Abstract
On May 2, 2009 the Canadian Food Inspection Agency notified the World Organization for Animal Health that an emerging novel influenza A virus (pandemic H1N1 2009) had been confirmed on a swine farm in Alberta. Over a 4-week period pigs in this farrow-to-finish operation were clinically affected by respiratory disease consistent with an influenza A virus infection and the presence of active viral infection was confirmed in all production areas by real-time polymerase chain reaction (RT-PCR). Despite clinical recovery of animals, there was reluctance by purchasers to receive animals from this operation due to concerns about the effect on both domestic and international markets. The owner decided to depopulate the entire herd due to impending welfare issues associated with overcrowding and economic concerns resulting from the inability to market these animals. Carcasses were rendered or composted and did not enter the human food or animal feed chain. The source of virus in this herd was determined to be an infected human. Zoonotic transmission to 2 individuals responding to the outbreak was suspected and recommendations to prevent occupational exposure are discussed.
Résumé
Enquête sur le virus de l’influenza pandémique humaine (H1N1) en 2009 dans une ferme porcine de l’Alberta. Le 2 mai 2009, l’Agence canadienne d’inspection des aliments a informé l’Organisation mondiale de la santé animale qu’un nouveau virus émergent de l’influenza A (H1N1 pandémique 2009) avait été confirmé dans une ferme porcine en Alberta. Pendant une période de 4 semaines, les porcs de cette exploitation de la parturition à la finition ont été cliniquement affectés par une maladie respiratoire présentant des symptômes conformes à l’infection du virus de l’influenza A et la présence d’une infection virale active a été confirmée dans toutes les aires de production par une réaction d’amplification en chaîne par la polymérase en temps réel. Malgré le rétablissement clinique des animaux, il y avait une réticence de la part des acheteurs à recevoir les animaux de cette exploitation en raison de préoccupations à propos de l’effet sur les marchés intérieurs et internationaux. Le propriétaire a décidé de dépeupler le troupeau au complet en raison d’enjeux imminents liés au bienêtre découlant du surpeuplement et de préoccupations économiques découlant de l’incapacité de vendre ces animaux sur le marché. Les carcasses ont été équarries et n’ont pas accédé à la chaîne alimentaire humaine ou animale. La source du virus dans ce troupeau a été déterminée comme étant un humain infecté. La transmission zoonotique à 2 intervenants lors de l’éclosion a été soupçonnée et des recommandations pour prévenir l’exposition au travail sont discutées.
(Traduit par Isabelle Vallières)
Introduction
Influenza virus infections of swine occur commonly worldwide on a year-round basis (1). Swine Influenza Virus (SIV) is a synergistic factor in the porcine respiratory disease complex (PRDC) and is an important cause of broncho-interstitial pneumonia and respiratory disease in pigs. Influenza virus is a zoonotic agent of concern on a global scale presenting economic and health challenges to human and animal populations (2). Although human H3N2 viruses have been isolated from pigs in Asia and Europe, historically there has been varied evidence of human H1N1 influenza viruses maintaining themselves in swine populations (3). Both H3N2 and H1N1 SIV infections have been reported in humans in Canada, the United States, Europe, and Asia (4). Humans occupationally exposed to pigs are at increased risk for sero-conversion and for influenza-like illness (ILI) attributable to SIV (5). The reported number of SIV infections in humans, however, is negligible compared to the number of people exposed to pigs (6). The true incidence and significance of zoonotic swine influenza infection is unknown, in part due to inconsistent diagnostic confirmation and reporting within and between jurisdictions.
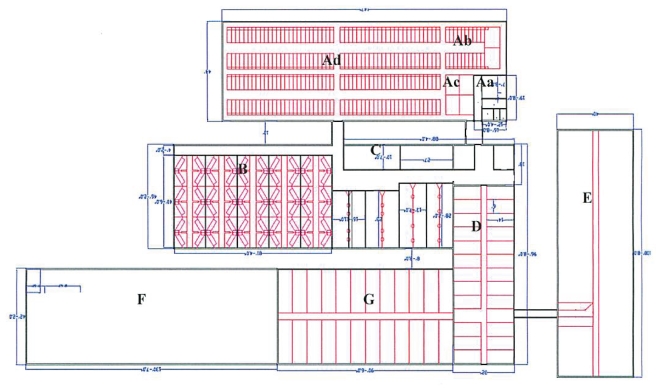
On April 28, 2009 the owner of a conventional 220-sow single site commercial farrow-to-finish swine operation in Alberta notified his herd veterinarian of an acute onset cough in his pre-grower and grower animals (Figure 1: D and E). A contract worker hired to rebuild the ventilation inlets and upgrade the exhaust fans in areas D and E had recently returned from Mexico and exhibited symptoms of ILI while working in the barn. Concerned about a potential public health risk the producer reported these findings to his herd veterinarian, who notified Alberta Agriculture and Rural Development (ARD) of this situation.
Figure 1.
Barn layout and design to scale.
Swine influenza is not a reportable disease under the federal Health of Animals Act and there is no national control program for this disease. However, authority is granted to the Canadian Food Inspection Agency (CFIA) under this Act to respond to any disease of animals including emerging diseases that may affect animals or that are zoonotic. There are also certain provinces, including Alberta, for which SIV is notifiable under provincial animal health regulations. A notifiable disease differs from a reportable one in that occurrences of a notifiable disease are recorded for surveillance purposes only and there is no government response to confirmed cases (no quarantine or disease control requirements) in Alberta. Based on the history of ILI in both the humans and swine associated with this operation, public concern, and the scientific uncertainty surrounding this emerging disease at the time, a joint decision was made by CFIA and ARD for CFIA to issue a precautionary quarantine under the Health of Animals Act for this herd and conduct a full epidemiological investigation.
Health history of herd
Prior to this disease investigation the health status of this swine operation was considered to be conventional stable. The herd was porcine reproductive and respiratory syndrome (PRRS) positive, Mycoplasma hyopneumoniae positive (MH), and Actinobacillus pleuropneumoniae (APP) 5b positive. Although PRRS positive for many years, the herd was stable for this virus. Performance of the reproductive herd was above industry average for farrowing rate and pigs weaned per sow per year. Routine postmortem examinations and bacteriology completed during regular herd visits revealed no gross pathology or bacterial isolates to suggest that APP was active within the herd. Prior to targeted vaccination of nursery pigs at 6 wk of age, MH had been clinically active in area E feeder pigs (Table 1). Barn E is the most challenged space with respect to air quality (dust, ventilation rate). Vaccination against MH (Respisure-One/ER Bac Plus; Pfizer Animal Health, Kirkland, Quebec) was routine at 6 wk of age and, through regular health and necropsy monitoring, vaccination had successfully reduced losses and pathology associated with this disease. Marketing records show that this was a high index herd with an excellent health check record.
Table 1.
Production areas and associated animal demographics
| Production area | Population | Description | Age range (weeks) | Flow |
|---|---|---|---|---|
| A | 175 | Breeding and gestation sows | ||
| 24 | Gilts | |||
| 2 | Boars | |||
| B | 48 | Lactation sows | ||
| 480 | Nursing piglets | 1–4 | AIAO | |
| C | 600 | Nursery | 4–10 | AIAO |
| D | 340 | Pre-grower | 10–14 | Continuous |
| E | 420 | Grower | 14–18.5 | Continuous |
| F | 440 | Grower/Finisher | 18.5–21 | Continuous |
| G | 300 | Finisher | 21–25 | Continuous |
In November 2004, shortly after the introduction of purchased gilts, there was a positive test on serology using the swine influenza (H1N1) IDEXX antibody enzyme-linked immunosorbent assay (ELISA). The parent herd of the gilts had experienced an acute episode of SIV-related illness that summer. Prior to shipping, these gilts had tested negative for SIV on ELISA but had begun showing ILI shortly after arrival. Subsequently, the breeding herd was vaccinated twice, 2 wk apart, with an inactivated swine influenza virus type A, subtype H1N1 vaccine (Maxivac H1N1; Schering-Plough Animal Health, Kirkland, Quebec). This vaccination strategy appeared to have resolved the clinical concerns attributable to SIV. In the subsequent 4.5 y, vaccination for SIV was not practiced and no further SIV cases were identified. Thus it is unlikely that any animals still present on this farm had been previously exposed to, or vaccinated for, influenza.
Barn design and pig flow
Barn design and pig flow are consistent with a conventional farrow-to-finish site. The 7 production areas (Figure 1) are all connected by short hallways or doorways; no truly separate airspaces exist within this facility. The main entrance (Aa) houses a boot change area only; there is no shower. Biosecurity for visitors consists of changing boots and donning cloth coveralls in an outbuilding prior to entering the main entrance. There is no quarantine associated with this site, purchased breeding stock (boars and gilts) are placed directly into Ac with immediate proximity to the gestating and breeding herd (Ad, Ab). Farrowing occurs in the newest area on site (B). Piglets are weaned at 28 days and then moved into 1 of 6 all-in-all-out (AIAO) hot nursery rooms (C). Pig flow from areas D through G is continuous (Figure 1).
Regulatory response
Upon laboratory confirmation of the emerging pandemic H1N1 2009 virus within this herd, public and animal health authorities at all levels became engaged in the development of a disease control strategy based on precautionary principles to address public health concerns. Animal and public health authorities supported the continuation of movement restrictions on this herd while there was evidence of live virus circulating to prevent spread of disease to human or animal populations, pending assessment of the behavior of the novel virus in swine populations. None of the authorities supported a policy of eradication of the entire pig herd on the basis of human or animal health risks. Additional sampling and regular health assessments provided information on the clinical course of disease and risk associated with pandemic H1N1 2009 virus in this herd.
With the entire herd under quarantine, finished hogs could not be shipped to slaughter and crowding became an animal welfare issue. To prevent distress in the animals and at the request of the producer, ARD undertook a limited cull of 475 grower/finisher animals on May 8th to alleviate animal welfare concerns and to allow time for repeat testing of the herd. Hogs were humanely destroyed on-site by captive bolt (“Cash Special” captive .22 and .25 caliber bolt stunner and “Cash Special” HD captive .25 calibre bolt stunner) by trained staff from ARD and the Alberta Society for the Prevention of Cruelty to Animals. Staff were trained on the use of the captive bolt stunners and veterinarians were present on-site at all times during the depopulation activities.
Carcasses were transported to a rendering establishment for disposal in an enclosed, leak-proof, fully covered conveyance via a pre-planned route to minimize exposure to human and pig populations. Routine biocontainment procedures, supervised by the CFIA, were followed for movement of a conveyance off an infected farm. Feeds division of the CFIA confirmed that rendered carcasses could be used in animal feeds as influenza A virus is heat labile and the time-temperature combination of the rendering process would inactivate any virus. Despite scientific evidence supporting the negligible risk associated with rendered product, the rendered material was buried in a landfill due to concerns by the rendering company about potential negative public perception and the marketability of the meat and bone meal produced. Disposal by burial was not an option because the large volume of carcasses could not be accommodated on the small farm property, there were concerns about public perception, and soil type precluded the use of other burial sites near the farm.
A controlled marketing approach allowing movement of test negative animals to slaughter was proposed, with culling only to address humane issues associated with overcrowding. The herd was monitored by both the CFIA staff and the private veterinarian, and sampling was completed in each production area to establish prevalence estimates and determine the clinical presentation within each population of animals. In consultation with stakeholders, criteria were developed to determine the clinical, laboratory, and epidemiological data needed to determine when the disease was no longer present on this farm and the time at which the quarantine could be released.
In spite of the uneventful clinical recovery of animals, no slaughter facility would accept animals from this farm after the quarantine was removed. Unfounded concerns about food safety and marketability from meat buyers were cited as the reason pork processors refused pigs from this farm. Due to impending overcrowding in the barn, the herd owner made an economic decision to depopulate the herd to allow him to escape the situation and resume operation with a replacement herd. The culling of the herd was not an ordered destruction by either the CFIA or ARD on the grounds of animal or human disease concerns. At the owner’s request, and with the assistance of ARD, approximately 3000 pigs were humanely destroyed between June 4th and 6th and either composted off site or disposed of via rendering. The quarantine was removed on July 29, 2009 when cleaning and disinfection measures developed by the private veterinarian and approved by CFIA had been completed.
Clinical and pathological findings
On April 28th, CFIA staff conducted an initial epidemiological investigation on the herd and obtained samples for testing at the National Centre for Foreign Animal Disease (NCFAD) in Winnipeg. An assessment completed by the CFIA District Veterinarian noted that approximately 25% of pigs in areas D and E were exhibiting clinical signs of respiratory disease. These signs were reported to have started around April 20th and included a deep non-productive cough with an abdominal effort and mild to moderate depression (Figure 2). A decrease in feed consumption was also noted. The records revealed an increase in percent mortality from 0.43% to 0.87% and 0.19% to 2.04% in areas D and E, respectively through to the end of April. Approximately 10% of pigs in areas D and E had been treated parenterally by the owner with trimethoprim-sulfadoxine (Trimidox; Vetoquinol Canada, Lavaltrie, Quebec). All other production areas were clinically unaffected when the initial site visit was completed by CFIA staff on April 28th. Under the direction of the herd veterinarian, chlortetracycline medicated premix (Aureomycin 110 G; Alpharma Canada, Mississauga, Ontario) was added at a rate of 2.5 kg/tonne of complete feed to the rations fed in areas D and E to alleviate concerns about known secondary bacterial pathogens endemic to the herd.
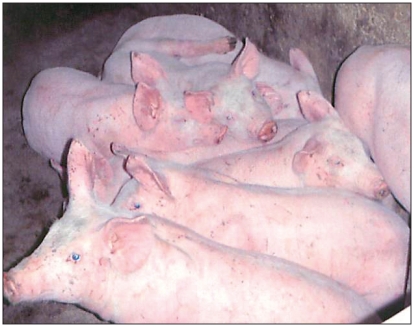
Figure 2.
Eleven-week-old pre-grower pigs huddling with mild depression and reluctance to rise (photo courtesy of Egan Brockhoff).
On May 5th, the herd veterinarian conducted a full health assessment. Ongoing daily communication with the producer had revealed that the cough described earlier was now present in other production areas. It was evident that the virus had spread rapidly throughout the facility. Individual pigs within the nursery-grow-finish (NGF) population within areas C through G presented with a sudden onset clear oculonasal discharge, sneezing, mild conjunctivitis, and a deep, dry, non-productive cough with significant abdominal effort. Clinically affected pigs in the NGF population were pyrexic, moderately depressed, anorexic, and mildly dehydrated. In areas D and E morbidity associated with ILI had declined significantly and was evident in only 10% of animals. Mortality had returned to historical levels less than 1%. In areas F and G only 5% of pigs presented with ILI and mortality in these areas remained unchanged from reports prior to the diagnosis of pandemic H1N1 2009 virus.
Approximately 10% of pigs in the nursery (area C) were coughing, depressed, and dehydrated; the cough was mildly productive suggesting a secondary bacterial infection. Mortality had not increased in the nursery and remained consistent with historical levels of well below 1%. As many as 10% of the oldest piglets in the farrowing rooms (area B) had a mild cough and were sneezing and only 1 sow showed any clinical signs of ILI. In area A, 2 of the 24 gilts had a mild cough and were slightly depressed; none of the sows showed any clinical signs of ILI. Feed consumption by the sows (areas A and B) was unchanged from previous visits.
Field necropsy examinations were performed on May 8th during the limited cull of 475 grower/finisher pigs. Grossly, pigs had poorly collapsed lungs with a rubbery texture and mild interlobular edema in the dorso-caudal lobes. Multifocal disseminated, dark red-purple, shrunken and firm individual lobules, sharply demarcated from adjacent lobules and coalescing in cranio-ventral regions were also noted. A gross diagnosis of lobular to coalescing broncho-pneumonia was made. There was evidence of secondary bacterial infections with copious purulent exudate in airways and severe consolidation with fibrin and abscessation was observed in some pigs but was not a consistent feature. Histopathologic examination of the trachea and lungs revealed mild, chronic, non-specific tracheitis, moderate broncho-interstitial pneumonia with perivascular and peribronchiolar lymphoid hyperplasia, mild multifocal necrotizing and suppurative alveolitis, and subacute to chronic necrotizing to hyperplastic bronchiolitis. Lesions were compatible with mild to moderately severe infection with multiple respiratory pathogens of PRDC including MH, PRRS, and secondary or opportunistic bacterial pathogens. The bronchiolar lesions were characterized by varying degrees of epithelial attenuation through to epithelial regenerative hyperplasia with microabscessation and mild peribronchiolar fibrosis, compatible with influenza infection in a subacute to chronic reparative stage.
There was evidence both histologically and by PCR that this herd dealt, on an ongoing basis, with M. hyopneumoniae and PRRSv activity with relatively mild lesions of both. Based on the herd history and these laboratory findings, the herd was fairly stable with respect to the background ongoing components of the PRDC, meaning that it did not typically experience dramatic mortality or morbidity attributable to these components. This influenza virus infection resulted in increased morbidity and mortality of short duration, with the acute infection passing rapidly through the herd to the regenerative stage seen in the pigs examined on May 8th.
During the week of May 11th, the herd veterinarian and the CFIA veterinarians and staff reassessed the medical condition of the herd and collected additional samples. Nasal swabs and blood samples were randomly collected from each of the 7 production areas for submission to NCFAD. The morbidity and mortality rates observed at the end of April and during the 1st week of May had declined to the pre-influenza levels. Feed consumption patterns within the herd were stable. The sow herd continued to show stability and there was no evidence of ILI in this group. The herd veterinarian and CFIA staff returned to the barn the week of May 25th to perform repeat diagnostic sampling and assess the health of the herd. Throughout areas A to G the health of the pigs was unremarkable. The majority of the pigs were bright, alert, and responsive. There was no cough in the piglet population in area B and < 2% of the piglets presented with a sneeze. There was no evidence of ILI in the nursery population. The week of June 1st was the last time that the herd was examined and diagnostic samples were procured. Prior to the initiation of the depopulation process on June 4th, the herd veterinarian detected no clinical evidence of ILI in any of the production areas.
Results of diagnostic tests
Following the initial on-site investigation by CFIA staff, nasal swab and serum samples from pigs in areas D, E, F, and G were submitted to NCFAD. Twenty-four nasal swab specimens were received on April 29th and RNA was immediately extracted for testing using the Spackman single tube real-time polymerase chain reaction (RT-PCR) assay targeting the M1 gene of influenza A viruses that has been developed for avian influenza testing (7). Preliminary results from this assay late in the evening of April 29th showed that 3 of the 24 specimens produced equivocal results and the remaining 21 were negative. As the sensitivity of this assay for the novel swine-origin virus had already been assessed as questionable by NCFAD, further testing using conventional RT-PCR assays specific for the M gene (8) and the H1 gene related to A/California/04/09 (National Microbiology Laboratory, Public Health Agency of Canada, unpublished protocol) was simultaneously performed. Results available in the morning of April 30th from the RT-PCR assays clearly identified that 19 and 15 of the 24 nasal swabs specimens produced positive results for the M gene and H1 gene, respectively, confirming infection with an H1 subtype influenza A virus.
A computer analysis of the M gene sequence for several of the human swine-origin H1N1 isolates indicated that the routinely used forward and reverse primers of the Spackman M1 gene RT-PCR assay (7) were less than optimal and these were therefore re-designed. Using the modified primers, results available in the early morning of May 2nd showed that 17 (71%) of the original 24 swab specimens gave a positive and another 4 (17%) samples gave suspicious results. This indicated that an influenza A virus with an M gene segment similar to that identified in several of the novel human isolates of pandemic H1N1 2009 was present (unpublished observations). The apparent prevalence (9) of pandemic H1N1 2009 in areas D, E, F, and G based on this sampling was estimated to be 87.5% [95% confidencial interval (CI): 69.0–95.7]. The CFIA immediately notified the World Organization for Animal Health (OIE) of these findings. By May 4th NCFAD determined the partial sequence of the M, H1, and N1 genes of the virus and confirmed that this was the pandemic H1N1 2009 virus; these additional findings were reported to the OIE on May 5th. Twenty-one of 24 nasal swab samples yielded influenza A virus isolates and 1 isolate was selected for full genome sequencing which was completed on May 7th. Phylogenetic analysis determined that the genome was 99% homologous to the novel H1N1 influenza A virus causing illness in humans around the world. Sequence results were submitted to GenBank on May 11th.
Of the 31 sera that were initially collected from barns D, E, F, and G, 8 (25.8%) tested positive for antibodies to influenza A virus. Five of these samples also had neutralizing antibody titers to the human isolate A/Mexico/InDRE4487/2009 (vH1N1 2009, kindly provided by the National Microbiology Laboratory). The nasal swab samples from these serologically positive animals gave either a negative or a weakly positive result with the modified M gene RT-PCR. Collectively these results suggest an initial infection 10 to 14 d earlier.
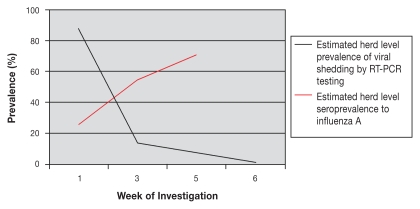
Follow-up testing using random sampling of the herd was carried out 2, 4, and 5 wk after the initial date of sampling as described under clinical findings. Although a proportion of nasal swab specimens collected subsequent to April 28th were positive for influenza A virus nucleic acid, no live virus was isolated from these samples. A decline in the proportion of samples that produced positive or suspicious results by RT-PCR over time was observed. Sampling completed during the week of May 11th identified an apparent herd level prevalence of 13.6% (95% CI: 9.6–18.8) with 29 out of 214 samples being positive or suspicious. Sampling completed the week of May 25th identified an apparent herd level prevalence of 7.9% (95% CI: 5.2–11.9) with 20 of 252 samples positive or suspicious. Sampling completed the week prior to depopulation (5 wk post initial sampling) identified an apparent herd level prevalence of 1.5% (95% CI: 0.5–4.4) with 3 of 198 samples being positive or suspicious. There was an increase in the proportion of pigs seropositive to influenza A nucleoprotein over the same time. On the date of initial sampling 8 of 31 (25.8%) samples were seropositive, indicating previous exposure to influenza A nucleoprotein in the sampled population. Two weeks later, this proportion had increased to 54.4% (95% CI: 47.1–61.4) and by 4 wk it had increased to 70.6 % (95% CI: 61.1–78.6). A review of the serological results suggested that pigs in all production areas were exposed to the virus within a relatively short period of time. These time lines are consistent with a predicted incubation period of 1 to 3 d with rapid recovery in 4 to 7 d that is typical for classical swine influenza. These data are presented in Figure 3.
Figure 3.
Estimated herd level prevalence of active viral shedding (as detected by PCR) and seroprevalence (to Influenza A) by week of investigation.
Epidemiological investigation
Tracing of the movement of all pigs, pig products, objects exposed to pig or pig products and humans associated with this farm during the 21-day period prior to the onset of clinical signs of respiratory disease observed was undertaken. The purpose was to identify other swine farms or humans at risk of having been exposed to pandemic H1N1 2009 virus and to attempt to confirm the source of introduction of virus. Although our initial hypothesis was that the contracted worker who returned from Mexico was the most likely source of virus on this farm, it was important to rule out the possibility of any other human or swine source and confirm that no other farms were at risk of being exposed.
The trace-out investigation did not identify any farms at risk of exposure via the direct or indirect movement of humans or animals. There was 1 shipment of 52 finished hogs to slaughter on April 23 but these animals were shipped from production areas F and G which were clinically unaffected on this date. Ante- and postmortem examinations on these animals at the slaughter plant were unremarkable. The most recent purchase of animals was breeding gilts in February of 2009 from a private purebred breeder. Prior to the delivery of gilts, and in the subsequent months, there had been no ILI in the source herd. The trace-in investigation did not identify any potential source farms, ruling out the possibility that an unidentified swine operation had been the source of virus.
It was confirmed that the individual hired to work on the ventilation system in areas D and E had experienced ILI while in the barn on April 14. This individual had returned from Mexico on April 12 prior to international awareness of this emerging disease. Retrospective investigation confirmed that this virus had been circulating in Mexico for at least several weeks prior to his return. Alberta Health Services (AHS) was contacted to investigate the human illness associated with this farm. The investigation of the hired individual, the farm family, and other community members revealed that several cases of pandemic H1N1 2009 were identified in the community in April and May. A number of community members had recently returned from travel to Mexico. Testing of the hired individual by RT-PCR using a nasopharyngeal swab was negative for influenza A using the probes, primers, and methods provided by the Centers for Disease Control and Prevention (CDC) Laboratory in Atlanta, Georgia. Of note, these nasopharyngeal swabs were collected well after onset of clinical signs of ILI, at days 11 and 19. Additional serological testing completed on June 26, 2009 using microneutralization assay performed at PHAC’s National Microbiology Laboratory (NML) and at the CDC laboratory met PHAC’s definition of a confirmed case of pandemic influenza virus (H1N1) 2009. There was also serological evidence of previous infection with seasonal H1N1 influenza virus. Although the hired individual wore a dust mask at times when in the barn, the protective measures were not consistent and did not suffice to prevent exposure of the pigs to the virus.
There were other individuals who had direct or indirect contact with the pigs and exhibited ILI prior to the first observed clinical signs in the pigs; however, it has not been possible to confirm or rule out the potential for these individuals to have been sources of the virus. There were H1N1-positive individuals with an established epidemiological link to this farm who had onset of symptoms after the swine were ill and the possibility of swine to human transmission cannot be excluded in this group. However, it is also possible that infection occurred as a result of person-to-person spread either via contact with the hired worker or other infected individuals in the community. The public health investigation concluded that the hired individual and possibly other individuals in direct contact with this herd introduced the virus to the swine.
Occupational health and safety
The zoonotic potential of swine influenza viruses is well-recognized (1). During the field epidemiological investigations, diagnostic sampling and humane destruction activities both CFIA and ARD staff entered the barn on multiple occasions. It was recognized that these staff could be at an increased risk for exposure to pandemic H1N1 2009 virus. Based on the specific situation, current scientific knowledge of the novel virus and after consultation with the Public Health Agency of Canada (PHAC), Health Canada’s Workplace Health and Public Safety Program (WHPSP) provided advice for all employees associated with the response. In consultation with WHPSP and the CFIA, Alberta Health and Wellness (AHW) and AHS provided advice to ARD staff.
Considering the novel nature of this infection in both human and swine populations, full personal protective equipment (PPE) was recommended by WHPSP on May 5th; this included N95 respirators, gloves, eye protection with seals around the eyes, boots, hair covers and coveralls or other body suit be used by all workers entering the barn. Due to the large amount of potentially contaminated fluids in the air, the possibility that the PPE may be dislodged, the perceived severity of human illness associated with this infection at the time, and the precedent set for anti-viral use during previous avian influenza responses, antiviral medications were recommended for prophylaxis for the duration of exposure plus 10 d. Prior to CFIA and ARD staff completing sampling and depopulation activities, seasonal influenza vaccine was recommended and antiviral medication (oseltamivir) offered to all staff in contact with the swine, but not all workers took the medication. Canadian Food Inspection Agency employees involved with the on-farm response were assessed by an Occupational Health Medical Officer from WHPSP for seasonal influenza vaccine, antiviral medication as well as Tetanus/Diphtheria vaccine. Antiviral medication was dispensed and the vaccines were administered by the Occupational Health Nurse from WHPSP. Ongoing follow-up was carried out to monitor adverse reactions to antiviral medication. Alberta Health Services provided similar occupational health and safety support to ARD staff. If workers experienced ILI after exposure to the quarantined premises they were advised to isolate themselves until 24 h after symptoms had resolved as a precautionary measure. As a nasopharyngeal swab is the most sensitive diagnostic sample to confirm influenza infection, exposed staff experiencing ILI were encouraged to contact the AHS Medical Officer of Health to arrange testing.
In order to assess the potential human exposure to influenza virus on this premises for both ARD and CFIA staff responding to the outbreak, a health surveillance questionnaire was administered by the public health division of AHS. The telephone questionnaire requested information on symptoms, onset and duration, antiviral and vaccine use, protective equipment, and breaches of the recommended protective practice. This public health investigation was undertaken to determine whether ILI was present in humans after being on the quarantined premises and to assess the effectiveness of personal protective equipment used to protect workers from exposure. It is recognized that pig-to-human and human-to-pig transmission of influenza virus occurs. It was also important to describe the type and duration of exposure that could be associated with infection. Response rates were excellent, with 93% of CFIA staff and 76% of ARD staff completing the questionnaire.
Two confirmed cases of pandemic H1N1 2009 occurred in workers who entered the barn on April 28th. These workers became symptomatic within the expected incubation period following exposure to the infected swine, and an investigation into their illness supported a common source of exposure. These individuals did not report any contact with a symptomatic human prior to developing ILI. The investigation revealed that there was an opportunity for transmission of the virus from the infected swine to the workers. Although it is possible that the exposed workers became infected from a human source that has not been identified, there is epidemiological support for zoonotic transmission in this situation.
The CFIA has determined that proper use of fitted full-face respirators with P100/chemical combination cartridges are indicated when working with influenza-infected animals. This is due to: the physical exertion and positioning required to carry out the required procedures, the heavy dust load of the swine or poultry housing environment, the need for eye protection (without fogging), and the common occurrence of ammonia and other manure gases. Subsequent to these identified cases, CFIA workers wore such protection. The CFIA workers involved in high-risk sampling activities who stated they followed the CFIA recommended procedures for working on influenza contaminated premises (10) did not become infected.
Anti-virals have been used in staff responding to previous outbreaks of avian influenza. Anti-virals were recommended in this outbreak for workers at risk of exposure due to the large amount of potentially contaminated fluids in the air and the possibility that PPE could be dislodged due to the working conditions. None of the workers became symptomatic when taking anti-viral medication at the time of exposure and in the 10 d following exposure. However, it is difficult to attribute this difference to the use of anti-virals in consideration of the small sample size. In addition, the infected workers were potentially exposed to higher concentrations of virus due to the higher prevalence of sick pigs in the confined area on April 28th compared with later workers. The importance of anti-virals for staff working with animals infected with influenza viruses should continue to be discussed, studied, and evaluated. Given that exposed staff are known and can be followed closely, early treatment on development of symptoms may be the best approach, as it minimizes potential side effects from possibly unnecessary medication and the promotion of resistance to anti-viral medication.
Approach to future cases of human pandemic H1N1 2009 virus infection in swine herds in Canada
The initial risk management decision by the CFIA and ARD to place this herd under federal movement restrictions under the Health of Animals Act was precautionary during a period of significant public and global concern and scientific uncertainty. At the time virus was confirmed on this farm, there was a lack of information on the virulence of this virus in human and pig populations. It was deemed prudent to conduct a full epidemiological investigation and restrict movement until such time as additional information was available and the risk to both the swine and human populations of North America could be assessed. As of August 7th other than Canada, only Argentina and Australia have identified and reported infection of swine with this virus.
Internationally, veterinary authorities are discussing the most appropriate approach to manage influenza infections in swine herds. Animal and public health authorities agree that influenza virus is not a food-borne zoonosis and does not affect the safety of properly cooked pork. Scientific evidence supports that live, infective virus is not present beyond the respiratory tract, and is most likely to be found in nasal and pharyngeal secretions during the febrile period of illness, of 1 to 3 d post-exposure (3); therefore, there is no risk of acquiring the virus from meat of recovered animals. As with any raw meat, pork should always be properly handled and cooked to eliminate a range of food safety concerns. Acutely ill pigs that are shedding virus could present a potential occupational risk to individuals handling live animals, but the obvious clinical manifestations of illness in affected animals (such as, respiratory signs, inactivity, decreased feed intake) should preclude their shipment to slaughter until they have recovered.
The OIE has stated that this virus is currently behaving in the same fashion as other swine influenza A viruses and does not require restrictive trade or disease control policies to be implemented. The Food and Agriculture Organization (FAO) of the United Nations approaches the management of this disease from a similar perspective. Public health authorities in Canada, in line with World Health Organization (WHO) recommendations, have indicated that no extraordinary response measures are needed or warranted in the human population to control the spread of the virus at this time. Public health instead provides advice about minimizing transmission, personal respiratory hygiene and the role of prescription anti-virals, where appropriate.
The number of humans who would have had contact with an infected swine herd is extremely limited when considering the opportunity for human-to-human transmission within any given infected community. Consequently, the imposition of strict control measures on swine herds while employing a more measured approach in people may create the impression that infected swine are more of a risk than infected people. This is clearly inconsistent with the observations to date. Based on current information, and the approach undertaken by public health authorities for humans, the CFIA has modified its initial approach of imposing federal quarantine restrictions on a swine herd infected with, or exposed to, the pandemic H1N1 2009 virus. Under this new policy, a federal quarantine will not be imposed unless there is evidence of a change in pathogenicity of the virus in humans or pigs. The CFIA will assist with the diagnostic characterization of any H1 influenza A virus isolated by a non-CFIA laboratory and offer advice and assistance to the provincial animal health authority if an infected herd is identified. The CFIA will lead a Federal/Provincial/Territorial consultation on the most appropriate manner to manage infected herds in collaboration with public health authorities, veterinary practitioners, and industry stakeholders. Under the authority in the Health of Animals Act and Regulations, the CFIA has the legislative mandate and capacity to implement stringent control measures should a change in the virus increase the risk to animal or public health and such measures become warranted. The CFIA is also working in collaboration with the veterinary authorities of the United States and Mexico on a protocol under which the 3 countries will agree on the notification framework and measures to be applied in a way to prevent trade restrictions. This approach is meant to minimize the economic impact of regulatory movement restrictions on swine producers while ensuring appropriate control mechanisms are in place.
Summary
This disease occurrence has highlighted the importance of ongoing collaboration between animal and public health authorities at all levels to ensure a timely and coordinated response to emerging zoonotic diseases. Given the continued spread of this virus in the human population, it is reasonable to predict that additional cases of pandemic H1N1 2009 in swine herds in Canada will be identified. Further dissemination of this virus in pig populations may pose an additional risk for transmission to humans in direct contact with clinically ill pigs. The need for appropriate PPE for workers investigating and sampling on suspect and confirmed infected farms is emphasized. Due to the potential for human-swine influenza virus reassortment and the development of a more virulent strain, the use of effective PPE needs to be addressed in these circumstances. In addition, it is important to emphasize that humans with ILI should avoid contact with any influenza susceptible animal species.
Although anti-viral prophylaxis was utilized in this specific situation due to global uncertainty surrounding this emerging virus at the time, early treatment of exposed individuals experiencing ILI may be sufficient and more closely parallels the response in human health care workers. Additional discussion and research are required to determine whether workers in a barn environment are more at risk of exposure and subsequent infection with this virus than those in a health care setting. From a human health perspective, the direct and indirect routes of human-to-human transmission will continue to account for the vast majority of new human infections. From an animal health perspective, evidence from this outbreak and findings from experimental studies suggest that in its current form this virus is unlikely to cause more significant clinical disease in pigs than commonly observed with classical SIV’s in Canada which is essentially a self-limiting infection confined to the respiratory tract with limited morbidity and eventual recovery.
Acknowledgments
The authors thank Drs. Brian Evans, Francine Lord, Jim Clark, Connie Argue, and Keith Campbell from the CFIA and Dr. Gerald Hauer from ARD for their review of the manuscript, and Drs. Kathleen Hooper-McGrevy, Carissa Embury-Hyatt, and James Neufeld for their diagnostic work at NCFAD. The authors acknowledge the diagnostic work completed at PHAC’s National Microbiology Laboratory and at the CDC Laboratory in Atlanta, Georgia. Diagnostic testing and advice provided by the Alberta Provincial Laboratory of Public Health was essential to the human investigation. Field epidemiologist Rachel McCormick contributed to the questionnaire design, data collection, and analysis with regard to exposed workers. The CFIA staff from Alberta North and other involved districts as well as ARD staff are acknowledged for their hard work and attention to detail during the investigation.
Footnotes
Reprints will not be available from the authors.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Olsen CW, Carey S, Hinshaw L, Karasin Al. Virologic and serologic surveillance for human, swine and avian influenza virus infections among pigs in the north-central United States. Arch Virol. 2000;145:1399–1419. doi: 10.1007/s007050070098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. Swine influenza viruses a North American Perspective. In: Maramorosch K, Shatkin AJ, Murphy FA, editors. Advances in Virus Research. Vol. 72. Burlington: Acad Pr; 2008. pp. 127–154. [DOI] [PubMed] [Google Scholar]
- 3.Olsen CW. Swine Influenza. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publ; 2006. pp. 469–482. [Google Scholar]
- 4.Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: A review of the literature. Clin Infect Dis. 2007;44:1084–1088. doi: 10.1086/512813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray GC, McCarthy T, Capuano AW, Setterquist SF, Olsen CW, Alavanja MC. Swine workers and swine influenza virus infections. Emerg Infect Dis. 2007;13:1871–1878. doi: 10.3201/eid1312.061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Reeth K. Avian and swine influenza viruses: Our current understanding of the zoonotic risk. Vet Res. 2007;38:243–260. doi: 10.1051/vetres:2006062. [DOI] [PubMed] [Google Scholar]
- 7.Spackman E, Senne DA, Myers TJ, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foucher RAM, Bestebroer TM, Herfst S, Van der Kemp L, Rimmelzwaan GF, Osterhaus ADME. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J Clin Microbiol. 2000;38:4096–4101. doi: 10.1128/jcm.38.11.4096-4101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown LD, Cai TT, DasGupta A. Confidence limits for apparent prevalence using the Wilson binomial approximation: Interval Estimation for a proportion. Stat Sci. 2001;16:101–133. [Google Scholar]
- 10.Canadian Food Inspection Agency, Field Biocontainment Working Group. Biocontainment and Safety Procedures for Working on Premises Contaminated with Notifiable Avian Influenza Viruses. 2007 Nov; RDIMS # 1305923. [Google Scholar]