Abstract
Alterations in gene expression in the midgut of female Culex pipiens quinquefasciatus exposed to blood meals containing 6.8 logs plaque-forming units/mL of West Nile virus (WNV) were studied by fluorescent differential display. Twenty-six different cDNAs exhibited reproducible differences after feeding on infected blood. Of these, 21 cDNAs showed an increase in expression, and 5 showed a decrease in expression as a result of WNV presence in the blood meal. GenBank database searches showed that one clone with increased expression, CQ G12A2, shares 94% identity with a leucine-rich repeat-containing protein from Cx. p. quinquefasciatus and 32% identity to Toll-like receptors from Aedes aegypti. We present the first cDNA clone isolated from female Cx. p. quinquefasciatus midgut tissue whose expression changes on exposure to WNV. This cDNA represents a mosquito gene that is an excellent candidate for interacting with WNV in Cx. p. quinquefasciatus and may play a role in disease transmission.
Introduction
Arboviruses are responsible for emerging and re-emerging infectious diseases throughout the world, thereby contributing to an increase in disease burden and impacting public health.1–3 West Nile virus (WNV; family Flaviviridae, genus Flavivirus) is the most widely spread arbovirus, occurring on all continents except for Antarctica.4 Since its introduction into North America in 1999, WNV has established itself across the region because of its ability to infect a variety of Culex spp. mosquitoes capable of virus transmission to local and migratory bird species.5–8Culex pipiens pipiens L., Cx. tarsalis Coquillett, Cx. p. quinquefasciatus Say, and Cx. nigripalpus Theobald are the primary enzootic vectors contributing to human WNV epidemics in the United States.5,9,10
The above-mentioned Culex spp. mosquitoes are all competent laboratory vectors of WNV. Competence of a mosquito to transmit an arbovirus is determined by internal and external factors.11,12 One of the important factors influencing the ability of a mosquito to transmit a virus is establishment of an infected midgut. The female mosquito midgut is involved in blood digestion, and thus, viruses ingested with the blood must enter and exit the midgut epithelial cells to infect salivary glands from which they can be transmitted to a naïve host.13,14 Elements of the midgut can act as a barrier to pathogens ingested with the blood that must be circumvented for an infection to be established.15–17 The barrier can either be physical or caused by interference by enzymes that function in blood digestion processes. Changes in mosquito midgut gene expression after blood ingestion has been documented.18–20 Blood ingestion up-regulates the expression of numerous genes involved in nutrient uptake, metabolism, stress responses, peritrophic matrix formation, and immune responses.18 Ingestion of a blood meal containing viruses also elicits a change in midgut morphology and gene expression.17,21
Studies investigating the morphologic alterations that take place after a mosquito becomes infected with virus have shown that Aedes aegypti L. cells infected with replicating yellow fever virus exhibited rough endoplasmic reticulum swelling, vesicle formation, dilation of perinuclear space, and vacuolization.22 Similar cellular changes were seen in Aedes albopictus Skuse C6/36 cells infected with the flavivirus, Kunjin virus.23 Girard and others24 studied the effects of virus replication on membrane induction, cellular organization, and cell viability in midgut and salivary gland tissues of Cx. p. quinquefasciatus and found that WNV induced significant membrane proliferation in the midgut epithelium, muscle, and salivary glands. Transmission electron microscopy showed a laboratory strain of Cx. p. pipiens refractory to WNV transmission caused by apoptosis of midgut cells, suggesting apoptosis as a potential antiviral mechanism.17 Antiviral responses, including the expression of genes encoding proteins of the innate immune pathway, have been detected in Ae. aegypti against Sindbis21 and dengue viruses25,26 and in Anopheles gambiae Giles against O'nyong-nyong virus.27 However, the effect of arbovirus infection on gene expression in midgut tissue of Culex spp. mosquitoes has not been studied.
This study investigated gene expression alterations in midgut tissue of Cx. p. quinquefasciatus after exposure to a WNV-infected blood meal. Results from this project will contribute to our understanding of the physiological process and molecular interactions affected in the midgut of Cx. p. quinquefasciatus after infection with WNV.
Materials and Methods
Mosquitoes and virus
Culex p. quinquefasciatus established in 1995 from a collection from Alachua County in north-central Florida (F > 50) were reared at 28°C and 70–75% humidity under a 14:10-hour light/dark cycle in a Harford Duracool Biochamber (Bio-Temp Scientific Inc., Sarasota, FL) with procedures described elsewhere.28
The WN-FL03p2-3 strain of WNV (passaged four times in Vero cells and one time in BHK cells) was isolated from a pool of Cx. nigripalpus mosquitoes from Indian River County, FL, in 2003 (A. Doumbouya, unpublished data).
Blood-meal preparation and mosquito feeding
Freshly propagated WNV stock was mixed with citrated bovine blood before mosquito feeding to create infected blood meals. Five-to 6-day-old mosquitoes were allowed to feed on cotton pledgets containing either infectious or non-infectious citrated bovine blood (Hemostat, Dixon, CA) warmed (35°C) for 10 minutes. After heating, two aliquots of 0.1 mL of infected blood were added to 1 mL BA-1 diluent29 and held at −80°C until processing to determine blood meal titer. Subsequent to feeding, mosquitoes were immobilized with cold, and fully engorged specimens were transferred to 1-L cardboard cages with mesh screening and maintained in incubators for experiment-specific extrinsic incubation periods of either 4 or 10 days after infection at 28°C and provided 20% sucrose ad libitum.
Mosquito RNA isolation
Total RNA was extracted from Cx. p. quinquefasciatus adult female mosquito midguts using the TRIzol Reagent (Invitrogen, Carlsbad, CA) following the included protocol with the following modifications: dissected midgut tissues were ground in 0.8 mL of TRIzol reagent. After homogenization, samples were allowed to incubate at 23°C for 10 minutes, followed by a debris clearing spin at 12,000g for 10 minutes at 4°C. Then, RNA samples were allowed to dry on ice for 1 hour and resuspended in 0.05 mL of diethylpyrocarbonate (DEPC)-treated water. To aid in resuspension, samples were incubated for 10 minutes at 60°C. All RNA samples were stored at −80°C until needed. All RNA samples were quantified using a SmartSpec Plus spectrophotometer (Bio-Rad Laboratories, Hercules, CA). RNA integrity was verified by separating the RNA on an agarose/formaldehyde gel, and the ribosomal bands were visualized using an In Genius gel documentation system (Syngene, Frederick, MD).
Fluorescent differential display gene expression
RNA from 200 pooled midguts dissected 4 days after female mosquitoes ingested either uninfected blood or WNV-infected blood was isolated using TRIzol Reagent (Invitrogen). Fifty micrograms of this female midgut RNA was sent to GenHunter (Nashville, TN), where differential display (DD) was performed using proprietary protocols.30 Before reverse transcription-polymerase chain reaction (RT-PCR), the RNA from each sample was treated with DNase to eliminate contamination from DNA. Using 48 13-mer arbitrary primers and oligo dT, RT-PCR reactions were performed and analyzed on a denaturing polyacrylamide gel. Fluorescence was analyzed using a digital Hitachi FMBIO II Fluorescence Imaging System (Hitachi Software Engineering America, Ltd., San Francisco, CA). We chose 11 of the 26 reproducible PCR amplified products showing an increase in expression after blood ingestion, and these were excised from the gel. The fragments were cloned by TA cloning into the pCR2.1 cloning vector (TA Cloning Kit; Invitrogen). Recombinant plasmids were transformed into TOP10 chemically competent Escherichia coli cells following the included protocol (TA Cloning Kit; Invitrogen) and grown overnight on Luria broth (LB) plates containing X-gal and ampicillin in a 37°C incubator. White colonies were used to inoculate LB broth containing ampicillin and grown under constant aeration overnight at 37°C. Recombinant plasmid DNA was isolated from the E. coli cells using the QIAprep Spin miniprep kit (Qiagen, Valencia, CA). Positive recombinant plasmids were identified by digestion with restriction enzymes, analyzed by agarose gel electrophoresis, and visualized on an InGenius gel documentation system (Syngene, Frederick, MD).
Sequence analysis
One of the PCR amplified products of interest, the cloned differentially expressed product, CQ G12A2, was sequenced using the CEQ DTCS Quick Start Kit (Beckman Coulter, Fullerton, CA) following the included protocol. The remaining 10 cloned amplified PCR products are presently being sequenced and will not be discussed in this manuscript. Sequencing reactions were analyzed on a Beckman Coulter CEQ 8000 Genetic Analysis System. BLAST was used to find regions of local similarity between the cloned sequence and sequences in the GenBank and VectorBase databases.
Semi-quantitative RT-PCR
In a separate experiment, temporal gene expression of the cloned differentially expressed product, CQ G12A2, was analyzed using semi-quantitative RT-PCR for midguts of mosquitoes fed uninfected or WNV-infected blood meals and allowed to incubate for 10 days. Clone-specific primers were designed (Integrated DNA Technologies, Coralville, IA) and included the following: CQ G12A2 forward primer, 5′-CTTGCAGGAGTCTATATTTGAGTC-3′, CQ G12A2 reverse primer, 5′-ATGAGTTTATCCTGTTGTTTGT GA-3′, to generate a PCR product of 362 bp. RT-PCR was performed on RNA isolated from midguts dissected at different time points (0, 3, 6, and 9 hours and 1–10 days; N = 20 midguts/time point) after feeding on uninfected blood or WNV-infected blood, using clone specific primers described previously and following the included protocol of the Enhanced Avian HS RT-PCR kit (Sigma Aldrich, St. Louis, MO). Total RNA from each sample was treated with RQ1 RNase-free DNase (Promega, Madison, WI) before RT-PCR to eliminate contamination from DNA. Semi-quantitative RT-PCR reactions were amplified on an MJ Mini Gradient Thermo Cycler (Bio-Rad Laboratories). All RT-PCR products were analyzed on 2% agarose gels, stained with ethidium bromide, and visualized on an InGenius gel documentation system. All semi-quantitative RT-PCR analyses were repeated three times.
Quantitative RT-PCR
The amount of WNV RNA in blood meals and midgut samples was determined using a LightCycler 480 system (Roche, Mannheim, Germany) and Superscript III One-Step Quantitative RT-PCR kit (Invitrogen) for quantitative real-time TaqMan RT-PCR (qRT-PCR) using methods described elsewhere.12 Standard curves were based on 10-fold serial dilutions of known WNV titers determined by plaque assay as described elsewhere.12
Statistical analysis
Box plots were used to test viral titers of pooled midgut samples for normality.31 The lack of normality was verified with Kolmogorov-Smirnov tests. Viral titers at each time point were log-transformed [log (x + 1)] before analysis of variance (ANOVA) with the GLM procedure in SAS.31 If significance was observed, the Duncan multiple range test was used to determine which means were significantly different.31
Results
Differential display of gene expression 4 days after ingesting uninfected or WNV-infected blood
The effect of WNV infection on gene expression in midgut tissue of Cx. p. quinquefasciatus mosquitoes was studied using a fluorescent DD approach to show broad changes in transcription.30 Mosquito midguts used in the DD analysis were dissected at 4 days after WNV exposure, a time shown to coincide with the presence of virus in midgut cells.32 A comparison of midgut transcription alterations 4 days after female mosquitoes ingested uninfected blood or blood meals containing ∼6.8 plaque-forming units (pfu) WNV/mL showed 26 amplification products with reproducible differences. Of these, 21 cDNAs showed an increase in expression, and 5 showed a decrease in expression as a result of WNV presence. Eleven cDNAs, ranging in size from 190 to 418 bp and exhibiting increased expression after infection with WNV, were cloned. Six of these 11 amplification products are shown on Figure 1. One of the 11 clones, CQ G12A2 (GenBank accession no. GO254244), contained a 418-bp insert and, through sequence analysis, was found to encode a putative translation product of 131 amino acids, and was incomplete at the 5′- and 3′- ends (Figure 2). The Cx. p. quinquefasciatus CQ G12A2 putative protein has about three repetitive stretches of amino acids containing leucine residues (Figure 2). VectorBase, Pfam, and BLAST tblastx database searches with the putative translation product of CQ G12A2 showed that it shares 94% identity with a leucine-rich repeat-containing protein from Cx. p. quinquefasciatus (accession no. XP_001846467; unpublished) and shares 32% identity with Toll-like receptors (TLRs) from Ae. aegypti (accession no. XP_001650338)33 (Figure 2; GenBank tblastx searches performed on April 23, 2009). Phylogenetic analysis of blastp results indicated that CQ G12A2 clusters with a TLR from Ae. aegypti (data not shown).
Figure 1.
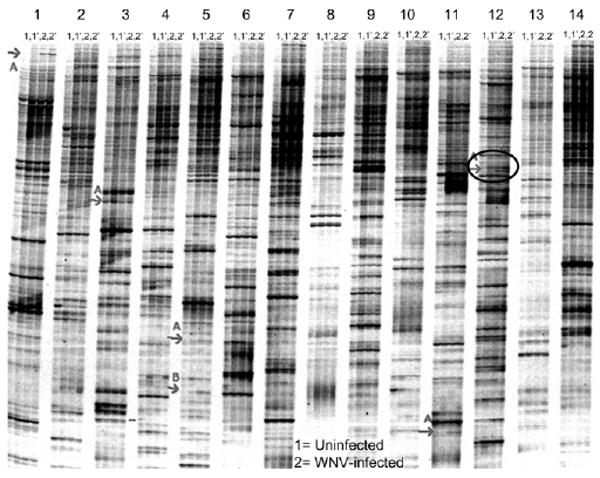
Results from the fluorescent differential display analysis of the gene expression differences between midgut tissue isolated from Cx. p. quinquefasciatus female mosquitoes 4 days after ingestion of uninfected (Sample 1) and WNV-infected (Sample 2) blood. Numbers 1–14 at the top of the figure represent 14 of the 48 primers used in the DD reaction. Each primer was used in a PCR reaction with RNA from uninfected midguts (Sample 1, first two lanes) and infected midguts (Sample 2, next two lanes). Each reaction was duplicated, as shown on the figure as Samples 1′ and 2′. The red arrows indicate those products with differential gene expression. The black circle points out the expression of the product CQ G12A resulting from fluorescent differential display.
Figure 2.
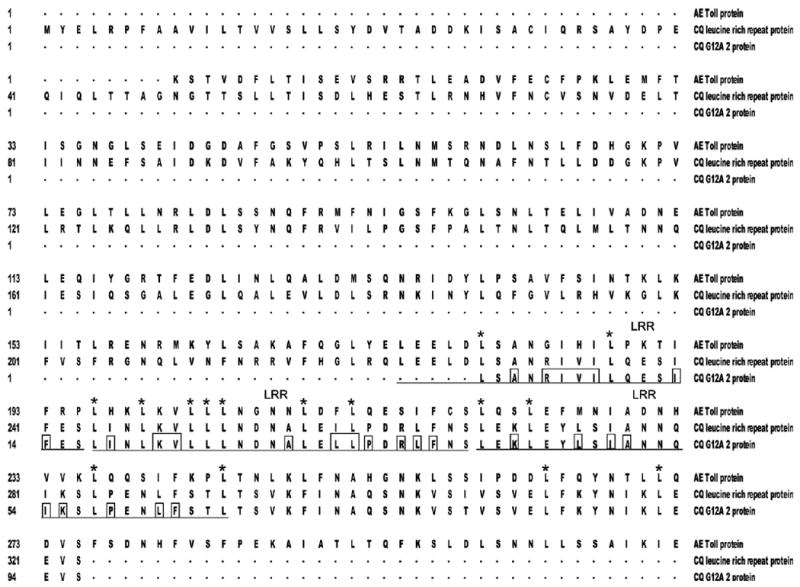
Multiple protein sequence alignment of a portion of the Cx. p. quinquefasciatus CQ G12A2 putative translation product (94 amino acids; CQ G12A2 protein; accession no. GO254244), with partial sequences representing the Toll protein (AE Toll protein; accession no. XP_001650338.1) and leucine-rich repeat protein (CQ leucine-rich repeat protein; accession no. XP_001846467.1) from Ae. aegypti and Cx. p. quinquefasciatus, respectively. The numbering represents the amino acid number. The amino acids that are underlined represent the putative conserved region containing leucine repeats in the CQ G12A2 translation product. The boxed residues represent the hydrophobic amino acids contained in the underlined region. The stars indicate leucine residues conserved in all three aligned amino acid sequences. LRR, leucine-rich repeat region.
Temporal gene expression in midgut tissue of mosquitoes fed uninfected or WNV-infected blood
To characterize the temporal expression of CQ G12A2 in midguts of blood-fed mosquitoes, we performed semi-quantitative RT-PCR on RNA extracted from midguts of female Cx. p. quinquefasciatus at different times after exposure to uninfected blood (i.e., 0, 3, 6, and 9 hours and 1–10 days after feeding). Expression of CQ G12A2 was detected at low levels in midgut tissues at each time; however, an increase in transcription was also detected (Figure 3). The up-regulation of transcription coincided with the early time periods after blood feeding, specifically at 3–6 hours, with an additional increase at 1 and 9 days after feeding (Figure 3A).
Figure 3.
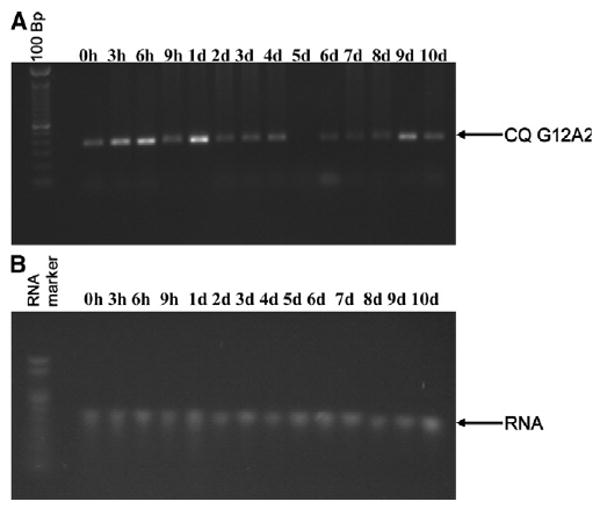
Semi-quantitative RT-PCR analyses of DD clone CQ G12A2 in Cx. p. quinquefasciatus mosquito midgut RNA isolated from females fed an uninfected blood meal (A). B, Integrity of the RNA. Bp, base pairs; h, hours; d, days.
To determine whether WNV infection affects temporal expression of CQ G12A2 in midgut tissue, we performed semi-quantitative RT-PCR on RNA extracted from midguts dissected from female Cx. p. quinquefasciatus at different times after exposure to blood containing 7.4 ± 0.1 logs pfu WNV/mL (i.e., 0, 3, 6, and 9 hours and 1–10 d after feeding; Figure 4). Expression of our gene of interest was seen in midguts at each of the times sampled. However, there were visible increases in mRNA in midguts dissected 6, 8, and 10 days after infection (Figure 4A).
Figure 4.
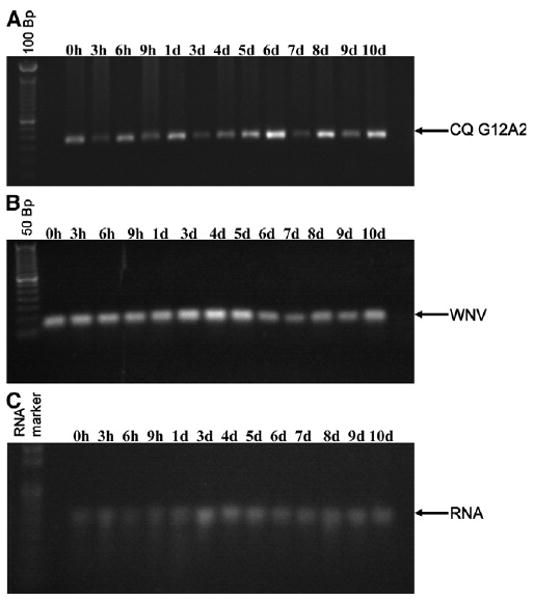
Semi-quantitative RT-PCR analyses of DD clone CQ G12A2 in Cx. p. quinquefasciatus mosquito midgut RNA isolated from females fed a WNV-infected blood meal (A). B, Agarose gel of the qRT-PCR analysis of WNV titer in the same RNA used in A. C, Integrity of the RNA. Bp, base pairs; h, hours; d, days.
To determine CQ G12A2's influence on WNV in midgut tissue, if any, we quantified the amount of WNV RNA in the midgut samples from female Cx. p. quinquefasciatus at different times after exposure to WNV-infected blood (Figure 5). Our qRT-PCR results showed that WNV was detected at all times tested, and significant differences in WNV titer were shown at different times after infection (F = 70.54; df = 12,38; P ≤ 0.001; Figure 5). We analyzed the qRT-PCR samples by agarose gel electrophoresis and found that the intensity of the PCR products coincided with the WNV titers observed using qRT-PCR analysis (Figure 4B). WNV titer increased when transcription of CQ G12A2 was low, as seen in samples dissected at 3–5 days after infection. However, the WNV titer in midguts dissected 6 days after infection showed the lowest titer, but this corresponded to the highest visible expression of CQ G12A2 (Figures 4A and B and 5). These findings are consistent with the possibility that CQ G12A2 plays a role in antiviral responses and consistent with its similarity with the TLR family.
Figure 5.
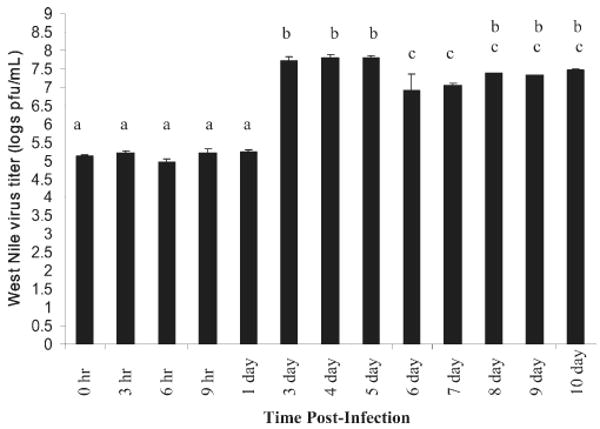
Culex p. quinquefasciatus midgut WNV titer at different time points after infection. When analyzed using analysis of variance, there is a significant difference in WNV titer in the mosquito midgut at different times after infection (F = 70.54, df = 12.38, P ≤ 0.001). Means with the same letter are not significantly different using the Duncan multiple range test.31
Discussion
Proteins with leucine-rich repeats (LRRs) are involved in protein–protein interactions and are of diverse structure, localization, and function.34 There are seven classes of leucine-rich repeats, and they share the characteristic structure of repetitive stretches of amino acids of variable length containing precisely positioned hydrophobic residues, usually leucines.35 The Cx. p. quinquefasciatus CQ G12A2 putative protein has about three repetitive stretches of amino acids containing leucine residues. Leucine-rich repeats are found in proteins, both intracellular and extracellular, that function in innate immunity and nervous system development in bacteria, fungi, plants, and animals.35–37 The similarity of CQ G12A2 to proteins containing LRR is consistent with the possibility that it plays a role in mosquito innate immunity or development. This will be assessed in future studies.
Extracellular LRR proteins in mammals involved in innate immunity include TLR and are characterized by an LRR region, a trans-membrane domain, and a cytoplasmic Toll/IL-1 receptor domain.37 Although it has been suggested that TLR proteins in insects such as Drosophila and mosquitoes evolved independently of mammals, dipteran TLRs retain some of the structure and function seen in vertebrate TLRs.38 Toll receptors in Drosophila function in development, antifungal, and antibacterial responses.39 Drosophila melanogaster Toll-1 has also been implicated in antiviral responses.40 Mosquito TLRs that function in antibacterial and antifungal responses have been characterized in Anopheles gambiae41 and Ae. aegypti.42 Additionally, the involvement of the Toll pathway has been shown in regulating resistance to dengue virus infection in Ae. aegypti mosquitoes.26 The similarity of the translation product of CQ G12A2 to a TLR of Ae. aegypti provides additional support for a probable role in innate immune responses in Cx. p. quinquefasciatus mosquitoes that needs further study.
We looked at the temporal gene expression in midgut tissue of mosquitoes fed uninfected blood and found visible increases in expression after the blood meal. The apparent change in expression at the earlier time points after blood ingestion suggests a role of CQ G12A2 early in digestion or in synthesis of proteins used for peritrophic matrix formation.43 Additionally, the putative expression product of CQ G12A2 may be involved in defense reactions to invading microorganisms ingested with the blood.18
The temporal expression of CQ G12A2 in midgut tissue of mosquitoes fed WNV-infected blood seemed to increase at 6, 8, and 10 days after infection. Expression of CQ G12A2 at 6 days after infection suggests that this gene is likely involved in more than blood digestion because all of the blood is already digested by this time.44 Activation of CQ G12A2 at these times is consistent with the possibility of a role in virus replication and dissemination, because WNV has been shown to replicate and even disseminate in Cx. p. quinquefasciatus by 4 days after infection (S. L. Anderson and others, unpublished data).32 However, because the putative protein product of CQ G12A2 shows similarity to proteins that play a role in immune functions, a role for this product in promoting viral replication is not strongly supported. We studied whether the expression of CQ G12A2 influenced WNV in midguts and found WNV in all time points tested. Although we did detect significant differences in WNV titer at different times after infection, we cannot entirely attribute these differences to the expression of CQ G12A2. There may be other unknown factors interacting with WNV and contributing to this phenomenon. Further studies are underway to address these possibilities. Interestingly, we did observe that WNV titer was lowest when the expression of CQ G12A2 seemed to be up-regulated. We believe that these findings are consistent with the possibility that CQ G12A2 plays a role in antiviral responses, which is comparable to other gene functions in the TLR family.
These studies showed that WNV infection alters the expression of genes that may be involved in antiviral responses in the midgut tissue of female Cx. p. quinquefasciatus. The similarity of the CQ G12A2 putative protein to LRR-containing proteins and its unique expression pattern in WNV-infected Cx. p. quinquefasciatus female mosquitoes suggests a potential role in antiviral responses, perhaps as a protein of the innate immune pathway. Further studies are warranted to characterize this gene and define its involvement in WNV infection of Cx. p. quinquefasciatus, which could enhance our understanding of Culex spp.–WNV interactions and contribute to our understanding of vector competence.
Acknowledgments
The authors thank Walter J. Tabachnick and Jonathan F. Day for editorial comments.
Financial support: This project was supported in part by a grant from the Florida Department of Agriculture and Consumer Services (Grants 00072154 and 00077263) to C.T.S. and in part by a grant from the National Institute of Health (Grant AI-49326) to C.C.L.
Contributor Information
Chelsea T. Smartt, Florida Medical Entomology Laboratory, 200 9th St. SE, Vero Beach, FL 32962, Tel: 772-778-7200, Fax: 772-778-7205.
Stephanie L. Richards, Email: slrichar@ufl.edu, Florida Medical Entomology Laboratory, 200 9th St. SE, Vero Beach, FL 32962
Sheri L. Anderson, Email: slander@ufl.edu, Florida Medical Entomology Laboratory, 200 9th St. SE, Vero Beach, FL 32962
Jennifer S. Erickson, Email: fishdoc_5@hotmail.com, Department of Biology, McMaster University, Hamilton, Ontario, Canada L8S 4M2
References
- 1.Murgue B, Murri S, Zientara S, Durand B, Durand JP, Zeller H. West Nile outbreak in horses in southern France, 2000: the return after 35 years. Emerg Infect Dis. 2001;7:692–696. doi: 10.3201/eid0704.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autorino GL, Battisti A, Deubel V, Ferrari G, Forletta R, Giovannini A, Lelli R, Murri S, Scicluna MT. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg Infect Dis. 2002;8:1372–1378. doi: 10.3201/eid0812.020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyrefitte CN, Pastorino B, Grau GE, Lou J, Tolou H, Couissinier-Paris P. Dengue virus infection of human microvascular endothelial cells from different vascular beds promotes both common and specific functional changes. J Med Virol. 2006;78:229–242. doi: 10.1002/jmv.20532. [DOI] [PubMed] [Google Scholar]
- 4.Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- 5.Sardelis MR, Turell MJ, Dohm DJ, O'guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day JF. Host-seeking strategies of mosquito disease vectors. J Am Mosq Control Assoc. 2005;21:17–22. doi: 10.2987/8756-971X(2005)21[17:HSOMDV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Hayes EB, Komar N, Nasci RS, Montgomery SP, O'leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 9.Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ. West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J Med Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- 10.Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- 11.DeFoliart GR, Grimstad PR, Watts DM. Advances in mosquito-borne arbovirus/vector research. Annu Rev Entomol. 1987;32:479–505. doi: 10.1146/annurev.en.32.010187.002403. [DOI] [PubMed] [Google Scholar]
- 12.Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy J, Houk E, Kramer L, Reeves W. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 14.Black WC, Bennett KE, Gorrochotegui-Escalante N, Barillas-Mury CV, Fernandez-Salas I, De Lourdes Munoz M, Farfan-Ale JA, Olson KE, Beaty BJ. Flavivirus susceptibility in Aedes aegypti. Arch Med Res. 2002;33:379–388. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- 15.Vlachou D, Schlegelmilch T, Christophides GK, Kafatos FC. Functional genomic analysis of midgut epithelial responses in Anopheles during Plasmodium invasion. Curr Biol. 2005;15:1185–1195. doi: 10.1016/j.cub.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Ebel GD, Rochlin I, Longacker J, Kramer LD. Culex restuans (Diptera: Culicidae) relative abundance and vector competence for West Nile virus. J Med Entomol. 2005;42:838–843. doi: 10.1603/0022-2585(2005)042[0838:CRDCRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Vaidyanathan R, Scott TW. Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis. 2006;11:1643–1651. doi: 10.1007/s10495-006-8783-y. [DOI] [PubMed] [Google Scholar]
- 18.Sanders HR, Evans AM, Ross LS, Gill SS. Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochem Mol Biol. 2003;33:1105–1122. doi: 10.1016/s0965-1748(03)00124-3. [DOI] [PubMed] [Google Scholar]
- 19.Smartt CT, Erickson JS. Bloodmeal-induced differential gene expression in the disease vector Culex nigripalpus (Diptera: Culicidae) J Med Entomol. 2008a;45:326–330. doi: 10.1603/0022-2585(2008)45[326:bdgeit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Smartt CT, Erickson JS. CNAct-1 gene is differentially expressed in the subtropical mosquito Culex nigripalpus (Diptera: Culicidae), the primary West Nile virus vector in Florida. J Med Entomol. 2008b;45:877–884. doi: 10.1603/0022-2585(2008)45[877:cgidei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Sanders HR, Foy BD, Evans AM, Ross LS, Beaty BJ, Olson KE, Gill SS. Sindbis virus induces transport processes and alters expression of innate immunity pathway genes in the midgut of the disease vector, Aedes aegypti. Insect Biochem Mol Biol. 2005;35:1293–1307. doi: 10.1016/j.ibmb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Deubel V, Digoutte JP, Mattei X, Pandare D. Morphogenesis of yellow fever virus in Aedes aegypti cultured cells. II. An ultrastructural study. Am J Trop Med Hyg. 1981;30:1071–1077. doi: 10.4269/ajtmh.1981.30.1071. [DOI] [PubMed] [Google Scholar]
- 23.Ng ML. Ultrastructural studies of Kunjin virus-infected Aedes albopictus cells. J Gen Virol. 1987;68:577–582. doi: 10.1099/0022-1317-68-2-577. [DOI] [PubMed] [Google Scholar]
- 24.Girard YA, Popov V, Wen J, Han V, Higgs S. Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2005;42:429–444. doi: 10.1093/jmedent/42.3.429. [DOI] [PubMed] [Google Scholar]
- 25.Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, Olson KE. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci USA. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards SL, Lord CC, Pesko KA, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for Saint Louis encephalitis virus. Am J Trop Med Hyg. 2009;81:264–272. [PMC free article] [PubMed] [Google Scholar]
- 29.Lanciotti R, Kerst A, Nasci R, Godsey M, Mitchell C, Savage H, Komar N, Panella N, Allen B, Volpe K, Davis B, Roehrig J. Rapid detection of West Nile virus from human clinical specimens, field collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 31.SAS Institute. SAS/STAT User's Guide for Personal Computers, Version 8.0. SAS Institute; Cary, NC: 2002. [Google Scholar]
- 32.Girard YA, Klinger KA, Higgs S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoonotic Dis. 2004;4:109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- 33.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O'leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11:725–732. doi: 10.1016/s0959-440x(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 35.Dolan J, Walshe K, Alsbury S, Hokamp K, O'keeffe S, Okafuji T, Miller SF, Tear G, Mitchell KJ. The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics. 2007;8:320. doi: 10.1186/1471-2164-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Aulia S, Li L, Tang BL. AMIGO and friends: an emerging family of brain-enriched, neuronal growth modulating, type I transmembrane proteins with leucine-rich repeats (LRR) and cell adhesion molecule motifs. Brain Res Brain Res Rev. 2006;51:265–274. doi: 10.1016/j.brainresrev.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Nurnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- 38.Imler JL, Zheng L. Biology of Toll receptors: lessons from insects and mammals. J Leukoc Biol. 2004;75:18–26. doi: 10.1189/jlb.0403160. [DOI] [PubMed] [Google Scholar]
- 39.Imler JL, Ferrandon D, Royet J, Reichhart JM, Hetru C, Hoffmann JA. Toll-dependent and Toll-independent immune responses in Drosophila. J Endotoxin Res. 2004;10:241–246. doi: 10.1179/096805104225005887. [DOI] [PubMed] [Google Scholar]
- 40.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci USA. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, Von Mering C, Xu J, Zheng L, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- 42.Shin SW, Bian G, Raikhel AS. A toll receptor and a cytokine, Toll5A and Spz1C, are involved in toll antifungal immune signaling in the mosquito Aedes aegypti. J Biol Chem. 2006;281:39388–39395. doi: 10.1074/jbc.M608912200. [DOI] [PubMed] [Google Scholar]
- 43.Marinotti O, Calvo E, Nguyen QK, Dissanayake S, Ribeiro JM, James AA. Genome-wide analysis of gene expression in adult Anopheles gambiae. Insect Mol Biol. 2006;15:1–12. doi: 10.1111/j.1365-2583.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 44.Clements AN. The Biology of Mosquitoes Volume 1: Development, Nutrition and Reproduction. New York: CABI Publishing; 2000. [Google Scholar]


