Abstract
Purpose/Objective(s)
HPV associated cancers of the head and neck (H&N) are increasing in frequency and are often treated with radiation. There is conflicting data in the literature regarding the radiation response in the presence of HPV infection with some data suggesting they may be more sensitive to radiation. There are few studies looking at in vitro effects of HPV and further sensitization by inhibitors of specific signaling pathways. We are in the process of starting a clinical trial in H&N cancer patients using NFV (which inhibits Akt) and it would be important to know the effect of HPV on radiation response plus/minus NFV.
Materials/Methods
Two naturally infected HPV-16 cell lines (UPCI-SCC90 and UMSCC47) and the HPV-negative SQ20B H&N squamous carcinoma cells were used. Western blots with or without 10 uM NFV were done to evaluate signaling from the PI3K-Akt pathway. Clonogenic assays were done in the three cell lines with or without NFV.
Results
Both UPCI-SCC90 and UMSCC47 cells were sensitive to radiation as compared to SQ20B and the degree corresponded to Akt activation. The SQ20B cell line has an activating mutation in EGFR resulting in phosphorylation (P) of Akt; UMSCC47 has decreased P-PTEN resulting in increased P-Akt; UPCI-SCC90 had over-expression of P-PTEN and decreased P-Akt. NFV resulted in down-regulation of Akt in all 3 cell lines resulting in sensitization to radiation.
Conclusions
HPV infected H&N cancers are sensitive to radiation. The degree of sensitivity correlates to Akt activation and they can be further sensitized by NFV.
Keywords: HPV, Radiation Sensitization, Akt, Nelfinavir, PTEN
INTRODUCTION
The role of the human papilloma virus (HPV) in virtually all cervical cancers has been well established (1). However it is only in the last few years, that an association and causal relation has been proven for HPV and head and neck (H&N) cancer (2). The rates of oropharynx cancers that contain HPV DNA has been reported to be as high as 72% (3) but most estimates are in the range of 40% (4). A recent phase II European Organisation for Research and Treatment of Cancer study (4) reported that in comparison to HPV negative cancers, HPV positive cancers had a better response to induction chemotherapy and chemoradiation. Their 2 year overall survival was also significantly improved to 95% as compared to 62%. All the patients in this study were treated with radiation.
Although these data imply an inherent sensitivity of HPV positive cancers to radiation therapy, this implication is controversial. Few studies have correlated HPV status with chemosensitivity or radiosensitivity in vitro. Integration of the HPV genome has been proposed to result in overexpression of the HPV oncoproteins. These oncoproteins are known to alter many cellular pathways (i.e. the p53 and RB1 pathways (5, 6)) and also cause polyploidy and chromosomal instability in keratinocytes (7). How the combination of these known HPV associated cellular alterations affects response to known therapies is not fully understood. Chemoresistance has been reported in both a HPV 16 positive cervical cell line (8) and a oropharyngeal cancer cell line (9). Two HPV positive cervical carcinoma cell lines have also been reported to be resistant to cis-diamminedichloroplatinum(CDDP), 5-FU, and radiation treatments (10). A literature search attempting to find in vitro data on ionizing radiation sensitization in the presence of HPV infection found no relevant results. While there are growing in vivo data that HPV positive tumors fare better (3, 11-14), it is difficult to reconcile this belief in the absence of any in vitro data on the impact of HPV infection on radiosensitivity.
Here we examined the radiation sensitivity of the 2 naturally HPV-16-transformed H&N cancer cell lines (UMSCC47 and UPCI-SCC90) in relation to a HPV-negative H&N cancer cell line (SQ20B). Both UMSCC47 and UPCI-SCC90 were more sensitive to radiation than the SQ20B cells. Radiation sensitivity has been correlated to activation of the PI3K-Akt pathway (15, 16). We found that SQ20B and UMSCC47 lines had similar levels of Akt phosphorylation and were closer in their radiation response than the UPCI-SCC90 line which had almost no activation of Akt and was exquisitely sensitive to radiation. To better understand the effect Akt phosphorylation played in radiation response we examined a known Akt signal inhibitor, Nelfinavir (NFV). This HIV protease inhibitor, has been shown to result in down-regulation of Akt signaling and sensitization to radiation (17, 18). We are in the process of initiating a clinical trial in non-HIV infected, (H&N)_cancer patients with NFV in combination with standard chemoradiation. The response of the HPV positive cell lines was evaluated to NFV and we found that NFV resulted in down-regulation of Akt in all 3 cell lines and further sensitization to radiation.
MATERIALS/METHODS
Cells
The SQ20B cell line was a gift from Dr. Ralph Weichselbaun (19). The UMUMSCC47 and UPCIUPCI-SCC90 cell lines had been obtained from Dr. Douglas Trask and Dr. Suzanne Gollin (20, 21). All cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Fisher Scientific, Pittsburgh, PA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA), penicillin (100 U/ml), and streptomycin (100 mg/ml) (Gibco/BRL, Gaithersburg, MD) at 37°C in humidified 5% CO2-95% air.
Western Blotting
Cells were lysed without trypsinization by rinsing culture dishes once with PBS followed by lysis with reducing Laemeli sample buffer. Samples were boiled, sheared, clarified by centrifugation, and stored at -20°C. Samples containing equal amounts of protein were separated on a 12% SDS polyacrylamide gel and blotted onto nitrocellulose membranes. Membranes were blocked in PBS containing 0.1% Tween-20 and 5% powdered milk before primary antibody addition. Monoclonal anti-phosphorylated EGFR (HER-1; Upstate Biotechnology, Waltham, MA), polyclonal anti-phosphorylated Ser 473 Akt, polyclonal anti-phosphorylated Thr-308 Akt, polyclonal total Akt, and polyclonal anti-phosphorylated Ser380 lipid Phosphatase and TENsin homologue (PTEN) antibodies, (Cell Signaling Technology, Danvers, MA) were all used at 1:2000 dilution. Polyclonal anti-GAPDH, (Sigma-Aldrich, St. Louis, MO) was used as a loading control at a dilution of 1:40,000. Antibody binding was detected using the ECL chemiluminescence kit (Amersham, Arlington Heights, IL). Images were digitized using an Arcus II scanner, and figures were assembled using Adobe Photoshop CS3 and Microsoft Power Point.
Radiation Survival Determination
Cells in exponential growth phase were counted and plated in 60-mm dishes containing 4 ml of media. The cells were allowed to attach and drugs were added to cultures at least one hour prior to radiation. Cells were irradiated with a Mark I cesium irradiator, (J.L. Shepherd, San Fernando, CA) at a dose rate of 1.6 Gy/min. Colonies were stained and counted 10-14 days after irradiation. A colony by definition had > 50 cells. The surviving fraction was calculated by dividing the number of colonies formed by the number of cells plated, multiplied by plating efficiency. Each point on the survival curve represents the mean surviving fraction from at least three replicates.
Nelfinavir
The HIV protease inhibitor nelfinavir (NFV) was purchased for research use from the inpatient pharmacy at the University of Iowa Hospitals and Clinics. It was provided as a solid caplet that was ground into a fine powder and subsequently dissolved in 100% ethanol.
RESULTS
Two HPV-16 positive cancer cell lines (UMSCC47 and UPCI-SCC90) were compared to a cell line that was HPV negative (SQ20B). To ascertain radiation sensitivity we performed clonogenic dose escalation radiation assays for the 3 cell lines. To determine the survival we examined the number of cells surviving that grew out colonies. Identical clonogenic assays were performed in the 3 cell lines (Figure 1). We found that SQ20B cells were the most resistant, followed by UMSCC47 but with UPCI-SCC90 being extremely sensitive to radiation (surviving fraction after 2 Gy, SF2, of 0.74, 0.55, and 0.13 respectively).
Figure 1.
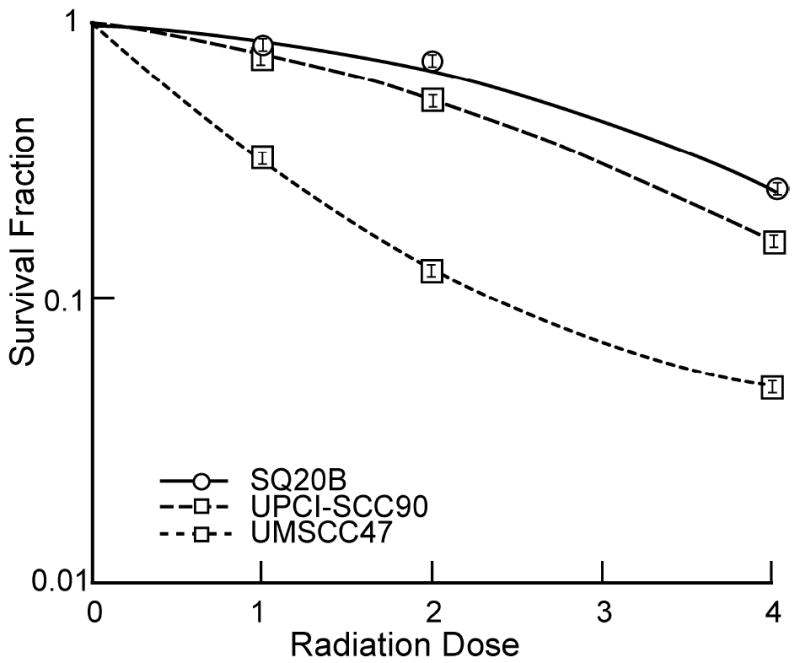
In vitro clonogenic survival after irradiation in the UMSCC47, UPCI-SCC90, and SQ20B cell lines.
SQ20B has a constitutively active mutation in the EGFR receptor as can be seen in Figure 2 compared to UMSCC47 and UPCI-SCC90 who have no appreciable amounts of phosphorylated EGFR. The active EGFR in SQ20B results in active signaling through Akt as visualized by phosphorylation of Akt at both the Ser473 and Thr308 sites. UMSCC47 also has abundant phosphorylated Akt at Ser473 but not as much at Thr308. In comparison, the UPCI-SCC90 cell line has little phosphorylation of both sites. It is known that full activation of Akt requires phosphorylation of both sites (22, 23), but Akt with defective phosphorylation on a single site can still be partially functional (24).
Figure 2.
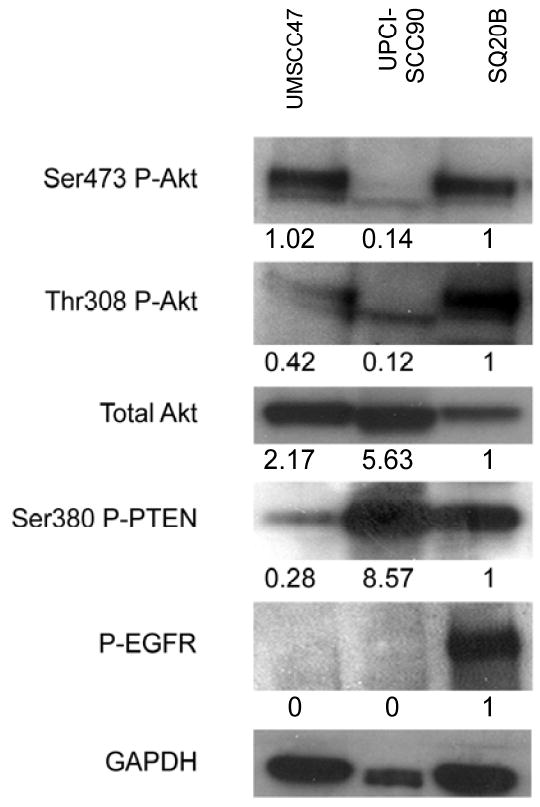
Western blots of the two naturally infected HPV 16 (UMSCC47, UPCI-SCC90) and the non-infected (SQ20B) H&N cancer cell lines. Levels of phosphorylation of Akt at Ser473 and Thr308, total Akt, phosphorylated Ser380 PTEN, and phosphorylated EGFR are compared. GAPDH is used as a protein loading control. The numbers below the gel refer to the densimetric analysis of the protein in comparison to the loading control and all have been normalized with SQ20B having a value of 1.
We also examined the phosphorylation status of PTEN at the Ser380 site in these cell lines (Figure 2). UPCI-SCC90, which has decreased protein loading as seen from the control Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), still has the most Ser380 P-PTEN, followed by SQ20B, and the least in UMSCC47. PTEN is the main negative regulator of the PI3K-Akt pathway. The exact mechanism by which PTEN is regulated is unknown, however, it has been postulated that phosphorylation at the Ser380 site promotes its stability (25) and thus decreased activation of Akt.
We are ultimately interested in sensitizing human tumors to the effects of ionizing radiation and one way to achieve this is by inhibiting Akt (15, 16). One clinically applicable way to inhibit Akt is by use of the HIV protease inhibitor Nelfinavir (NFV) (17, 18). We tested whether NFV can inhibit Akt in this system (Figure 3A) and found that 24 hour treatment with 10 μM NFV did inhibit Akt in all 3 cell lines. With longer exposure (30 mins) of the film to the membrane, UPCI-SCC90 cells do have some Ser473 P-Akt which is inhibited by NFV. SQ20B cells have 60% inhibition but we have previously reported that inhibition is dose and drug exposure time dependent. For SQ20B cells to have 90% inhibition, treatment for 3 days is required (17). UMSCC47 cells were more resistant to NFV even with longer drug exposure time (data not shown) possibly due to the lack of active PTEN providing negative regulation of the PI3K-Akt inhibition. With 15 μM NFV, however, 80% inhibition of Ser473 P-Akt was seen at 24 hours (Figure 3B).
Figure 3.
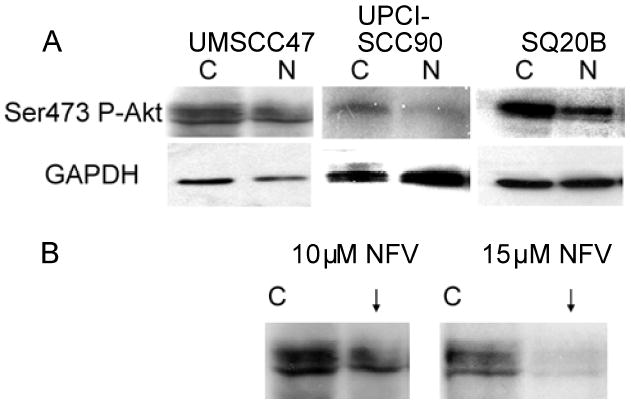
Western blots of cells treated with and without nelfinavir (NFV). (A) The 3 cell lines are treated with 10 μM NFV for 24 hours and probed with phosphorylated Ser473 Akt. (B) UMSCC47 cells are treated with 15 μM NFV for 24 hours resulting in down-regulation of Akt at Ser473.
In Figure 4, we performed clonogenic assays in the cells with and without 10 μM NFV. NFV by itself was not toxic to the cells as the plating efficiency with and without NFV were equivalent in the cell lines. All 3 cell lines were sensitized to radiation. The SF2 values are shown in Table 1. The dose modification factor (DMF) was calculated. This was defined as the radiation dose resulting in 50% cell kill of control vs. NFV treated. The DMF for SQ20B is 1.65 and that of UMSCC47 and UPCI-SCC90 is 1.60 (Table 1), suggesting that NFV resulted in sensitization of all 3 cell lines to an equivalent degree regardless of Akt status.
Figure 4.
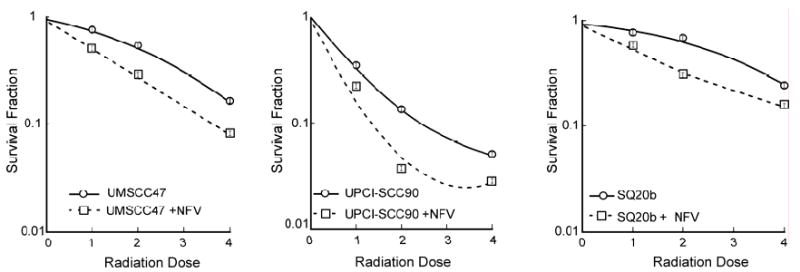
Effect of NFV on in vitro clonogenic survival after irradiation. The 3 cell lines were plated with 4 ml of media in 60-mm dishes and allowed to attach. The cells were then treated with 1 ml of 50 μM NFV resulting in final concentration of 10 μM NFV for at least one hour prior to irradiation. Control dishes received an equal volume of drug carrier. The NFV was left on for the duration of the experiment.
Table 1.
Surviving fraction after 2 Gray (SF2) in UMSCC47, UPCI-SCC90, and SQ20B cell lines with and without 10 μM Nelfinavir (NFV).
| Cell Line | SF2 Control | SF2 NFV | DMF* |
|---|---|---|---|
| SQ20B | 0.74+0.08 | 0.42+0.04 | 1.65 |
| UMSCC47 | 0.55±0.07 | 0.29±0.05 | 1.6 |
| UPCI-SCC90 | 0.13±0.03 | 0.04±0.008 | 1.6 |
Dose modification factor with NFV for 50% cell kill.
In Figure 5, we show a possible schematic of how Akt is being regulated in the three cell lines. SQ20B cells which have increased signaling through EGFR result in positive signaling through Akt. In UPCI-SCC90 cells which have phosphorylated PTEN resulting in stabilization, there is negative signaling through Akt. Whereas in UMSCC47 cells with little phosphorylation of PTEN, there is resulting in degradation and positive signaling through the Akt pathway.
Figure 5.
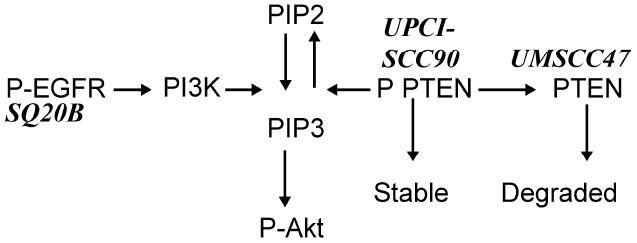
Proposed schematic of Akt pathway control in the UMSCC47, UPCI-SCC90, and the SQ20B cell lines.
DISCUSSION
In this study, we examined the radiation sensitivity of the 2 naturally HPV-16-transformed H&N cancer cell lines (UMSCC47 and UPCI-SCC90) in relation to a HPV negative H&N cancer cell line (SQ20B). Both UPCI-SCC90 and UMSCC47 cells were sensitive to radiation as compared to SQ20B and the degree corresponded to Akt activation. Nelfinavir (NFV), a known inhibitor of Akt, resulted in down-regulation of Akt in all 3 cell lines resulting in sensitization to radiation with nearly the same dose modification factor and no toxicity by itself.
These results are in accordance with the clinical data available that there is a positive prognostic significance of HPV infected H&N cancers. This raises the issue of what treatment would be the most efficacious and yet result in decreased toxicity in these patients. Fakhry et al. recently reported the results of a phase II prospective trial (E2399) of locally advanced HNSCC treated with induction chemotherapy followed by concurrent chemoradiotherapy in tumors demonstrating a favorable response (4). HPV positive tumors defined by the presence of oncogenic HPV DNA (primarily HPV-16) had superior response rates following induction chemotherapy (p=0.01), concurrent chemoradiation (p=0.007), overall survival (p=0.005) and progression-free survival (p=0.02). The mechanism for this favorable treatment response and survival may not simply be due to increased chemo- and radiosensitivity as a recent study by Licitra et al noted an improved survival in oropharyngeal carcinomas treated by surgery alone (12). Treatment de-intensification has been favored in light of the increased acute and potential long-term swallowing complications that exist with concurrent chemoradiotherapy approaches. One approach may be via the use of biologic agents. Although Cetuximab in combination with radiotherapy offers the promise of radiosensitization with less acute toxicity (26), in HPV patients, this needs to be further evaluated as these cancers are not driven by their EGFR status. The sensitization that was observed in these experiments suggest that even with the increased intrinsic radiosensitivity observed with HPV infection, further sensitization is possible. This suggests an alternative clinical strategy for HPV infected head and neck carcinomas which may offer the promise of reduced toxicities.
Although HPV infection is common in H&N cancer, it is difficult to find naturally infected cell lines(21, 27). UMSCC47 and UPCI-SCC90(21) have integrated HPV-16 DNA and express high levels of E6, which is important for cellular transformation and thus may most accurately be representative of HPV associated carcinogenesis and subsequently response to therapy. One mechanism by which HPV E6 leads to carcinogenesis is by ubiquitination and degradation of the tumor suppressor protein p53(28). UPCI-SCC90 has been shown to contain wild-type (wt) p53 which is rapidly degraded in untreated cells, but is recoverable by the action of specific proteasome inhibitors and E6 knockdown by siRNA(21). The effect of p53 on the radiation response is controversial and depends on the system studied(29) and the radiation dose(30). At lower doses of radiation (<15 Gy single dose), mutant p53 has been shown to be protective to radiation; whereas, at higher doses, it results in increased cell death in mice(30).
Another pathway that contributes to radiation resistance is activation of the PI3K-Akt (15, 16) pathway. Phosphorylation of Akt at Ser473 has been shown to result in a poor outcome in both H&N (31) and lung (32) cancers when treated with chemoradiation. Akt is involved in the regulation of diverse cellular processes, including glucose metabolism, cell growth, cell proliferation, angiogenesis, and apoptosis (22). Deregulated Akt activity has been linked to the formation of numerous human malignancies including breast, ovarian, gastric, and thyroid cancers (33). Full activation of Akt requires phosphorylation of both the Thr308 and Ser 473 sites (22, 23), but Akt with defective phosphorylation on a single site can still be partially functional (24). This is consistent with our data that SQ20B cells with robust phosphorylation at both sites are the most resistant, followed by UMSCC47 cells with mostly Ser473 P-Akt, but the most sensitive are the UPCI-SCC90 with limited amounts of P-Akt at either site.
We further hypothesize that the different response to radiation between the UMSCC47 and UPCI-SCC90 cell lines is due to the stability of PTEN. It is a tumor suppressor protein that functions as a phosphatase converting inositol-containing lipids, known as phosphatidylinositols (PtdIns), at their 3-position PtdIns(3,4,5)P3 (PIP3) to PtdIns(4,5)P2 (PIP2). The reverse reaction of converting PIP2 to PIP3 is via phosphatidylinositol 3-kinase (PI3K). PIP3 in turn functions as a secondary messenger to activate Akt (Figure 5) (33). Odriozola et al. have examined mutating the different phosphorylation sites of PTEN and found that mutating Ser380 enhances membrane binding and decreases its phosphorylation state (25). It has been proposed that phosphorylation at the c-terminal tail of PTEN results in a closed conformation and stabilization of the protein (34). Figure 2 shows that UPCI-SCC90 cells have the most phosphorylation of Ser380 PTEN, followed by SQ20B, with barely detectable levels in UMSCC47. Although our data is preliminary, we propose that phosphorylation of PTEN at Ser380 leads to stabilization of PTEN, increased PIP2, and decreased Akt activation which is observed in the UPCI-SCC90 cells; whereas, the reverse is seen in the UMSCC47 cells. SQ20B cells, however, have mutated EGFR, resulting in increased activity of PI3K, more PIP3, and increased signaling through Akt (Figure 5).
Nelfinavir (NFV) is an HIV protease inhibitor that has demonstrated down-regulation of Akt signaling leading to radiation sensitization (17, 18). The mechanism by which this occurs has not been completely elucidated. We have proposed that NFV may also result in the un-folded protein response and endoplasmic reticulum stress caused by proteasome inhibition (35). It has been shown that proteasome inhibition leads to growth inhibition, whether it is through a p53 dependent (21, 36) or independent (37) mechanism is controversial. We also demonstrated that NFV decreased phosphorylation of Akt (Figure 3) and increased radiation sensitization (Figure 4) in all 3 cell lines. Whether the sensitization was directly due to proteasome inhibition and stabilization of p53 or due to Akt pathway inhibition still needs to be determined. Nelfinavir has been used continuously on patients since 1997 with well characterized pharmacokinetics and is well tolerated. A recent phase I study in patients with unresectable pancreatic cancer with NFV and chemoradiotherapy resulted in resectability in 6/10 patients (38). A trial of NFV in H&N cancer patients with stratification based on HPV status should be tried and could have implications on the treatment of patients both in terms of outcome and toxicity.
Acknowledgments
Grant Support: This work was supported by NIH grant R21 CA121580-01A1 (A.K.G.).
Footnotes
Meeting Presentation: This work was presented at 50th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Boston, MA, September 21-25, 2008.
Conflicts of Interest Notification. Anjali K. Gupta has a use patent pending on Nelfinavir as a radiation sensitizer.
No other conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Dahlstrom KR, Adler-Storthz K, Etzel CJ, et al. Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never-smokers: a matched pair analysis. Clin Cancer Res. 2003;9:2620–2626. [PubMed] [Google Scholar]
- 3.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 5.Williams AT, Sexton CJ, Sinclair AL, et al. Retention of low copy number human papillomavirus DNA in cultured cutaneous and mucosal wart keratinocytes. J Gen Virol. 1994;75(Pt 3):505–511. doi: 10.1099/0022-1317-75-3-505. [DOI] [PubMed] [Google Scholar]
- 6.Atula S, Grenman R, Kujari H, et al. Detection of human papillomavirus (HPV) in laryngeal carcinoma cell lines provides evidence for a heterogeneic cell population. Eur J Cancer. 1999;35:825–832. doi: 10.1016/s0959-8049(98)00424-9. [DOI] [PubMed] [Google Scholar]
- 7.Incassati A, Patel D, McCance DJ. Induction of tetraploidy through loss of p53 and upregulation of Plk1 by human papillomavirus type-16 E6. Oncogene. 2006;25:2444–2451. doi: 10.1038/sj.onc.1209276. [DOI] [PubMed] [Google Scholar]
- 8.Padilla LA, Leung BS, Carson LF. Evidence of an association between human papillomavirus and impaired chemotherapy-induced apoptosis in cervical cancer cells. Gynecol Oncol. 2002;85:59–66. doi: 10.1006/gyno.2002.6604. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann TK, Sonkoly E, Hauser U, et al. Alterations in the p53 pathway and their association with radio- and chemosensitivity in head and neck squamous cell carcinoma. Oral Oncol. 2008 doi: 10.1016/j.oraloncology.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Saxena A, Yashar C, Taylor DD, et al. Cellular response to chemotherapy and radiation in cervical cancer. Am J Obstet Gynecol. 2005;192:1399–1403. doi: 10.1016/j.ajog.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Thompson CH, Cossart YE, et al. The expression of key cell cycle markers and presence of human papillomavirus in squamous cell carcinoma of the tonsil. Head Neck. 2004;26:1–9. doi: 10.1002/hed.10335. [DOI] [PubMed] [Google Scholar]
- 12.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 13.Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 14.Sisk EA, Soltys SG, Zhu S, et al. Human papillomavirus and p53 mutational status as prognostic factors in head and neck carcinoma. Head Neck. 2002;24:841–849. doi: 10.1002/hed.10146. [DOI] [PubMed] [Google Scholar]
- 15.Gupta AK, Bakanauskas VJ, Cerniglia GJ, et al. The Ras radiation resistance pathway. Cancer Res. 2001;61:4278–4282. [PubMed] [Google Scholar]
- 16.Grana TM, Rusyn EV, Zhou H, et al. Ras mediates radioresistance through both phosphatidylinositol 3-kinase-dependent and Raf-dependent but mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-independent signaling pathways. Cancer Res. 2002;62:4142–4150. [PubMed] [Google Scholar]
- 17.Gupta AK, Cerniglia GJ, Mick R, et al. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65:8256–8265. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 18.Cuneo KC, Tu T, Geng L, et al. HIV protease inhibitors enhance the efficacy of irradiation. Cancer Res. 2007;67:4886–4893. doi: 10.1158/0008-5472.CAN-06-3684. [DOI] [PubMed] [Google Scholar]
- 19.Weichselbaum RR, Dahlberg W, Beckett M, et al. Radiation-resistant and repair-proficient human tumor cells may be associated with radiotherapy failure in head- and neck-cancer patients. Proc Natl Acad Sci U S A. 1986;83:2684–2688. doi: 10.1073/pnas.83.8.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spanos WC, Hoover A, Harris GF, et al. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. J Virol. 2008;82:2493–2500. doi: 10.1128/JVI.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferris RL, Martinez I, Sirianni N, et al. Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): a natural disease model provides insights into viral carcinogenesis. Eur J Cancer. 2005;41:807–815. doi: 10.1016/j.ejca.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Scheid MP, Woodgett JR. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 2003;546:108–112. doi: 10.1016/s0014-5793(03)00562-3. [DOI] [PubMed] [Google Scholar]
- 24.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Odriozola L, Singh G, Hoang T, et al. Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem. 2007;282:23306–23315. doi: 10.1074/jbc.M611240200. [DOI] [PubMed] [Google Scholar]
- 26.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 27.Shillitoe EJ, Noonan S. Strength and specificity of different gene promoters in oral cancer cells. Oral Oncol. 2000;36:214–220. doi: 10.1016/s1368-8375(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 28.Scheffner M, Whitaker NJ. Human papillomavirus-induced carcinogenesis and the ubiquitin-proteasome system. Semin Cancer Biol. 2003;13:59–67. doi: 10.1016/s1044-579x(02)00100-1. [DOI] [PubMed] [Google Scholar]
- 29.Sionov RV, Haupt Y. The cellular response to p53: the decision between life and death. Oncogene. 1999;18:6145–6157. doi: 10.1038/sj.onc.1203130. [DOI] [PubMed] [Google Scholar]
- 30.Komarova EA, Kondratov RV, Wang K, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–3271. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- 31.Gupta AK, McKenna WG, Weber CN, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8:885–892. [PubMed] [Google Scholar]
- 32.Gupta AK, Soto DE, Feldman MD, et al. Signaling pathways in NSCLC as a predictor of outcome and response to therapy. Lung. 2004;182:151–162. doi: 10.1007/s00408-004-0310-8. [DOI] [PubMed] [Google Scholar]
- 33.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 34.Leslie NR, Batty IH, Maccario H, et al. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008;27:5464–5476. doi: 10.1038/onc.2008.243. [DOI] [PubMed] [Google Scholar]
- 35.Gupta AK, Li B, Cerniglia GJ, et al. The HIV protease inhibitor nelfinavir downregulates Akt phosphorylation by inhibiting proteasomal activity and inducing the unfolded protein response. Neoplasia. 2007;9:271–278. doi: 10.1593/neo.07124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbert NL, Sedman SA, Schiller JT. Human papillomavirus type 16 E6 increases the degradation rate of p53 in human keratinocytes. J Virol. 1992;66:6237–6241. doi: 10.1128/jvi.66.10.6237-6241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris GFt, Anderson ME, Lee JH. The effect of proteasome inhibition on p53 degradation and proliferation in tonsil epithelial cells. Arch Otolaryngol Head Neck Surg. 2008;134:157–163. doi: 10.1001/archoto.2007.37. [DOI] [PubMed] [Google Scholar]
- 38.Brunner TB, Geiger M, Grabenbauer GG, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26:2699–2706. doi: 10.1200/JCO.2007.15.2355. [DOI] [PubMed] [Google Scholar]


