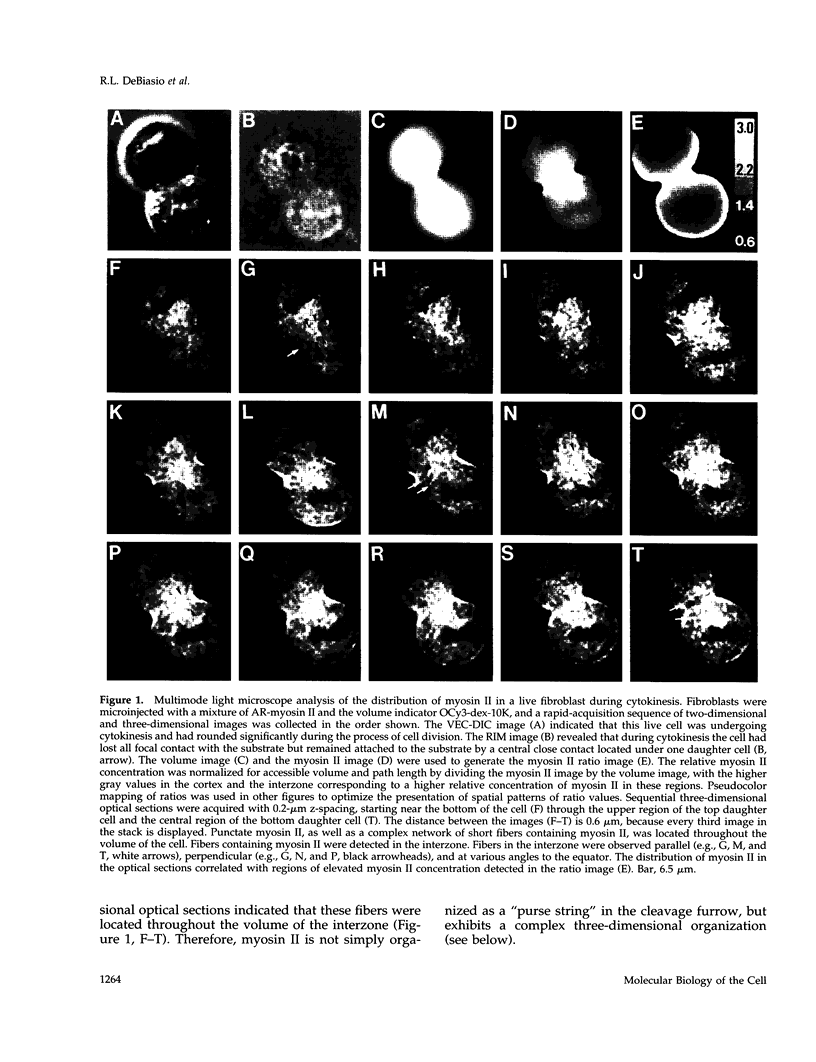
Abstract
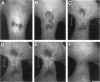
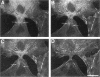
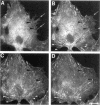
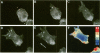
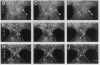
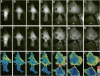
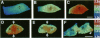
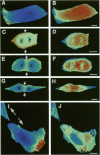
The mechanism of cytokinesis has been difficult to define because of the short duration and the temporal-spatial dynamics involved in the formation, activation, force production, and disappearance of the cleavage furrow. We have investigated the structural and chemical dynamics of myosin II in living Swiss 3T3 cells from prometaphase through the separation and migration of daughter cells. The structural and chemical dynamics of myosin II have been defined using the semiautomated, multimode light microscope, together with a fluorescent analogue of myosin II and a fluorescent biosensor of myosin II regulatory light chain (RLC) phosphorylation at serine 19. The correlation of image data from live cells using different modes of light microscopy allowed interpretations not possible from single-mode investigations. Myosin II transported toward the equatorial plane from adjacent regions, forming three-dimensional fibers that spanned the volume of the equator during anaphase and telophase. A global phosphorylation of myosin II at serine 19 of the RLC was initiated at anaphase when cortical myosin II transport started. The phosphorylation of myosin II remained high near the equatorial plane through telophase and into cytokinesis, whereas the phosphorylation of myosin II at serine 19 of the RLC decreased at the poles. The timing and pattern of phosphorylation was the same as the shortening of myosin II-based fibers in the cleavage furrow. Myosin II-based fibers shortened and transported out of the cleavage furrow into the tails of the two daughter cells late in cytokinesis. The patterns of myosin II transport, phosphorylation, and shortening of fibers in the migrating daughter cells were similar to that previously defined for cells migrating in a wound in vitro. The temporal-spatial patterns and dynamics of myosin II transport, phosphorylation at serine 19 of the RLC, and the shortening and disappearance of myosin II-based fibers support the proposal that a combination of the cortical flow hypothesis and the solation-contraction coupling hypothesis explain key aspects of cytokinesis and polarized cell locomotion.
Full text
PDF





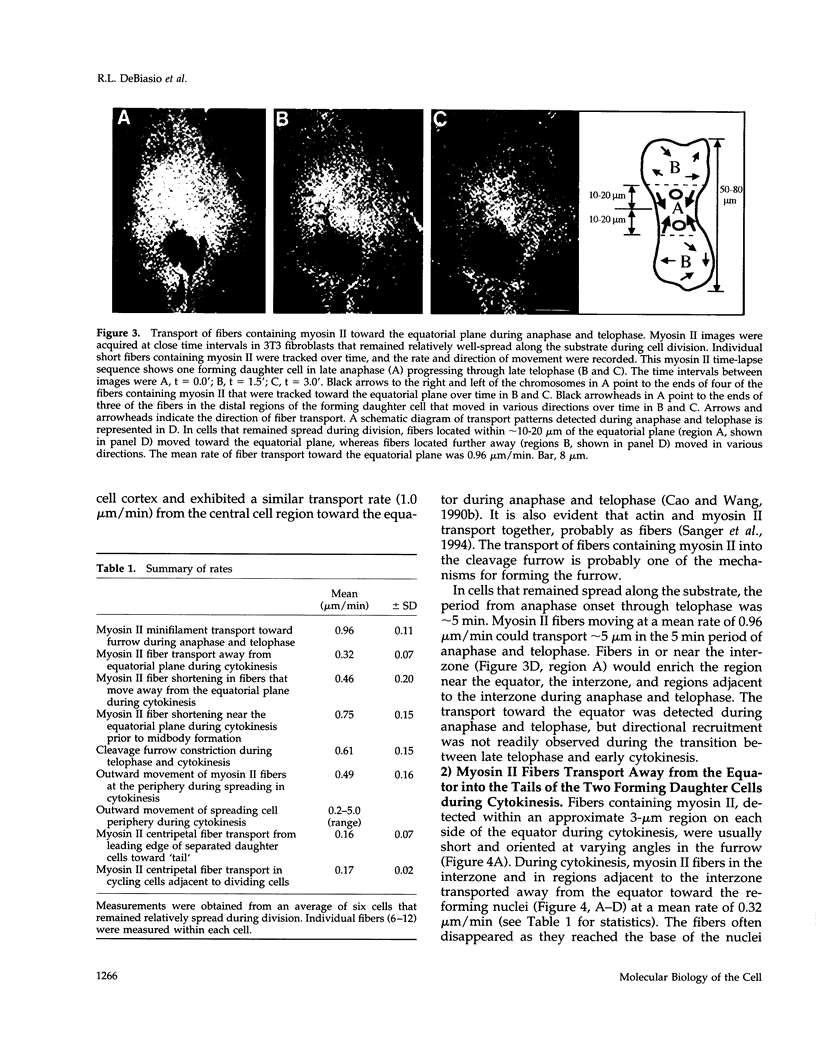

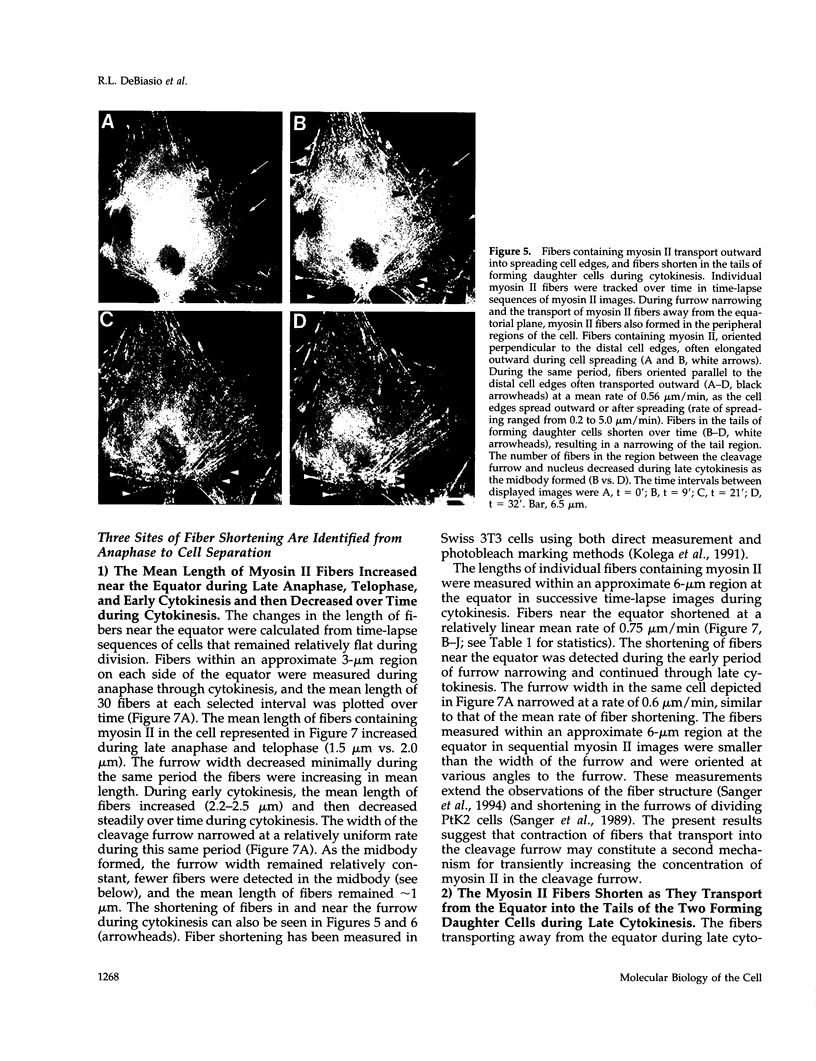
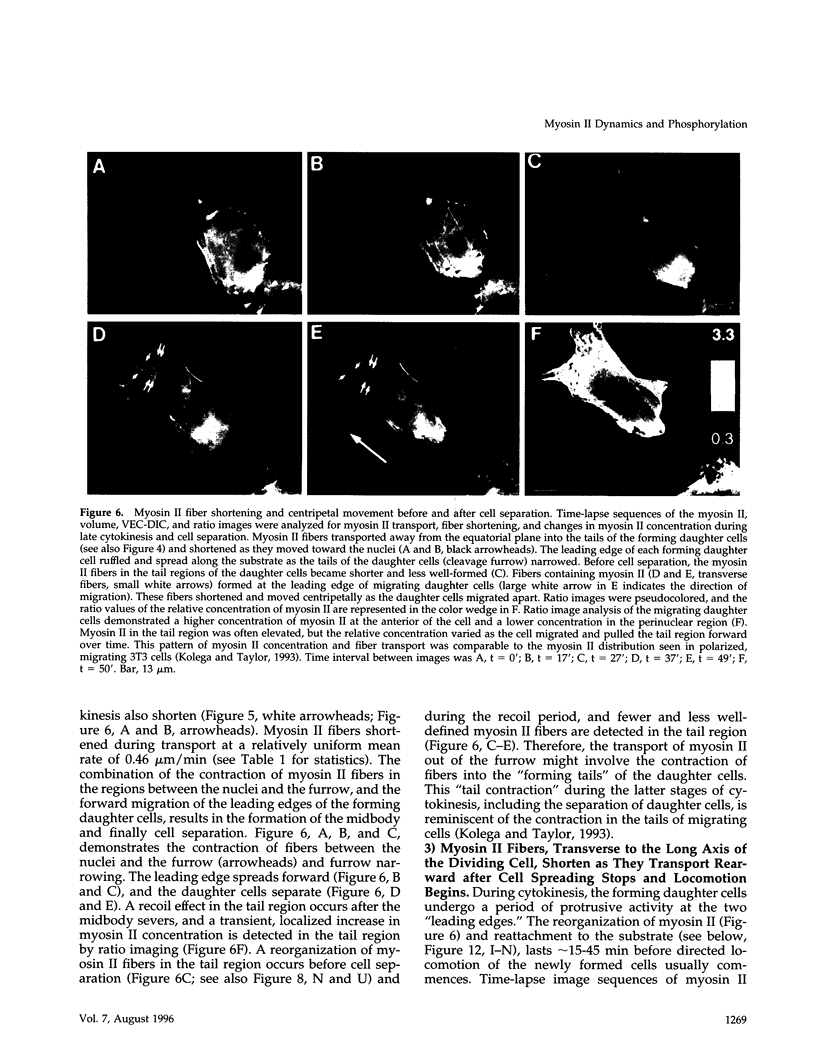
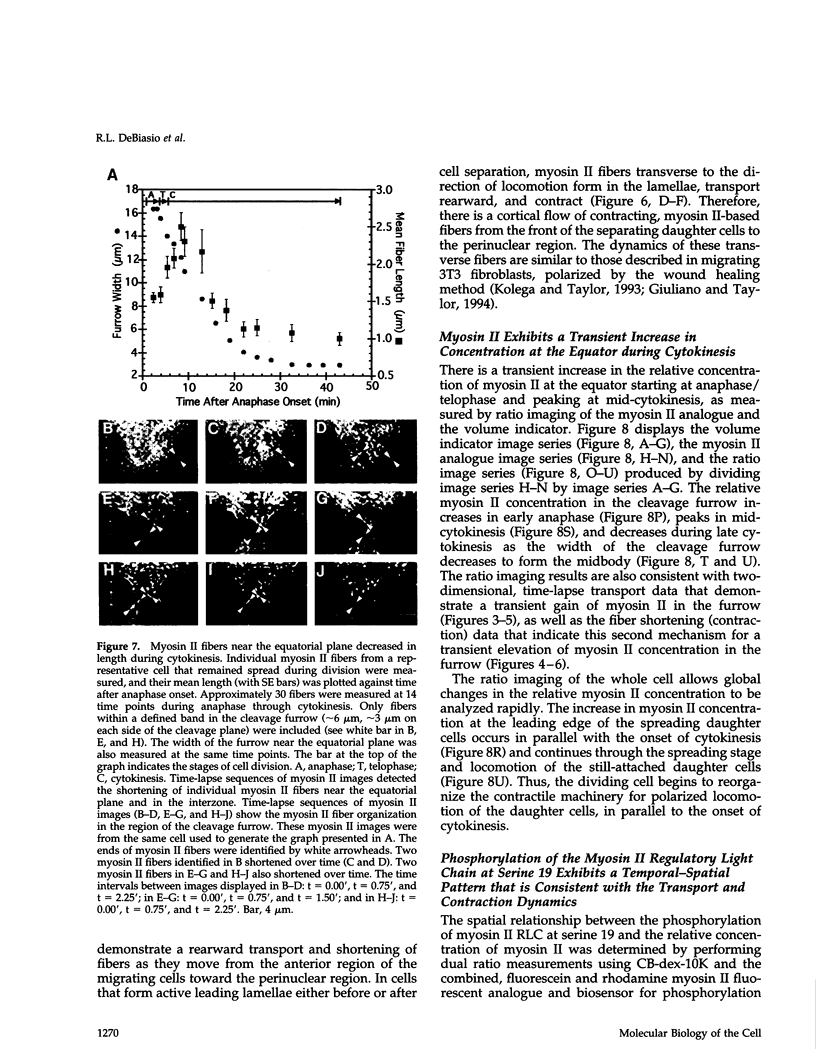








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A. Optical sectioning microscopy: cellular architecture in three dimensions. Annu Rev Biophys Bioeng. 1984;13:191–219. doi: 10.1146/annurev.bb.13.060184.001203. [DOI] [PubMed] [Google Scholar]
- Amato P. A., Unanue E. R., Taylor D. L. Distribution of actin in spreading macrophages: a comparative study on living and fixed cells. J Cell Biol. 1983 Mar;96(3):750–761. doi: 10.1083/jcb.96.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn J., Fox C. H., Thorell B. Quantitative reflection contrast microscopy of living cells. J Cell Biol. 1979 Sep;82(3):767–779. doi: 10.1083/jcb.82.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., White J. G. Cortical flow in animal cells. Science. 1988 Feb 19;239(4842):883–888. doi: 10.1126/science.3277283. [DOI] [PubMed] [Google Scholar]
- Breckler J., Burnside B. Myosin I localizes to the midbody region during mammalian cytokinesis. Cell Motil Cytoskeleton. 1994;29(4):312–320. doi: 10.1002/cm.970290404. [DOI] [PubMed] [Google Scholar]
- Bright G. R., Fisher G. W., Rogowska J., Taylor D. L. Fluorescence ratio imaging microscopy: temporal and spatial measurements of cytoplasmic pH. J Cell Biol. 1987 Apr;104(4):1019–1033. doi: 10.1083/jcb.104.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L. G., Wang Y. L. Mechanism of the formation of contractile ring in dividing cultured animal cells. I. Recruitment of preexisting actin filaments into the cleavage furrow. J Cell Biol. 1990 Apr;110(4):1089–1095. doi: 10.1083/jcb.110.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L. G., Wang Y. L. Mechanism of the formation of contractile ring in dividing cultured animal cells. II. Cortical movement of microinjected actin filaments. J Cell Biol. 1990 Nov;111(5 Pt 1):1905–1911. doi: 10.1083/jcb.111.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Chang D. C., Meng C. A localized elevation of cytosolic free calcium is associated with cytokinesis in the zebrafish embryo. J Cell Biol. 1995 Dec;131(6 Pt 1):1539–1545. doi: 10.1083/jcb.131.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciapa B., Pesando D., Wilding M., Whitaker M. Cell-cycle calcium transients driven by cyclic changes in inositol trisphosphate levels. Nature. 1994 Apr 28;368(6474):875–878. doi: 10.1038/368875a0. [DOI] [PubMed] [Google Scholar]
- Condeelis J. S., Taylor D. L. The contractile basis of amoeboid movement. V. The control of gelation, solation, and contraction in extracts from Dictyostelium discoideum. J Cell Biol. 1977 Sep;74(3):901–927. doi: 10.1083/jcb.74.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad P. A., Giuliano K. A., Fisher G., Collins K., Matsudaira P. T., Taylor D. L. Relative distribution of actin, myosin I, and myosin II during the wound healing response of fibroblasts. J Cell Biol. 1993 Mar;120(6):1381–1391. doi: 10.1083/jcb.120.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer L. P., Mitchison T. J. Myosin is involved in postmitotic cell spreading. J Cell Biol. 1995 Oct;131(1):179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- DeBiasio R. L., Wang L. L., Fisher G. W., Taylor D. L. The dynamic distribution of fluorescent analogues of actin and myosin in protrusions at the leading edge of migrating Swiss 3T3 fibroblasts. J Cell Biol. 1988 Dec;107(6 Pt 2):2631–2645. doi: 10.1083/jcb.107.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBiasio R., Bright G. R., Ernst L. A., Waggoner A. S., Taylor D. L. Five-parameter fluorescence imaging: wound healing of living Swiss 3T3 cells. J Cell Biol. 1987 Oct;105(4):1613–1622. doi: 10.1083/jcb.105.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas D. L., Baxter G., DeBiasio R. L., Gough A., Nederlof M. A., Pane D., Pane J., Patek D. R., Ryan K. W., Taylor D. L. Multimode light microscopy and the dynamics of molecules, cells, and tissues. Annu Rev Physiol. 1993;55:785–817. doi: 10.1146/annurev.ph.55.030193.004033. [DOI] [PubMed] [Google Scholar]
- Fishkind D. J., Cao L. G., Wang Y. L. Microinjection of the catalytic fragment of myosin light chain kinase into dividing cells: effects on mitosis and cytokinesis. J Cell Biol. 1991 Sep;114(5):967–975. doi: 10.1083/jcb.114.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishkind D. J., Wang Y. L. New horizons for cytokinesis. Curr Opin Cell Biol. 1995 Feb;7(1):23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Fishkind D. J., Wang Y. L. Orientation and three-dimensional organization of actin filaments in dividing cultured cells. J Cell Biol. 1993 Nov;123(4):837–848. doi: 10.1083/jcb.123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck R. A., Miller A. L., Jaffe L. F. Slow calcium waves accompany cytokinesis in medaka fish eggs. J Cell Biol. 1991 Dec;115(5):1259–1265. doi: 10.1083/jcb.115.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Inoué S. Cell division in Dictyostelium with special emphasis on actomyosin organization in cytokinesis. Cell Motil Cytoskeleton. 1991;18(1):41–54. doi: 10.1002/cm.970180105. [DOI] [PubMed] [Google Scholar]
- Fukui Y. Toward a new concept of cell motility: cytoskeletal dynamics in amoeboid movement and cell division. Int Rev Cytol. 1993;144:85–127. doi: 10.1016/s0074-7696(08)61514-4. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Post P. L., Hahn K. M., Taylor D. L. Fluorescent protein biosensors: measurement of molecular dynamics in living cells. Annu Rev Biophys Biomol Struct. 1995;24:405–434. doi: 10.1146/annurev.bb.24.060195.002201. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Taylor D. L. Fluorescent actin analogs with a high affinity for profilin in vitro exhibit an enhanced gradient of assembly in living cells. J Cell Biol. 1994 Mar;124(6):971–983. doi: 10.1083/jcb.124.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano K. A., Taylor D. L. Formation, transport, contraction, and disassembly of stress fibers in fibroblasts. Cell Motil Cytoskeleton. 1990;16(1):14–21. doi: 10.1002/cm.970160104. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Taylor D. L. Measurement and manipulation of cytoskeletal dynamics in living cells. Curr Opin Cell Biol. 1995 Feb;7(1):4–12. doi: 10.1016/0955-0674(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Hahn K., DeBiasio R., Taylor D. L. Patterns of elevated free calcium and calmodulin activation in living cells. Nature. 1992 Oct 22;359(6397):736–738. doi: 10.1038/359736a0. [DOI] [PubMed] [Google Scholar]
- Hepler P. K. The role of calcium in cell division. Cell Calcium. 1994 Oct;16(4):322–330. doi: 10.1016/0143-4160(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Mechanical properties of the cortex before and during cleavage. Ann N Y Acad Sci. 1990;582:22–30. doi: 10.1111/j.1749-6632.1990.tb21664.x. [DOI] [PubMed] [Google Scholar]
- Hird S. N., White J. G. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J Cell Biol. 1993 Jun;121(6):1343–1355. doi: 10.1083/jcb.121.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson L. W., Kolega J., Taylor D. L. Modulation of contraction by gelation/solation in a reconstituted motile model. J Cell Biol. 1991 Sep;114(5):1005–1015. doi: 10.1083/jcb.114.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson L. W., Taylor D. L. In vitro models of tail contraction and cytoplasmic streaming in amoeboid cells. J Cell Biol. 1993 Oct;123(2):345–356. doi: 10.1083/jcb.123.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart D. P., Mabuchi I., Inoué S. Evidence that myosin does not contribute to force production in chromosome movement. J Cell Biol. 1982 Jul;94(1):165–178. doi: 10.1083/jcb.94.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanishi-Yumura T., Fukui Y. Actomyosin organization during cytokinesis: reversible translocation and differential redistribution in Dictyostelium. Cell Motil Cytoskeleton. 1989;12(2):78–89. doi: 10.1002/cm.970120203. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Kolega J., Janson L. W., Taylor D. L. The role of solation-contraction coupling in regulating stress fiber dynamics in nonmuscle cells. J Cell Biol. 1991 Sep;114(5):993–1003. doi: 10.1083/jcb.114.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J., Taylor D. L. Gradients in the concentration and assembly of myosin II in living fibroblasts during locomotion and fiber transport. Mol Biol Cell. 1993 Aug;4(8):819–836. doi: 10.1091/mbc.4.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Oliver J. M., Berlin R. D. Surface functions during mitosis. III. Quantitative analysis of ligand-receptor movement into the cleavage furrow: diffusion vs. flow. J Cell Biol. 1982 Jun;93(3):950–960. doi: 10.1083/jcb.93.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Watrin A., Labbé J. C., Cavadore J. C. Microinjection of p34cdc2 kinase induces marked changes in cell shape, cytoskeletal organization, and chromatin structure in mammalian fibroblasts. Cell. 1990 Jan 12;60(1):151–165. doi: 10.1016/0092-8674(90)90725-t. [DOI] [PubMed] [Google Scholar]
- Mabuchi I. Cleavage furrow: timing of emergence of contractile ring actin filaments and establishment of the contractile ring by filament bundling in sea urchin eggs. J Cell Sci. 1994 Jul;107(Pt 7):1853–1862. doi: 10.1242/jcs.107.7.1853. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin P., Phillips C. L., Adelstein R. S., Pollard T. D. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J Cell Sci. 1994 Nov;107(Pt 11):3077–3090. doi: 10.1242/jcs.107.11.3077. [DOI] [PubMed] [Google Scholar]
- Maupin P., Pollard T. D. Arrangement of actin filaments and myosin-like filaments in the contractile ring and of actin-like filaments in the mitotic spindle of dividing HeLa cells. J Ultrastruct Mol Struct Res. 1986 Jan;94(1):92–103. doi: 10.1016/0889-1605(86)90055-8. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Kiehart D. P. Fly division. J Cell Biol. 1995 Oct;131(1):1–5. doi: 10.1083/jcb.131.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal B., Sanger J. M., Sanger J. W. Visualization of myosin in living cells. J Cell Biol. 1987 Oct;105(4):1753–1760. doi: 10.1083/jcb.105.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post P. L., DeBiasio R. L., Taylor D. L. A fluorescent protein biosensor of myosin II regulatory light chain phosphorylation reports a gradient of phosphorylated myosin II in migrating cells. Mol Biol Cell. 1995 Dec;6(12):1755–1768. doi: 10.1091/mbc.6.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post P. L., Trybus K. M., Taylor D. L. A genetically engineered, protein-based optical biosensor of myosin II regulatory light chain phosphorylation. J Biol Chem. 1994 Apr 29;269(17):12880–12887. [PubMed] [Google Scholar]
- Rappaport R. Cytokinesis in animal cells. Int Rev Cytol. 1971;31:169–213. doi: 10.1016/s0074-7696(08)60059-5. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- Salmon E. D. Cytokinesis in animal cells. Curr Opin Cell Biol. 1989 Jun;1(3):541–547. doi: 10.1016/0955-0674(89)90018-5. [DOI] [PubMed] [Google Scholar]
- Sanders S. L., Field C. M. Cell division. Septins in common? Curr Biol. 1994 Oct 1;4(10):907–910. doi: 10.1016/s0960-9822(00)00201-3. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Dome J. S., Hock R. S., Mittal B., Sanger J. W. Occurrence of fibers and their association with talin in the cleavage furrows of PtK2 cells. Cell Motil Cytoskeleton. 1994;27(1):26–40. doi: 10.1002/cm.970270104. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Mittal B., Dome J. S., Sanger J. W. Analysis of cell division using fluorescently labeled actin and myosin in living PtK2 cells. Cell Motil Cytoskeleton. 1989;14(2):201–219. doi: 10.1002/cm.970140207. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Changing patterns of actin localization during cell division. Proc Natl Acad Sci U S A. 1975 May;72(5):1913–1916. doi: 10.1073/pnas.72.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite L. L., Pollard T. D. Cytokinesis. Curr Opin Cell Biol. 1992 Feb;4(1):43–52. doi: 10.1016/0955-0674(92)90057-j. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E. The contractile ring and furrowing in dividing cells. Ann N Y Acad Sci. 1990;582:78–87. doi: 10.1111/j.1749-6632.1990.tb21669.x. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Pato M. D., Adelstein R. S. Reversible phosphorylation of smooth muscle myosin, heavy meromyosin, and platelet myosin. J Biol Chem. 1981 Dec 25;256(24):13137–13142. [PubMed] [Google Scholar]
- Tanasugarn L., McNeil P., Reynolds G. T., Taylor D. L. Microspectrofluorometry by digital image processing: measurement of cytoplasmic pH. J Cell Biol. 1984 Feb;98(2):717–724. doi: 10.1083/jcb.98.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S., Moore P. L., Allen R. D. The contractile basis of amoeboid movement. I. The chemical control of motility in isolated cytoplasm. J Cell Biol. 1973 Nov;59(2 Pt 1):378–394. doi: 10.1083/jcb.59.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Fechheimer M. Cytoplasmic structure and contractility: the solation--contraction coupling hypothesis. Philos Trans R Soc Lond B Biol Sci. 1982 Nov 4;299(1095):185–197. doi: 10.1098/rstb.1982.0125. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Wang Y. L. Fluorescently labelled molecules as probes of the structure and function of living cells. Nature. 1980 Apr 3;284(5755):405–410. doi: 10.1038/284405a0. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Wang Y. L. Molecular cytochemistry: incorporation of fluorescently labeled actin into living cells. Proc Natl Acad Sci U S A. 1978 Feb;75(2):857–861. doi: 10.1073/pnas.75.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky A. B., Borisy G. G. Non-sarcomeric mode of myosin II organization in the fibroblast lamellum. J Cell Biol. 1993 Nov;123(3):637–652. doi: 10.1083/jcb.123.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Silverman J. D., Cao L. G. Single particle tracking of surface receptor movement during cell division. J Cell Biol. 1994 Nov;127(4):963–971. doi: 10.1083/jcb.127.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Taylor D. L. Distribution of fluorescently labeled actin in living sea urchin eggs during early development. J Cell Biol. 1979 Jun;81(3):672–679. doi: 10.1083/jcb.81.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Borisy G. G. On the mechanisms of cytokinesis in animal cells. J Theor Biol. 1983 Mar 21;101(2):289–316. doi: 10.1016/0022-5193(83)90342-9. [DOI] [PubMed] [Google Scholar]
- Yamakita Y., Yamashiro S., Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J Cell Biol. 1994 Jan;124(1-2):129–137. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Ernst L., Wagner M., Waggoner A. Sensitive detection of RNAs in single cells by flow cytometry. Nucleic Acids Res. 1992 Jan 11;20(1):83–88. doi: 10.1093/nar/20.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]