Abstract
IL-27 has recently been identified as a differentiation factor for the generation of IL-10-producing regulatory type 1 (Tr1) T cells. However, how IL-27 induces the expansion of Tr1 cells has not been elucidated. Here we demonstrate that IL-27 drives expansion and differentiation of IL-10-producing murine Tr1 cells by inducing three key elements: the transcription factor c-Maf, cytokine IL-21 and costimulatory receptor ICOS. IL-27-driven c-Maf expression transactivates IL-21 production, which acts as an autocrine growth factor for the expansion and/or maintenance of IL-27-induced Tr1 cells. ICOS further promotes IL-27-driven Tr1 cells. Each of those elements is essential since loss of c-Maf, IL-21-signaling or ICOS decreases the frequency of IL-27-induced differentiation of IL-10-producing Tr1 cells.
Introduction
IL-27, a member of the IL-12/IL-23 heterodimeric family of cytokines produced by APCs, is composed of two chains, IL-27p28 and EBV-induced gene 3 (1). Activated T cells and NK cells have the highest expression of IL-27R, which is composed of two chains, a specific IL-27Ra chain (WSX-1 or TCCR) and a signaling chain, gp130, that it shares with IL-6R (1). Initial studies have suggested that, similar to IL-12, IL-27 induces expansion of proinflammatory Th1 cells by activating the STAT-1-mediated T-bet pathway (1). However, analysis of the IL-27Ra-/- (WSX-1-/-) mice infected with various pathogens resulted in clearance of the parasites with exaggerated T cell responses and enhanced proinflammatory cytokine production (1). Furthermore, IL-27Ra-/- mice developed severe experimental autoimmune encephalomyelitis with enhanced Th17 responses (1) and treatment with rIL-27 suppressed disease and decreased frequency of Th17 cells (1). These paradoxical observations led to the hypothesis that IL-27 may not be necessary for the generation of proinflammatory T cells (Th1 or Th17), but rather play a crucial role in regulating T cell responses. Subsequently, three groups, including ours, reported that IL-27, not only inhibited the generation of Th17 cells, but also induced differentiation of IL-10-producing regulatory T cell type 1 (Tr1 cells) from naïve T cells (2-4).
Tr1 cells are a subset of T cells that have strong immunosuppressive properties, predominantly produce IL-10 with variable amounts of IFN-γ, but do not express Foxp3 (5). Adoptive transfer of Tr1 cells have been shown to suppress autoimmunity, colitis, graft versus host disease and tissue inflammation (6). Although initial studies suggested that Tr1 cells are induced by repetitive antigenic stimulation of T cells in the presence of IL-10 (6), T cells differentiated in the presence of IL-10 could not be propagated long-term in culture. The identification of IL-27 as a differentiating factor for the generation of Tr1 cells provided a means by which they could be grown in large numbers and facilitated their functional analysis. However, the molecular mechanisms by which IL-27-mediated generation and/or expansion of Tr1 cells is not well understood. Thus we analyzed the expression of various key cytokines and transcription factors induced by IL-27. Our results show that IL-27 is a potent inducer of three essential elements: the transcription factor c-Maf, cytokine IL-21 and costimulatory receptor ICOS, which coordinately work together to promote differentiation of Tr1 cells.
Materials and methods
Mice and reagents
IL-10-eGFP reporter mice (Vert-X), Foxp3.gfp ‘knock-in’ mice, ICOS-/- mice on C57Bl/6 background were generated as described (7) (8). WSX-1-/- mice on C57Bl/6 background were obtained from Chris Saris (Amgen Inc., Thousand Oaks, CA) and IL-21R-deficient mice on NOD background from Nora Sarvetnick (9) (Scripps Institute, CA). C-Maf-/- mice on N5 BALB/c background have been described (10). Mice were housed in conventional, pathogen free facilities at the Harvard Institute of Medicine. All experiments were undertaken in accordance with guidelines from the Committee on Animals at Harvard Medical School.
T cell differentiation and proliferation in vitro
Naïve CD4+ T cells (CD4+CD62LhiCD25-) pooled from both spleen and lymph nodes or Memory cells (CD4+CD44+) obtained from lymph nodes were purified by flow cytometry and stimulated with plate-bound antibody against CD3 (145-2C11, 1μg ml-1) and CD28 (PV-1, 1μg ml-1). Cells were cultured as previously described (8). Monoclonal anti-TGF-β1 antibody (10μg ml-1), mouse IL-21 (80 ng ml-1), mouse IL-27 (25 ng ml-1), anti-mouse IL-21 antibody (25 ug ml-1) were all purchased from R&D Systems. Proliferation assay was performed as previously described (8).
Measurement of cytokines
Secreted cytokines were measured after 48 h by cytometric bead array (BD Biosciences) or ELISA. Intracellular cytokine staining was performed by as previously described (8).
Quantitative RT-PCR
RNA was extracted with RNAeasy minikits (Qiagen) and were analyzed by real-time PCR (RT-PCR) according to the manufacturer’s instructions (Applied Biosystems). Primers-probe mixtures were purchased from Applied Biosystems: IL-10 (catalog no Mm00439615-g1); ICOS (catalog no Mm004497600-m1); c-Maf (catalog No Mm 02581355-S1); IL-21 (catalog No Mm00517640-m1); IL-21R (catalog No Mm00600319-M1).
Luciferase assay
HEK 293T cells (105) were co-transfected with pGL3-IL-21-Luc reporter plasmid and Renilla luciferase reporter plasmid (pRL-TK) and vector pcDNA3.1 or pcDNA3.1(HA-c-Maf) or pcDNA3.1(T-bet) or pcDNA3.1(hGATA3). Cells were collected 24 h. post transfection, and IL-21 promoter activities were analyzed using Dual-Glo™ Luciferase Assay System (Promega) according to the manufacturer’s instructions. The luciferase activities were normalized against the Renilla luciferase activity.
Fetal Thymic Organ Culture
Thymi from c-Maf-/- fetuses were removed on E15 and individual lobes were cultured for 7 days. Genotyping was performed using DNA isolated from the fetal limbs. Thymocytes were recovered on day 7 of culture after collagenase digestion. CD4+CD8-CD25-cells were sorted and cultured for 4 days on a anti-CD3 (2 μg ml1) and anti-CD28 (2 μg ml-1) coated plate.
Statistics
Statistical analysis was performed using the unpaired Student’s t-test.
Results and Discussion
Our previous studies showed that TGF-β and IL-27 acted synergistically to generate Tr1 cells. However, under such culture conditions, low Foxp3 expression was also induced by TGF-β. Using IL-10.eGFP reporter mice (Vert-X), we developed culture conditions under which IL-27 alone could induce Tr1 cells. These in vitro derived Tr1 cells were as suppressive as natural Foxp3+ regulatory T (Treg) cells in inhibiting T cell proliferation in vitro (supplementary Fig. 1).
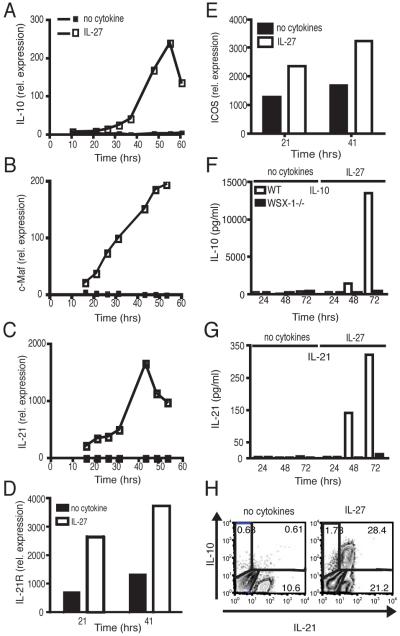
To understand the molecular mechanisms by which IL-27 induces and expands Tr1 cells, we analyzed expression of genes upregulated by IL-27 at multiple time points following T cell activation in the presence of IL-27. As expected, IL-27 induced IL-10 mRNA expression that peaked 48 h post-activation (Fig. 1A). Interestingly, IL-27 induced the expression of transcription factor c-Maf, at early time points, which progressively increased over time (Fig. 1B). Consistent with our previous observation that c-Maf regulates IL-21 expression in Th17 cells (8), we observed that IL-27 also induced IL-21 mRNA and that IL-10 and IL-21 showed similar kinetics of mRNA expression (Fig. 1A and C). Although IL-21R expression was low in unactivated T and B cells, TCR-driven activation upregulated this expression, IL-27 further upregulated IL-21R expression in activated T cells (Fig. 1D). We (8) and others (11) have shown that c-Maf transcription factor is downstream of ICOS, and since IL-10-producing T cells were first shown to be preferentially costimulated by ICOS (12), we examined the expression of ICOS mRNA and observed that the addition of IL-27 indeed induced higher ICOS expression than T cell activation without IL-27 (Fig. 1E). Thus, IL-27, in addition to inducing IL-10 production, induced c-Maf, IL-21, IL-21R and ICOS expression. At the protein level, T cells activated in the presence of IL-27 produced both IL-10 and IL-21, thus confirming mRNA expression (Fig. 1F, G). Besides the mRNA expression, we also observed an increase in ICOS expression induced by IL-27 at the protein level (data not shown). Furthermore, IL-27Ra-/- (WSX-1-/-) mice produced no detectable IL-10 or IL-21 and did not show an increased ICOS expression upon activation, indicating that IL-10 and IL-21 production and increased ICOS expression were specifically induced by IL-27 (Fig. 1F, G and data not shown). To analyze the cells that produce both IL-10 and IL-21, we undertook intracellular cytokine staining for IL-10 and IL-21 after 3 days of culture in vitro with IL-27 and found that the IL-10-producing cells also produced IL-21 (Fig. 1H).
Figure 1. IL-27 induces c-Maf, IL-21, IL-21R and ICOS.
RNA isolated from Naïve CD4+CD62LhiCD25- cells cultured with IL-27 (open squares) or without IL-27 (closed squares) were subjected to RT-PCR relative to the expression of mRNA encoding β-actin (2 -ΔCT×100000) to examine expression of cytokines at different time points following activation. RT-PCR of (A) IL-10, (B) c-Maf, (C) IL-21, (D) IL-21R and (E) ICOS induction by IL-27. (F) IL-10 and (G) IL-21 production measured by cytokine bead array as induced by IL-27 in WT (open bars) and IL-27ra-/- (WXS-1-/-) (filled bars) CD4+CD62LhiCD25- cells. (H) Intracellular cytokine staining of IL-10 and IL-21 by T cells following activation in the presence of IL-27.
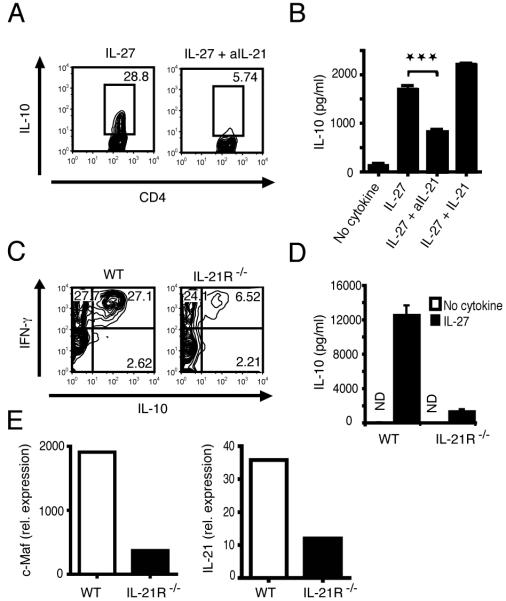
Since IL-21 belongs to the IL-2 cytokine family and utilizes the common γ chain receptor, we hypothesized that IL-27-driven IL-21 production from T cells may be an autocrine growth factor for the generation of Tr1 cells. To test this, we first added a neutralizing IL-21 antibody in the presence of IL-27 and found that blocking IL-21 reduced the frequency of IL-10-producing T cells significantly by over 75% (Fig. 2A) and IL-10 cytokine production in the culture supernatants by over 50% (Fig. 2B). Further addition of IL-21 together with IL-27 increased IL-10 production, but this increase with exogenous IL-21 was modest (Fig. 2B). These data raised the issue of whether IL-21 could directly expand IL-10-producing T cells. However, activation of T cells from mice lacking IL-27Ra (WSX-1) signaling in the presence of IL-21 and IL-27 did not expand Tr1 cells (supplementary Fig. 2). We further confirmed the role of IL-21 in the expansion of Tr1 cells using CD4+ T cells from IL-21R-deficient mice. Loss of IL-21 signaling resulted in inhibition of IL-27-driven generation of IL-10-producing T cells by over 75% (Fig. 2C) and IL-10 cytokine production in the culture supernatants by over 90% (Fig. 2D). However, loss of IL-21 signaling had no effect on IL-27-driven IFN-γ production (data not shown). Furthermore, IL-21R-deficient CD4+ T cells stimulated with IL-27 expressed lower levels of c-Maf and IL-21, as determined by RT-PCR (Fig. 2E). These data suggest that IL-21 may be an important growth factor induced by IL-27 to expand Tr1 cells without affecting expansion of IFN-γ-producing cells. Since IL-27 not only induces IL-21 production but also induces IL-21R expression, these data suggest that IL-27-mediated IL-21R up-regulation might be required for IL-21 to expand Tr1 cells.
Figure 2. IL-21 is necessary for IL-10 production in Tr1 cells.
(A) IL-10.GFP expression as analyzed by flow cytometry in naïve T cells activated in the presence of IL-27 for 72 hours with or without addition of neutralizing anti-IL-21 antibody. (B) ELISA to detect IL-10 production in the supernatant of naive T cells differentiated with IL-27 and anti-IL-21 or IL-21 (mean and s.d.; p=0.0003). (C) IL-10 and IFN-γ production by WT and IL-21R-/- naïve T cells activated in the presence of IL-27 for 72 hours as determined by intracellular cytokine staining and analyzed by flow cytometry. (D) Supernatants from IL-27 differentiated naïve T cells from WT and IL-21R-/- mice analyzed by IL-10 cytokine ELISA (mean and s.d.) ND not detected. (E) RT-PCR analysis of c-Maf and IL-21 in WT and IL-21R-/- CD4+ cells stimulated with IL-27.
To study the relevance of IL-21 in expanding Tr1 cells in vivo, we examined the frequency of IL-10-producing Tr1 cells generated in vivo in IL-21R-/- mice. We found that the fraction of IL-10-producing CD4+CD44+ memory T cells was significantly reduced in IL-21R-/- mice showing only 10% as many IL-10-producing T cells compared to WT mice (Fig. 3A). In contrast, the frequency of IFN-γ producers was similar in WT and IL-21R-/- cells. To further determine whether IL-27 could correct the defect in Tr1 cell development, CD4+CD44+ memory T cells were activated in the presence of IL-27, but IL-21R-/- mice continued to show a significant reduction in IL-10-producing T cells (data not shown). CD4+CD44+ T cells purified from IL-21R-/- mice also showed a lower expression of c-Maf and IL-21 mRNA (Fig. 3B), highlighting the importance of this amplification loop in generating Tr1 cells. In addition to the induction of c-Maf, IL-21 and IL-21R, IL-27 also enhanced the expression of ICOS. Since IL-10-producing T cells were first shown to be preferentially costimulated by ICOS (12), we analyzed the effect of lack of ICOS signaling on the induction of Tr1 cells by IL-27. In vitro differentiation of T cells from ICOS-deficient mice demonstrated that the IL-10 production by ICOS-/- T cells was similar at 48 h, but by 72 h, there was a significant defect in IL-10 production induced by IL-27, as determined by cytometric bead array (Fig. 3C) and by intracellular staining (data not shown). In the plate bound antibody system employed here, ICOS-L is most likely provided by the CD4+ T-cells since T cells can express ICOS-L when activated (13). When Tr1 cells were differentiated from ICOS-/- mice, it was clear that ICOS-/- mice had a defect in sustaining growth/expansion of Tr1 cells in vitro.
Figure 3. Memory CD4+CD44+ cells from IL-21R-/- mice are defective in IL-10 production in vivo.
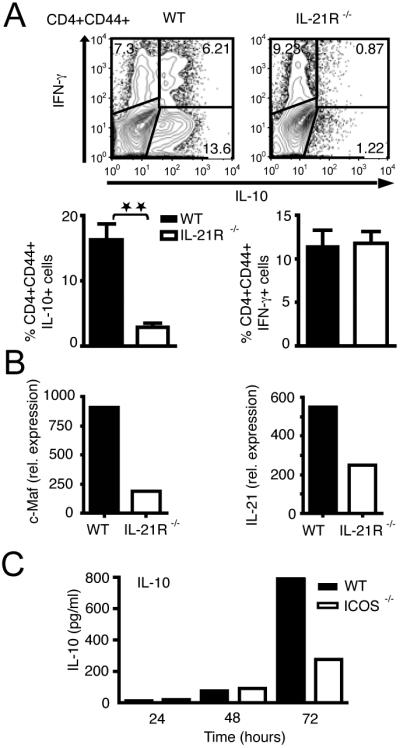
(A) IL-10 and IFN-γ production as detected by intracellular cytokine staining in memory CD4+CD44+ T cells after 72 hours in vitro activation (n=5 mice per group). IL-21R-/- CD4+CD44+ T cells from lymph nodes are defective in IL-10 production (P= 0.0077) but not for IFN-γ compared to WT controls. (B) RT-PCR analysis of IL-21 and c-Maf in WT and IL-21R-/- CD4+CD44+ T cells cultured in vitro for 48 h. (C) IL-10 production induced by IL-27 in WT mice (filled bars) and ICOS-/- (open bars) CD4+CD62L+ CD25- T-cells as measured by cytokine bead array.
Our results clearly demonstrated that IL-27 induces expression of c-Maf and that expression of c-Maf and IL-21 mRNA appeared to be coexpressed in differentiating Tr1 cells under various differentiation conditions (Fig. 1, 2E and 3B). Therefore, we compared c-Maf expression in T cell subsets (Th0, Th1, Th2, Th17 and Tr1), and observed that Tr1 cells had approximately 500-fold higher expression than Th1 or Th2 cells (Fig. 4A). We have observed that c-Maf and IL-21 mRNA were co-expressed by Tr1 cells suggesting that c-Maf may be a transcription factor for IL-21, which in turn expands Tr1 cells. Our analysis of the IL-21 promoter revealed four putative conserved binding sites, located 1070 bp (half MARE), 370 bp (V-MARE), 260 bp (half MARE) and 200 bp (V-MARE) upstream of the transcriptional start site. To test this, an IL-21 promoter-luciferase reporter construct was co-transfected with a c-Maf expression plasmid into HEK 293T cells. Interestingly, c-Maf could transactivate IL-21 promoter-luciferase in a dose-dependent manner but transcription factors T-bet and GATA-3, involved in Th1 and Th2 differentiation, could not (Fig. 4B), suggesting that c-Maf may expand Tr1 cells by inducing IL-21 production. Indeed we have previously shown that c-Maf-deficient mice have a defect in IL-21 production (8). To address whether c-Maf-deficient mice have a defect in the IL-27-mediated IL-10 and IFN-γ production, we activated c-Maf-deficient T cells in the presence of IL-27 and analyzed the expression of IL-10 and IFN-γ. IL-27 was not able to induce either IL-10 or IL-21 production in c-Maf-deficient CD4+ T cells (Fig. 4C and data not shown), but the IL-27-mediated IFN-γ response was not affected (data not shown). As c-Maf has been described to directly transactivate IL-10 promoter (14) (15), we further added IL-21 to IL-27 activated c-Maf-deficient CD4+ T cells and showed that exogenous IL-21 can partially rescue IL-10 production in c-Maf-/- T cells (Fig. 4D) highlighting the importance of IL-21 transactivation by c-Maf.
Figure 4. IL-27 induces c-Maf, which transactivates IL-21.
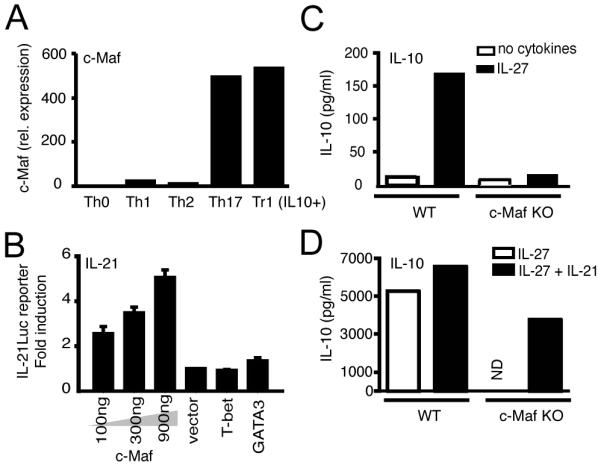
(A) Expression of c-Maf in Th0, Th1, Th2 and Th17 cells as detected by Real-time PCR. (B) C-Maf transactivates the IL-21 promoter in HEK 293T cells as detected by co-transfection of IL-21-luciferase reporter plasmid (IL-21Luc reporter) with c-Maf expression vector (c-Maf) transfected at three different plasmid concentrations (100, 300 or 900 ng) or the control empty expression vector (vector, 900 ng) or T-bet (900 ng) or GATA3 (900 ng) vectors. Promoter activity was quantified by luciferase assay 24 hr post transfection. The promoter-luciferase activity observed by transfection of the empty expression vector was normalized to 1. IL-10 production induced by IL-27 (C) or IL-27 plus IL-21 (D) in WT and c-Maf-/- CD4+CD8-CD25- T cells, after 4 days in culture, as detected by cytokine bead array. ND not detectable.
These data clearly show that IL-27 induces c-Maf, a transcription factor previously identified in Th2 cells, to transactivate IL-21 that then drives the expansion of Tr1 cells. Therefore, similar to Th17 and T follicular helper (TFh) cells, Tr1 cells express c-Maf and IL-21 and utilize IL-21 for autocrine growth and expansion. It is interesting to note that three different T cell subsets, Tr1, Th17 and TFh cells, that express c-Maf and IL-21, also produce IL-10, albeit at different levels (8, 16, 17). IL-21 acts as an autocrine growth/differentiation factor for all three subsets of T cells. It bears to reason that IL-21, which belongs to the family of IL-2 growth factors and utilizes the common γ chain for signaling, may act as an expansion/growth factor for cells that do not produce IL-2. Consistent with this idea, loss of IL-21 or IL-21 signaling results in a defect in all the three T cell subsets, Th17, TFh and Tr1 cells (8, 16). Our data are consistent with a recent study showing that IL-21 mediates its inhibitory effects by inducing IL-10 production (18). Similar to IL-6, which induces IL-21 by inducing p-STAT-3, IL-27 also induces p-STAT-3 and IL-21, this is most likely due to the fact that IL-6 and IL-27 both share the gp130 chain for signaling.
IL-27 enhanced expression of ICOS is of interest since ICOS was initially shown to costimulate IL-10-producing T cells (12). We and others have shown that ICOS/ICOS ligand interaction induces c-Maf expression that may further enhance stable IL-21 production from developing Tr1 cells (8, 11). As for Th17 and TFh cells, ICOS appears to be crucial in maintaining IL-27-driven Tr1 cells. These results are supported by the observation that ICOS-/- mice indeed have a defect in IL-10-producing T cells and therefore develop more severe autoimmunity (8, 19). IL-27 also upregulates ICOS for maintenance and survival of Tr1 cells.
In summary, we have demonstrated that IL-27 drives expansion and differentiation of Tr1 cells by inducing expression of three key elements: the transcription factor c-Maf, growth factor IL-21 and costimulatory receptor ICOS which coordinately act to mediate Tr1 differentiation (supplementary Fig. 3). Loss of any of these factors results in a defective IL-27-driven IL-10-producing Tr1 cells.
Supplementary Material
Acknowledgments
We thank D. Kozoriz and R. Chandwaskar for cell sorting and technical assistance.
Footnotes
This work was supported by grants from the Swiss National Science Fondation (C.P.), the National Multiple Sclerosis Society (RG2571 to V.K.K.), National Institutes of Health (R01NS045937, R01NS035685, R37NS030843, R01AI044880, P01AI039671 and P01NS038037 to V.K.K. and R37A138310 to A.H.S). V.K.K. is a recipient of the Javits Neuroscience Investigator Award from the National Institutes of Health.
- Tr1 cells
- Regulatory type 1 cells
- Foxp3
- Fork head box 3
- c-Maf
- c-Maf protooncogene
- RT-PCR
- Real-time PCR
- WT
- Wild type
Disclaimer
“This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
Disclosures
The authors declare no competing financial interests.
References
- 1.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 3.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 5.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O’Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 6.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta S, Sarvetnick NE. IL-21 limits peripheral lymphocyte numbers through T cell homeostatic mechanisms. PLoS ONE. 2008;3:e3118. doi: 10.1371/journal.pone.0003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745–751. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- 11.Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I, Flavell RA, Dong C. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 12.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 13.Ling V, Wu PW, Finnerty HF, Bean KM, Spaulding V, Fouser LA, Leonard JP, Hunter SE, Zollner R, Thomas JL, Miyashiro JS, Jacobs KA, Collins M. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J Immunol. 2000;164:1653–1657. doi: 10.4049/jimmunol.164.4.1653. [DOI] [PubMed] [Google Scholar]
- 14.Cao S, Liu J, Song L, Ma X. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. J Immunol. 2005;174:3484–3492. doi: 10.4049/jimmunol.174.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, Ochando JC, Bromberg JS, Ding Y. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 18.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.