Abstract
The EGFR has been targeted through the development of selective tyrosine kinase inhibitors (TKIs) that have proven effective in a subset of non-small cell lung cancer (NSCLC) patients, many bearing gain-of-function EGFR mutations or egfr gene amplification. However, the majority (~80–90%) of NSCLC patients do not respond to EGFR-specific TKIs and a high rate of acquired resistance to these therapeutics is observed in those that do respond. Thus, EGFR-specific TKIs will not, as single agents, make a high impact on overall lung cancer survival. A number of studies support the activities of other receptor tyrosine kinase pathways including cMet, IGF-1R and FGFRs as mechanisms for both intrinsic and acquired resistance to EGFR TKIs. While the role of cMet and IGF-1R signaling systems as mechanisms of resistance to EGFR TKIs has been widely reviewed in recent years, the potential role of FGFR-dependent signaling as a mechanism for EGFR TKI resistance has more recently emerged and will be highlighted herein. Due to the high degree of homology of FGFRs with VEGFRs and PDGFRs, FGFR-active TKIs already exist via development of VEGFR-targeted TKIs as angiogenesis inhibitors. Thus, these agents could be rapidly advanced into clinical investigations as FGFR inhibitors, either alone or in combination with TKIs selective for EGFR, cMet or IGF-1R as a means to expand the spectrum of NSCLC patients that can be effectively targeted with TKI-directed therapies.
Keywords: FGF, FGFR, NSCLC, intrinsic resistance, receptor tyrosine kinase, tyrosine kinase inhibitors
1. Introduction
Lung cancer, the leading cause of cancer deaths in the United States and worldwide (Jemal et al., 2008), is divided into non-small cell lung cancer (NSCLC), accounting for ~85% lung cancers and small-cell lung cancer (SCLC), accounting for ~15% of all lung cancers. Despite advances in early detection and continued incremental improvement in standard cytotoxic therapy-based care, the five-year survival rate for lung cancer has changed minimally over the past twenty-five years from 13% for those diagnosed between 1975–1977 to 16% for those diagnosed between 1996–2003 (Jemal et al., 2008).
The failure of advancements in standard cytotoxic chemotherapy to markedly prolong lung cancer patient survival has provided an impetus to pursue novel targets in lung cancer. The six hallmarks of cancer as outlined by Hanahan and Weinberg present a useful structure for considering the dominant pathways and cell programs controlling cancer cell behavior (Hanahan and Weinberg, 2000). Among these hallmarks, “self-sufficiency in growth” is frequently driven by receptor tyrosine kinase-dependent growth factor pathways operating in an autocrine fashion. In this regard, the high frequency of epidermal growth factor receptor (EGFR) expression in NSCLC (Hirsch et al., 2003) highlighted this receptor tyrosine kinase (RTK) as an attractive target for the development of small-molecule tyrosine kinase inhibitors (TKIs), including gefitinib and erlotinib (Dancey, 2004). In 2005, a placebo-controlled phase III trial, the National Cancer Institute of Canada BR.21 (NCIC BR.21) evaluated erlotinib in stage IIIB/IV NSCLC patients with prior chemotherapy exposure. Erlotinib yielded an objective response rate of ~9% versus <1% for placebo, increased overall survival (6.7 vs. 4.7 months), and reduced cancer-related symptoms. Independent predictors of survival were Asian ethnicity, adenocarcinoma histology, and never smoker status (Shepherd et al., 2005). In light of these aforementioned independent survival predictors in the NCIC BR.21 trial, a slight benefit did exist in an unselected population. However, a significant number of patients who received erlotinib do not respond despite reassuring demographic factors (i.e. female, Asian, adenocarcinoma, never smokers) (Shepherd et al., 2005). Clearly, demographics alone are not sufficient to define who will respond to erlotinib. Moreover, EGFR expression is necessary for EGFR TKI responsiveness, but is also not sufficient to predict response as a single measurement. However, the presence of activating EGFR mutations (Lynch et al., 2004; Mitsudomi and Yatabe, 2007; Paez et al., 2004) or egfr gene amplification (Cappuzzo et al., 2005) are highly predictive of response to EGFR TKI therapy. Activating EGFR mutations are present in ~10% of lung adenocarcinomas in Western populations and 30–50% in Asian populations (Herbst et al., 2008). Importantly, response to gefitinib or erlotinib is not dictated simply by gain-of-function EGFR mutations as a significant number of EGFR TKI-responsive patients bear lung tumors with wild-type EGFR (Sequist et al., 2007). Combined, these studies demonstrate the necessity of using EGFR TKIs on selected patient populations. Also, as indicated in Figure 1, the majority of NSCLC patients do not respond to EGFR TKIs, indicating that additional target pathways mediating self-sufficiency in growth will need to be identified and appropriate inhibitors deployed to impact the outcome of these patients.
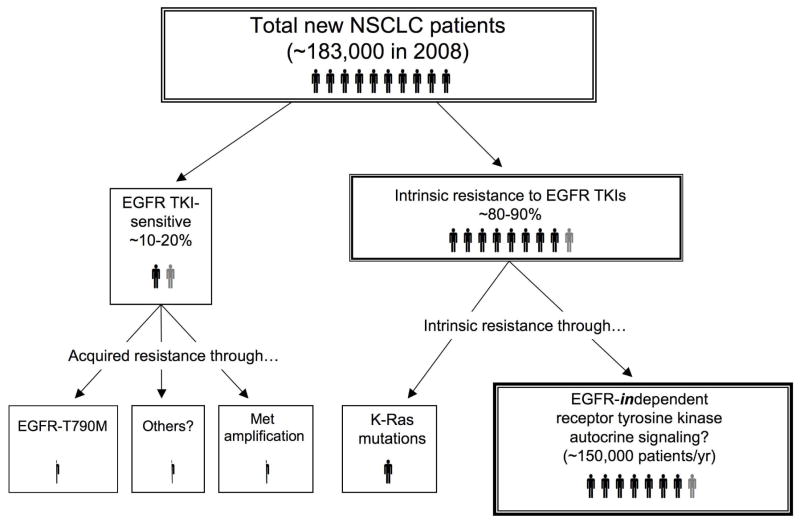
Figure 1. Frequencies of intrinsic resistance to EGFR-specific TKIs relative to sensitivity/acquired resistance in NSCLC.
The diagram indicates the relative frequencies of EGFR TKI sensitivity (10–20% in the United States) versus intrinsic resistance (80–90%). Mutation of K-Ras occurs in ~10–30% of adenocarcinomas (but rarely in squamous and large cell carcinoma) and accounts for a known resistance mechanism to EGFR TKIs (Herbst et al., 2008). We hypothesize that autocrine signaling through EGFR-independent receptor tyrosine kinases functions as a mechanism of intrinsic resistance to EGFR TKIs in NSCLC not bearing EGFR or K-Ras mutations.
All patients with tumors which are initially sensitive to EGFR TKIs will relapse (reviewed in (Camp et al., 2005; Engelman et al., 2008; Morgillo et al., 2005)). Of the patients with acquired resistance to EGFR TKI treatments, approximately 50% bear the EGFR T790M mutation that resides within an analogous position to previously-defined acquired resistance mutations in Abl, PDGFRα and cKit. In addition, c-Met amplification is likely to account for approximately 20% of acquired resistance to EGFR TKIs (Engelman et. al, 2007, Bean et. al., 2007). The mechanism(s) that account for the remaining 30% of acquired resistance to EGFR TKIs remains ill-defined. If intrinsic resistance is defined as having an initial clinical response followed by disease progression within 6 months, or having no initial response to treatment, then the majority (~80–90%) of NSCLC patients from Western populations who harbor a tumor not bearing an activating EGFR mutation are likely to exhibit intrinsic resistance to EGFR TKI therapy (Figure 1). One established mechanism for intrinsic resistance is seen in lung tumors bearing gain-of-function K-Ras mutations leading to EGFR-independent activation of multiple effector pathways including the ERK MAP kinase signaling pathway (Cox and Der, 2003). K-Ras mutations are detected in 10–30% of lung adenocarcinomas (Herbst et al., 2008), but rarely in squamous and large cell carcinomas, and present in a mutually exclusive pattern with regard to EGFR activating mutations (Eberhard et al., 2005; Pao et al., 2005).
The NSCLC tumors that exhibit intrinsic resistance to EGFR TKIs distinct from K-Ras mutation, in fact, represent the majority of lung cancers (Figure 1). Moreover, no specific targeted therapies presently exist for the treatment of this group. It is increasingly evident, from both clinical and biological perspectives, that EGFR is only one of many important growth factor pathways that function in lung cancer. We and others have considered the hypothesis that EGFR-independent receptor tyrosine kinase signaling pathways dominate in EGFR TKI-insensitive NSCLC (Marek et al., 2009; Morgillo and Lee, 2005; Thomson et al., 2008). Therefore, continued progress towards successful therapeutics of NSCLC will ultimately depend on identification and inhibition of additional receptor tyrosine kinases and their downstream signaling pathways dominant in individual NSCLC tumors. As proof-of-principle for this hypothesis, we will briefly consider the role of the HGF/c-Met and insulin-growth factor receptor (IGF-1R) signaling pathways in the intrinsic resistance of NSCLC to EGFR TKIs and more extensively discuss the role of the fibroblast growth factors (FGFs) and their receptors as components of a novel autocrine growth pathway in lung cancer.
2. Alternative receptor tyrosine kinase pathways as mechanisms for intrinsic resistance to EGFR TKIs
As indicated in Figure 1, 80 to 90 percent of NSCLC patients bear tumors that lack activating EGFR mutations and/or egfr gene amplification and less likely to be responsive to EGFR TKIs. Besides mutations in K-Ras that contribute to EGFR TKIs intrinsic resistance (Herbst et al., 2008), a simple explanation for the insensitivity of the majority of NSCLC to EGFR inhibitors is the dominant activity of alternative RTK systems distinct from EGFR. In this regard, it has been demonstrated that 33 of the 58 defined RTKs are expressed at the mRNA level in at least 25 percent of a panel of primary NSCLC tumors (Muller-Tidow et al. 2005). An independent phosphoproteomic approach confirms the extensive array of RTKs that are expressed and active in NSCLC cell lines and tumors (Rikova et al., 2007). Thus, there is no shortage of candidates for RTKs that may function as alternatives to EGFR in signal transduction of growth and transformation in NSCLC. Among these, the literature highlights roles for cMet (Engelman et al., 2007; Lutterbach et al., 2007), Axl (Shieh et al., 2005; Wimmel et al., 2001), IGF-1R (Morgillo and Lee, 2005) and PDGFR (Thomson et al., 2008; McDermott et al., 2009) as well as novel tyrosine kinases such as Alk and Ros (Rikova et al., 2007). Among these, a role for c-Met and IGF-1R has been most extensively explored (Camp et al., 2005; Engelman and Janne, 2008; Morgillo and Lee, 2005) (Figure 2).
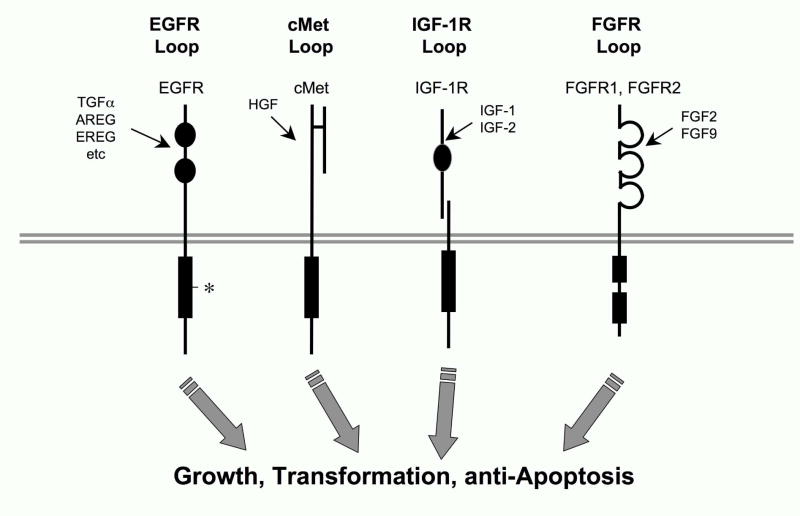
Figure 2. Autocrine and paracrine growth loops that may contribute to growth and transformation in NSCLC independent of EGFR.
The literature (see accompanying text) supports the involvement of cMet, IGF-1R and FGFR receptor tyrosine kinases in the autocrine and paracrine-stimulated growth of NSCLC, thereby conferring intrinsic resistance to EGFR TKIs. In addition to autocrine signaling through EGFR via expression and release of distinct EGF family ligands (TGFα, amphiregulin (AREG), epiregulin (EREG)), the asterisk indicates the presence of activating somatic mutations that occur in EGFR.
The RTK c-Met, is expressed and active in 60% of NSCLC as measured by IHC staining (Ma et al., 2005). High levels of HGF, the c-Met ligand, have also been correlated with poor prognosis of NSCLC patients (Siegfried et al., 1998). Recent studies have demonstrated c-Met amplification and over-expression as a mechanism for both intrinsic and acquired gefitinib resistance in NSCLC (Bean et al., 2007). Autocrine signaling via the c-Met/HGF loop leads to intrinsic gefitinib resistance by restoring the PI3K/AKT signaling pathway independent of EGFR or ErbB3 activation in adenocarcinoma NSCLC lines harboring EGFR activating mutations (Yano et al., 2008). Furthermore c-Met gene amplification serves as a mechanism for acquired gefitinib resistance in both NSCLC lines and ~20% of NSCLC tumors (Engelman and Janne, 2008). In response to gefitinib treatment, NSCLC lines underwent amplification of chromosomal region 7q31.1–33.3 containing the c-Met gene, allowing c-Met activation of the PI3K/AKT pathway in an ErbB3-dependent manner, but independent of either EGFR or ErbB2 activation (Engelman et al., 2007). Importantly, inhibition of c-Met restored gefitinib sensitivity in these cells. Thus, c-Met signaling likely contributes to reduced EGFR-specific TKI response in NSCLC.
The IGF-IR and its ligands insulin, insulin-like growth factor I (IGF-I), and insulin-like growth factor II (IGF-II) have also been implicated as components of an active growth factor pathway in NSCLC. NSCLC cell lines and primary tumors express IGF-IR (Cappuzzo et al., 2006; Morgillo et al., 2007; Quinn, 1996), although one study suggests that IGF-1R protein, by itself, does not correlate with EGFR TKI resistance (Cappuzzo et al., 2006). Primary NSCLC tumors have been shown to express higher concentrations of circulating IGF-I than surrounding normal lung tissue (Minuto et al., 1986), suggesting a paracrine signaling mechanism between tumor stromal fibroblasts secreting IGF-I and neoplastic epithelial cells. Clearly NSCLC cell lines are responsive to exogenous IGF-I (Ankrapp and Bevan, 1993) and knockdown of IGF-IR in NSCLC cell lines with antisense or RNAi strategies inhibited cell proliferation and in vitro and in vivo estimates of transformed growth (Ankrapp and Bevan, 1993; Dong et al., 2007; Lee et al., 1996). Evidence also suggests that inhibition of IGF-IR in NSCLC cells lines may increase their sensitivity to gefitinib (Morgillo et al., 2007). Together, this evidence suggests that the IGF-I/IGF-IR and HGF/cMet signaling pathways may contribute to both intrinsic and acquired resistance to EGFR TKIs in NSCLC.
Despite an extensive literature demonstrating a role for FGF receptors (FGFRs) in human disease including cancer (see Section 3), these receptors have not been widely considered as a putative autocrine growth factor pathway in lung cancer. The literature supporting the contribution of specific FGFs and FGFRs to EGFR TKI resistance in NSCLC is reviewed in Section 4.
3. FGF and FGFR signaling in cancer
The mammalian fibroblast growth factors (FGFs) are comprised of 22 family members and mediate numerous developmental programs during embryogenesis as well as critical roles in adult tissue repair and maintenance (reviewed in (Eswarakumar et al., 2005; Grose and Dickson, 2005; Mohammadi et al., 2005)). FGFs initiate signal transduction by binding members of a family of RTKs (FGFR1-4), usually in the context of heparan sulfate proteoglycans (HSPG), inducing receptor dimerization and commencement of downstream signaling. FGFRs contain two or three extracellular Ig-like loops that comprise the FGF and HSPG binding sites. Particularly important, alternative splicing in the membrane-proximal Ig loop of FGFR1-3 dictates ligand specificity (Zhang et al., 2006). The third loop is encoded by an invariant N-terminal portion (exon IIIa) and either exon IIIb or IIIc for the C-terminal portion. FGFR2-IIIb and FGFR3-IIIb splice variants bind FGF7 and FGF10 with high affinity while FGFR1-3 IIIc splice variants bind FGF2 and FGF9. Generally, these isoforms are also cell type specific with FGFR-IIIb expression on epithelial cells and FGFR-IIIc expression on mesenchymal cells. In addition, FGF7 and FGF10, which bind to epithelial FGFR-IIIb, are expressed in mesenchymal cell types while FGF2 is expressed in epithelial cells and binds to FGFR-IIIc presented on mesenchymal cells, establishing paracrine signaling pathways (Grose and Dickson, 2005).
Aberrant signaling by FGFs and FGFRs has been implicated in diverse human cancers (Eswarakumar et al., 2005; Grose and Dickson, 2005). In many cancers, FGF and FGFR signaling is enhanced by acquisition of somatic gain-of-function mutations in specific FGFRs. In urothelial cancers, mutations in the ligand binding domain of FGFR3 cause ligand-independent dimerization or stabilization of the active conformation of the receptor while mutations in the FGFR3 kinase domain can render the receptor constitutively active (Grose and Dickson, 2005). Somatic mutations in FGFR2 conferring gain-of-function are observed in ~10% of primary endometrial cancers as well as endometrial tumor cell lines (Dutt et al., 2008; Pollock et al., 2007). Moreover, blocking FGFR2 activity inhibits transformation and survival of endometrial carcinoma lines (Dutt et al., 2008). Similar somatic mutations in FGFR2 and FGFR3 are also observed in gastric cancer and colorectal cancers, respectively (Jang et al., 2001). Blocking FGFR2 activity with an FGFR-active TKI inhibited growth of gastric cancer cell lines (Takeda et al., 2007).
In the hematologic malignancy 8p11 myeloproliferative syndrome (EMS), genomic sequences encoding the kinase domain of FGFR1 are fused with one of several donor genes causing constitutive activation of the FGFR1 kinase domain (reviewed in (Grose and Dickson, 2005)). Also, a similar gene translocation event occurs with FGFR3 in some multiple myelomas (Grose and Dickson, 2005).
FGFRs 1, 2, and 4 are frequently over-expressed in breast cancers and a selective inhibitor of FGFR activity caused G1 growth arrest in breast cancer cell lines (Koziczak et al., 2004). FGFRs have also been shown to be physically associated with N-cadherin in various cancer cells, resulting in cell survival and motility (Cavallaro and Christofori, 2004; Suyama et al., 2002). It is hypothesized that N-cadherin facilitates FGF2 binding while preventing ligand-induced internalization, thereby yielding sustained signaling (Cavallaro and Christofori, 2004; Suyama et al., 2002). Finally, tumorigenesis via FGFs and FGFRs does not always depend on the accumulation of receptor mutations or marked over-expression. In the mouse, FGF3, FGF4 and FGF8 were initially identified as mouse mammary tumor virus-activated genes leading to mouse mammary gland tumorigenesis, presumably through an autocrine mechanism mediated by the deregulated FGFs (Payson et al., 1996; van Leeuwen and Nusse, 1995). Similarly, FGF2 and FGF9 are expressed by stromal cells within the prostate as well as by epithelial carcinoma cells. These growth factors can then signal in a paracrine and autocrine fashion by binding to FGFR1-IIIc and FGFR2-IIIc detected on prostate epithelial cancer cells (Kwabi-Addo et al., 2004; Kwabi-Addo et al., 2001). These studies provide examples where FGF/FGFR-mediated transformation does not require somatic mutation, but can occur simply through inappropriate expression of a specific FGF.
Thus, FGFRs can be activated in the setting of human cancer through somatic mutations, gene fusion events or induction of ligands and/or FGFRs, thereby resulting in autocrine activation.
4. FGF and FGFR autocrine signaling in non-small cell lung cancer
Accumulating evidence implicates specific FGFs and FGFRs as components of an autocrine signaling pathway in NSCLC tumors and cell lines. While rare somatic mutations in different FGFRs have been detected in NSCLC ((Ding et al., 2008), http://www.sanger.ac.uk/genetics/CGP/cosmic/), numerous in vitro studies employing NSCLC cell lines reveal that specific FGFs (FGF2 and FGF9) as well as FGFR1 and FGFR2 are frequently co-expressed (Berger et al., 1999; Chandler et al., 1999; Fischer et al., 2008; Kuhn et al., 2004; Marek et al., 2009; Thomson et al., 2008). Several independent studies also demonstrated frequent expression of FGF2, FGFR1 and FGFR2 mRNA and protein in primary NSCLC specimens as well (Behrens et al., 2008; Donnem et al., 2009; Muller-Tidow et al., 2005; Volm et al., 1997). Molecular analysis of the FGFR1 and FGFR2 splice variant status in NSCLC cell lines revealed that the relevant FGFR-IIIc forms are expressed (Fischer et al., 2008; Marek et al., 2009), a requirement for serving as a receptor for FGF2 and FGF9 (Figure 3). Moreover, application of anti-sense RNA or RNAi approaches (Kuhn et al., 2004; Marek et al., 2009), neutralizing FGF2 antibodies (Kuhn et al., 2004) or FGFR-active TKIs (Fischer et al., 2008; Marek et al., 2009; Thomson et al., 2008) caused inhibition of cell proliferation or tumor growth, suggesting that the co-expressed FGF2 and FGFRs function as an autocrine growth pathway in some NSCLC cell lines. The FGF-FGFR pathway has also been suggested to mediate EGFR TKI resistance (Marek et al., 2009; Thomson et al., 2008). In general, these studies show that an FGF-FGFR autocrine pathway dominates in NSCLC cell lines of squamous cell (SCC) and large cell carcinoma (LC) histologies that frequently exhibit a more mesenchymal differentiation state (Marek et al., 2009; Thomson et al., 2008). This contrasts with the enrichment of adenocarcinoma (AC) and bronchoalveolar histologies observed in primary tumors and NSCLC cell lines sensitive to EGFR-specific TKIs ((Coldren et al., 2006; Herbst et al., 2008) and Figure 3). Moreover, Thomson et al suggest that a switch to FGFR and PDGFR signaling occurs in concert with an epithelial to mesenchymal transition (EMT) (Thomson et al., 2008), a malignant phenotype (Sabbah et al., 2008) that has also been correlated with EGFR-specific TKI resistance (Coldren et al., 2006).
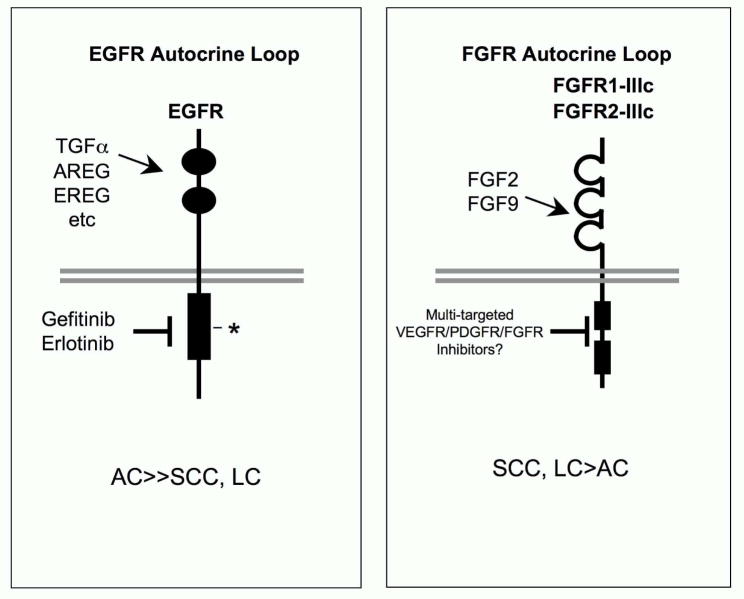
Figure 3. Specific FGFs and FGFRs comprise an autocrine growth loop in NSCLC cell lines.
While adenocarcinoma is the dominant NSCLC histology sensitive to EGFR TKIs (Herbst et al., 2008), studies with NSCLC cell lines indicate more frequent autocrine signaling in squamous and large cell carcinoma-derived cells through FGF2, FGF9, FGFR1-IIIc and FGFR2-IIIc (Marek et al., 2009; Thomson et al., 2008). Importantly, the homology of FGFRs with VEGFRs and PDGFRs results in frequent activity of VEGFR-targeted TKIs on FGFRs as well (see Table 1). Thus, TKIs in this class target multiple RTKs including VEGFRs, PDGFRs and FGFRs.
5. FGFR inhibitors as therapeutics in NSCLC
5.1. Receptor TKIs
Both small-molecule TKIs and inhibitory FGFR1 monoclonal antibodies have been developed as potential therapeutics to disrupt FGFR signaling in cancer cells. Due to extensive sequence homology, FGFRs, VEGFRs and PDGFRs constitute a sub-family of receptor tyrosine kinases (Manning et al., 2002). As a result, many TKIs developed as inhibitors for VEGFRs will also inhibit PDGFR and FGFR family members at similar potencies (see Table 1). In our recent study demonstrating an autocrine role for FGFR1 and FGFR2 in NSCLC cells, RO4383596 served as an effective FGFR inhibitor (Marek et al., 2009). Likewise, AZD2171 (cediranib) was developed as a VEGFR inhibitor (Nikolinakos and Heymach, 2008), but exhibits good potency for FGFRs (Table 1) and has been employed as an effective inhibitor of growth of FGFR2-driven gastric cancer cell lines (Takeda et al., 2007). Additionally, a multi-kinase targeted TKI, TKI-258, has been used to inhibit activated FGFR3 in multiple myeloma (Xin et al., 2006). It is possible that this class of TKIs with broad-spectrum activity against multiple members of the FGFR/VEGFR/PDGFR family will serve as superior anti-cancer agents. However, it may also be possible to develop TKIs with higher selectivity for the FGFR family relative to VEGFRs and PDGFRs. An advantage of such inhibitors might be the lack of various side-effects caused by VEGFR or PDGFR inhibition.
Table 1.
Receptor Tyrosine Kinase Targets for Anti-Angiogenic TKIs
| IC50, nM | |||||||
|---|---|---|---|---|---|---|---|
| TKI | VEGFR-1 | VEGFR-2 | PDGFR-β | cKit | FGFR1 | Others | Reference: |
| RO4383596 | - | 44 | 33 | - | 29 | (McDermott et al., 2005) | |
| PD173074 | 100–200 | 18 | - | 22 | cSrc, 20 nM | (Mohammadi et al., 1998) | |
| TKI-258 | 10 | 13 | 27 | 2 | 8 | (Lee et al., 2005) | |
| Cediranib (AZD2171) | 5 | 1 | 5 | 2 | 26 | (Wedge et al., 2005) | |
| Sorafenib | - | 90 | 57 | 68 | 580 | Raf-1, 6 nM | (Wilhelm et al., 2004) |
| Sunitinib (SU11248) | - | 80 | 2 | - | 2900 | (Sun et al., 2003) | |
| Vandetanib ZD6474) | 1600 | 40 | - | - | 3600 | EGFR, 500 nM | (Hennequin et al., 2002) |
| Pazopanib | 10 | 30 | 84 | 74 | 140 | (Kumar et al., 2007) | |
| Brivanib (BMS540215) | 380 | 25 | >6000 | - | 148 | (Bhide et al., 2006) | |
5.2. Monoclonal antibodies
Based on the effectiveness of inhibitory monoclonal antibody reagents targeting EGFR or ErbB2 (Croce, 2008), similar antibody-base reagents targeting members of the FGFR family may serve as excellent therapeutics for cancers such as NSCLC where these receptors may function in autocrine growth signaling. An advantage of monoclonal antibody-based agents is their exquisite specificity for distinct FGFR proteins as well as avoiding activity on VEGFR and PDGFR family members. In this regard, fully-human FGFR1 inhibitory monoclonal antibodies have been developed with specificity for either FGFR1-IIIb or FGFR1-IIIc (Sun et al., 2007). These monoclonal antibodies potently inhibited FGF signaling mediated by the respective FGFR1 isoforms. Based on the observation that FGFR2-IIIc appears to be a dominant FGFR2 isoform active in NSCLC cell lines (Marek et al., 2009), inhibitory FGFR2-IIIc antibodies could also be developed and assessed as inhibitors of FGFR signaling. Human single-chain Fv antibodies to FGFR3-IIIc have been shown to inhibit growth of bladder cancer cells in vitro (Martinez-Torrecuadrada et al., 2005). Finally, as an alternative approach, soluble FGFR1 reagents have been developed as ligand traps that exhibit potent inhibitory actions on human NSCLC cells grown as xenografts in immunodeficient mice (Ogawa et al., 2002).
5.3. Identification of lung tumors bearing an activate FGFR pathway
Based on the clinical experiences with gefitinib and erlotinib, FGFR TKIs and/or FGFR monoclonal antibodies should be targeted to lung cancer patients whose tumors bear active FGFR signaling pathways. While gain-of-function FGFR mutations have been identified in certain malignancies, studies to date have failed to reveal frequent mutation or amplification of FGFRs in lung cancer. Rather, it is likely that the FGFR pathway is activated through autocrine production of the ligands including FGF2 and FGF9 (Fischer et al., 2008; Marek et al., 2009; Thomson et al., 2008). Our own study revealed enrichment of the squamous cell and large cell lung carcinoma histologies in the FGFR-dependent NSCLC cell lines (Marek et al., 2009) although not all squamous cell and large cell carcinoma cell lines were sensitive to FGFR inhibition. Another potential identifier of FGFR-dependent NSCLC cell lines is the ligand, FGF2. In this regard, elevated FGF2 levels are noted in serum and urine from diverse cancer patients (Nguyen et al., 1994; Ueno et al., 2001) as well as NSCLC patients (Brattstrom et al., 2002; Brattstrom et al., 2004; Ueno et al., 2001). Secreted FGF2 levels have not yet been directly linked to FGFR-dependence in the primary tumor. However, if high serum or urinary levels of FGF2 accurately predict FGFR dependence, then the growth factor itself may provide a simple biomarker. Yet, our recent experience with NSCLC cell lines reveals a number of lines that express FGF2, but not FGFR1 or FGFR2 (Marek et al., 2009 and unpublished observations). Thus, measurements that relate tumor co-expression of FGF2 and FGFRs are likely to be a more reliable measure of functional activity of an FGF-FGFR pathway in NSCLC.
5.4. Re-evaluation of VEGFR TKI clinical trial results
As previously noted, many VEGFR TKIs also inhibit FGFRs at similar potency (Table 1) due to the inherent homology of FGFRs and VEGFRs (Manning et al., 2002). A relatively high potency for FGFRs is especially apparent with TKI-258, AZD2171 (cediranib), BMS-540215 (brivanib) and pazopanib. All four of these TKIs have entered clinical trial for evaluation and tumor responses have been noted (Kumar et al., 2007; Nikolinakos and Heymach, 2008; Platero et al., 2008; Sarker et al., 2008). While the results from these trials are interpreted in the context of the activity of the TKIs on VEGFRs, it is possible that both tumor responses and side-effects may be mediated, in part, through FGFR antagonism. In fact, evaluation of tumor response in a brivanib trial revealed that FGF2-positive tumors showed increased response to brivanib relative to FGF2-negative tumors (Platero et al., 2008). This study, in particular, provides impetus for reevaluating tumor responsiveness to VEGFR TKIs to determine if an active FGF and FGFR pathway in the tumor is better correlated with response.
6. Conclusions and future perspective: therapeutic approaches involving simultaneous inhibition of multiple receptor tyrosine kinases
Ultimately, effective treatment of NSCLC will likely require therapeutic strategies involving the combined use of two or more TKIs. Despite dramatic tumor responses to erlotinib and gefitinib in NSCLC patients whose tumors bear activating EGFR mutations, all sensitive tumors eventually undergo one or more mechanisms of acquired resistance (reviewed in (Engelman and Janne, 2008; Morgillo and Lee, 2005)). In addition to the ability of FGFR signaling to mediate intrinsic resistance to EGFR inhibitors as discussed above, our preliminary findings reveal that expression of FGFR2 and FGFR3 are induced at the mRNA and protein level following gefitinib treatment of EGFR-dominant NSCLC cell lines and head-and-neck cancer cell lines (Ware, Marshall and Heasley, unpublished observations), thus providing yet another mechanism for acquired resistance to EGFR TKIs. If induction of FGFRs in response to treatment with EGFR TKIs is proven to be a significant mechanism for acquired resistance to this class of TKIs in patients, then combined treatment with EGFR and FGFR-specific TKIs could represent a strategy to prolong or enhance the action of drugs like gefitinib and erlotinib. Moreover, a recent study (Fischer et al., 2008) as well as our own unpublished observations demonstrate that many NSCLC cell lines appear to employ baseline autocrine signaling through both EGFRs and FGFRs without pretreatment as evidenced by additive or synergistic growth inhibition by combinations of TKIs inhibiting EGFR and FGFRs. In a similar fashion, Stommel et al demonstrated coactivation of multiple RTKs in glioblastoma cell lines and showed that combinations of RTK inhibitors are required to decrease signaling, cell survival and anchorage-independent growth (Stommel et al., 2007). It is likely, therefore, that multiple RTKs including EGFR, c-Met, IGF-1R and FGFRs will be engaged simultaneously in specific NSCLCs such that specific combinations of selective TKIs will be required to completely inhibit signaling and cell transformation. As the repertoire of TKIs and antibody-based inhibitors of RTKs continues to expand, it will be important to also generate gene expression signatures (Coldren et al., 2006), genotype signatures (McDermott et al., 2007) or phosphoproteomic signatures (Rikova et al., 2007) for human cancers such as NSCLC that delineate the activated and dominant pathways in a given tumor so that appropriate combinations of inhibitors can be deployed.
In conclusion, the high frequency of intrinsic resistance to EGFR-specific TKIs as well as the occurrence of acquired-resistance to these drugs indicates that additional receptor tyrosine kinase-dependent pathways must be targeted to bring about effective inhibition of tumor growth. The literature reviewed here indicates that FGFs and FGFRs function in an autocrine fashion in NSCLC. Because of the inherent homology between FGFRs and VEGFRs, a number of TKIs with activity on FGFRs are already available and can be quickly tested as therapeutics, either alone or in combination with EGFR TKIs.
Acknowledgments
The studies were supported by NIH grants R01 CA127105 and P50 CA58187.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ankrapp DP, Bevan DR. Insulin-like growth factor-I and human lung fibroblast-derived insulin-like growth factor-I stimulate the proliferation of human lung carcinoma cells in vitro. Cancer Res. 1993;53:3399–3404. [PubMed] [Google Scholar]
- Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci (USA) 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens C, Lin HY, Lee JJ, Raso MG, Hong WK, Wistuba II, et al. Immunohistochemical expression of basic fibroblast growth factor and fibroblast growth factor receptors 1 and 2 in the pathogenesis of lung cancer. Clin Cancer Res. 2008;14:6014–6022. doi: 10.1158/1078-0432.CCR-08-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W, Setinek U, Mohr T, Kindas-Mugge I, Vetterlein M, Dekan G, et al. Evidence for a role of FGF-2 and FGF receptors in the proliferation of non-small cell lung cancer cells. Int J Cancer. 1999;83:415–423. doi: 10.1002/(sici)1097-0215(19991029)83:3<415::aid-ijc19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Bhide RS, Cai ZW, Zhang YZ, Qian L, Wei D, Barbosa S, et al. Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5- methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan- 2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J Med Chem. 2006;49:2143–2146. doi: 10.1021/jm051106d. [DOI] [PubMed] [Google Scholar]
- Brattstrom D, Bergqvist M, Hesselius P, Larsson A, Lamberg K, Wernlund J, et al. Elevated preoperative serum levels of angiogenic cytokines correlate to larger primary tumours and poorer survival in non-small cell lung cancer patients. Lung Cancer. 2002;37:57–63. doi: 10.1016/s0169-5002(02)00027-2. [DOI] [PubMed] [Google Scholar]
- Brattstrom D, Bergqvist M, Hesselius P, Larsson A, Wagenius G, Brodin O. Serum VEGF and bFGF adds prognostic information in patients with normal platelet counts when sampled before, during and after treatment for locally advanced non-small cell lung cancer. Lung Cancer. 2004;43:55–62. doi: 10.1016/j.lungcan.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Camp ER, Summy J, Bauer TW, Liu W, Gallick GE, Ellis LM. Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin Cancer Res. 2005;11:397–405. [PubMed] [Google Scholar]
- Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- Cappuzzo F, Toschi L, Tallini G, Ceresoli GL, Domenichini I, Bartolini S, et al. Insulin-like growth factor receptor 1 (IGFR-1) is significantly associated with longer survival in non-small-cell lung cancer patients treated with gefitinib. Ann Oncol. 2006;17:1120–1127. doi: 10.1093/annonc/mdl077. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Chandler LA, Sosnowski BA, Greenlees L, Aukerman SL, Baird A, Pierce GF. Prevalent expression of fibroblast growth factor (FGF) receptors and FGF2 in human tumor cell lines. Int J Cancer. 1999;81:451–458. doi: 10.1002/(sici)1097-0215(19990505)81:3<451::aid-ijc20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Coldren CD, Helfrich BA, Witta SE, Sugita M, Lapadat R, Zeng C, et al. Baseline gene expression predicts sensitivity to gefitinib in non-small cell lung cancer cell lines. Mol Cancer Res. 2006;4:521–528. doi: 10.1158/1541-7786.MCR-06-0095. [DOI] [PubMed] [Google Scholar]
- Cox AD, Der CJ. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- Croce CM. Oncogenes and cancer. N Engl J Med. 2008;358:502–511. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- Dancey JE. Predictive factors for epidermal growth factor receptor inhibitors--the bull’s-eye hits the arrow. Cancer Cell. 2004;5:411–415. doi: 10.1016/s1535-6108(04)00122-9. [DOI] [PubMed] [Google Scholar]
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong AQ, Kong MJ, Ma ZY, Qian JF, Xu XH. Down-regulation of IGF-IR using small, interfering, hairpin RNA (siRNA) inhibits growth of human lung cancer cell line A549 in vitro and in nude mice. Cell Biol Int. 2007;31:500–507. doi: 10.1016/j.cellbi.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Donnem T, Al-Shibli K, Al-Saad S, Busund LT, Bremnes RM. Prognostic Impact of Fibroblast Growth Factor 2 in Non-small Cell Lung Cancer: Coexpression with VEGFR-3 and PDGF-B Predicts Poor Survival. J Thorac Oncol. 2009 doi: 10.1097/JTO.0b013e31819f2e38. In press. [DOI] [PubMed] [Google Scholar]
- Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci (USA) 2008;105:8713–8717. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Fischer H, Taylor N, Allerstorfer S, Grusch M, Sonvilla G, Holzmann K, et al. Fibroblast growth factor receptor-mediated signals contribute to the malignant phenotype of non-small cell lung cancer cells: therapeutic implications and synergism with epidermal growth factor receptor inhibition. Mol Cancer Ther. 2008;7:3408–3419. doi: 10.1158/1535-7163.MCT-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose R, Dickson C. Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev. 2005;16:179–186. doi: 10.1016/j.cytogfr.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hennequin LF, Stokes ES, Thomas AP, Johnstone C, Ple PA, Ogilvie DJ, et al. Novel 4-anilinoquinazolines with C-7 basic side chains: design and structure activity relationship of a series of potent, orally active, VEGF receptor tyrosine kinase inhibitors. J Med Chem. 2002;45:1300–1312. doi: 10.1021/jm011022e. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. New Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch FR, Varella-Garcia M, Bunn PA, Jr, Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- Jang JH, Shin KH, Park JG. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001;61:3541–3543. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Koziczak M, Holbro T, Hynes NE. Blocking of FGFR signaling inhibits breast cancer cell proliferation through downregulation of D-type cyclins. Oncogene. 2004;23:3501–3508. doi: 10.1038/sj.onc.1207331. [DOI] [PubMed] [Google Scholar]
- Kuhn H, Kopff C, Konrad J, Riedel A, Gessner C, Wirtz H. Influence of basic fibroblast growth factor on the proliferation of non-small cell lung cancer cell lines. Lung Cancer. 2004;44:167–174. doi: 10.1016/j.lungcan.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004;11:709–724. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- Kwabi-Addo B, Ropiquet F, Giri D, Ittmann M. Alternative splicing of fibroblast growth factor receptors in human prostate cancer. Prostate. 2001;46:163–172. doi: 10.1002/1097-0045(20010201)46:2<163::aid-pros1020>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Lee CT, Wu S, Gabrilovich D, Chen H, Nadaf-Rahrov S, Ciernik IF, et al. Antitumor effects of an adenovirus expressing antisense insulin-like growth factor I receptor on human lung cancer cell lines. Cancer Res. 1996;56:3038–3041. [PubMed] [Google Scholar]
- Lee SH, Lopes de Menezes D, Vora J, Harris A, Ye H, Nordahl L, et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res. 2005;11:3633–3641. doi: 10.1158/1078-0432.CCR-04-2129. [DOI] [PubMed] [Google Scholar]
- Lutterbach B, Zeng Q, Davis LJ, Hatch H, Hang G, Kohl NE, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res. 2007;67:2081–2088. doi: 10.1158/0008-5472.CAN-06-3495. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Marek L, Ware KE, Fritzsche A, Hercule P, Helton WR, Smith JE, et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharmacol. 2009;75:196–207. doi: 10.1124/mol.108.049544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torrecuadrada J, Cifuentes G, Lopez-Serra P, Saenz P, Martinez A, Casal JI. Targeting the extracellular domain of fibroblast growth factor receptor 3 with human single-chain Fv antibodies inhibits bladder carcinoma cell line proliferation. Clin Cancer Res. 2005;11:6280–6290. doi: 10.1158/1078-0432.CCR-05-0282. [DOI] [PubMed] [Google Scholar]
- McDermott LA, Simcox M, Higgins B, Nevins T, Kolinsky K, Smith M, et al. RO4383596, an orally active KDR, FGFR, and PDGFR inhibitor: synthesis and biological evaluation. Bioorg Med Chem. 2005;13:4835–4841. doi: 10.1016/j.bmc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- McDermott U, Sharma SV, Dowell L, Greninger P, Montagut C, Lamb J, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad USA. 2007;104:19936–19941. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott U, Ames RY, Iafrate AJ, Maheswaran S, Stubbs H, Greninger P, et al. Ligand-dependent platelet-derived growth factor receptor (PDGFR)-α activation sensitizes rare lung cancer and sarcoma cells to PDGFR kinase inhibitors. Cancer Res. 2009;69:3937–3946. doi: 10.1158/0008-5472.CAN-08-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuto F, Del Monte P, Barreca A, Fortini P, Cariola G, Catrambone G, et al. Evidence for an increased somatomedin-C/insulin-like growth factor I content in primary human lung tumors. Cancer Res. 1986;46:985–988. [PubMed] [Google Scholar]
- Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, et al. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, Lee HY. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–2803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- Morgillo F, Lee HY. Resistance to epidermal growth factor receptor-targeted therapy. Drug Resist Updat. 2005;8:298–310. doi: 10.1016/j.drup.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Muller-Tidow C, Diederichs S, Bulk E, Pohle T, Steffen B, Schwable J, et al. Identification of metastasis-associated receptor tyrosine kinases in non-small cell lung cancer. Cancer Res. 2005;65:1778–1782. doi: 10.1158/0008-5472.CAN-04-3388. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Watanabe H, Budson AE, Richie JP, Hayes DF, Folkman J. Elevated levels of an angiogenic peptide, basic fibroblast growth factor, in the urine of patients with a wide spectrum of cancers. J Natl Cancer Inst. 1994;86:356–361. doi: 10.1093/jnci/86.5.356. [DOI] [PubMed] [Google Scholar]
- Nikolinakos P, Heymach JV. The tyrosine kinase inhibitor cediranib for non-small cell lung cancer and other thoracic malignancies. J Thorac Oncol. 2008;3:S131–134. doi: 10.1097/JTO.0b013e318174e910. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Takayama K, Takakura N, Kitano S, Ueno H. Anti-tumor angiogenesis therapy using soluble receptors: enhanced inhibition of tumor growth when soluble fibroblast growth factor receptor-1 is used with soluble vascular endothelial growth factor receptor. Cancer Gene Ther. 2002;9:633–640. doi: 10.1038/sj.cgt.7700478. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payson RA, Wu J, Liu Y, Chiu IM. The human FGF-8 gene localizes on chromosome 10q24 and is subjected to induction by androgen in breast cancer cells. Oncogene. 1996;13:47–53. [PubMed] [Google Scholar]
- Platero S, Mokliatchouk O, Jayson GC, Jonker DJ, Rosen LS, Luroe S, et al. Correlation of FGF2 tumor expression with tumor response, PFS, and changes in plasma pharmacodynamic (PD) markers following treatment with brivanib alaninate, an oral dual inhibitor of VEGFR and FGFR tyrosine kinases. J Clin Oncol. 2008;26:3506. [Google Scholar]
- Pollock PM, Gartside MG, Dejeza LC, Powell MA, Mallon MA, Davies H, et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene. 2007;26:7158–7162. doi: 10.1038/sj.onc.1210529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KA, Treston AM, Unsworth EJ, Miller MJ, Vos M, Grimley C, Battey J, Mulshine JL, Cuttitta F. Insulin-like growth factor expression in human cancer cell lines. J Biol Chem. 1996;271:11477–11483. doi: 10.1074/jbc.271.19.11477. [DOI] [PubMed] [Google Scholar]
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Sabbah M, Emami S, Redeuilh G, Julien S, Prévost G, Zimber A, et al. Molecular signature and therapeutic perspective of the epitelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123–151. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Sarker D, Molife R, Evans TR, Hardie M, Marriott C, Butzberger-Zimmerli P, et al. A phase I pharmacokinetic and pharmacodynamic study of TKI258, an oral, multitargeted receptor tyrosine kinase inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2008;14:2075–2081. doi: 10.1158/1078-0432.CCR-07-1466. [DOI] [PubMed] [Google Scholar]
- Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. New Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, et al. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005;7:1058–1064. doi: 10.1593/neo.05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried JM, Weissfeld LA, Luketich JD, Weyant RJ, Gubish CT, Landreneau RJ. The clinical significance of hepatocyte growth factor for non-small cell lung cancer. Ann Thorac Surg. 1998;66:1915–1918. doi: 10.1016/s0003-4975(98)01165-5. [DOI] [PubMed] [Google Scholar]
- Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- Sun HD, Malabunga M, Tonra JR, DiRenzo R, Carrick FE, Zheng H, et al. Monoclonal antibody antagonists of hypothalamic FGFR1 cause potent but reversible hypophagia and weight loss in rodents and monkeys. Am J Physiol Endocrinol Metab. 2007;292:E964–976. doi: 10.1152/ajpendo.00089.2006. [DOI] [PubMed] [Google Scholar]
- Sun L, Liang C, Shirazian S, Zhou Y, Miller T, Cui J, et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J Med Chem. 2003;46:1116–1119. doi: 10.1021/jm0204183. [DOI] [PubMed] [Google Scholar]
- Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–314. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- Takeda M, Arao T, Yokote H, Komatsu T, Yanagihara K, Sasaki H, et al. AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res. 2007;13:3051–3057. doi: 10.1158/1078-0432.CCR-06-2743. [DOI] [PubMed] [Google Scholar]
- Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis. 2008;25:843–854. doi: 10.1007/s10585-008-9200-4. [DOI] [PubMed] [Google Scholar]
- Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. New Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- Ueno K, Inoue Y, Kawaguchi T, Hosoe S, Kawahara M. Increased serum levels of basic fibroblast growth factor in lung cancer patients: relevance to response of therapy and prognosis. Lung Cancer. 2001;31:213–219. doi: 10.1016/s0169-5002(00)00187-2. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F, Nusse R. Oncogene activation and oncogene cooperation in MMTV-induced mouse mammary cancer. Semin Cancer Biol. 1995;6:127–133. doi: 10.1006/scbi.1995.0018. [DOI] [PubMed] [Google Scholar]
- Volm M, Koomagi R, Mattern J, Stammler G. Prognostic value of basic fibroblast growth factor and its receptor (FGFR-1) in patients with non-small cell lung carcinomas. Eur J Cancer. 1997;33:691–693. doi: 10.1016/s0959-8049(96)00411-x. [DOI] [PubMed] [Google Scholar]
- Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer. 2001;37:2264–2274. doi: 10.1016/s0959-8049(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Xin X, Abrams TJ, Hollenbach PW, Rendahl KG, Tang Y, Oei YA, et al. CHIR-258 is efficacious in a newly developed fibroblast growth factor receptor 3-expressing orthotopic multiple myeloma model in mice. Clin Cancer Res. 2006;12:4908–4915. doi: 10.1158/1078-0432.CCR-06-0957. [DOI] [PubMed] [Google Scholar]
- Yano S, Wang W, Li Q, Matsumoto K, Sakurama H, Nakamura T, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]