Summary
Introduction
Avian H5N1 influenza viruses currently circulating in southeast Asia could potentially cause the next pandemic. However, currently licensed human vaccines are subtype-specific and do not protect against these H5N1 viruses. We aimed to develop an influenza vaccine and assessed its immunogenicity and efficacy to confer protection in BALB/c mice.
Methods
We developed an egg-independent strategy to combat the avian influenza virus, because the virus is highly lethal to chickens and the maintenance of a constant supply of embryonated eggs would be difficult in a pandemic. We used a replication-incompetent, human adenoviral-vector-based, haemagglutinin subtype 5 influenza vaccine (HAd-H5HA), which induces both humoral and cell-mediated immune responses against avian H5N1 influenza viruses isolated from people.
Findings
Immunization of mice with HAd-H5HA provided effective protection from H5N1 disease, death, and primary viral replication (p<0.0001) against antigenically distinct strains of H5N1 influenza viruses. Unlike the recombinant H5HA vaccine, which is based on a traditional subunit vaccine approach, HAd-H5HA vaccine induced a three-fold to eight-fold increase in HA-518-epitope-specific interferon-)-secreting CD8 T cells (p=0.01).
Interpretation
Our findings highlight the potential of an Ad-vector-based delivery system, which is both egg-independent and adjuvant-independent and offers stockpiling options for the development of a pandemic influenza vaccine.
Introduction
Highly pathogenic avian influenza viruses of subtype H5N1 are now endemic in domestic poultry in southeast Asia. Since early 2004, human infections with H5N1 viruses have been frequently reported in the region with increasing frequency and high fatality rates. H5N1 viruses were first recognized to cause respiratory disease in people in 1997, when 18 documented cases, including six deaths, occurred after outbreaks of highly pathogenic avian influenza in poultry farms and markets in Hong Kong.1,2 Two additional human H5N1 infections were identified in a family in Hong Kong in 2003.3 Since then, H5N1 viruses have spread to nine Asian countries, and recently have reached several countries in eastern Europe. At least 152 laboratory confirmed cases of human infection with a fatality rate of greater than 50% have been reported to WHO since January, 2004.4
So far, most human H5N1 infections have been due to direct transmission of the virus from infected poultry, although possible cases of human-to-human transmission have been reported.5 Genetic reassortment between a human and avian influenza virus or mutations in the avian H5N1 virus genome could result in the generation of a novel influenza virus of the haemagglutinin subtype 5 (H5) that could initiate a pandemic if it acquired the ability to undergo sustained transmission in an immunologically naive human population. Therefore, effective vaccines against highly pathogenic avian influenza H5N1 viruses are an urgent and global public-health priority.6–8 Vaccines developed and investigated in response to the 1997 human outbreak of H5N1 influenza were only modestly immunogenic in people, and needed multiple and higher doses of antigen or adjuvant (or both) to elicit detectable seroconversions.9,10 The H5N1 viruses isolated from human beings in 2004 were genetically and antigenically distinct from those in 1997 and 2003,11 necessitating the development of new vaccines currently undergoing clinical investigation.12 Current H5N1 vaccines depend on a supply of embryonated eggs to produce inactivated sub-virion vaccines.13–16 To produce enough egg-derived pandemic vaccine for 1–2 billion people worldwide at high risk would need 4 billion embryonated eggs. However, this type of vaccine will not be effective against the genetic drift variants, and therefore precludes the stockpiling option. Moreover, in the event of a pandemic, maintenance of the availability of embryonated eggs would be a potential problem since H5N1 viruses are highly virulent in poultry. Hence, alternative vaccine manufacturing strategies are needed.
We have developed an Ad-vector-based strategy for pandemic influenza vaccine using the haemagglutinin from an H5N1 human isolate and assessed its immunogenicity and efficacy to confer protection in BALB/c mice against heterologous challenges, including recent highly pathogenic avian influenza viruses.
Methods
The Cre-recombinase recombinase-mediated site-specific recombination system17 was used to construct a replication-defective, recombinant human Ad vector expressing H5 (HAd-H5HA). This vector carries the full-length coding region of the H5 gene of the avian H5N1 influenza virus (A/Hong Kong/156/197 [HK/156/97]) inserted into the early region (E) 1 of the human Ad genome (webfigure 1, A),18 under the control of cytomegalovirus immediate early promoter, and the H5 gene was expressed efficiently in human embryonic kidney cells (webfigure 1, B). A human Ad genome with deletions of the E1 and E3 regions (HAd-ΔE1E3) served as a negative control.19
To determine whether the Ad vector H5 vaccine was immunogenic and conferred protection against lethal challenge with a homotypic H5N1 virus, 10-week-old BALB/c mice (five per group) were immunized intramuscularly with 1×108 plaque-forming units (pfu) of HAd-H5HA vaccine or HAd-ΔE1E3. This pfu is equivalent to a 1.2×1010 virus particle number, which is an optimum dose for the induction of protective immune response (webfigure 2).20,21 Doses were given twice at a 4-week interval. Another group of animals was inoculated with phosphate-buffered saline (PBS) as a negative control. Two additional groups of mice were immunized to compare the human Ad vaccine strategy with a traditional subunit vaccine approach used in a previous clinical trial,10 the findings of which indicated that a baculovirus-expressed recombinant H5 vaccine (rH5HA), based on the HK/156/97 virus, is modestly immunogenic even after two doses of 90 µg of vaccine. These mice were immunized twice at 4 weeks apart with 3 µg of rH5HA, either in the presence or absence of 1% alum adjuvant. Serum samples were obtained 3 weeks after the second intramuscular immunization from all five groups, to monitor the development of haemagglutinin-specific antibodies by haemagglutination inhibition (HI) assays21 using horse red blood cells and virus neutralization assays before challenge.22 These serological assays determine the concentration (titre) of haemagglutinin-specific antibodies that bind to haemagglutinin on the virus and prevent the agglutination of red blood cells (HI assays) or infection of Madin-Darby canine kidney (MDCK) cells (virus neutralization assays) in vitro.
Since HK/156/97 virus is not lethal to mice, to assess protection, we have used the A/Hong Kong/483/97 (HK/483/97) virus for challenges since it is not only genetically and antigenically similar to HK/156/97, but also lethal to mice.
The efficacy of vaccine was determined by challenging all five groups of mice with 100×50% lethal dose (LD50), which is equivalent to 103–7 EID50 (egg infectivity dose; the amount of virus needed to kill 50% of fertilized chicken embryos) of H5N1 HK/483/97 virus, 4 weeks after the second immunization.18, 20 Mice were monitored for clinical signs and changes in bodyweight every day for 14 days postchallenge. The maximum mean weight loss was the weight loss on day 6 postchallenge.
We investigated whether the serological responses induced by HAd-H5HA vaccine would cross-react with more recent H5N1 viruses isolated from human beings, and whether the route of inoculation (systemic vs mucosal) would affect vaccine immunogenicity. BALB/c mice (20 per group) were immunized either intramuscularly or intranasally with 1×108 pfu of HAd-H5HA twice every 4 weeks. Other groups of mice (20 per group) were intramuscularly immunized with 108 pfu of HAd- Δ E1E3 or 3 µg of rH5HA with alum. The serum samples were obtained 4 weeks after the second immunization and analyzed by virus neutralization assays to assess their ability to react with a homologous virus (HK/156/97), or with antigenically heterologous viruses (A/Hong Kong/213/2003 [HK/213/03] and A/Vietnam/1203/04 [VN/1203/04]). Compared with HK/156/97, the aminoacid homology in the haemagglutinin subunit is 94.8% for HK/213/03 and 95.5% for VN/1203/04.
In addition to neutralizing antibody responses against haemagglutinin, CD8 T cell responses have been shown to contribute to viral clearance.23,24 Although the genome of influenza A virus has many potential epitopes that could generate CD8 T cell responses, antiviral CD8 T cell responses are actually restricted to only a few epitopes. Furthermore, some epitopes seem to generate a robust response whereas others tend to generate a weaker response leading to a hierarchy, known as immuno-dominance. CD8 T cell responses to nucleoprotein (NP) 147 are dominant followed by haemagglutinin (HA) 518 responses in influenza-virus-infected BALB/c mice.
HA 518 was originally described for the haemagglutinin of an H1N1 virus, A/Puerto Rico/8/34 (PR/8/34). This epitope is conserved in all three H5N1 viruses used in this study, including currently circulating avian and human H5N1 viruses, and some H9N2 viruses (webtable).25 The HA 518 epitope has also been shown to be the dominant epitope in H5N3-infected animals.26
To determine whether HAd-H5HA vaccine induced functional CD8 T cells, mice (nine per group) were inoculated intramuscularly or intranasally twice every 4 weeks with 108 pfu of HAd-H5HA. Similarly, groups of mice were immunized with 108 pfu of HAd- ΔE1E3 (as a negative control) or 3 µg of rH5HA with alum. An additional group was inoculated intraperitonally with one dose of 500 haemagglutination units (HAU) of HK/213/03 virus as a positive control. Splenocytes from the immunized animals were stained with a murine MHC-encoded allele Kd -specific pentamer for the immunodominant HA 518 epitope conjugated with phycoerythrin (PE). Spleen cells were also stained with anti-CD8 antibodies conjugated with allophycocyanin (APC). PE and APC fluorochrome-conjugated reagents were used to identify the number of HA-518-specific CD8 T cells among all CD8 T cells of different specificities by use of a flow cytometer.
After recognition of peptide epitopes on the virus-infected cells, CD8 T cells from virus-infected or vaccinated animals kill infected cells via cytotoxic mechanisms and aid in viral clearance, as well as secreting interferon γ. To assess the functionality of CD8 T cells, splenocytes from immunized mice were cultured with syngeneic γ-irradiated spleen cells presenting either HA 518 or NP 147 peptides on anti-mouse interferon-γ-coated filter plates for 60 h and developed according to an ELISpot protocol. Splenocytes treated with phorbol myristate acetate and ionomycin were used as positive controls within every group.
To assess the protective efficacy against challenge with the variant H5N1 strains, mice (15 per group) were inoculated intramuscularly or intranasally twice every 4 weeks with 108 pfu of HAd-H5HA or intramuscularly with 108 pfu of HAd-ΔE1E3 (as a negative control). Five animals from every group were challenged 4 weeks after the second immunization with 100×50% LD50 of HK/483/97 (6.3×103 EID50), VN/1203/04 (6.9×103 EID50), or 100×50% mouse infectious dose (MID50; 6.3×103 EID50) of HK/213/03. Mice challenged with HK/483/97 or VN/1203/04 were monitored for clinical signs and changes in bodyweight daily. Since HK/213/03 is not lethal for mice, to assess the vaccine efficacy we measured the viral titres in the lungs on day 4 after challenge. Viral lung titres were expressed as the mean EID50 (log10/mL). Sensitivity of detection was 1.5 log10 EID50.
We used the Kruskall-Wallis test for all calculations of significance in this study.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Mice immunized with either HAd-H5HA or rH5HA and alum produced significantly higher HI titres (p=0.05) and virus neutralization titres (p=0.001) than titres from controls receiving PBS, HAd-ΔE1E3, or rH5HA alone. These mice were protected from death and weight loss when challenged with H5N1 virus (HK/483/97), and the HAd-H5HA vaccine was better than the rH5HA-alone vaccine and at least as good or better than the rH5HA and alum vaccine (table). Although four of five animals that received rH5HA alone were protected against death, they showed substantial weight loss, a marker for morbidity. These results are consistent with previous findings that rH5HA without an adjuvant has suboptimum immunogenicity, hence we did not include it in subsequent studies.9,10
Table 1.
Serological response and survival after lethal H5N1 virus challenge in mice immunized with Had-H5HA vaccine
| HI titre (geometric mean) | Neutralization titre (geometric mean) | Number of animals that survived / total | Maximum mean weight loss (% [SD]) | |
|---|---|---|---|---|
| PBS | <25 | <20 | 0/5 | 24.7% (0.5) |
| Had-ΔE1E3 | <25 | <20 | 0/5 | 24.8% (1.1) |
| Had-H5HA | 696* | 3146† | 5/5 | 1.3% (1.5) |
| rH5HA | 38‡ | 69†∫ | 4/5 | 15.4% (9.5) |
| rH5HA + alum | 696‡ | 2633∫ | 5/5 | 3.3% (1.6) |
Data are listed by immunogen.
* and ‡: differences between marked data are p=0.05.
† and ∫ differences between marked data are p=0.001.
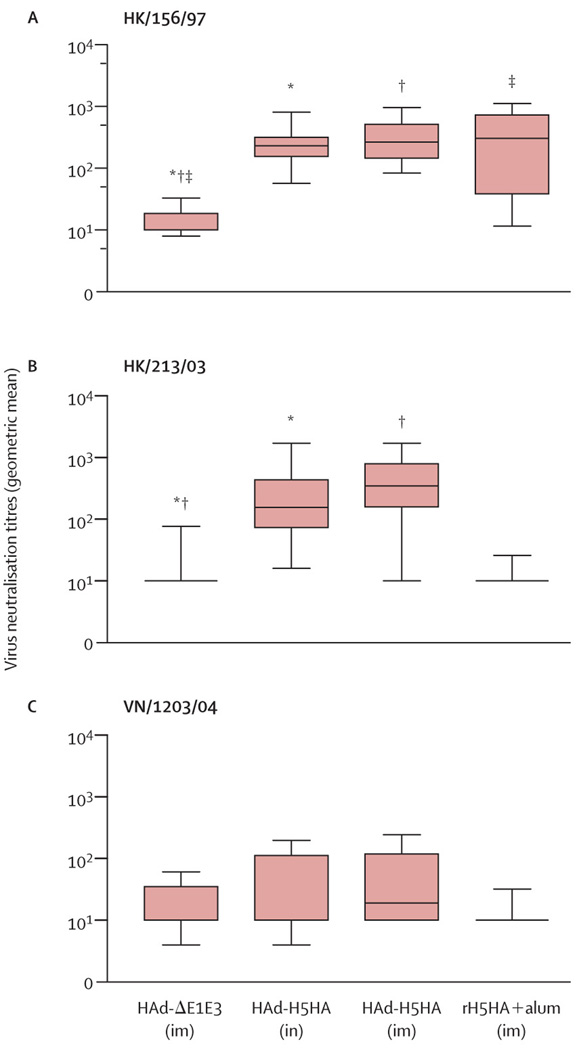
Serological responses in mice immunized with HAd-H5HA either intramuscularly or intranasally showed significantly high neutralizing antibody titres against HK/156/97 virus, and reduced titres against heterologous HK/213/03 or VN/1203/04 virus (figure 1). However, mice immunized with rH5HA and alum showed high antibody titres against homologous HK/156/97 virus, but failed to neutralize HK/213/03 or VN/1203/04 virus. No significant cross-reactivity was recorded by any vaccine against VN/1203/04 (figure 1).
Figure 1. Virus-neutralizing antibody response against homologous and heterologous avian influenza virus strains in mice immunized with HAd-H5HA vaccine.
Box-whisker plots showing mean, IQRs, and range of the antibody response against (A) HK/156/97, (B) HK/213/03, and (C) VN/1203/04 strains. *, †, and ‡: differences between marked data are p=0.001. im=intramuscular immunization. in=intranasal immunization.
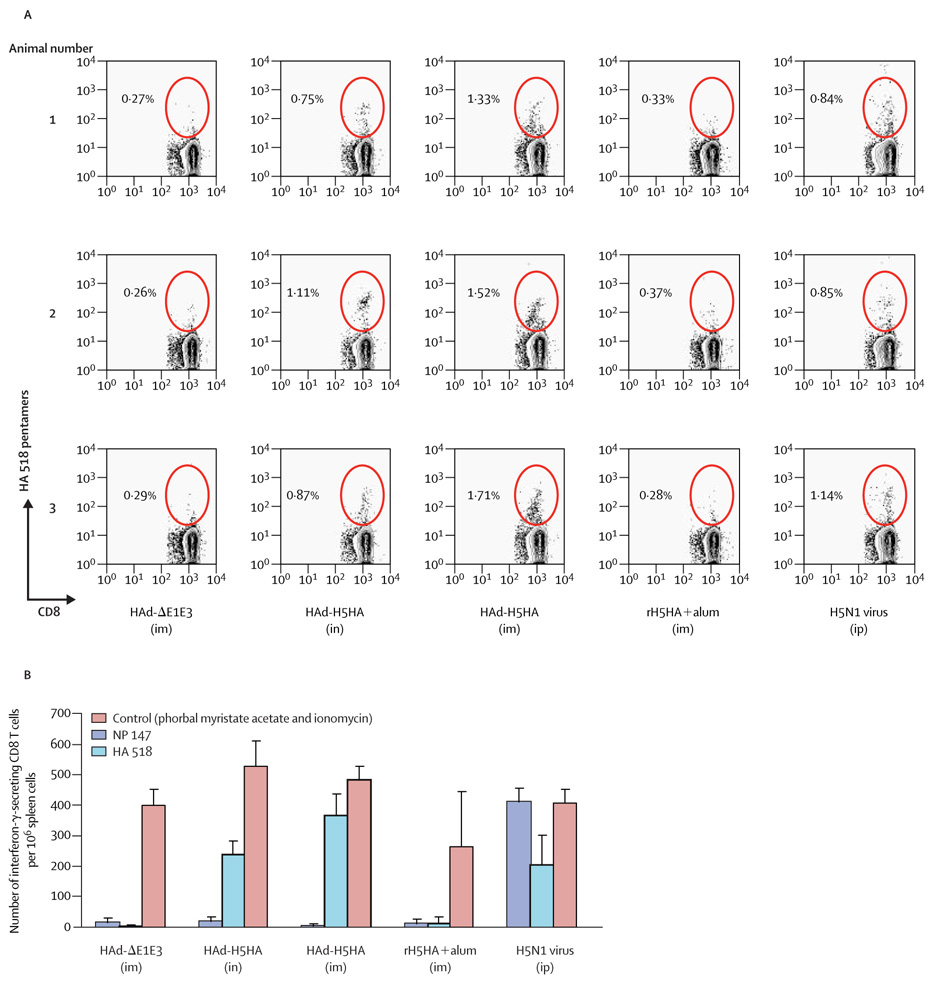
Mice that received the HAd-H5HA vaccine had a three-fold to eight-fold higher frequency of HA-518-specific CD8 T cells than mice immunized with HAd-ΔE1E3 or with rH5HA and alum, when delivered intranasally (p=0.01) or intramuscularly (p=0.001, figure 2, A). The vaccine contains only haemagglutinin and not nucleoprotein; therefore, as expected, we recorded no detectable increase in NP-147-epitopespecific CD8 T cells in animals immunized with HAd-H5HA vaccine (data not shown). By contrast, control mice infected with H5N1 virus showed a predominant NP-147-epitope-specific CD8 T cell response (data not shown), followed by an HA-518-epitope-specific response (figure 2, A), in a manner similar to that recorded with an H1N1 virus infection.25 None of the mice vaccinated with HAd-AE1E3 or rH5HA with alum showed an increase in HA-518-epitope-specific CD8 T cell frequencies (figure 2, A).
Figure 2. Induction of HA-518-epitope-specific and interferon - - secreting CD8 T cells in mice immunized with HAd-H5HA vaccine.
(A) Flow cytometric analysis of spleen cells from immunized mice (three per group) stained with HA 518 pentamer epitope. Pentamer-positive cells (circled) are shown as a percentage of CD8 Tlymphocytepopulation. (B) ELISpots measurements of interferon in spleen cells of immunized mice. Data are mean and SDs (error bars). im=intramuscular immunization. in=intranasal immunization. ip=intraperitoneal immunization.
Furthermore, significantly increased numbers of interferon-γ-secreting cells were detected in spleen cells taken from HAd-H5HA-vaccinated mice compared with those from mice vaccinated with HAd-ΔE1E3 or rH5HA and alum (by either delivery method; p=0.001), when stimulated with syngeneic γ-irradiated spleen cells pulsed with the conserved HA 518 epitope, but not with the NP 147 epitope (figure 2, B). Therefore, despite the induction of only low concentrations of neutralizing antibodies against heterologous viruses, HAd-H5HA vaccine induced high cell-mediated immune responses to the HA 518 epitope. Overall, intramuscular delivery of HAd-H5HA vaccine induced consistently higher humoral and cellular immune responses, including cytotoxic T lymphocytes (data not shown) than intranasal delivery.
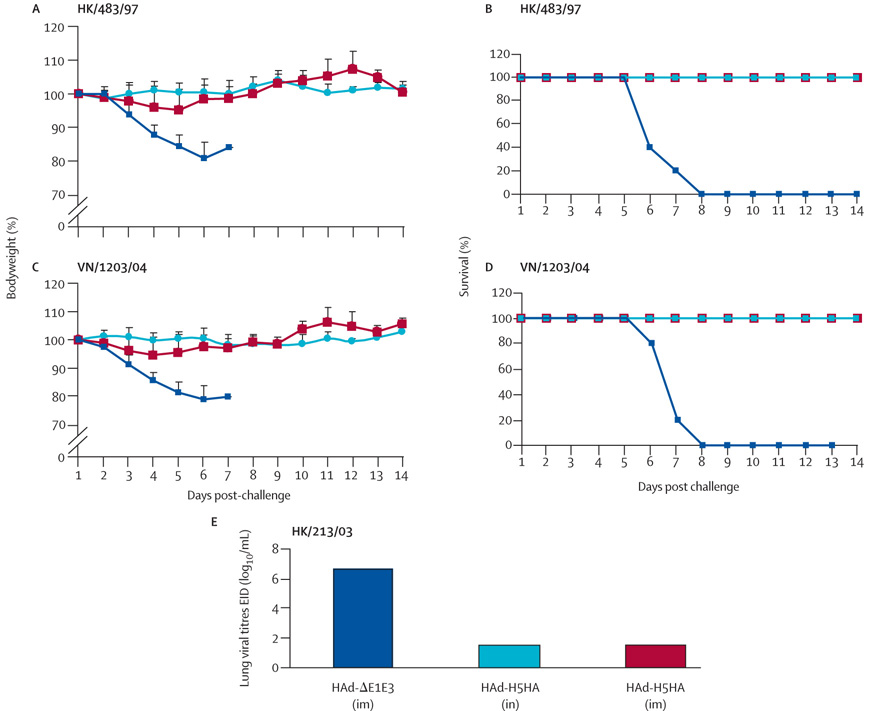
Intramuscular or intranasal immunization of mice with HAd-H5HA showed minimum morbidity and provided complete protection against death after challenge with HK/483/97 (figure 3, A and B). All mice vaccinated with HAd-H5HA also survived and exhibited very low morbidity, as measured by weight loss, after a lethal challenge with the more recent H5N1 virus, VN/1203/04 (figure 3, C and D). Furthermore, mice vaccinated with HAd-H5HA by either route of inoculation and challenged with HK/213/03 virus had no detectable virus in the lungs on day 4 postinfection (figure 3, E), whereas mice vaccinated with the control vector HAd-ΔE1E3 had a mean lung viral titre of greater than 106 EID50/mL (p<0.0001). Therefore, the HAd-H5HA vaccine induced significant protection against challenge with heterologous H5N1 viruses, even in the presence of low concentrations of cross-neutralizing serum antibody titres. Since we did not detect any correlates of functional immune responses (ie, neutralizing antibodies against HK/213/03 or VN/1203/04) or HA-518-epitope-specific CD8 T cells by pentamer staining or ELISpot in animals immunized with rH5HA and alum, we did not assess protection efficacy of this subunit vaccine against heterotypic challenge.
Figure 3. Conferred protection from immunization of BALB/c mice with HAd-H5HA vaccine against challenge with recent H5N1 viruses.
Red=intramuscular delivery of HAd-H5HA. Light blue=intranasal delivery of HAd-H5HA. Dark blue=intramuscular delivery of HAd-ΔE1E3. Line graphs show percentages of initial bodyweight and survival after challenges from (A, B) HK/483/97 and (C, D) VN/1203/04. Bar graph shows (E) lung viral titres after HK/213/03 challenge. Error bars represent SDs.
Discussion
Our results show that BALB/c mice immunized withHAd-H5HA vaccine were effectively protected from H5N1 disease, death, and primary viral replication against antigenically distinct strains of H5N1 influenza viruses. Our data highlight the potential of an Ad-vector-based delivery system for the development of a pandemic influenza vaccine. This strategy has the advantage of inducing strong humoral and cellular immunity and conferring cross-protection against continuously evolving H5N1 viruses without the need of an adjuvant. To further enhance the cross-reactive immunity and effectiveness induced by Ad-vector-based vaccines, genes of highly conserved proteins such as the nucleoprotein or matrix proteins could be included in the vaccine formulation, since CD8 T cells against influenza viral antigens have been shown to provide protection in animal models as well as in people.24,27–31 Furthermore, inclusion of haemagglutinin from the genetic drift variants of H5N1 viruses and other potential pandemic strains such as H7N7 and H9N2 could be considered for the development of Ad-vector-based vaccines that could cover a wide range of pandemic influenza viruses.
Replication-incompetent adenoviral vectors are a viable egg-independent approach to pandemic influenza vaccines because they can be grown to very high titres in available qualified cell lines; they can overcome glycosylation issues that might contribute to poor immunogenicity; they can be effectively delivered by either a parenteral or mucosal route; and they replicate episomally and do not insert their genome into that of the host cell, thereby ensuring less disruption of vital cellular genes. These vectors are highly immunogenic because of preferential targeting of antigen-presenting cells, and several National Institute of Health (NIH)-sponsored or independent human clinical trials for several infectious diseases and cancer targets are in progress, ensuring that Ad vector delivery and expression of therapeutic antigens is safe in people.32,33
Studies with animals have shown that pre-existing immunity to human adenoviruses could be a potential problem in the generation of immune response against a foreign gene of interest.34,35 However, the results from a phase I study, albeit in a small number of recruits, indicated little correlation between the titres against adenoviruses and the immune response to the vaccine antigen.36 In our mouse inoculation studies, we always recorded enhanced immune response after the second immunization, indicating that immune response generated against the Ad vector during the primary immunization was not critically interfering with the booster dose. Furthermore, several human phase I to phase III clinical trials with Ad-vector-based vaccine strategies are currently underway, and pre-existing immunity to adenoviruses has not so far affected the progression of clinical trials to phase III. Nevertheless, the availability of several non-human Ad vectors, such as porcine Ad type 3, bovine Ad type 3, canine Ad type 2, ovine Ad, and chimpanzee Ad, could also serve as alternative or supplemental vectors to overcome human Ad-specific immunity, if it becomes a serious problem.32,37
In recent years, avian H5N1 viruses have under gone rapid genetic evolution and have expanded their host range and level of virulence in mammalian species.38 Viruses from two genetic clades have caused human infections and death in Asia in 2005.1,8 In this study, the HAd-H5HA vaccine induced H5 cross-reactive humoral and cellular immunity and conferred cross-protection against lethal challenge of viruses with less than 96% aminoacid identity in the haemagglutinin subunit.
This approach is a feasible vaccine strategy against existing and newly emerging viruses of highly pathogenic avian influenza to prepare against a potential pandemic. This approach also provides a viable option for potential vaccine stockpiling for the influenza pandemic.
Supplementary Material
Acknowledgments
We thank Kristy Szretter, Catherine Smith, Randy Albrecht, and Nancy Cox for their advice and critical reading of this manuscript; and Po-Yng Cheng, and Soyoun Park for advice on statistical analyses of the data. Work at Purdue University was supported by public-health service grant AI059374 from the National Institute of Allergy and work at the Centers for Disease Control and Prevention was funded by the Infectious Diseases and National Vaccine Program Office. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
Footnotes
Conflict of interest statement
I Stephenson has received travel grants to attend scientific meetings from several pharmaceutical companies including Roche, Chiron, Solvay, GSK, and Sanofi. He has grant awards from Roche Hoffman to conduct scientific-investigator-led studies. All the other authors declare that they have no conflict of interest.
References
- 1.de Jong JC, Claas EC, Osterhaus AD, Webster RG, Lim WL. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subbarao K, Klimov A, Katz J, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 3.Peiris JSM, Yu WC, Leung CW, et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. [accessed Jan 26, 2006];Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2006_01_25/en/index.html.
- 5.Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Update: influenza activity—United States and worldwide, 2003–04 season, and composition of the 2004–05 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2004;53:547–552. [PubMed] [Google Scholar]
- 7.Hien TT, de Jong M, Farrar J. Avian influenza—a challenge to global health care structures. N Engl J Med. 2004;351:2363–2365. doi: 10.1056/NEJMp048267. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Outbreak news. Avian influenza A(H5N1) Wkly Epidemiol Record. 2004;79:65–70. [Google Scholar]
- 9.Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomized trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 10.Treanor JJ, Wilkinson BE, Masseoud F, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- 11.The World Health Organization Global Influenza Program Surveillance Network 1. Evolution of H5N1 avian influenza viruses in Asia. Emerg Infect Dis. 2005;11:1515–1521. doi: 10.3201/eid1110.050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler D. [accessed Jan 26,2006];Bird flu vaccine not up to scratch. http://www.nature.com/news/2005/050808/pf/050808-9_pf.html.
- 13.Webby RJ, Perez DR, Coleman JS, et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363:1099–1103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann E, Lipatov AS, Webby RJ, Govorkova EA, Webster RG. Role of specific hemagglutinin amino acids in the immunogenicity and protection of H5N1 influenza virus vaccines. Proc Natl Acad Sci USA. 2005;102:12915–12920. doi: 10.1073/pnas.0506416102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipatov AS, Webby RJ, Govorkova EA, Krauss S, Webster RG. Efficacy of H5 influenza vaccines produced by reverse genetics in a lethal mouse model. J Infect Dis. 2005;191:1216–1220. doi: 10.1086/428951. [DOI] [PubMed] [Google Scholar]
- 16.Tian G, Zhang S, Li Y, et al. Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics. Virology. 2005;341:153–162. doi: 10.1016/j.virol.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Ng P, Parks RJ, Cummings DT, Evelegh CM, Sankar U, Graham FL. A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum Gene Ther. 1999;10:2667–2672. doi: 10.1089/10430349950016708. [DOI] [PubMed] [Google Scholar]
- 18.Gao P, Watanabe S, Ito T, et al. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noblitt LW, Bangari DS, Shukla S, et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. CancerGeneTher. 2004;11:757–766. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- 20.Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004;103:91–95. doi: 10.1016/j.virusres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender BS, Small PA., Jr Influenza: pathogenesis and host defense. Semin Respir Infect. 1992;7:38–45. [PubMed] [Google Scholar]
- 24.Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic Tlymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Anton LC, Bennink JR, Yewdell JW. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity. 2000;12:83–93. doi: 10.1016/s1074-7613(00)80161-2. [DOI] [PubMed] [Google Scholar]
- 26.Hoelscher M, Yewdell J, Katz JM, Sambhara S. Avian influenza viruses influence CTL immunodominance. Proceedings of Research Conference on Orhomyxoviruses; Nov 2–4, 2001; Texel, Netherlands. p. 36. [Google Scholar]
- 27.Epstein SL, Kong WP, Misplon JA, et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23:5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 28.Fries LF, Montemarano AD, Mallett CP, Taylor DN, Hale TL, Lowell GH. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect Immun. 2001;69:4545–4553. doi: 10.1128/IAI.69.7.4545-4553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambhara S, Woods S, Arpino R, et al. Heterotypic protection against influenza by immunostimulating complexes is associated with the induction of cross-reactive cytotoxic T lymphocytes. J Infect Dis. 1998;177:1266–1274. doi: 10.1086/515285. [DOI] [PubMed] [Google Scholar]
- 31.Sambhara S, Kurichh A, Miranda R, et al. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol. 2001;211:143–153. doi: 10.1006/cimm.2001.1835. [DOI] [PubMed] [Google Scholar]
- 32.Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24(7):849–862. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morelli AE, Larregina AT, Ganster RW, et al. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappa B-dependent pathway. J Virol. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanda A, Lynch DM, Goudsmit J, et al. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J Virol. 2005;79:14161–14168. doi: 10.1128/JVI.79.22.14161-14168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sailaja G, HogenEsch H, North A, Hays J, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. GeneTher. 2002;9:1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Kampen KR, Shi Z, Gao P, et al. Safety and immunogenicityof adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23:1029–1036. doi: 10.1016/j.vaccine.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 37.Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors inhuman, murine, bovine, and porcine cells in culture. Biochem Biophys Res Commun. 2005;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. [accessedOct25, 2006];Avian influenza A(H5N1)—update 28: reports of infection in domestic cats (Thailand),situation (human) in Thailand, situation (poultry) in Japan andChina. http://www.who.int/csr/don/2004_02_20/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.