ref. 19,20,38,39 | +++ in B1-B? Ref. 27 | proliferation, differentiation, migration Ref. 18,25,35 |
| TLR6 | ++ | co-receptor for TLR2 | + | co-receptor for TLR2 | |
| TLR10 | +++ | ? Ref. 22 | − | − | |
| TLR4 | LPS | (+) | − Ref. 16 | + | proliferation, differentiation, CSR, migration Ref. 13–15,25,33–35 |
| TLR5 | Flagellin | + (PP B cells) Ref. 40,41 | CSR Ref. 40 | (+) Ref. 27,32 | differentiation Ref. 32 |
| Endosomal TLR | |||||
| TLR3 | dsRNA | + (mucosal B cells) Ref. 8 | CSR Ref. 8 | MZ B cells Ref. 25,26 | proliferation, differentiation, migration Ref. 7,35 |
| TLR7 | RNA | ++ | proliferation, differentiation upregulation of TLR7 by IFN-I Ref. 16,20 | ++ | proliferation, differentiation CSR, migration Ref. 25,35 |
| TLR8 | ssRNA | (+) | − | B1-B? Ref. 25 | ? |
| TLR9 | CpG DNA | ++ protein expression Ref. 43–45 | proliferation, differentiation CSR Ref. 16,40,42,49 | +++ | proliferation, differentiation CSR Ref. 25,33 |
The table summarizes the ligands, expression levels and function of TLR1–10 in human and murine B cells. Expression levels relate to messenger RNA expression. They are given as − (absent), (+) (weak to absent), + (weak), ++ (average) and +++ (strong). The term ‘differentiation’ accounts for plasma blast formation and immunoglobulin secretion. The reference numbers refer to those given in reference list.
BCR, B-cell receptor; CSR, class switch recombination; IFN-I, interferon type I; LPS, lipopolysaccharide.
Nevertheless, murine and human B cells express TLR7 and TLR9,16,17,23–27 and can be activated with specific DNA (TLR9) or RNA (TLR7) sequence motifs. Interestingly, in human B cells TLR7 expression depends on type I interferon (IFN-I) priming that up-regulates the de novo synthesis of TLR7 messenger RNA (mRNA) and sensitizes B cells for TLR7 ligands.16 More recently, expression of UNC-93B, a transmembrane endoplasmic reticulum protein, was found to be a prerequisite for the functional integrity of all nucleic acid-sensing TLR.28–30 Interestingly, UNC-93B is highly expressed in murine B cells30 but was not detected in human B cells.31 Further data indicated that human nucleic acid-sensing TLR may at least partially act independently of UNC-93B.31
Taken together, murine B cells are more prone to respond to TLR stimulation than their human counterparts because of their expression of the easily accessible surface TLR (TLR2 and TLR4). On the contrary, the exclusive presence of the poorly accessible endosomal TLR (TLR7 and TLR9) accompanied by the lack of UNC-93B in human B cells suggests that in the human immune system TLR-mediated activation of B lymphocytes may be tightly regulated to avoid an overshooting immune response. We can only speculate whether this implies that TLR fulfil a highly specialized and crucial effector function in human B-cell activation.
TLR expression and responsiveness of murine B-cell subsets
TLR expression and responsiveness vary depending on the B-cell subset. Not surprisingly, most of the experimental data available on this topic are derived from studies on murine B-cell subsets. Three detailed studies show that TLR4 and TLR1 mRNA expression is detected in all B-cell subpopulations with only a little variability.25–27 In marked contrast, TLR7 and TLR9 are present in all subpopulations although expression levels vary depending on the B-cell subset and the study. Interestingly, in two studies TLR3 mRNA expression was only detectable in marginal zone (MZ) B cells25,26 although in one study TLR3 mRNA was present in all B-cell subpopulations. Moreover, one report showed that TLR2 expression is most prominent in B-1 B cells.27 Also, TLR8 mRNA expression was detected in B-1 B cells in another report.25 Furthermore, TLR5 mRNA expression was absent in two studies but was described in the third study and elsewhere.27,32
Functional analyses of TLR responsiveness in different murine B-cell subpopulations revealed that proliferative responses upon activation of TLR2, TLR7 and TLR9 were detectable in follicular and MZ B cells.25 However, significant proliferation rates in response to stimulation with LPS were only measured in MZ B cells. Interestingly, differentiation into antibody-secreting cells was not linked to proliferation:25 despite only low proliferation levels in response to all TLR stimuli tested in B-1 B cells, TLR2, TLR4, TLR7 and TLR8 activation resulted in a strong induction of immunoglobulin M (IgM) secretion25 and correlated with rapid induction of Blimp-1, a transcription factor promoting plasma cell differentiation in this B-cell subpopulation.33 Furthermore, TLR9-triggered differentiation was found to be restricted to B1 and MZ B-cell subsets,25 although TLR9 agonists induced proliferation in follicular B cells. This was in line with the finding that significant IgM and IgG secretion from follicular B cells was only observed after stimulation with TLR2 and TLR4 agonists, but not in response to TLR7 or TLR9 ligands.33 Moreover, MZ B cells migrated in vivo in response to TLR2, TLR3, TLR4 and TLR7 activation,34,35, and were potently triggered to secrete IgM and IgG upon engagement of TLR2, TLR4, TLR7 and TLR9 in vitro.25
Overall, major differences in TLR expression patterns in murine B-cell subsets exist despite significant variability among the available studies. Differences in the sorting procedures, or in the the age, infection status or genetic background of the mice used in these studies may explain these variations. Most importantly, stimulation of the same TLR in two different B-cell subpopulations does not necessarily result in identical effector functions. This is exemplified by TLR9 activation that promotes a proliferative response in the absence of differentiation in follicular B cells, and terminal differentiation in the absence of proliferation in B-1 B cells. Future studies will have to clarify the underlying mechanisms.
Cellular targets of TLR stimulation in human B cells
Although a contribution of TLR activation to the progression of autoimmune disease and B-cell leukaemia has been propagated,36,37 only few studies address the role of TLR in human B-lymphocyte function. Interestingly, in the human body the local environment seems to shape the TLR repertoire: TLR2, TLR3 and TLR9 expression and responsiveness towards the respective ligands is increased in B cells isolated from tonsils when compared with those isolated from peripheral blood.8,38,39 Furthermore, despite TLR5 mRNA expression having only been reported in peripheral blood B cells, functionally relevant TLR5 expression was only described in B cells from Peyer’s patches.40,41 Furthermore, naïve human B cells are barely responsive to TLR stimulation, and, according to one study, express only low levels of TLR, whereas memory B cells are more reactive and more prone to proliferate and differentiate into plasma blasts upon TLR stimulation.4,16,23,42
Although the analyses of TLR expression patterns are most frequently based on mRNA expression studies, recent reports have included protein expression.8,43–45 Among these, two studies have described surface expression of TLR9 on two subsets of human B cells.43,45 Nevertheless, the functional significance of this finding and the B-cell subpopulations where this occurs remain ill-defined.43,45
Although CpG-containing oligodeoxynucleotides (ODN) have been propagated as polyclonal B-cell stimulators it is becoming more and more evident that only a few B-cell subsets respond to TLR9 stimulation with CpG-ODN. Antonio Lanzavecchia’s group was among the first to define the role of TLR in human naïve B-cell activation.20,23 They proposed that full-blown naïve B-cell activation requires three synergistically acting stimuli: (i) antigen-mediated BCR activation, (ii) T-cell co-stimulation via CD40 activation and (iii) TLR engagement, as depicted in Fig. 2(b). In spite of this, several groups have described human naïve B-cell activation by single TLR stimulation, mainly using TLR9-activating ODN.4,16,46–48 Furthermore, CpG-ODN supported productive CSR in naïve human B cells in the presence of recombinant interleukin-10 (IL-10).46
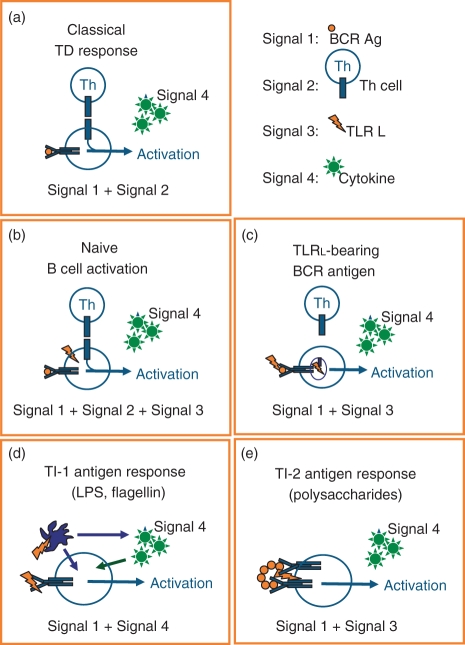
Figure 2.
Models for Toll-like receptor (TLR) -mediated B-cell activation. The graphs summarize different models for the role of TLR in B-cell activation in different B-cell subsets or environments that have been presented in this review. Because cytokines can modulate all types of B-cell responses they are depicted in all graphs as Signal 4. (a) This graph depicts the classical T-cell-dependent response where sole B-cell receptor (BCR) ligation (Signal 1) induces apoptosis and sets the requirement for T-cell co-stimulation via CD40–CD40 ligand interaction (Signal 2) which then allows B-cell expansion. (b) Based on the concept proposed by Lanzavecchia and colleagues naïve B cells require three signals for potent activation: (i) BCR (Signal 1), (ii) CD40 ligation (Signal 2) and (iii) TLR (Signal 3). (c) The model suggested by Marshak-Rothstein and colleagues implies that a TLR ligand (nucleic acid) -bearing BCR stimulus promotes the internalization of BCR antigen (Signal 1) together with the TLR-activating nucleic acid (Signal 3), which thereby gains access to endosomally localized TLR. Additional co-stimulation via CD40–CD40 ligand (Signal 2) interaction is, of course, possible. (d) T-cell-independent type I (TI-1) antigens such as lipopolysaccharide (LPS) or flagellin concomitantly activate specific BCRs as BCR antigens (Signal 1) and the respective surface TLR on accessory cells, e.g. antigen-presenting cells (APC). The APC will either directly interact with B cells or indirectly modulate B-cell function via cytokine secretion (Signal 4). (e) T-cell-independent type II (TI-2) antigens are carbohydrate polymers that are recognized by specific BCRs in B-cell subsets such as marginal zone B cells where BCR bind capsular polysaccharides (Signal 1). As a result of the nature of the antigen surface, TLR ligands are co-expressed and provide B-cell co-stimulation via TLR Signal 3.
In a recent report Rita Carsetti’s group characterized transitional (CD24bright CD27− CD38+) human B cells as the main non-memory B-cell subset responsive to TLR9 activation within the CD27-negative B-cell fraction.49 Stimulation of TLR9 triggered differentiation of this B-cell subpopulation to antibody-secreting cells. These findings are further supported by a report stating that the stimulation of TLR4 or TLR9 in murine transitional 1 B cells promotes CSR and the development of antibody-secreting cells, and a second study suggesting that TLR9 activation triggers proliferation of murine transitional 2 B cells.50,51
Recently, we and others have described human IgM+ CD27+ B cells as the main target cells for immunostimulatory DNA ODN.4,49 It has previously been proposed that these cells represent the human equivalent of the murine MZ B-cell compartment.52–54 The TLR9 stimulation induced ZAP-70 expression, sustained proliferation and PKB/Akt expression as well as differentiation into plasma blasts (CD27high CD38high).4 Most importantly, class-switched memory B cells were irresponsive to CpG-ODN in this study. Additionally, CD27– IgD– effector B cells were described to accumulate in patients with autoimmune diseases, and were also shown to respond to CpG-ODN.55
In summary, both naïve and transitional human B cells have been demonstrated to be responsive to sole TLR7 or TLR9 stimulation. Engagement of TLR induces differentiation in the absence of significant proliferation in these B-cell subsets. A proliferative response to TLR7/9 ligands is predominantly observed in IgM+ memory B cells. Hence, B-cell activation by CpG-ODN in vivo may also be restricted to defined B-cell subpopulations, which may limit their clinical efficacy. Based on the model for human naïve B-cell activation depicted in Fig. 2(b) CpG-ODN-based tumour therapies could be improved by sensitizing antigen-specific B lymphocytes for TLR agonists through co-delivery of tumour-derived BCR antigens.
Accessing the endosome for TLR7/9 activation
Although the concept that BCR activation synergizes with TLR co-stimulation is well-acknowledged, the molecular basis for this synergy remains unexplained. Human B-cell activation initiated by BCR cross-linking, CD40–CD40 ligand interaction or TLR9 engagement has been reported to temporarily increase TLR7, TLR9 and TLR10 expression levels on resting human B cells.23,24 Hence, activation-induced de novo synthesis of TLR or TLR adaptor molecules may facilitate TLR signalling. Alternatively, intracellular redistribution of TLR has been discussed in this context.
Among those who addressed this topic the group of Ann Marshak-Rothstein defined the hallmarks of TLR-mediated B-cell activation in the autoimmune context. Using a murine cell line expressing a transgenic BCR that specifically binds the Fc portion of IgG2a, it was demonstrated that BCR-mediated uptake of an immune complex containing an anti-nucleic acid autoantibody with endogenous chromatin or RNA provided access to the endosome and thereby licensed the activation of TLR9 or TLR7, respectively.56–60 Most importantly, this process was independent of Fcγ receptor engagement, although this receptor is involved in nucleic acid uptake of immune complexes, subsequently triggering TLR7/9 activation in other cell types.61–64 The model proposed therefore implies that physiological TLR7/9 stimulation of B cells occurs upon BCR-mediated uptake of nucleic acid-containing antigens, as schematically depicted in Fig. 2(c). More recent results obtained in this experimental system indicate that simultaneous activation of the receptor for advanced glycosylated end products (RAGE) in the presence of its ligand HMGB1, a DNA-binding protein, may enhance BCR-triggered TLR9 activation.65
By comparing dual stimulation with anti-immunoglobulin for BCR activation and CpG-ODN to stimulation with chemically linked anti-immunoglobulin and CpG-ODN two further studies in murine B cells found that TLR9 stimulation is much more effective when TLR9 activation occurs subsequent to BCR-mediated endocytosis.66,67 Uptake of a nucleic acid-bearing BCR antigen was shown to be followed by an intracellular redistribution of TLR9 to the BCR-induced autophagosome.66 Interestingly, c-Jun N-terminal kinase (JNK) was found to be crucial for BCR and TLR9 entry to the late endosome, which provides the acidic and kathepsin-rich environment required for TLR7/9 activation.10
Although this work should be viewed as a continuation of the B-cell activation model proposed by Marshak-Rothstein and colleagues, it extends their findings from a primarily pathological setting, an autoimmune disorder, to a physiological context such as infection. In line with this concept it was very recently demonstrated that BCR-mediated uptake of whole bacteria into human B cells enables nucleic-acid-driven TLR9 activation and subsequent cell cycle entry.68
TLR-induced cytokine secretion
Many different cell types contribute to the regulation of immune responses by secreting cytokines that affect cell function in an autocrine or paracrine fashion. The TLR-stimulated B cells have been proposed to exert a regulatory function that prevents overshooting immune responses in murine autoimmune models and neonates, and this tolerogenic role has been attributed to IL-10 production by MZ and B-1 B cells.27,69–73 Moreover, murine IL-10 secretion was detected in MZ B cells in response to single TLR2, TLR4, TLR9 or combined TLR stimulation but was absent in splenic follicular B cells whereas IL-6 was detectable in both populations.27,69,70
Furthermore, among the murine B-cell subpopulations tested, only follicular B cells synthesized IFN-γ in response to stimulation with TLR9 ligands combined with TLR2, TLR4 or TLR7 agonists, indicating that cytokine secretion may depend on the differentiation stage.27 Several reports further indicate that murine B cells produce tumour necrosis factor, IL-6, IL-12, IL-23 and IL-27 in response to TLR engagement.51,69,71,74,75 Although TLR-induced secretion of pro-inflammatory cytokines and of IL-10 was shown to depend on the PI3K p110δ subunit,76 only IL-10 secretion was demonstrated to occur in a Btk-dependent manner.51,74,75
Human B cells are generally considered poor cytokine producers. Stimulation with TLR7 and TLR9 ligands results in the secretion of IL-10, IL-6 and IL-8.16,41,77,78 Further, IL-1β and IL-2 secretion have been detected in response to B-cell-specific CpG-ODN.41 In contrast to murine B cells, secretion of biologically active IL-12p70 can only be achieved by combining TLR9 ligand CpG DNA with CD40 ligation.79 Despite similar mechanisms of action B cell activating factor belonging to the TNF family (BAFF/BlyS) or a proliferation-inducing ligand (APRIL) cannot substitute for CD40 ligand in this setting (Bekeredjian-Ding, unpublished data), indicating that IL-12 production in human B cells remains a T-cell-dependent event.
Taken together these reports indicate that TLR elicit cytokine production in murine and human B cells. B lymphocytes can thereby control the ongoing immune response as demonstrated for IL-10. As a further example, IL-6 and IL-10 have been shown to block memory B-cell proliferation and to promote plasma cell differentiation.80,81 Toll-like receptor-induced secretion of these cytokines may therefore contribute to terminal differentiation.25,82,83
B-cell differentiation
Toll-like receptors were proposed to play a role in B-cell differentiation at early stages of B-cell development.84 Subsequently, it was demonstrated that B-cell precursor maturation in vitro is supported by the early exposure to TLR4 ligands but arrested upon stimulation of TLR2.85 Nevertheless, B-cell subpopulation analyses in MyD88-deficient mice did not reveal any important alterations.86 TRIF-deficient mice, however, displayed a modest reduction in B-1a cells, a defect that is well compatible with the finding that MZ B cells and B-1a cells are moderately reduced in IFNaR−/− and STAT1−/− mice.86 Therefore, TLR-induced type I interferons may influence the development and/or survival of B-cell subsets that are yet to be defined.
In marked contrast, there is strong evidence that TLR regulate the differentiation of transitional and mature B cells. Differences in plasma cell differentiation in the murine B-cell subsets have already been addressed above. TLR-mediated activation of the transcription factor NF-κB is believed to be sufficient to promote the expression of Blimp-1, a transcription factor crucial for plasma cell and pre-plasma memory B-cell differentiation.81,87 Additionally, a recent study suggested a role for TRAF3 in TLR-mediated and antigen-mediated plasma cell differentiation and IgG secretion.88 In spite of these findings, the reports on TLR-induced B-cell differentiation remain controversial because TLR4-induced or TLR9-induced plasma cell formation was not observed in an in vivo study.89
There are no publications available on the persistence of TLR expression and the role of TLR in plasma cells. Our own unpublished analyses, however, indicate that TLR1, TLR7, TLR9 and TLR10 are retained in plasma cells generated in vitro and that stimulation with CpG-ODN increases plasma cell survival and IgG secretion. Previously published findings on the role of TLR in their malignant counterpart multiple myeloma cells further support these observations: TLR expression in multiple myeloma cells resembles that described in B cells and stimulation of the multiple myeloma cells with synthetic TLR ligands results in proliferation and survival.36,90,91 Direct plasma cell stimulation by TLR ligands may therefore contribute to the amplification and the maintenance of humoral immune responses.
Modulation of CSR in vitro
Toll-like receptor activation has been reported to promote the expression of activation-induced cytidine deaminase and CSR in murine and human B cells.13,14,46,49 In murine B cells the TLR4 ligand LPS promotes the CSR of naïve B cells to IgG2b and IgG3, and to IgG1 and IgE when combined with IL-4.13–15 In the human immune system Andrea Cerutti’s group was the first to find that CSR to IgG is induced when naïve B cells are cultured in the presence of IL-10 and stimulated with CpG-ODN.46 In their study, CSR was enhanced when B cells were co-incubated with BAFF/BlyS or CD40 ligand. Furthermore, CSR to IgG and IgA was observed when human B cells isolated from tonsils were challenged with TLR3 ligand PolyI : C and BAFF.8 Moreover, the same group reported that T-cell-independent CSR to IgA2 is most efficient in human B cells treated with the TLR5 ligand flagellin or TLR9 ligand CpG DNA in the presence of APRIL.8,40
Additionally, in murine B cells, TLR9 stimulation can inhibit LPS + IL-4-mediated CSR to IgG1 and IgE and promote IgG2a, IgG2b and IgG3 production.92–94 The latter finding is believed to be the result of direct induction of the transcription factor T-bet.95 Suppression of IgG1 and IgE synthesis may be mediated by an inhibition of NF-κB and/or IFN regulatory factor 4 activity.92 This finding is compatible with the observation that mice deficient in different NF-κB subunits display reduced switching to IgG1, IgG3, IgE and IgA, depending on the missing subunit.96–100 In spite of this, the exact molecular mechanisms regulating these TLR effects remain elusive.
Modulation of antibody production in vivo
Adjuvanticity
The TLR ligands have become integral components of antimicrobial and anti-tumoral vaccines, but explaining their efficacy is not within the scope of this review and has been extensively reviewed elsewhere.101–104 Highlighting the adjuvant potential of TLR2 in infection with Streptococcus pneumoniae, TLR2-dependent secretion of pro-inflammatory cytokines was found to play a key role in promoting both anti-protein and anti-polysaccharide responses of all IgG isotypes.105 Moreover, Won et al.21 observed a reduction in anti-phosphocholine (PC) IgM when TLR2-deficient mice were challenged with S. pneumoniae, and severely decreased anti-PC IgG in TLR2−/− CD36−/− double knockout mice, indicating that co-operation of these receptors may be important for plasma cell generation or IgG secretion in response to pneumococci. In spite of these studies, this issue remains controversial because normal IgM and IgG responses to Borrelia have been reported in TLR2-deficient and MyD88-deficient mice.106,107
Only recently, two studies asking whether TLR activation is required for antibody responses in adjuvant-supported vaccination against a specific antigen were conducted. The first study was performed by David Nemazee’s group.108,109 MyD88−/− TRIF−/− double knockout mice were challenged with trinitrophenol (TNP)-haemocyanin or TNP-keyhole limpet haemocyanin for T-cell-dependent responses or with TNP-Ficoll for TI-2 responses in the presence of four classical adjuvants: all antibody responses were intact. Notably, IgG2a/c and IgG2b levels were slightly decreased when compared with wild-type animals. However, IgG1 and IgE levels were consistently elevated and IgM levels persisted longer in one experiment although this was not commented on by the authors.
In the second study David Rawlings’ group included experiments evaluating the humoral memory response.110 Here, B-cell-deficient mice were used for adoptive transfer of MyD88−/− or wild-type B cells. After transfer of wild-type B cells LPS enhanced anti-NP (4-hydroxy-3-nitrophenylacetyl)-chicken gamma globulin (CGG)-specific IgM and IgG titres. Interestingly, the LPS-induced elevation of IgM was dependent on MyD88 expression in B cells and independent of MyD88 expression in accessory cells. However, increased IgG titres were dependent on TLR-mediated activation of accessory cells, and independent of B-cell expression of MyD88, a model that we propose for T-cell-independent type I responses (TI-1) as depicted in Fig. 2(d).
Taken together these studies supported the notion that TLR serve as adjuvants that accelerate early antigen-specific antibody responses but are dispensable for the long-term outcome of the antigen-specific immunoglobulin response. In the meantime this has also been confirmed by other groups.35 However, in an infection model using Borrelia hermsii pathogen-specific IgM production was fully intact in Btk-deficient and MyD88-deficient mice, but was abolished in MyD88−/− Btk−/− double knockout mice although these mice responded normally to alum-precipitated T-cell-dependent antigen.106 This indicated that recognition of microbial antigens via the BCR and via TLR is redundant under these circumstances. These findings may therefore provide the decisive key to the understanding of the vaccination trials described above.
Antibody diversity in the absence of MyD88 or nucleic acid-sensing TLR7 and TLR9
Moreover, the vaccination studies contradicted the initial report that MyD88 and TLR are essential for the regulation of adaptive immune responses, including the B-cell response.94 Using an OVA immunization model an elegant study by Ruslan Medhitzov’s group had provided evidence that immunized MyD88−/− mice display a skewed immunoglobulin isotype pattern completely lacking IgG2a, whereas IgG1 post-vaccination titres were only slightly reduced, and total IgE was markedly increased. Most importantly, a role of IL-1β or IL-18, which rely on MyD88 signalling, was excluded by studying caspase-1-deficient mice. In support of this study, MyD88-deficient mice also displayed elevated IgM and IgG1 titres but diminished type I antibodies (IgG2a/c, IgG2b and IgG3) after challenge with intact pathogens such as Borrelia burgdorferi, Leishmania major, influenza virus or S. pneumoniae.111–114
A subsequent publication from the Medhitzov group demonstrated that the humoral defect was not the result of disrupted T helper cell function or failure to produce IFN-γ in MyD88-deficient mice compared with wild-type mice.32 Adoptive transfer experiments of wild-type and MyD88-deficient B cells to μmT mice, that lack mature B cells, allowed the following conclusions: (i) IgE and IgA production were found to be independent of MyD88; (ii) IgM, IgG1 and IgG2c responses were dependent on the expression of MyD88 in B cells; (iii) IgG3 production in response to flagellin turned out to be independent of B-cell expression of MyD88, but dependent on MyD88 expression in accessory cells.
The following principles were derived from this study: T-cell-dependent B-cell responses require direct TLR-mediated B-cell activation as suggested by Lanzavecchia and colleagues (Fig. 2b). In marked contrast, TLR-mediated activation of non-B cells is a prerequisite for the T-cell-independent B-cell response to flagellin. B-cell activation itself is independent of MyD88 in this context. Taking into account that in Rawlings’ studies LPS-induced increases in IgG levels were dependent on MyD88 expression in accessory cells but not in B cells, that LPS primarily induces IgG3 production,115 and that LPS and flagellin are both TI-1 antigens, we propose that TI-1 responses are mediated via BCR recognition of the TI antigen and activation of accessory cells, most likely antigen-presenting cells, via the TI-1 antigen acting as a TLR ligand as depicted in Fig. 2(d).
Similar functions in the humoral immune response have been described for TLR7 and TLR9. First Jegerlehner et al.116 provided evidence that the T-cell-dependent response to virus-like particles is shifted to IgG2a, IgG2b and IgG3 with IgG1 titres being nearly absent when either TLR9 ligand CpG DNA or TLR7 ligand single-strand RNA were coupled to the virus-like particles. Second, Ravetch et al.117 used a murine lupus model to show that MyD88-deficiency and TLR9-deficiency both abrogated CSR to IgG2a and IgG2b, and so prevented disease manifestation that was mediated by deposition of autoreactive IgG2a and IgG2b. The data further suggested that defective switching could be the result of insufficient T-bet recruitment upon TLR9 engagement. These findings were supported by the observation that T-bet-deficient mice lack CSR to IgG2a, IgG2b and IgG3, but display elevated IgG1 levels,118 and a further publication that showed that TLR9-active ODN stimulate T-bet expression and promote CSR to IgG2a, but block CSR to IgG1 and IgE.119 Third, the finding that B-cell-deficient mice succumb to influenza virus infection120,121 indicated that antibody-mediated immune defence is crucial for the host response. It was subsequently demonstrated that TLR7-deficient mice display a partial defect in anti-influenza immunoglobulin responses that resembles that observed in MyD88-deficient mice, whereas T-cell responses were not affected by the targeted disruption of myd88 or tlr7 genes.112 Importantly, CSR to IgG2a/c turned out to be dependent on dual B-cell stimulation via CD40 and TLR7 in this context.
Consequently, TLR can be viewed as central players in fine-tuning the antibody-mediated defence. This function is not limited to an adjuvant effect accelerating immunoglobulin production, but rather represents an active intervention in the process of CSR.
TLR-mediated type I IFN induction
When challenged with influenza virus IFN-α/β−/− and IFN-αR−/− mice displayed a reduction in IgM, IgG2a, IgG2b and IgG3-secreting cells similar to that observed in MyD88-deficient and TLR7-deficient mice.112,122 This was accompanied by impaired plasma blast differentiation, which was previously described as a direct effect of IFN-I on B cells in response to TLR-mediated or influenza-mediated B-cell activation.42,83 Additionally, type I IFN-mediated induction of T-bet has been implicated in CSR to IgG,118 a finding that may result in the skewing of the IgG isotype distribution. Direct targeting of B cells via TLR and indirect TLR-mediated activation of IFN-α-secreting cells may therefore synergize in shaping the immunoglobulin isotype response to influenza virus. However, the exact role of type I IFN in direct or indirect regulation of the humoral immune response remains a controversial issue and according to the available data may depend on the type of infection or vaccination.122–125
Evidence for a role of TLR in human antibody responses
Three genetically defined immunodeficiency disorders resulting in impaired TLR signalling have been described: (i) interleukin-1 receptor-associated kinase 4 (IRAK4) deficiency, (ii) MyD88 deficiency and (iii) NF-κB essential modulator (NEMO) deficiency.126–130 Although all three molecules are important mediators of TLR signalling, it should be noted that MyD88 deficiency also affects IL-1R and IL-18R signalling, and that NEMO (also known as IKKγ), a key molecule in NF-κB activation, influences many other cellular signalling pathways. Clinically these immunodeficiencies are mainly associated with severe infections with Gram-positive bacteria, mostly S. aureus and S. pneumoniae, during childhood.
Taken together the two clinical case studies describing humoral immune defects in IRAK4-deficient and NEMO-deficient patients revealed a defective antibody response to pneumococcal polysaccharide vaccination but an intact primary response to T-cell-dependent antigen with normal responses to tetanus, diphtheria toxin and haemophilus influenzae B conjugate vaccines.126,131 The most striking abnormality, though, lay in the failure to maintain high levels of antigen-specific antibody titres, with pneumococcal polysaccharide antibody titres falling back to baseline within 5–6 months after vaccination boosts.
Interestingly, there is also one study describing a defect in long-term humoral immunity in MyD88-deficient mice.132 The authors observed a decrease in type I antibodies 2 weeks after immunization, and no further increase in antibody titres over 1·5 years. Levels of IgG1 were unaffected. Despite a normal germinal centre (GC) reaction they found reduced numbers and delayed migration of antigen-specific IgG-secreting cells to the bone marrow. In agreement with the observations made in IRAK-4-deficient patients, the authors concluded that MyD88 deficiency leads to a defect in mounting T-cell-independent IgG responses.
Although skewing of IgG isotypes to IgG1 may be interpreted in the supplemental data of the initial publication on MyD88-deficient patients the authors do not report any alterations. The data available only allow the conclusion that these patients mount antigen-specific antibody responses post vaccination and have normal CSR.130 Similarly, there is only scarce information available on the integrity of the humoral immune responses in patients with UNC-93B mutations.
Nucleic acid-sensing TLR are closely linked to type I IFN production. In the murine immune system mice defective in TLR7/9 signalling phenotypically resemble those lacking IFN-α/β receptors. As these patients fail to mount type I IFN responses it could be suspected that they also display altered antibody responses.28 Co-ordinated clinical studies will be crucial for the revelation of the mechanisms underlying increased susceptibility to infection with well-defined microbial pathogens in these patients.
Conclusions and perspectives
Over the past 10 years the awareness that TLR represent important regulators of a broad variety of B-cell effector functions has received increased consent. In spite of this, it remains obscure whether their role is essential to the humoral immune response in complex organisms such as the human or the mouse. In our opinion it is crucial to accept that TLR may play distinct roles in different B-cell subsets, differentiation stages or depending on the environment. This implies that the importance of TLR may vary depending on the immunological context. Figure 2 compares the classical model of B-cell activation through BCR antigen and T-cell co-stimulation mediated via CD40–CD40 ligand interaction (Fig. 2a) to four different models for B-cell responses using TLR activation (Fig. 2b–e).
The model proposed for the TI-1 response highlights the importance of related BCR and TLR ligand specificities (Fig. 2d). It thereby integrates the adjuvant properties of cytokines derived from TLR-activated accessory cells as modulating factors into the classical BCR-mediated B-cell activation pathways. Alternatively, accessory cells, such as antigen-presenting cells or epithelial cells, may influence B-cell function by directly interacting with the B lymphocyte. These possibilities will have to be evaluated in future experiments.
A spatial relationship of TLR ligands to BCR antigens is pivotal for endosomal TLR activation upon BCR-mediated uptake (Fig. 2c) as well as for the model depicting the T-cell-independent type 2 (TI-2) response (Fig. 2e): here, TLR ligands, e.g. TLR2-active bacterial lipopeptides, are co-expressed with multivalent TI-2 antigens such as pneumococcal polysaccharides. Clifford Snapper and colleagues favour the hypothesis that BCR engagement by TI-2 antigens sets the requirement for co-stimulation that is subsequently provided via a TLR signal. This implies that both substances directly target the B lymphocyte (Fig. 2e). Although it is an elegant model the experimental evidence does not exclude the possibility that B-cell differentiation could be driven by TLR-activated non-B cells, similarly to the model proposed for the TI-1 response.114–116 These discrepancies emphasize the need for further studies addressing this question.
Overall, future work will prove the validity of these models and provide a deeper insight into the exact role of TLR in the humoral immune response. From the authors’ point of view, studies using pathogenic organisms, e.g complex antigens, will be of particular value for a better understanding of the molecular events integrating TLR into the physiological and pathological regulation of B-cell activity and function. Major differences between mammalian species can be anticipated so studies in the human immune system will be of central interest.
Acknowledgments
I.B.D. is supported by the Olympia-Morata programme of the Medical faculty of the University of Heidelberg, the Fritz-Bender-Stiftung and a grant from the German Research Foundation DFG BE3841/2-1.
References
- 1.Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008;20:420–5. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 4.Bekeredjian-Ding I, Doster A, Schiller M, Heyder P, Lorenz HM, Schraven B, Bommhardt U, Heeg K. TLR9-activating DNA up-regulates ZAP70 via sustained PKB induction in IgM+ B cells. J Immunol. 2008;181:8267–77. doi: 10.4049/jimmunol.181.12.8267. [DOI] [PubMed] [Google Scholar]
- 5.Gerondakis S, Grumont RJ, Banerjee A. Regulating B-cell activation and survival in response to TLR signals. Immunol Cell Biol. 2007;85:471–5. doi: 10.1038/sj.icb.7100097. [DOI] [PubMed] [Google Scholar]
- 6.Richards S, Watanabe C, Santos L, Craxton A, Clark EA. Regulation of B-cell entry into the cell cycle. Immunol Rev. 2008;224:183–200. doi: 10.1111/j.1600-065X.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall-Clarke S, Downes JE, Haga IR, Bowie AG, Borrow P, Pennock JL, Grencis RK, Rothwell P. Polyinosinic acid is a ligand for toll-like receptor 3. J Biol Chem. 2007;282:24759–66. doi: 10.1074/jbc.M700188200. [DOI] [PubMed] [Google Scholar]
- 8.Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, He B, Chen K, Cerutti A. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol. 2008;181:276–87. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dye JR, Palvanov A, Guo B, Rothstein TL. B cell receptor cross-talk: exposure to lipopolysaccharide induces an alternate pathway for B cell receptor-induced ERK phosphorylation and NF-kappa B activation. J Immunol. 2007;179:229–35. doi: 10.4049/jimmunol.179.1.229. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill SK, Veselits ML, Zhang M, et al. Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic cells. Proc Natl Acad Sci USA. 2009;106:6262–7. doi: 10.1073/pnas.0812922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilker PR, Kohyama M, Sandau MM, Albring JC, Nakagawa O, Schwarz JJ, Murphy KM. Transcription factor Mef2c is required for B cell proliferation and survival after antigen receptor stimulation. Nat Immunol. 2008;9:603–12. doi: 10.1038/ni.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minguet S, Dopfer EP, Pollmer C, Freudenberg MA, Galanos C, Reth M, Huber M, Schamel WW. Enhanced B-cell activation mediated by TLR4 and BCR crosstalk. Eur J Immunol. 2008;38:2475–87. doi: 10.1002/eji.200738094. [DOI] [PubMed] [Google Scholar]
- 13.Lutzker S, Rothman P, Pollock R, Coffman R, Alt FW. Mitogen- and IL-4-regulated expression of germ-line Ig gamma 2b transcripts: evidence for directed heavy chain class switching. Cell. 1988;53:177–84. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- 14.Mandler R, Finkelman FD, Levine AD, Snapper CM. IL-4 induction of IgE class switching by lipopolysaccharide-activated murine B cells occurs predominantly through sequential switching. J Immunol. 1993;150:407–18. [PubMed] [Google Scholar]
- 15.Severinson E, Fernandez C, Stavnezer J. Induction of germ-line immunoglobulin heavy chain transcripts by mitogens and interleukins prior to switch recombination. Eur J Immunol. 1990;20:1079–84. doi: 10.1002/eji.1830200520. [DOI] [PubMed] [Google Scholar]
- 16.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–50. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 17.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 18.Borsutzky S, Kretschmer K, Becker PD, Muhlradt PF, Kirschning CJ, Weiss S, Guzman CA. The mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B lymphocytes via the TLR2 without the need of accessory cells. J Immunol. 2005;174:6308–13. doi: 10.4049/jimmunol.174.10.6308. [DOI] [PubMed] [Google Scholar]
- 19.Bekeredjian-Ding I, Inamura S, Giese T, Moll H, Endres S, Sing A, Zahringer U, Hartmann G. Staphylococcus aureus protein A triggers T cell-independent B cell proliferation by sensitizing B cells for TLR2 ligands. J Immunol. 2007;178:2803–12. doi: 10.4049/jimmunol.178.5.2803. [DOI] [PubMed] [Google Scholar]
- 20.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–6. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 21.Won WJ, Bachmann MF, Kearney JF. CD36 is differentially expressed on B cell subsets during development and in responses to antigen. J Immunol. 2008;180:230–7. doi: 10.4049/jimmunol.180.1.230. [DOI] [PubMed] [Google Scholar]
- 22.Hasan U, Chaffois C, Gaillard C, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174:2942–50. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 23.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 24.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–63. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 25.Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–86. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 26.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS ONE. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–53. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casrouge A, Zhang SY, Eidenschenk C, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–12. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 29.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–8. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 30.Tabeta K, Hoebe K, Janssen EM, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–64. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 31.Isnardi I, Ng YS, Srdanovic I, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–57. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 33.Fairfax KA, Corcoran LM, Pridans C, Huntington ND, Kallies A, Nutt SL, Tarlinton DM. Different kinetics of blimp-1 induction in B cell subsets revealed by reporter gene. J Immunol. 2007;178:4104–11. doi: 10.4049/jimmunol.178.7.4104. [DOI] [PubMed] [Google Scholar]
- 34.Gunn KE, Brewer JW. Evidence that marginal zone B cells possess an enhanced secretory apparatus and exhibit superior secretory activity. J Immunol. 2006;177:3791–8. doi: 10.4049/jimmunol.177.6.3791. [DOI] [PubMed] [Google Scholar]
- 35.Rubtsov AV, Swanson CL, Troy S, Strauch P, Pelanda R, Torres RM. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008;180:3882–8. doi: 10.4049/jimmunol.180.6.3882. [DOI] [PubMed] [Google Scholar]
- 36.Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, Bataille R, Jego G. Toll-like receptors: lessons to learn from normal and malignant human B cells. Blood. 2008;112:2205–13. doi: 10.1182/blood-2008-02-140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganley-Leal LM, Liu X, Wetzler LM. Toll-like receptor 2-mediated human B cell differentiation. Clin Immunol. 2006;120:272–84. doi: 10.1016/j.clim.2006.04.571. [DOI] [PubMed] [Google Scholar]
- 39.Mansson A, Adner M, Hockerfelt U, Cardell LO. A distinct Toll-like receptor repertoire in human tonsillar B cells, directly activated by PamCSK, R-837 and CpG-2006 stimulation. Immunology. 2006;118:539–48. doi: 10.1111/j.1365-2567.2006.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He B, Xu W, Santini PA, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–26. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Hanten JA, Vasilakos JP, Riter CL, Neys L, Lipson KE, Alkan SS, Birmachu W. Comparison of human B cell activation by TLR7 and TLR9 agonists. BMC Immunol. 2008;9:39. doi: 10.1186/1471-2172-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poeck H, Wagner M, Battiany J, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 43.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Pozzetto B, Richard Y, Garraud O. Identification of two subpopulations of purified human blood B cells, CD27(–) CD23(+) and CD27(high) CD80(+), that strongly express cell surface Toll-like receptor 9 and secrete high levels of interleukin-6. Immunology. 2008;125:430–37. doi: 10.1111/j.1365-2567.2008.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H. Expression of toll-like receptors on B lymphocytes. Cell Immunol. 2005;2:140–5. doi: 10.1016/j.cellimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 45.Eaton-Bassiri A, Dillon SB, Cunningham M, Rycyzyn MA, Mills J, Sarisky RT, Mbow ML. Toll-like receptor 9 can be expressed at the cell surface of distinct populations of tonsils and human peripheral blood mononuclear cells. Infect Immun. 2004;72:7202–11. doi: 10.1128/IAI.72.12.7202-7211.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–91. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 47.Huggins J, Pellegrin T, Felgar RE, et al. CpG DNA activation and plasma-cell differentiation of CD27-naive human B cells. Blood. 2007;109:1611–9. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37:2205–13. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 49.Capolunghi F, Cascioli S, Giorda E, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–8. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 50.Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178:3593–601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasan M, Lopez-Herrera G, Blomberg KE, Lindvall JM, Berglof A, Smith CI, Vargas L. Defective Toll-like receptor 9-mediated cytokine production in B cells from Bruton’s tyrosine kinase-deficient mice. Immunology. 2008;123:239–49. doi: 10.1111/j.1365-2567.2007.02693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein U, Kuppers R, Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood. 1997;89:1288–98. [PubMed] [Google Scholar]
- 53.Weller S, Braun MC, Tan BK, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–54. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willenbrock K, Jungnickel B, Hansmann ML, Kuppers R. Human splenic marginal zone B cells lack expression of activation-induced cytidine deaminase. Eur J Immunol. 2005;35:3002–7. doi: 10.1002/eji.200535134. [DOI] [PubMed] [Google Scholar]
- 55.Wei C, Anolik J, Cappione A, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178:6624–33. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 56.Busconi L, Bauer JW, Tumang JR, et al. Functional outcome of B cell activation by chromatin immune complex engagement of the B cell receptor and TLR9. J Immunol. 2007;179:7397–405. doi: 10.4049/jimmunol.179.11.7397. [DOI] [PubMed] [Google Scholar]
- 57.Lau CM, Broughton C, Tabor AS, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–7. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 59.Rifkin IR, Leadbetter EA, Beaudette BC, Kiani C, Monestier M, Shlomchik MJ, Marshak-Rothstein A. Immune complexes present in the sera of autoimmune mice activate rheumatoid factor B cells. J Immunol. 2000;165:1626–33. doi: 10.4049/jimmunol.165.3.1626. [DOI] [PubMed] [Google Scholar]
- 60.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–47. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 61.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin–immunoglobulin G complexes. J Exp Med. 2004;199:1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–72. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 63.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody–DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–17. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parcina M, Wendt C, Goetz F, Zawatzky R, Zahringer U, Heeg K, Bekeredjian-Ding I. Staphylococcus aureus-induced plasmacytoid dendritic cell activation is based on an IgG-mediated memory response. J Immunol. 2008;181:3823–33. doi: 10.4049/jimmunol.181.6.3823. [DOI] [PubMed] [Google Scholar]
- 65.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 66.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9-ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969–77. doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]
- 68.Jendholm J, Morgelin M, Perez Vidakovics ML, Carlsson M, Leffler H, Cardell LO, Riesbeck K. Superantigen- and TLR-dependent activation of tonsillar B cells after receptor-mediated endocytosis. J Immunol. 2009;182:4713–20. doi: 10.4049/jimmunol.0803032. [DOI] [PubMed] [Google Scholar]
- 69.Lampropoulou V, Hoehlig K, Roch T, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–73. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 70.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 71.Ng LG, Ng CH, Woehl B, Sutherland AP, Huo J, Xu S, Mackay F, Lam KP. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur J Immunol. 2006;36:1837–46. doi: 10.1002/eji.200635956. [DOI] [PubMed] [Google Scholar]
- 72.Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 2005;22:467–77. doi: 10.1016/j.immuni.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Deriaud E, Jiao X, Braun D, Leclerc C, Lo-Man R. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J Exp Med. 2007;204:1107–18. doi: 10.1084/jem.20062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee KG, Xu S, Wong ET, Tergaonkar V, Lam KP. Bruton’s tyrosine kinase separately regulates NFκB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B cells. J Biol Chem. 2008;283:11189–98. doi: 10.1074/jbc.M708516200. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt NW, Thieu VT, Mann BA, Ahyi AN, Kaplan MH. Bruton’s tyrosine kinase is required for TLR-induced IL-10 production. J Immunol. 2006;177:7203–10. doi: 10.4049/jimmunol.177.10.7203. [DOI] [PubMed] [Google Scholar]
- 76.Dil N, Marshall AJ. Role of phosphoinositide 3-kinase p110δ in TLR4- and TLR9-mediated B cell cytokine production and differentiation. Mol Immunol. 2009;46:1970–8. doi: 10.1016/j.molimm.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 77.Glaum MC, Narula S, Song D, Zheng Y, Anderson AL, Pletcher CH, Levinson AI. Toll-like receptor 7-induced naive human B-cell differentiation and immunoglobulin production. J Allergy Clin Immunol. 2009;123:224–30. doi: 10.1016/j.jaci.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 78.Malaspina A, Moir S, DiPoto AC, Ho J, Wang W, Roby G, O’Shea MA, Fauci AS. CpG oligonucleotides enhance proliferative and effector responses of B cells in HIV-infected individuals. J Immunol. 2008;181:1199–206. doi: 10.4049/jimmunol.181.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wagner M, Poeck H, Jahrsdoerfer B, et al. IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA. J Immunol. 2004;172:954–63. doi: 10.4049/jimmunol.172.2.954. [DOI] [PubMed] [Google Scholar]
- 80.Choe J, Choi YS. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur J Immunol. 1998;28:508–15. doi: 10.1002/(SICI)1521-4141(199802)28:02<508::AID-IMMU508>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 81.Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–69. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- 82.Chen-Kiang S. Regulation of terminal differentiation of human B-cells by IL-6. Curr Top Microbiol Immunol. 1995;194:189–98. doi: 10.1007/978-3-642-79275-5_23. [DOI] [PubMed] [Google Scholar]
- 83.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 84.Su TT, Guo B, Wei B, Braun J, Rawlings DJ. Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunol Rev. 2004;197:161–78. doi: 10.1111/j.0105-2896.2004.0102.x. [DOI] [PubMed] [Google Scholar]
- 85.Hayashi EA, Akira S, Nobrega A. Role of TLR in B cell development: signaling through TLR4 promotes B cell maturation and is inhibited by TLR2. J Immunol. 2005;174:6639–47. doi: 10.4049/jimmunol.174.11.6639. [DOI] [PubMed] [Google Scholar]
- 86.Wei B, Su TT, Dalwadi H, et al. Resident enteric microbiota and CD8(+) T cells shape the abundance of marginal zone B cells. Eur J Immunol. 2008;38:3411–25. doi: 10.1002/eji.200838432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–20. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 88.Zapata JM, Llobet D, Krajewska M, Lefebvre S, Kress CL, Reed JC. Lymphocyte-specific TRAF3-transgenic mice have enhanced humoral responses and develop plasmacytosis, autoimmunity, inflammation, and cancer. Blood. 2009;113:4595–603. doi: 10.1182/blood-2008-07-165456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richard K, Pierce SK, Song W. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo. J Immunol. 2008;181:1746–52. doi: 10.4049/jimmunol.181.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bohnhorst J, Rasmussen T, Moen SH, Flottum M, Knudsen L, Borset M, Espevik T, Sundan A. Toll-like receptors mediate proliferation and survival of multiple myeloma cells. Leukemia. 2006;20:1138–44. doi: 10.1038/sj.leu.2404225. [DOI] [PubMed] [Google Scholar]
- 91.Jego G, Bataille R, Geffroy-Luseau A, Descamps G, Pellat-Deceunynck C. Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia. 2006;20:1130–7. doi: 10.1038/sj.leu.2404226. [DOI] [PubMed] [Google Scholar]
- 92.Kusunoki T, Sugai M, Gonda H, et al. CpG inhibits IgE class switch recombination through suppression of NF kappa B activity, but not through Id2 or Bcl6. Biochem Biophys Res Commun. 2005;328:499–506. doi: 10.1016/j.bbrc.2004.12.192. [DOI] [PubMed] [Google Scholar]
- 93.Lin L, Gerth AJ, Peng SL. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004;34:1483–7. doi: 10.1002/eji.200324736. [DOI] [PubMed] [Google Scholar]
- 94.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 95.Gerth AJ, Lin L, Peng SL. T-bet regulates T-independent IgG2a class switching. Int Immunol. 2003;15:937–44. doi: 10.1093/intimm/dxg093. [DOI] [PubMed] [Google Scholar]
- 96.Doi TS, Takahashi T, Taguchi O, Azuma T, Obata Y. NF-κB RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J Exp Med. 1997;185:953–61. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horwitz BH, Zelazowski P, Shen Y, Wolcott KM, Scott ML, Baltimore D, Snapper CM. The p65 subunit of NF-κB is redundant with p50 during B cell proliferative responses, and is required for germline CH transcription and class switching to IgG3. J Immunol. 1999;162:1941–6. [PubMed] [Google Scholar]
- 98.Snapper CM, Rosas FR, Zelazowski P, Moorman MA, Kehry MR, Bravo R, Weih F. B cells lacking RelB are defective in proliferative responses, but undergo normal B cell maturation to Ig secretion and Ig class switching. J Exp Med. 1996;184:1537–41. doi: 10.1084/jem.184.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Snapper CM, Zelazowski P, Rosas FR, Kehry MR, Tian M, Baltimore D, Sha WC. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J Immunol. 1996;156:183–91. [PubMed] [Google Scholar]
- 100.Zelazowski P, Carrasco D, Rosas FR, Moorman MA, Bravo R, Snapper CM. B cells genetically deficient in the c-Rel transactivation domain have selective defects in germline CH transcription and Ig class switching. J Immunol. 1997;159:3133–9. [PubMed] [Google Scholar]
- 101.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 102.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–17. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 103.Krieg AM. Antiinfective applications of toll-like receptor 9 agonists. Proc Am Thorac Soc. 2007;4:289–94. doi: 10.1513/pats.200701-021AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–63. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 105.Khan AQ, Shen Y, Wu ZQ, Wynn TA, Snapper CM. Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae. Infect Immun. 2002;70:749–61. doi: 10.1128/iai.70.2.749-761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alugupalli KR, Akira S, Lien E, Leong JM. MyD88- and Bruton’s tyrosine kinase-mediated signals are essential for T cell-independent pathogen-specific IgM responses. J Immunol. 2007;178:3740–9. doi: 10.4049/jimmunol.178.6.3740. [DOI] [PubMed] [Google Scholar]
- 107.Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168:348–55. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 108.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–8. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nemazee D, Gavin A, Hoebe K, Beutler B. Immunology: Toll-like receptors and antibody responses. Nature. 2006;441:E4. doi: 10.1038/nature04875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007;204:3095–101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Debus A, Glasner J, Rollinghoff M, Gessner A. High levels of susceptibility and T helper 2 response in MyD88-deficient mice infected with Leishmania major are interleukin-4 dependent. Infect Immun. 2003;71:7215–8. doi: 10.1128/IAI.71.12.7215-7218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–91. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 113.Khan AQ, Chen Q, Wu ZQ, Paton JC, Snapper CM. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect Immun. 2005;73:298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu N, Montgomery RR, Barthold SW, Bockenstedt LK. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect Immun. 2004;72:3195–203. doi: 10.1128/IAI.72.6.3195-3203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Quintana FJ, Solomon A, Cohen IR, Nussbaum G. Induction of IgG3 to LPS via Toll-like receptor 4 co-stimulation. PLoS ONE. 2008;3:e3509. doi: 10.1371/journal.pone.0003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–20. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 117.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–61. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci USA. 2002;99:5545–50. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–93. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 120.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–8. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kopf M, Brombacher F, Bachmann MF. Role of IgM antibodies versus B cells in influenza virus-specific immunity. Eur J Immunol. 2002;32:2229–36. doi: 10.1002/1521-4141(200208)32:8<2229::AID-IMMU2229>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 122.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–51. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 123.Bach P, Kamphuis E, Odermatt B, Sutter G, Buchholz CJ, Kalinke U. Vesicular stomatitis virus glycoprotein displaying retrovirus-like particles induce a type I IFN receptor-dependent switch to neutralizing IgG antibodies. J Immunol. 2007;178:5839–47. doi: 10.4049/jimmunol.178.9.5839. [DOI] [PubMed] [Google Scholar]
- 124.Proietti E, Bracci L, Puzelli S, et al. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol. 2002;169:375–83. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]
- 125.van den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69:4792–6. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ku CL, Picard C, Erdos M, et al. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J Med Genet. 2007;44:16–23. doi: 10.1136/jmg.2006.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ku CL, von Bernuth H, Picard C, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–22. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ku CL, Yang K, Bustamante J, et al. Inherited disorders of human Toll-like receptor signaling: immunological implications. Immunol Rev. 2005;203:10–20. doi: 10.1111/j.0105-2896.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 129.Qin J, Jiang Z, Qian Y, Casanova JL, Li X. IRAK4 kinase activity is redundant for interleukin-1 (IL-1) receptor-associated kinase phosphorylation and IL-1 responsiveness. J Biol Chem. 2004;279:26748–53. doi: 10.1074/jbc.M400785200. [DOI] [PubMed] [Google Scholar]
- 130.von Bernuth H, Picard C, Jin Z, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–6. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Day N, Tangsinmankong N, Ochs H, Rucker R, Picard C, Casanova JL, Haraguchi S, Good R. Interleukin receptor-associated kinase (IRAK-4) deficiency associated with bacterial infections and failure to sustain antibody responses. J Pediatr. 2004;144:524–6. doi: 10.1016/j.jpeds.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 132.Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol. 2007;178:5124–31. doi: 10.4049/jimmunol.178.8.5124. [DOI] [PubMed] [Google Scholar]