Abstract
The ε4 allele of ApoE is associated with an earlier onset and faster progression of Alzheimer’s disease in patients with the familial form of this neurodegenerative condition. Although ApoE4 has been repeatedly associated with altered sphingomyelin and cholesterol levels in tissue culture and rodent models, there has not been a direct quantification of sphingomyelin or sterol levels in the brains of patients with different forms of ApoE. We measured the sphingolipid and sterol content of human brain tissues and found no evidence of perturbed sterol or sphingolipid biochemistry in the brains of individuals expressing ApoE4 who did not have a preexisting neurodegenerative condition. Nevertheless, ApoE4 was associated with gross abnormalities in the sterol and sphingolipid content of numerous brain regions in patients with Alzheimer’s diseease. The findings suggest that ApoE4 may not by itself alter sterol or sphingolipid metabolism in the brain under normal conditions, but that other neuropathologic changes of Alzheimer’s are required to unmask the effect of ApoE4, and to perturb sterol and sphingolipid biochemistry.
Keywords: Alzheimer’s disease, apolipoprotein, ApoE, ApoE4, sterol, cholesterol, sphingomeylin, ceramide, sphingolipid
1. Introduction
ApoE4 has been identified as a major risk factor for Alzheimer’s disease (AD). There are three major isoforms of ApoE that result from single amino acid substitutions: ApoE2 has cysteines at positions 112 and 158; ApoE3 has a cysteine at position 112 and an arginine at position 158; and ApoE4 has arginines at positions 112 and 158. These single amino acid changes result in functional differences in ApoE isoforms, including their relative binding affinities for lipoproteins and ApoE receptors. Although a number of ApoE-initiated receptor signaling events have been identified, the primary function for ApoE is sterol transport. Deficits in sterol transport associated with the ε4 allele of ApoE are thought to be responsible for the enhanced rate of cell death and greater volume loss in hippocampus and amygdala structures of AD patients expressing ApoE4 (Lehtovirta et al. 1996). In tissue culture and rodent models it has been demonstrated that ApoE4 is associated with dysfunctions in neurite outgrowth and synaptogenesis, disordered placement of committed neurons during development, disrupted neurotrophic effects, abnormal regulation of nitric oxide and reduced production of antioxidants (Ignatius et al. 1987; Nathan et al. 1994; Fagan et al. 1996; Narita et al. 1997; Trommsdorff et al. 1999; Mauch et al. 2001; Veinbergs et al. 2001; Brown et al. 2002; Colton et al. 2002; Nathan et al. 2002; Levi et al. 2003; Love et al. 2005). Despite numerous observations suggesting that ApoE4-associated deficits in sterol transport can result in neuronal dysfunction, there has not been direct evidence for abnormal sterol metabolism in the brain parenchyma of AD patients who express ApoE4, although several studies have used indirect measures to correlate brain cholesterol with ApoE polymorphisms. In one study, ApoE4 was associated with reduced levels of cholesterol, phospholipids, and fatty acids in the CSF of AD patients (Mulder et al. 1998) and in a second study, 24S-hydroxycholesterol, a major cholesterol ester that is transported out of brain, was found to be elevated in the CSF and serum of AD patients with an apparent gene dosing effect of ApoE4 (Lutjohann et al. 2000; Papassotiropoulos et al. 2002). Although these findings suggest that ApoE4 perturbs cholesterol metabolism, it is not clear if more or less cholesterol is retained in the brain of AD patients expressing ApoE4. In the present study we directly measured the levels of cholesterol and cholesterol esters, sphingomyelin, ceramide, and 4-hydroxynonenal (4HNE) levels in normal and AD brain to determine whether ApoE polymorphism modifies brain sterol and lipid metabolism. We found that ApoE4 was not associated with changes in brain sterol or sphingolipid levels in brain tissues from cognitively normal subjects, but was associated with large accumulations of cholesterol and sphingolipids in particular brain regions of AD patients. These findings suggest that ApoE4 alone may not appreciably modify brain sterol and sphingolipid metabolism, instead, the effect of ApoE4 in disrupting sterol and sphingolipid balance may be manifest only in the presence of additional perturbations that increase free cholesterol.
2. METHODS
2.1 Brain Tissues
All brain tissues were obtained from the Alzheimer's Disease Research Center at Johns Hopkins University School of Medicine. Fresh frozen tissue blocks of middle frontal gyrus (MFG), middle temporal gyrus (MTG), and cerebellum were obtained from normal subjects and cases of definite AD diagnosed according to CERAD criteria (Mirra et al. 1991). Patients were divided into four groups on the basis of neurological status and ApoE genotype determined by polymerase chain reaction (PCR) (Hixson and Vernier 1990): In the AD group, E3 (ApoE 2/3, n = 2 and ApoE 3/3, n = 13) and E4 (ApoE 3/4, n = 10 and ApoE 4/4, n = 5). In the normal group, E3 (ApoE 2/3 n = 5 and ApoE 3/3 n = 15) and ApoE4, (ApoE3/4 n = 4 and ApoE 4/4 n = 2). Subject demographics are listed in Table 1. All tissues were frozen without fixative at −80°C until homogenization.
Table 1.
Patient Demographics and Neuropathological findings for Control and Alzheimer’s Disease subjects. PMD, postmortem delay. CERAD, Consortium to Establish A Registry for Alzheimer's Disease. Braak, Braak & Braak staging system.
| Control | AD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 68 | M | W | 14 | 3/3 | unk | unk | 63 | F | W | 11 | 2/3 | 0 | C | 6 | |
| 79 | F | W | 24 | 3/3 | unk | unk | 75 | F | B | 8 | 2/3 | 2 | C | 6 | |
| 80 | F | W | 6 | 3/3 | unk | 1 | 75 | M | W | 12 | 3/3 | 5 | C | 6 | |
| 80 | M | B | 21 | 3/3 | unk | 0 | 85 | F | B | 12 | 3/3 | 1 | C | 5 | |
| 88 | M | W | 10 | 3/3 | unk | unk | 71 | F | W | 22 | 3/3 | 1 | C | 6 | |
| 73 | M | W | 9 | 2/3 | unk | unk | 83 | F | W | 31 | 3/3 | 2 | C | 6 | |
| 83 | F | W | 8 | 3/3 | unk | 0 | 76 | M | W | 25 | 3/3 | 5 | C | 6 | |
| 66 | M | W | 10 | 2/3 | unk | 0 | 82 | F | W | 19 | 3/3 | 12 | C | 6 | |
| 83 | M | W | 5 | 2/3 | unk | unk | 92 | F | W | 14 | 3/3 | 5 | C | 6 | |
| 72 | M | W | 16 | 3/3 | RARE | 2 | 79 | F | W | 7 | 3/3 | 13 | C | 6 | |
| 66 | M | W | 12 | 3/3 | unk | 3 | 91 | F | W | 14 | 3/3 | 13 | C | 6 | |
| 68 | M | W | 10 | 3/3 | unk | 2 | 87 | M | W | 16 | 3/3 | 27 | B | 4 | |
| 87 | M | W | 17 | 3/3 | unk | 4 | 54 | F | W | 14.5 | 3/3 | 28 | unk | unk | |
| 80 | M | W | 22 | 3/3 | unk | 2 | |||||||||
| 92 | F | W | UNK | 3/3 | unk | 1 | 79 | F | W | 12 | 3/4 | 5 | C | 6 | |
| 87 | M | W | UNK | 2/3 | 0 | 2 | 85 | M | W | 18 | 4/4 | 15 | C | 4 | |
| 74 | M | W | 4 | 3/3 | unk | 2 | 77 | M | W | UKN | 4/4 | 3 | C | 6 | |
| 91 | F | W | 8 | 2/3 | unk | 1 | 92 | F | B | 13 | 3/4 | 2 | C | 6 | |
| 99 | M | B | 24 | 3/3 | 0 | 4 | 76 | F | W | 21 | 3/4 | unk | C | 5 | |
| 86 | M | W | 7 | 3/3 | 0 | 4 | 80 | F | W | 7 | 3/4 | 18 | C | 6 | |
| 81 | F | W | 7 | 3/4 | 20 | C | 6 | ||||||||
| 66 | M | W | 6 | 4/4 | unk | 1 | 68 | M | W | 4 | 4/4 | 3 | C | 6 | |
| 67 | M | W | 8 | 3/4 | B | 2 | 78 | F | W | 10.5 | 4/4 | 21 | C | 6 | |
| 81 | M | W | 20 | 3/4 | unk | unk | 80 | F | W | 6.5 | 3/4 | 8 | C | 6 | |
| 80 | F | W | 8 | 3/4 | unk | 0 | 85 | F | W | 18 | 3/4 | 10 | C | 6 | |
| 71 | F | W | 16 | 4/4 | A | 2 | 87 | F | B | 16 | 3/4 | 7 | C | 6 | |
| 94 | M | W | 16 | 3/4 | A | 3 | 94 | F | W | 4 | 4/4 | unk | C | 6 | |
| 89 | M | W | 9.5 | 3/4 | 14 | C | 6 | ||||||||
| 62 | F | W | 11 | 3/4 | unk | C | 6 | ||||||||
2.2 Lipid extraction of tissue and cells
Total lipids from samples were prepared according to a modified Bligh and Dyer procedure (Haughey et al. 2004). Briefly, each sample was homogenized at room temperature in 10 volumes of deionized water containing 300 nM EDTA, followed by 3 volumes of 100% methanol containing 53 mM ammonium formate and vortexed. Four volumes of chloroform then were added, and the mixture was vortexed and then centrifuged at 1,000 g for 10 minutes. The bottom (chloroform) layer was removed and analyzed by direct injection into a tandem mass spectrometer. Borosilicate-coated glass tubes, pipettes, and injectors were used for lipid extraction.
2.3 Measurement of sphingolipids, phospholipids, cholesterol and lipid peroxides
ESI/MS/MS analyses were performed by methods similar to those used in previous studies (Cutler et al. 2004b; Haughey et al. 2004). Samples were injected using a Harvard Apparatus pump at 5 µl/min into an electrospray ionization (Turbo Ion Spray) Sciex API 3,000 triple stage quadrupole tandem mass spectrometer (ESIMS/MS) from Sciex Inc. (Thornhill, Ontario, Canada) operated in the positive mode. The ion spray voltage (V) was 5,500 at a temperature of 80°C with a nebulizer gas of 8 psi, curtain gas of 8 psi, and the collision gas set at 4 psi. The declustering potential was 80 V, the focusing potential 400 V, the entrance potential –10 V, the collision energy 30 V, and the collision cell exit potential was 18 V. MS/MS scanned from 300 to 2,000 atomic mass units (amu) per second with steps of 0.1 amu. Each species of sphingolipids, cholesterol esters, and lipid peroxides initially was identified by a Q1 mass scan, followed by precursor ion scanning or neutral loss scanning of a purified standard. Samples were injected into the ESI/MS/MS for 3 minutes, where the mass counts accumulated and the sum of the total counts under each peak was used to quantify each species. Cholesterol, and cholesterol ester standards C16:0, C18:0, and C18:1 were purchased from Sigma. Sphingomyelin and ceramides C16:0, C18:0, C20:0, C22:0, C24:0, C24:1 were purchased from Avanti Polar Lipids (Alabaster, AL). 4-Hydroxynonenol and adducts were purchased from Cayman Chemicals (Ann Arbor, MI).
3. Results
3.1 Sphingolipid and sterol balance are disrupted in the brains of Alzheimer’s patients
We first grouped data based on disease status (without regard to the patients’ ApoE genotype) and found prominent changes of sphingolipid and sterol levels in the middle frontal gyrus (MFG) of AD patients compared with those of neurologically normal subjects. In the MFG grey matter, we found significant increases of sphingomyelin C16:0, C18:0, C22:0 and C24:0, steraoyl, cholesterol, and the cholesterol ester C18:1 (range 1.8- to 4.9-fold). Exact levels are given in the legend to Figure 1. In contrast, in MFG whie matter, we found no difference between sphingomyelin and sterol levels between AD and normal subjects, but significant decreases of ceramide C16:0, C22:0, C24:1, sterolyl, and sulfatide (range 3.7- to 9.2-fold), Figure 2. These data indicate that in the MFG, ceramide and sterol levels were increased in the cell bodies (grey matter) and ceramide was decreased in the fiber tracks (white matter) of AD brain compared with similar tissues from normal subjects. In the middle temporal gyrus grey matter of AD patients there were trends toward decreased levels of sphingomyelin C24:0, and increased levels of ceramide C24:0 and cholesterol esters, although these values were not significantly different from those in normal subjects (data not shown). There were likewise no significant differences in levels of sphingomyelin, ceramide, or sterols in the temporal white matter or cerebellum of AD compared with levels in normal subjects (data not shown).
Figure 1. Sphingomyelin, ceramide and sterol levels are increased in the grey matter of middle frontal gyrus in AD brain.
Total lipids were extracted from the MFG grey matter and sphingomyelin, ceramide and sterols were identified and quantified by ESI/MS/MS. In each panel, representative spectra are presented on the left, and bar graphs comparing quantification of constituents from AD and normal brain are on the right. (A) Spectra from MFG grey matter of an AD patient, showing the identification and exact mass of sphingomyelin C16:0, C18:0, C20:0, C22:0 and C24:0. Bar graph compares quantification of sphingomyelin constituents from total ApoE3 and ApoE4 in AD and normal brains. Increases in AD brain are: sphingomyelin C16:0 (1.8-fold); C18:0 (4.9-fold); C22:0 (4.4-fold); C24:0 (2.4-fold). (B) Spectra of ceramides from the MFG grey matter of an AD patient showing the exact mass of C16:0, C18:0, C22:0, C24:1, C24:0, steraoyl and sulfatide. Bar graph compares quantification of ceramides in AD and normal brain. Decreases in ceramide are: 24:0 (3.6-fold) and steraoyl (2.7-fold). (C) Spectrum from the MFG grey matter showing the identification and exact mass of free cholesterols and the cholesterol esters C16:0 and C18:1. Bar figures compare the quantification of cholesterol and cholesterol esters in AD and normal brain. Data are mean and S.D. of counts per second (cps). n = 30 patient samples per group. ** = p < 0.01 and *** = p < 0.001. ANOVA with Tukey post hoc comparisons.
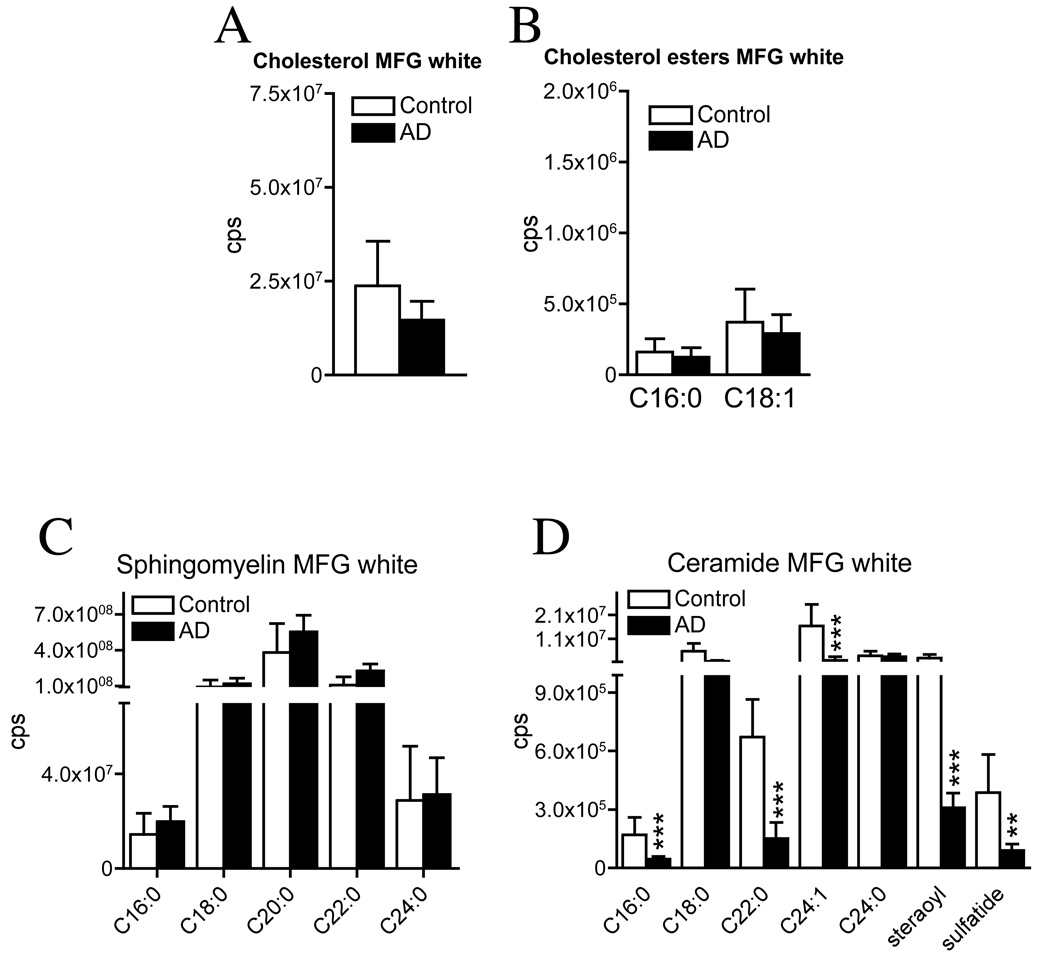
Figure 2. Ceramide levels are decreased in white matter of middle frontal gyrus in AD brain.
White matter was dissected from the middle frontal gyrus (MFG) of AD and normal brains and sterol, sphingomyelin and ceramide levels were determined by ESI/MS/MS. (A) Levels of free cholesterol (monomer, dimmer and trimer combined), (B) the cholesterol esters C16:0 and C18:1 (mono-, di- and trimer combined) and (C) sphingomyelins: none were significantly different in AD vs. normal brain. (D) Ceramides were separated by carbon chain length. Whereas C18:0 and C24:0 were not significantly different, there were significant decreases in ceramide C16:0 (3.7-fold), C22:0 (4.5-fold), C24:1 (9.2-fold), steraoyl (8.4-fold) and sulfatide (4.4-fold) in AD compared with normal brains. Data are mean and S.D. of counts per second (cps). n = 30 patient samples per group. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001. Statistical tests include: Students T-test for cholesterol and cholesterol ester and ANOVA with Tukey post hoc comparisons for sphingomeylins and ceramides.
3.2 ApoE4 is associated with the accumulation of sterols in the brains of Alzheimer’s patients
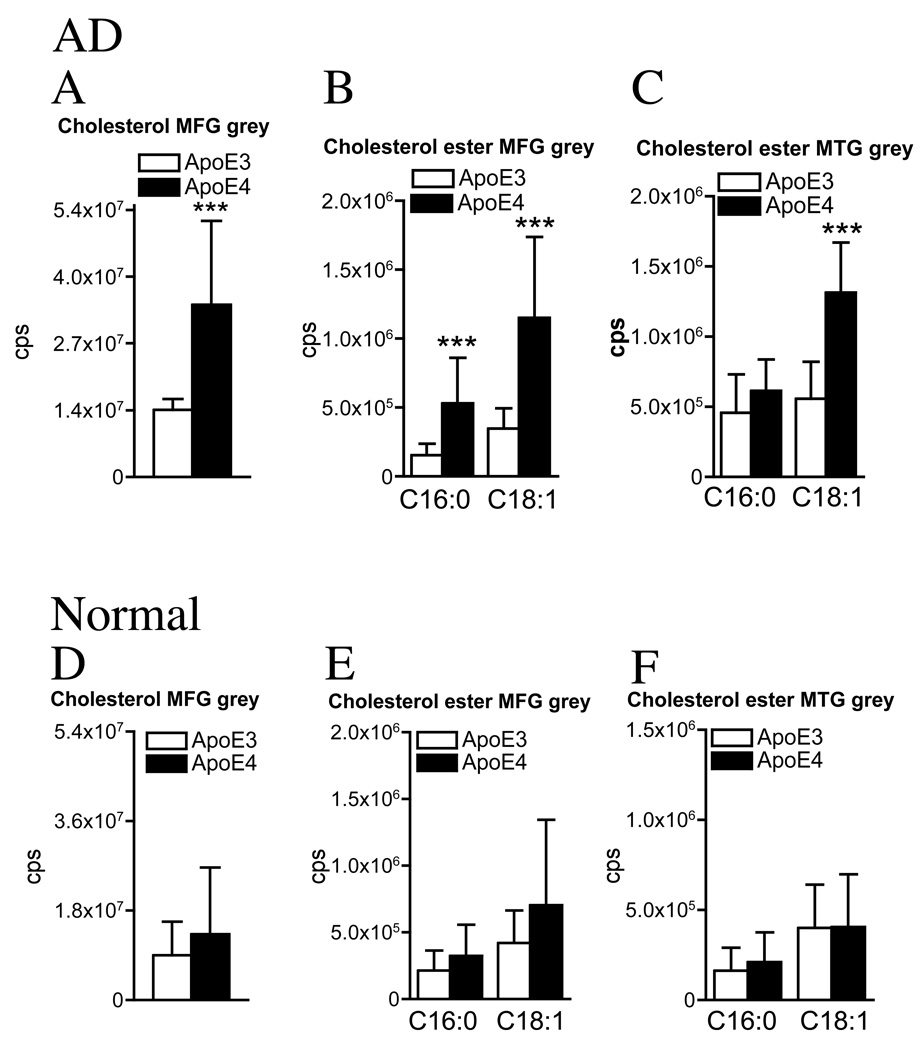
We next separated patients based on their ApoE genotype and compared AD patients expressing ApoE3 with AD patients expressing ApoE4 and normal subjects expressing ApoE3 with normal subjects expressing ApoE4. During data analysis a gene dosing effect of ApoE4 was not apparent in AD or normal (see supplementary Figures 1–3), and data were combined into two groups: ApoE3 (ApoE3/3), and ApoE4 (ApoE3/4 & ApoE4/4). Cholesterol accumulated in grey matter and relatively few changes were observed in the white matter of AD patients expressing ApoE4 compared with AD patients expressing ApoE3. In the MFG grey matter of AD patients with an ApoE4 genotype, there were increased levels of cholesterol, the cholesterol esters C16:0, and C18:1 (range 2.6- to 3.4-fold), and in the MTG grey matter, only the cholesterol ester C18:1 was elevated (2.4-fold) in AD patients expressing ApoE4 compared with AD patients expressing ApoE3 (Fig 3B, C). There were no differences in the levels of cholesterol, or cholesterol esters in the MFG or MTG white matter or cerebellum of AD patients expressing either the ε3 or ε4 allele (data not shown). Thus, in the MFG and MTG of AD patients expressing ApoE4, cholesterol and esterfied cholesterol accumulated in the cell bodies but not in the fiber tracks, and no ApoE allele-dependent differences were observed in the white matter of AD patients.
Figure 3. ApoE4 is associated with increased sterol levels in grey matter of AD but not normal subjects.
White and grey matter were separated from the MFG and MTG before extracting total lipids for analysis by ESI/MS/MS. (A) In the MFG, there were significant increases of cholesterol (2.6-fold), (B) cholesterol esters C16:0 (3.4-fold), and C18:1 (3.3-fold), and (C) in the MTG, the cholesterol ester C18:1 (2.4-fold), in ApoE4 compared with ApoE3 AD brain. (D) In the MFG, cholesterol, (E) cholesterol esters, and (F) in the MTG cholesterol esters, were similar in ApoE4 compared with ApoE3 normal brain. Graphs are summary data of cholesterol (mono-, di- and trimer) and the indicated cholesterol esters (mono-, di- and trimer). Data are mean and S.D. of counts per second (cps). n = 15 patient samples per group. *** = p < 0.001. Students T-test.
In normal subjects, there were no ApoE allele-associated differences in the levels of cholesterol or esters of cholesterol in any brain region tested (Fig. 3D–F). These unexpected findings suggest that ApoE4 may disrupt brain sterol metabolism only in the setting of a neurodegenerative condition.
3.3 ApoE4 is associated with dysfunctions in sphingolipid metabolism in the brains of Alzheimer’s patients
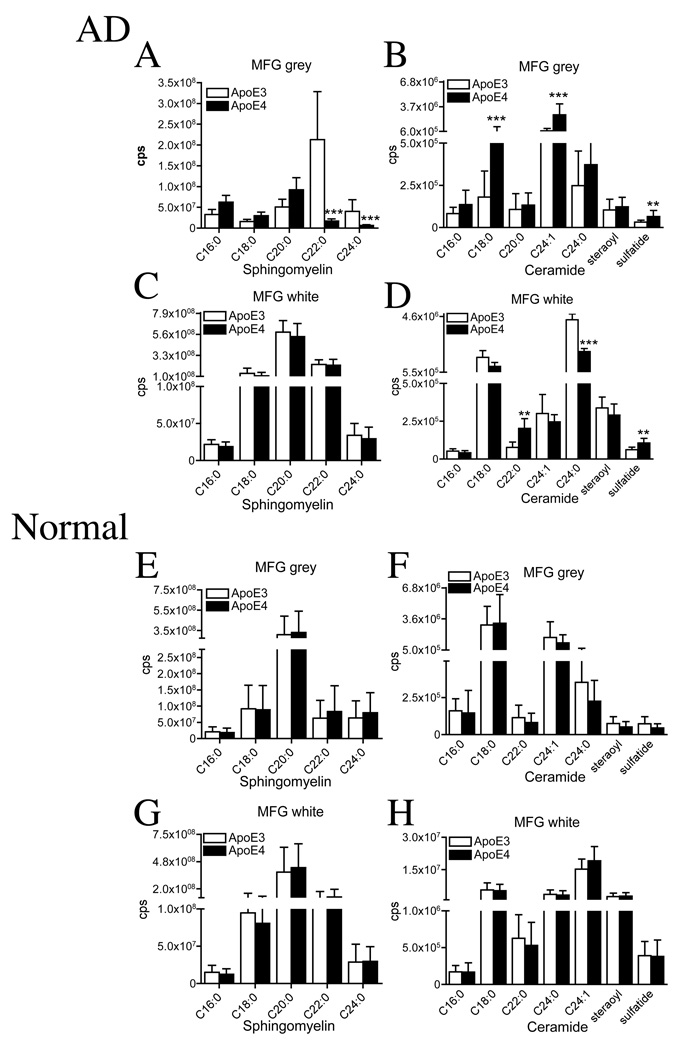
When we compared AD patients expressing ApoE4 with AD patients expressing ApoE3, we found ApoE-allele associated changes in the sphingolipid content of both grey and white matter. In the MFG grey matter of ApoE4 AD patients there were decreases in sphingomylein C22:0 (12.5-fold), and C24:0 (6.2-fold; Fig 4A), compared with ApoE4 AD patients. In contrast, ceramides C18:0, C24:1 and sulfatide were increased in the MFG grey matter of ApoE4 AD patients (1.8- to 2.0-fold; Fig 4B). In the MFG white matter, sphingomeylin content was similar in ApoE3 and ApoE4 AD patients (Fig 4C), but ceramide C22:0 and sulfatide were increased (2.6- and 1.7-fold respectively), and ceramide C24:0 was decreased (2.1-fold; Fig 4D). Sphingomyelin and ceramide levels of the MTG grey and white matter were similar in ApoE3 and ApoE4 AD patients and there were likewise no differences in the cerebellar content of sphingomyelin or ceramide in either patient population (data not shown).
Figure 4. ApoE4 is associated with depletion of sphingomyelins and accumulation of ceramides in AD but not in normal subjects.
White and grey matter were dissected from the MFG of AD and normal brain. Total lipids were extracted for identification of sphingomyelins and ceramides by ESI/MS/MS. (A) In the MFG grey matter, there were significant decreases of sphingomyelin C22:0 (12.5-fold), and C24:0 (6.2-fold), and (B) significant increases of ceramide C18:0 (2.0-fold) and C24:1 (1.8-fold) in ApoE4 compared with ApoE3 AD brain. (E) In the MFG white matter, while there were no significant differences in sphingomeylin, there were significant differences in ceramide C22:0 (2.6-fold increase), C24:0 (1.2-fold decrease), and sulfatide (1.7-fold increase), in ApoE4 vs. ApoE3 brain. (E) In the MFG grey matter there were no significant differences in sphingomyelin or (F) ceramide, and (G) in the MFG white matter there were no differences in sphingomyelin or (H) ceramide of ApoE4 compared with ApoE4 normal brain. Data are mean and S.D. of counts per second (cps). n = 15 patient samples per group. ** = p < 0.01 and *** = p < 0.001. ANOVA with Tukey post hoc comparisons for sphingomyelins and ceramides.
In brain tissues from normal subjects, there were no ApoE allele-associated differences in sphingomyelin (Fig 4E, G) or ceramide (Fig 4F, H) levels of MFG grey or white matter. There were likewise no ApoE allele-dependent differences in the MTG grey or white matter or in the cerebellum of normal patients (data not shown).
3.4 Evidence of increased oxidative damage in Alzheimer’s patients expressing ApoE4
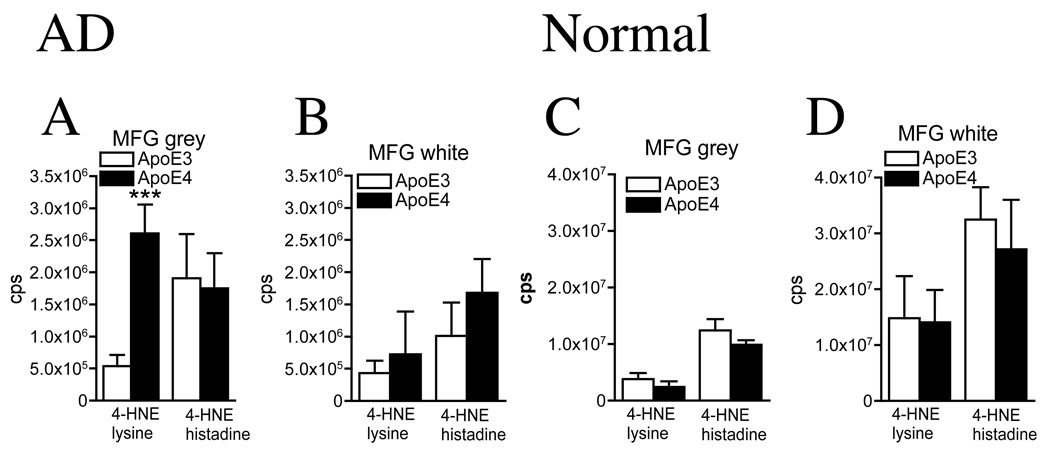
Disruptions in sterol and sphingolipid metabolism can increase cellular oxidation levels and increase the amounts of lipid peroxidation products, including 4-HNE. Coincident with increased cholesterol, cholesterol esters, and ceramide in the MGF grey matter of AD patients with an ApoE4 genotype, we found a 4.9-fold increase in the lysine adduct of 4-HNE compared to that inAD patients expressing ApoE3 (Fig 5A). Consistent with increased ceramide levels of the MFG white matter of ApoE4 vs. ApoE3 AD patients, there were trends toward increased levels of the lysine and histidine adducts of 4-HNE in ApoE4 AD (Fig 5B). Concentrations of 4-HNE were similar in the MTG white and grey matter of ApoE4 and ApoE3 AD patients (data not shown). In normal subjects there were no significant differences in levels of cellular lipid peroxidation products in any brain region tested of ApoE3 compared with subjects expressing ApoE4 (Fig 5C, D and data not shown).
Figure 5. 4-hydroxynonenal is increased in the frontal grey matter of AD patients expressing ApoE4, but not in normal subjects expressing one or more ApoE4 alleles.
The MFG of AD and normal brain was dissected into white and grey matter and 4-HNE was measured by ESI/MS/MS. The lysine and histadine adducts of 4-HNE are shown. (A) In the MFG grey matter there was a significant increase in the lysine adduct of 4-HNE (4.9-fold) and, (B) no significant difference in MFG white matter content of ApoE4 compared with ApoE3 AD brain. (C) In the MFG grey, (D) and MFG white matter, there were no significant differences in 4-HNE of ApoE4 compared with ApoE3 normal brain. Data are mean and S.D. of counts per second (cps)n = 15 patient samples per group. *** = p< 0.001. Students T-test.
4. Discussion
Our results suggest that the ε4 allele of ApoE is not associated with disordered brain sterol or sphingolipid biochemistry in patients without an underlying neurological disorder. Although ApoE4 binds lipoprotein receptors and clears triglyceride-rich lipoprotein remnants with similar efficacy to ApoE3 (Gregg et al. 1986; Bohnet et al. 1996; Knouff et al. 1999), compared with ApoE3, ApoE4 is less efficient at promoting cholesterol efflux in fibroblasts and astrocytes (Huang et al. 1995; Michikawa et al. 2000), binds preferentially very low density lipoproteins (Weisgraber 1990; Dong et al. 1994) and is recycled inefficiently after internalization (Heeren et al. 2004). Despite these deficits, ApoE4 may effectively transport cholesterol in brain under steady state conditions when cholesterol turnover is low. Perturbations that increase the amount of free cholesterol, however, may overwhelm the ability of ApoE4 to transport cholesterol and result in the accumulation of sterols.
Several lines of evidence suggest that dysregulated sphingolipid metabolism in AD could increase free cholesterol. In AD there is a disruption in brain sphingolipid balance, with increased levels of long chain ceramides ((Han et al. 2002; Cutler et al. 2004a);Fig 1). This shift in sphingolipid balance is thought to be the result of an enhanced sphingomyelinase (Smase) mediated catabolism of sphingomyelin to ceramide (Cutler et al. 2004a). There are at least five types of Smases, including the ubiquitous lysosomal acid SMase, the zinc-dependent secreted acid SMase, a neutral, Mg2+-dependent SMase, a neutral Mg2+-independent SMase, and an alkaline SMase. Neutral sphingomyelinase 2 (nSMase2) is a brain-specific nSMase that is thought to be located on the inner leaflet of the plasma membrane and is activated by phosphatidylserine and cellular stressors including the inflammatory cytokines TNFα, IL-1, Fas-L, UV irradiation, the neurotoxic HIV-proteins gp120 and Tat, and amyloid β (Brann et al. 1999; Sortino et al. 1999; Haughey et al. 2003; Castiglione et al. 2004; Lee et al. 2004; Sanchez-Alavez et al. 2006). Once nSMase2 is active, sphingomyelin shifts to the innerleaflet of the plasma membrane, where it can be catabolized to ceramide. Cholesterol that is normally tightly associated with sphingomyelin is released during the membrane translocation of sphingomyelin, thereby creating a pool of free cholesterol. Under normal conditions, free cholesterol would be esterified or cleared by cholesterol binding proteins, of which ApoE is the most abundant in brain. Because ApoE4 has altered binding capacity, and reduced affinity for low-density lipoproteins (Perugini, et al. 2002; Guillaume, et al. 1996), it may be unable to bind effectively and to transport this enlarged pool of cholesterol. Sterols may then accumulate in the brain parenchyma where they could disrupt biophysical properties of membranes and perturb cellular functions, including amyloid processing.
Cholesterol, sphingomyelin, ceramide, and gangliosides are the primary components of specialized membrane domains known as lipid rafts. Cholesterol levels are thought to modulate the processing of amyloid precursor protein by controlling secretase activity. Current evidence suggests that α-secretase resides in phospholipid-rich domains, while γ and β-secretases are found in lipid rafts (Riddell et al. 2001; Wahrle et al. 2002; Cordy et al. 2003). Experimentally reducing cholesterol in tissue culture has been shown to enhance α-secretase activity and to inhibit γ-secretase activity and reduce the production of pathogenic forms of Aβ (Bodovitz and Klein 1996; Kojro et al. 2001; Ehehalt et al. 2003). Use of a glycosylphosphatidylinositol (GPI) anchor to target β-secretase to lipid rafts increased Aβ formation, and this amyloidogenic effect was inhibited when lipid rafts were disrupted by depleting cholesterol from the membrane (Cordy et al. 2003). Nonetheless, there have been reports that lowering cholesterol increases Aβ production (Abad-Rodriguez et al. 2004), suggesting that maintaining homeostatic levels of cholesterol in brain may be important for normal neuronal function. In addition, Aβ may enhance neutral sphingomyelinase activity, increase ceramide levels, and further the production of pathogenic forms of Aβ (Lee et al. 2004). Thus, one potential mechanism for the earlier onset and faster progression of AD in patients with an ApoE4 genotype may involve a positive feed-back effect where Aβ-induced disruption of sphingolipid metabolism increases free cholesterol that enhances the production of pathogenic forms of Aβ. These observations suggest that pharmacologic modulation of sphingomyelin metabolism may protect neurons and reduce the Aβ load in AD patients with ApoE4 genotype.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Pamela Knapp for editorial assistance. This work was supported by NIH/NIA grants AG023471 and AG24984 to N.J.H., and NIH/NIA grant AG05146 to J.T., and by the Johns Hopkins University Alzheimer’s Disease Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement. The authors have no actual or potential conflicts of interest related to this manuscript.
REFERENCES
- Abad-Rodriguez J, Ledesma MD, Craessaerts K, Perga S, Medina M, Delacourte A, Dingwall C, De Strooper B, Dotti CG. Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol. 2004;167:953–960. doi: 10.1083/jcb.200404149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodovitz S, Klein WL. Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- Bohnet K, Pillot T, Visvikis S, Sabolovic N, Siest G. Apolipoprotein (apo) E genotype and apoE concentration determine binding of normal very low density lipoproteins to HepG2 cell surface receptors. J Lipid Res. 1996;37:1316–1324. [PubMed] [Google Scholar]
- Brann AB, Scott R, Neuberger Y, Abulafia D, Boldin S, Fainzilber M, Futerman AH. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J Neurosci. 1999;19:8199–8206. doi: 10.1523/JNEUROSCI.19-19-08199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Wright E, Colton CA, Sullivan PM, Laskowitz DT, Vitek MP. Apolipoprotein E isoform mediated regulation of nitric oxide release. Free Radic Biol Med. 2002;32:1071–1075. doi: 10.1016/s0891-5849(02)00803-1. [DOI] [PubMed] [Google Scholar]
- Castiglione M, Spinsanti P, Iacovelli L, Lenti L, Martini F, Gradini R, Di Giorgi Gerevini V, Caricasole A, Caruso A, De Maria R, Nicoletti F, Melchiorri D. Activation of Fas receptor is required for the increased formation of the disialoganglioside GD3 in cultured cerebellar granule cells committed to apoptotic death. Neuroscience. 2004;126:889–898. doi: 10.1016/j.neuroscience.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Colton CA, Brown CM, Czapiga M, Vitek MP. Apolipoprotein-E allelespecific regulation of nitric oxide production. Ann N Y Acad Sci. 2002;962:212–225. doi: 10.1111/j.1749-6632.2002.tb04070.x. [DOI] [PubMed] [Google Scholar]
- Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci U S A. 2004a;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Haughey NJ, Tammara A, McArthur JC, Nath A, Reid R, Vargas DL, Pardo CA, Mattson MP. Dysregulation of sphingolipid and sterol metabolism by ApoE4 in HIV dementia. Neurology. 2004b;63:626–630. doi: 10.1212/01.wnl.0000134662.19883.06. [DOI] [PubMed] [Google Scholar]
- Dong LM, Wilson C, Wardell MR, Simmons T, Mahley RW, Weisgraber KH, Agard DA. Human apolipoprotein E. Role of arginine 61 in mediating the lipoprotein preferences of the E3 and E4 isoforms. J Biol Chem. 1994;269:22358–22365. [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Bu G, Sun Y, Daugherty A, Holtzman DM. Apolipoprotein Econtaining high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:30121–30125. doi: 10.1074/jbc.271.47.30121. [DOI] [PubMed] [Google Scholar]
- Gregg RE, Zech LA, Schaefer EJ, Stark D, Wilson D, Brewer HB., Jr Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest. 1986;78:815–821. doi: 10.1172/JCI112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume D, Bertrand P, Dea D, Davignon J, Poirier J. Apolipoprotein E and low-density lipoprotein binding and internalization in primary cultures of rat astrocytes: isoform-specific alterations. J Neurochem. 1996;66:2410–2418. doi: 10.1046/j.1471-4159.1996.66062410.x. [DOI] [PubMed] [Google Scholar]
- Han XDMH, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Involvement of Perturbed Sphingolipid Metabolism and Ceramide Production in the Pathogenesis of HIV Dementia. Annals of Neurology. 2003;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Heeren J, Grewal T, Laatsch A, Becker N, Rinninger F, Rye KA, Beisiegel U. Impaired recycling of apolipoprotein E4 is associated with intracellular cholesterol accumulation. J Biol Chem. 2004;279:55483–55492. doi: 10.1074/jbc.M409324200. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Huang Y, von Eckardstein A, Wu S, Assmann G. Effects of the apolipoprotein E polymorphism on uptake and transfer of cell-derived cholesterol in plasma. J Clin Invest. 1995;96:2693–2701. doi: 10.1172/JCI118336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius MJ, Shooter EM, Pitas RE, Mahley RW. Lipoprotein uptake by neuronal growth cones in vitro. Science. 1987;236:959–962. doi: 10.1126/science.3576212. [DOI] [PubMed] [Google Scholar]
- Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579–1586. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004;164:123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovirta M, Soininen H, Laakso MP, Partanen K, Helisalmi S, Mannermaa A, Ryynanen M, Kuikka J, Hartikainen P, Riekkinen PJ., Sr SPECT and MRI analysis in Alzheimer's disease: relation to apolipoprotein E epsilon 4 allele. J Neurol Neurosurg Psychiatry. 1996;60:644–649. doi: 10.1136/jnnp.60.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi O, Jongen-Relo AL, Feldon J, Roses AD, Michaelson DM. ApoE4 impairs hippocampal plasticity isoform-specifically and blocks the environmental stimulation of synaptogenesis and memory. Neurobiol Dis. 2003;13:273–282. doi: 10.1016/s0969-9961(03)00045-7. [DOI] [PubMed] [Google Scholar]
- Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Lutjohann D, Papassotiropoulos A, Bjorkhem I, Locatelli S, Bagli M, Oehring RD, Schlegel U, Jessen F, Rao ML, von Bergmann K, Heun R. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41:195–198. [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Michikawa M, Fan QW, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. 2000;74:1008–1016. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mulder M, Ravid R, Swaab DF, de Kloet ER, Haasdijk ED, Julk J, van der Boom JJ, Havekes LM. Reduced levels of cholesterol, phospholipids, and fatty acids in cerebrospinal fluid of Alzheimer disease patients are not related to apolipoprotein E4. Alzheimer Dis Assoc Disord. 1998;12:198–203. doi: 10.1097/00002093-199809000-00012. [DOI] [PubMed] [Google Scholar]
- Narita M, Bu G, Holtzman DM, Schwartz AL. The low-density lipoprotein receptor-related protein, a multifunctional apolipoprotein E receptor, modulates hippocampal neurite development. J Neurochem. 1997;68:587–595. doi: 10.1046/j.1471-4159.1997.68020587.x. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Jiang Y, Wong GK, Shen F, Brewer GJ, Struble RG. Apolipoprotein E4 inhibits, and apolipoprotein E3 promotes neurite outgrowth in cultured adult mouse cortical neurons through the low-density lipoprotein receptorrelated protein. Brain Res. 2002;928:96–105. doi: 10.1016/s0006-8993(01)03367-4. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Lutjohann D, Bagli M, Locatelli S, Jessen F, Buschfort R, Ptok U, Bjorkhem I, von Bergmann K, Heun R. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J Psychiatr Res. 2002;36:27–32. doi: 10.1016/s0022-3956(01)00050-4. [DOI] [PubMed] [Google Scholar]
- Perugini MA, Schuck P, Howlett GJ. Differences in the binding capacity of human apolipoprotein E3 and E4 to size-fractionated lipid emulsions. Eur J Biochem. 2002;269:5939–5949. doi: 10.1046/j.1432-1033.2002.03319.x. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Christie G, Hussain I, Dingwall C. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr Biol. 2001;11:1288–1293. doi: 10.1016/s0960-9822(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc Natl Acad Sci U S A. 2006;103:2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sortino MA, Condorelli F, Vancheri C, Canonico PL. Tumor necrosis factor-alpha induces apoptosis in immortalized hypothalamic neurons: involvement of ceramide-generating pathways. Endocrinology. 1999;140:4841–4849. doi: 10.1210/endo.140.10.7062. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Veinbergs I, Van Uden E, Mallory M, Alford M, McGiffert C, DeTeresa R, Orlando R, Masliah E. Role of apolipoprotein E receptors in regulating the differential in vivo neurotrophic effects of apolipoprotein E. Exp Neurol. 2001;170:15–26. doi: 10.1006/exnr.2001.7684. [DOI] [PubMed] [Google Scholar]
- Wahrle S, Das P, Nyborg AC, McLendon C, Shoji M, Kawarabayashi T, Younkin LH, Younkin SG, Golde TE. Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis. 2002;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res. 1990;31:1503–1511. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.