Abstract
Hydrogels are an important class of biomaterials for cell encapsulation and delivery, providing a physical barrier or “immuno-isolation” between the host tissue and encapsulated cells. The semipermeable gel protects the encapsulated cells from host immune cells and/or antibody recognition while allowing facile diffusion of nutrients. However, a previously un-addressed problem is that highly permissive hydrogels cannot exclude the infiltration of soluble immune-mediators, such as pro-inflammatory cytokines that are highly expressed in wounded environments in vivo. When encountered with pro-inflammatory cytokines, encapsulated cells fail to perform their desired functions. Here, we report the synthesis, characterization, and application of peptide-functionalized, cytokine-antagonizing poly(ethylene glycol) (PEG) hydrogels capable of sequestering the pro-inflammatory cytokine, tumor necrosis factor α (TNFα). Results demonstrate that the survival, function, and differentiation of encapsulated cells (e.g., rat adrenal pheochromocytoma cells – PC12s, mouse pancreatic islets, and human mesenchymal stem cells or hMSCs) are significantly hindered in un-modified PEG hydrogels under in vitro TNFα treatments. In contrast, cells encapsulated in TNFα-antagonizing hydrogels are un-affected by the infiltrated TNFα. This study demonstrates the importance of controlling the availability of pro-inflammatory cytokines in highly permissive hydrogels.
Keywords: Hydrogels, inflammation, cytokine, apoptosis, mesenchymal stem cells, TNFα, tissue engineering
1. Introduction
Poly(ethylene glycol) (PEG) hydrogels have been used to encapsulate a wide variety of cell types for tissue engineering and regenerative medicine applications [1]. PEG hydrogels present unique advantages, as well as challenges, as cell delivery vehicles due to their high water content and short diffusion time scales. Further, peptide-functionalized PEG hydrogels mimic aspects of the extracellular matrix to support the survival and function of encapsulated cells [2-4], thus providing an excellent bioinspired platform for research and clinical applications. The semipermeable PEG networks not only permit facile molecular transport, but also serve as an immuno-isolation barrier that prohibits the infiltration of immune cells and antibodies [5], two critical mediators in the host immune response. Due to these preferential properties, PEG hydrogels are considered excellent materials for cell delivery and therapy, such as the encapsulation of pancreatic islets for the treatment of type 1 diabetes [6, 7], the delivery of neuronal cells for the treatment of neurodegenerative diseases [8], and the encapsulation of mesenchymal stem cells for controlled differentiation [9-13].
Although the survival and function of cells encapsulated in PEG hydrogels can be stringently controlled in most in vitro studies, these cells encounter additional challenges when they are transplanted in vivo. The host innate immunity responds rapidly to the injury caused by the implantation procedure and triggers normal wound healing cascades, during which the pro-inflammatory cytokines secreted by inflammatory cells recruit more inflammatory/immune cells as well as activate apoptotic pathways in the transplanted foreign cells. Although the semipermeable PEG hydrogels effectively prevent direct contact between immune cells and encapsulated cells, they cannot prevent the diffusion of small cytotoxic molecules, such as reactive oxygen species (ROS) and pro-inflammatory cytokines (e.g., tumor necrosis factor-α (TNFα, 17.4kDa); interleukin-1β (IL-1β, 17kDa), etc). Once these cytotoxic molecules have penetrated into the hydrogel barrier, they can trigger apoptotic pathways that lead to apoptosis or impaired cell function. Although theoretically the infiltration of small cytotoxic molecules in PEG gels can be decreased through increasing the crosslinking density of the gels, this approach is not practical as it also significantly decreases the water content of the gels and diffusion of nutrients, hence hindering the survival of the encapsulated cells. Thus, challenges and opportunities exist for improving the survival and function of the cells encapsulated in highly swollen PEG hydrogels in the presence of small cytotoxic molecules.
In this regard, Anseth and co-workers recently synthesized a polymerizable superoxide dismutase (SOD) mimetic metalloporphyrin macromer that was co-polymerized with PEG-diacrylate (PEGDA) to form PEG hydrogel networks that actively decrease the activity of ROS [14]. This approach decreases ROS-mediated cell damage and prolongs the survival and function of encapsulated cells. Unfortunately, to date, there is no methodology for counteracting the cytotoxic effects induced by the infiltration of pro-inflammatory cytokines, such as TNFα, in crosslinked and highly permissive PEG hydrogels. One facile approach is to immobilize cytokine-antagonizing antibodies in PEG hydrogels. However, the conjugation of modified antibodies in PEG hydrogels can be problematic due to their large molecular size (hundreds of kDa) and protein stability issues. Furthermore, antibodies produced from animals can give rise to concerns of immunogenicity when used in humans. In contrast to large antibodies, small molecules, such as peptides, that exhibit specific and high affinity for cytokines are excellent alternatives, in part due to their small and defined molecular structures that can be easily modified to fulfill the needs of specific applications. For example, a soluble peptide mimic derived from a critical TNFα recognition loop on TNF-receptor 1 (TNFR1), namely WP9QY (YCWSQYLCY, di-sulfide form), has been identified as a potent peptide antagonist that binds to human TNFα and inhibits its binding to cell surface TNFR1, thereby decreasing TNFα-mediated cell apoptosis [15] and bone destruction [16].
Taking advantage of the highly specific TNFα-WP9QY binding, we report herein the synthesis, characterization, and applications of TNFα-antagonizing PEG hydrogels for enhancing the survival and function of encapsulated cells against TNFα challenge. We first describe the design and characterization of affinity binding of human TNFα to WP9QY peptide-functionalized PEG hydrogels using fluorescent resonance energy transfer (FRET), followed by the characterization of the physical properties of the PEG-WP9QY hydrogels, including the degree of hydrogel swelling, the diffusivity of small molecules, and cytokine uptake in gels. Since TNFα is a potent pro-inflammatory cytokine that impacts broadly on many major cell types in the body [17-22], we encapsulated the following three cell types that are sensitive to TNFα challenge, including rat adrenal pheochromocytoma cells – PC12s, mouse pancreatic islets, and human mesenchymal stem cells (hMSCs), in the PEG-WP9QY functionalized hydrogels to demonstrate the ability to inhibit the impairment of the encapsulated cell fate induced by TNFα.
2. Materials and Methods
2.1 Materials
Amino acids and reagents for solid phase peptide synthesis were obtained from Anaspec. Cytokines and ELISA kits were purchased from Peprotech. AlexaFluor-555 protein labeling kit was obtained from Invitrogen. All other chemicals were supplied by Sigma-Aldrich unless noted otherwise.
2.2 PEGDA macromers and peptide synthesis
PEGDA macromers (6kDa and10kDa) were synthesized as described elsewhere [23]. TNFα-antagonizing peptide WP9QY (YCWSQYLCY) was synthesized on a solid phase peptide synthesizer (Applied Biosystems, Model 433A) using standard Fmoc chemistry. After synthesis, resin-bound peptide was acrylated by conjugating the N-terminus of the peptides with excess amount of HATU-activated acrylic acid in the presence of diisopropylethylamine (DIEA). Acrylated peptides were then cleaved in a cleavage cocktail (5 wt% phenol in 95% trifluoroacetic acid (TFA), 2.5% triisopropylsilane (TIPS), and 2.5% water) for two hours at room temperature, followed by precipitation in cold ether. The crude peptide was then dissolved in 50% DMSO at 5mM for intramolecular disulfide bond formation. The reaction was continued at room temperature with agitation until no free thiol groups were detected as determined by Ellman's reagent (PIERCE). For FRET experiments, the peptide analog K(Mtt)YCWSQYLCY was synthesized, followed by N-terminal acrylation as described above, selective removal of Mtt protecting group from Lys(Mtt) residue by 5% TFA, FITC conjugation to the deprotective lysine, and finally peptide deprotection and cleavage. The cleaved peptide was also dissolved in 50% DMSO for disulfide bond formation in the dark. The resulting peptide analogs (acryl-WP9QY or acryl-(FITC)WP9QY) were diluted at least 5 times to decrease the DMSO concentration for HPLC purification. The molecular weights of the purified peptides (purity > 90%) were analyzed with matrix-assisted laser desorption/ionization – time of flight (MALDI-TOF).
2.3 FRET experiments
To examine TNFα binding to immobilized WP9QY, FRET experiments were designed in PBS, or in the presence of PEGDA macromer solutions or hydrogels. Briefly, TNFα was labeled with alexafluor 555 according to the manufacturer's protocol and used as a FRET acceptor (AF555-TNFα), while acryl-(FITC)WP9QY was used as a FRET donor. Samples for FRET measurements, including acceptor-only samples, donor-only samples, and acceptor and donor samples, were prepared in a 96-well plate. The fluorescence of all the samples was quantified in a microplate reader with three filter sets, including donor (Ex: 485nm, Em: 535nm), acceptor (Ex: 560nm, Em: 595nm), and FRET (Ex: 485nm, Em: 595nm) filters. The obtained fluorescence signals (in PBS or PEGDA solution before and after photopolymerization) were calibrated as described in a previous report [24]. The calibrated FRET for each acceptor concentration was then normalized by the FRET in the absence of acceptor (donor-only) to obtain normalized FRET (see Supporting information).
2.4 PEG-WP9QY hydrogel synthesis and characterization
To form WP9QY functionalized PEG hydrogels, PEGDA (final concentration: 10 wt%), selected concentration of acryl-WP9QY, and photoinitiator I-2959 (0.025 wt% Irgacure-2959, Ciba chemical) were dissolved in PBS and sterile-filtered through a 0.2 μm filter. The macromer solutions were exposed to UV light (365 nm, 8 mW/cm2) for 10 minutes to obtain PEG-WP9QY hydrogels (hydrogel dimension: 5mm dia. × 1mm thick). Dose-dependent incorporation of WP9QY peptide in PEG hydrogels was visualized using a Zeiss LSM 5 Pascal confocal microscope. hTNFα absorption in hydrogels was accomplished via incubating pre-swelled PEG-WP9QY hydrogels (gel volume: 30μL) in 1mL of recombinant hTNFα solution (50ng/mL). The concentrations of hTNFα in the supernatants were quantified with hTNFα ELISA kit (Peprotech) and the differences between the initial amount and the remaining amount in the supernatants were calculated as TNFα absorption in the gels.
2.5 Degree of equilibrium binding of TNFα and WP9QY
The equilibrium binding between TNFα and its affinity peptide, WP9QY, was determined by the following equations:
Here, CT and CW are respectively the total concentration of TNFα and WP9QY peptide in the gel, and x is the equilibrium concentration of bound complex determined by:
Thus, the bound TNFα concentration is [x], while the free TNFα concentration is [CT-x].
2.6 Cell culture and encapsulation
PC12 cells were maintained in RPMI1640 media containing 15% horse serum, 5% fetal bovine serum, 1% penicillin-streptomycin, and 0.5μg/mL fungizone at 37°C in humid conditions with 5% CO2. For encapsulation, PC12 cells were trypsinized from the flask and mixed with 10wt% PEGDA macromer solution containing 0.025wt% I-2959 and required amount of the acryl-WP9QY peptide. The cell adhesive peptide CGRGDS (2mM) was also incorporated to support cell survival in the PEG gels. Photo-encapsulation was achieved via exposing the cell-containing macromer solutions (40μL/gel, 150,000 cells/gel) under UV similar to the photopolymerization process described above. After encapsulation, the cell-laden gels were maintained in PC12 differentiation media containing 1% horse serum and 50ng/mL 2.5S nerve growth factor (NGF, Invitrogen) for 6 days for neuronal differentiation. Isolated mouse islets (adult Balb/c mice, obtained from the Diabetes and Endocrinology Research Center at the Barbara Davis Center for Childhood Diabetes, Denver, CO) were suspended in 10 wt% PEGDA macromer solutions containing the desired amount of acryl-WP9QY and 0.025 wt% I-2959. The islet-macromer solutions containing approximately 20 islets each (30μL) were injected in a perfusion chamber mold (9 mm dia. × 0.5 mm thick, Grace Bio-Labs, Bend, OR) and photopolymerized under UV exposure for 10 minutes. Encapsulated islets were maintained in RMPI 1640 media supplemented with 10 % FBS, 1% penicillin-streptomycin, and 0.5 μg/mL fungizone. Culture media was changed every other day. hMSCs (Cambrex) were maintained in growth media (low-glucose DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 0.5 μg/mL fungizone, and 1ng/mL bFGF) and passage twice every week. All hMSCs used in encapsulations (similar procedure as described above; 150,000 cells/gel) were from passage 3 and maintained in osteogenic differentiation media (high-glucose DMEM containing 10% FBS, 1% penicillin-streptomycin, and 0.5 μg/mL fungizone, 0.01 μM Dexamethasone, 0.2mM ascorbic acid 2-phosphate, 10mM glycerol 2- phosphate).
2.7 TNFα challenge and caspase 3/7 activity measurement
PEG hydrogels and PEG-WP9QY hydrogels containing cells were exposed to recombinant human TNF-α (50 ng/mL) for 24 hours. After which, the cell-laden gels were transferred to 0.6 mL of fresh media containing 10% of AlamarBlue regent (AbD Serotec) and incubated for 3 hours. 200 μL of the solution were transferred to a 96-well clear plate, and the AlamarBlue fluorescence (Ex: 560nm; Em: 590nm) signals were measured to determine the relative cell number in each gel. The gels were then transferred to fresh media containing 50% CaspasGlo 3/7 reagent (Promega) and incubated for 1 hour at room temperature on an orbital shaker. 200 μL of the solutions were transferred to a white-wall 96-well plate for luminescence measurements in a microplate reader. The obtained luminescence signals were normalized to the previously obtained AlamaBlue fluorescence for the respective gel samples and expressed as relative caspase 3/7 activity. Encapsulated PC12 cells with or without TNFα challenge were stained with a Live/Dead staining kit (Invitrogen) and imaged with confocal microscopy.
2.8 Insulin secretion from encapsulated islets
Gel samples encapsulated with islets were exposed to glucose solutions for insulin secretion. Briefly, encapsulated islets were first incubated in a Kreb-Ringer buffer solution containing 0.2 g/L glucose for 45min, followed by incubation in a 4.5 g/L glucose buffer solution for 1 hour. Buffer solutions containing secreted insulin were collected and stored at -80°C until the quantification of insulin concentration by a mouse/rat insulin ELISA kit. ATP production (CellTiter-Glo cell viability kit) was used as an indication of the metabolic activity of the encapsulated islets.
2.9 Alkaline phosphatase (ALP) activity assay
Before measuring the ALP activity, hMSC-laden gel samples were incubated in media containing 10% AlamarBlue reagent for 3 hours to determine the relative cell numbers. Then, the gel samples were washed extensively in PBS to remove residual culture medium and manually homogenized in CelLytic M cell lysis buffer (Sigma). The supernatants were collected and reacted with equal volume of alkaline phosphatase substrate solution (Sigma). The absorbance at 405nm was recorded continuously at a 1-min interval for 10-min. The slope of the absorbance curve was normalized to the AlamarBlue fluorescence signal for each respective gel sample to obtain a specific ALP activity. The specific ALP activity of TNFα treated cells was normalized to that of un-treated cells to obtain the normalized ALP activity.
3. Results and discussion
While it has been demonstrated that the TNFα antagonist, WP9QY, binds to TNFα and inhibits its binding of TNFR-1 [15], it is not clear whether or not WP9QY retains its affinity for binding to TNFα after conjugation to 3D PEG hydrogel networks. To examine the TNFα-binding capability of immobilized WP9QY, we synthesized a crosslinkable and FITC-labeled WP9QY peptide analog, namely acryl-(FITC)WP9QY (Fig. 1a). FRET experiments, an indication of affinity binding, were first carried out in the presence of acryl-(FITC)WP9QY peptide (donor) and AlexaFluor555-labeled TNFα (AF555-TNFα, acceptor) in PBS or PEGDA macromer solutions. As shown in Fig. 2a, an increased normalized FRET was observed as a function of acceptor (AF555-TNFα) concentration at a constant donor (acryl-(FITC)WP9QY) concentration of 500nM. The increased FRET in PBS demonstrates that the synthesized WP9QY analog retains its binding affinity to TNFα. The degree of TNFα-WP9QY binding further increased in PEGDA (both 6kDa and 10kDa) macromer solutions. This was attributed to the high exclusion volume of PEG macromer that increased the local TNFα and WP9QY concentrations, which leads to a movement of the binding equilibrium to TNFα-WP9QY complexes. Note that the increase in the normalized FRET in the presence of PEG macromers cannot be attributed to the auto-fluorescence of PEG molecules, as the FRET signals were normalized to that of PEG/donor solutions without the presence of acceptor.
Figure 1.
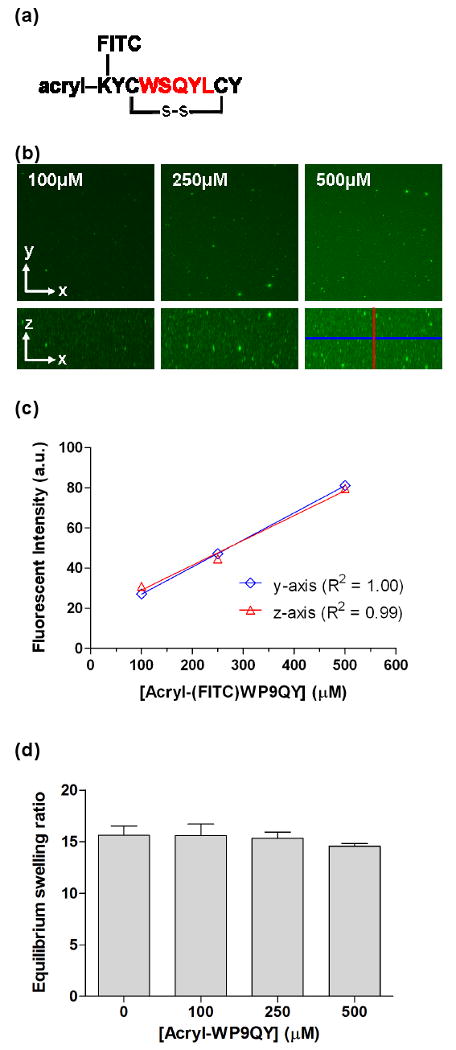
(a) Structure of a polymerizable TNFα binding peptide – WP9QY. The TNFα binding sequence WSQYL is color-coded. FITC was conjugated on an additional lysine residue for FRET analysis. (b) Representative confocal stacking images of acryl-(FITC)WP9QY peptide incorporated in PEG hydrogels. (c) Average fluorescent intensity of the cross-section (y-axis) and longitudinal-section (z-axis) in the PEG-WP9QY hydrogels. (d) Equilibrium mass swelling ratio of PEG-WP9QY hydrogels (10wt% PEGDA-10kDa). No statistical difference is observed between groups with or without the incorporation of the WP9QY peptide.
Figure 2.
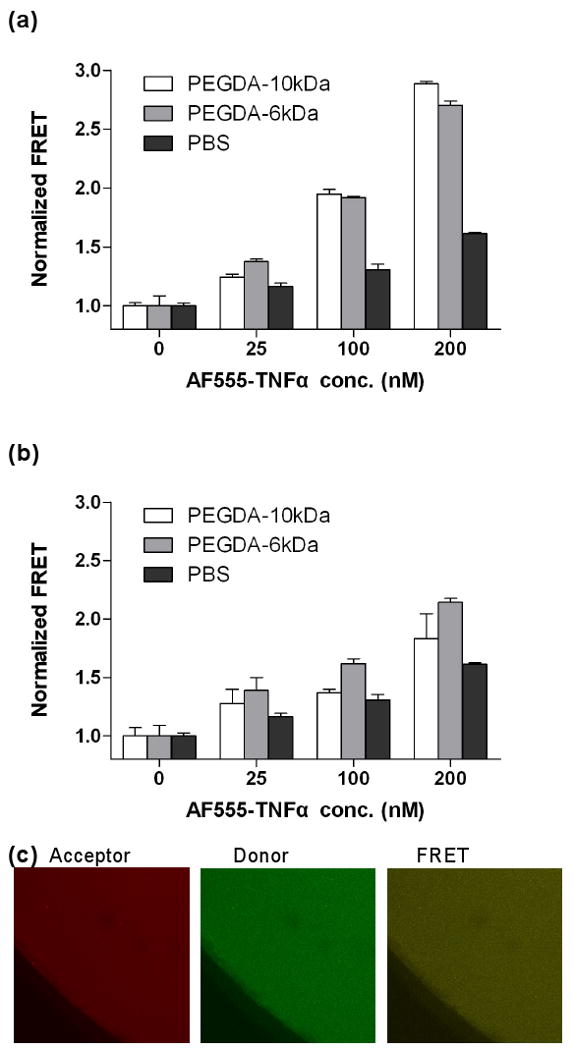
(a) FRET analysis of affinity binding between soluble acryl-(FITC)WP9QY and AlexaFluor555-TNFα in solutions of 10wt% PEGDA-6kDa, PEGDA-10kDa, or PBS. (b) FRET analysis of affinity binding between immobilized acryl-(FITC)WP9QY and soluble AlexaFluor555-TNFα in hydrogels photocrosslinked from 10wt% PEGDA-6kDa or PEGDA-10kDa. Donor concentrations in the prepolymer solutions were fixed at 500nM. (N=3, Mean ± SEM) (c) Representative confocal z-stack images of 10wt% PEG (10kDa) hydrogels copolymerized with acceptor (200nM soluble AlexFluor555-TNFα) and donor (500nM acryl-(FITC)-WP9QY peptide). Hydrogels were swelled for two days before image analysis.
To examine the binding of TNFα to immobilized peptide antagonist WP9QY, PEGDA solutions were photopolymerized in the presence of acryl-(FITC)WP9QY and soluble AF555-TNFα to form PEG-WP9QY hydrogels. As shown in Figure 1b & 1c, the incorporation of acryl-(FITC)WP9QY in PEG hydrogels can be rigorously controlled by adjusting the concentration of the functionalized peptide in the PEGDA prepolymer solutions. Further, the FRET results (Figure 2b) show that the normalized FRET of TNFα and WP9QY again increases as a function of acceptor concentration, indicating that the immobilized WP9QY retains its binding ability to soluble TNFα. It is worth noting that the extent of TNFα-WP9QY binding in PEGDA hydrogels was lower than the binding in PEGDA solutions, where both TNFα and WP9QY are soluble. In previous work [24], we demonstrated that the affinity binding of hydrogel network-immobilized peptides to soluble protein is decreased due to the reduced mobility of the immobilized peptide and the “steric hindrance” resulting from the surrounding PEG chains. Nonetheless, the binding of TNFα to immobilized WP9QY in PEG hydrogels is still higher than its binding in PBS (Figure 2b), suggesting a similar, if not higher, TNFα antagonistic effects of PEG-WP9QY hydrogels. The FITC-labeled PEG-WP9QY hydrogels, the presence of AlexaFluor555-TNFα, and the FRET phenomena were all visualized with confocal microscopy (Figure 2c).
Next, the swelling of the PEG-WP9QY hydrogels was characterized as a function of the peptide content. Hydrogel swelling is important in that it dictates the transport properties that affect the time scale of diffusion of important molecules and correlates to the survival of encapsulated cells. To verify that the incorporation of acryl-WP9QY within PEGDA hydrogels does not adversely affect the swelling and diffusional properties of PEG hydrogels, we characterized the equilibrium mass swelling ratio of, as well as insulin (MW: 5.5kDa) diffusion from, PEG-WP9QY hydrogels at physiological pH (7.4). Results indicate that both hydrogel swelling (Figure 1d) and insulin release (data not shown) are unaffected by the incorporation of the acryl-WP9QY peptide at concentrations up to 500μM.
Another factor that would significantly affect the outcome of encapsulated cell fate in the PEG-WP9QY hydrogels is the amount of absorbed TNFα in the gels. In 2D culture, the concentrations of soluble factors can be easily controlled via media supplements. However, when cells are encapsulated in 3D PEG hydrogels, the actual cytokine concentration bound within the gel cannot be assumed equal to that in the media, especially when an affinity peptide is copolymerized within PEG hydrogels to interact with the cytokine. To understand the extent of TNFα penetration/absorption in the affinity PEG-WP9QY hydrogels, we incubated gels in concentrated recombinant TNFα solutions and quantified the concentration of TNFα in the supernatant. As shown in Figure. 3a, the amount of TNFα penetrated/absorbed in PEG-WP9QY hydrogels increases with increasing incubation time, as well as increasing acryl-WP9QY concentrations. However, significant differences were only found at higher WP9QY concentrations (>250μM), indicating that the incorporation of a small quantity of WP9QY peptide (<100μM) in PEG hydrogels does not significantly affect the amount of TNFα absorption, as compared to unmodified PEG hydrogels. Even at high WP9QY peptide incorporation, such as 250μM and 500μM, the absorbed TNFα in the gels are in the bound state. Figure 3b shows the theoretical calculation of the equilibrium concentrations of bound versus free TNFα in PEG-WP9QY hydrogels. While all the TNFα absorbed into the un-modified PEG hydrogels (∼6.8ng/gel after 72hr, Figure 3a) is freely diffusible and able to bind to the TNF-R1 on the encapsulated cells, almost all TNFα is bound to the immobilized WP9QY peptide (when KD<10-5M). It is worth noting that TNFα has a low dissociation constant for TNF-R1 (KD ∼ 1.9 × 10-11 M [25]), implying a strong binding of infiltrated TNFα to the TNF receptor binding loop-derived WP9QY peptide. Although Figure 3b does not take into account the competitive binding of TNFα-TNFR1 nor does it account for TNFα proteolysis/degradation, it should be noted that the incorporated WP9QY peptides are distributed homogenously throughout the PEG gels (Figure 1b) while the encapsulated cells are discretely distributed (Figure 4c & 4d). Further, the WP9QY peptide incorporated in the PEG gels (100 – 500μM) is in large molar excess compared to TNFα level in this study (∼ 50 ng/mL or 2.87 nM) or that found in healing (∼ 0.9 ng/mL or 0.05 nM) or non-healing wound sites (∼ 2.4 ng/mL or 0.14 nM) [26]. Therefore, when the infiltrated TNFα is bound to the abundant WP9QY peptide in the gel, it is unable to interact with the encapsulated cells in the WP9QY functionalized gels.
Figure 3.
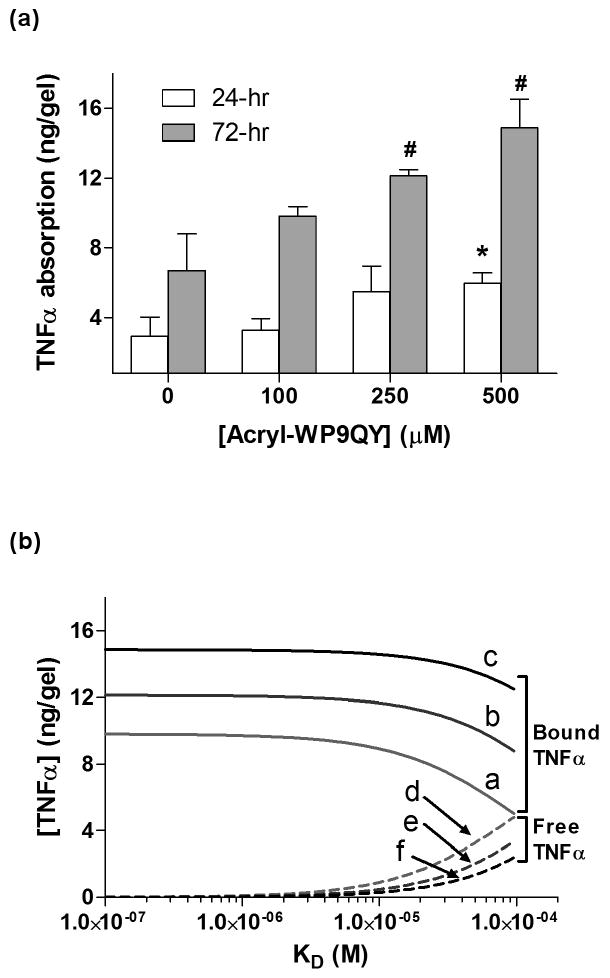
(a) TNFα absorption in PEG hydrogels incorporated with different amounts of acryl-WP9QY peptide after 24 or 72 hours. Asterisks indicate statistical significance (p<0.05) compared to the amount of TNFα absorbed in the un-modified PEG gels at each respective time point (N=3, Mean ± SEM). (b) Theoretical calculation of bound (curves a-c) versus free (curves d-f) TNFα concentration in PEG-WP9QY hydrogels as a function of the dissociation constant (KD). Curves a, d: 100μM acryl-WP9QY; Curves b, e: 250μM acryl-WP9QY; Curves c, f: 500μM acryl-WP9QY. The amounts of total TNFα absorption in the gels after 72-hr incubation (Fig. 2a) were used as the total TNFα concentration (Volume of gels: 30μL).
Figure 4.
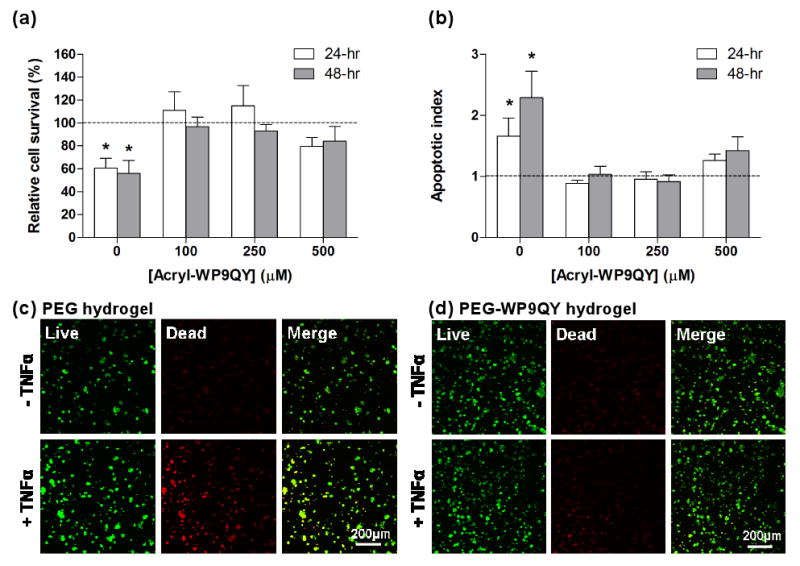
(a) Cyto-protective effects of PEG-WP9QY hydrogels to encapsulated PC12 cells under TNFα (50ng/mL) challenge. Viability of PC12s was determined with AlamarBlue regent. (b) Apoptotic index of PC12 cells encapsulated in PEG-WP9QY hydrogels. Apoptotic index was obtained by first normalizing the luminescence signals (caspase 3/7 activity) to the AlamarBlue fluorescence signals (cell survival) in respective cell-laden gel samples, then to the values obtained from cell-laden gel samples without TNFα challenge. All gels for PC12 encapsulation were incorporated with 2mM CGRGDS peptide. Dashed lines represent cell viability without TNFα challenge. Asterisks indicate statistical significance (p<0.05) compared to non-(TNFα) challenged cells in the same gel composition (N=3, Mean ± SEM). No statistical difference was found between groups of 250μM and 500μM WP9QY peptides. (c) Representative confocal microscopy images of PC12s live/dead staining in PEG hydrogels copolymerized with RGD only. (d) Representative confocal microscopy images of PC12s live/dead staining in PEG hydrogels copolymerized with RGD only.
As described above, the pro-inflammatory cytokine TNFα induces apoptosis in a wide variety of cell types. To elucidate the effects of PEG-WP9QY hydrogels in protecting encapsulated cells from TNFα-induced apoptosis, rat pheochromocytoma cells, or PC12 cells, were encapsulated in PEG hydrogels functionalized with the WP9QY peptide. A cell adhesive peptide, CGRGDS, was also immobilized in PEG gels via a thiol-acrylate photopolymerization [9, 27]. The RGD peptide functionality is required to support PC12 survival in a 3D hydrogel [28, 29]. Prior research has revealed that TNFα induces significant apoptosis in differentiated, but not naïve, PC12 cells in 2D culture [30, 31]. We observed a similar phenomenon in 3D hydrogel culture, where un-differentiated PC12 cells in PEG-RGD hydrogels remained viable and nonapoptotic, even with the challenge of TNFα (data not shown). When encapsulated PC12 cells were differentiated with nerve growth factor (2.5S NGF) for 6 days, however, an ∼40% reduction in viability of differentiated PC12 cells was observed in PEG-RGD hydrogels, as compared to the viability of un-challenged cells (Figure 4a). On the other hand, the survival of PC12 cells under TNFα challenge was not affected and maintained a viability comparable to the unchallenged cells in PEG-RGD-WP9QY hydrogels (100 and 250μM WP9QY). In addition, qualitative live/dead staining and confocal imaging results also revealed an increased number of dead cells upon TNFα challenge in un-modified PEG gels, but not in PEG-WP9QY (100μM acryl-WP9QY) gels (Figure 4c & 4d), demonstrating an important role of immobilized WP9QY peptide in protecting encapsulated cells from cytokine-induced cell death. Further examination of caspase 3/7 activation in encapsulated PC12 cells with or without TNFα challenge reveals that the reduced cell survival under TNFα challenge is mediated by an apoptotic pathway (Fig. 4b).
To further explore the efficacy of PEG-WP9QY hydrogels in promoting encapsulated cell function, we photo-encapsulated mouse pancreatic islets within PEG or PEG-WP9QY (100μM) hydrogels and studied the effects of immobilized WP9QY in protecting islet cells from apoptosis and reduced insulin secretion due to the infiltrated TNFα. Pancreatic islets have been shown to undergo apoptotic pathways when challenged with pro-inflammatory cytokines, such as TNFα [21]. Although islet encapsulation and transplantation have been suggested as a solution for curing type 1 diabetes, current encapsulation techniques cannot prevent β-cells damage induced by small pro-inflammatory cytokines. To this end, we successfully fabricated permissive PEG-WP9QY hydrogel environments that support the survival and function of photo-encapsulated pancreatic islets. As shown in Figure S1, although less potent than mouse cytokines, human cytokines do induce significant mouse β-cell apoptosis, suggesting that the PEG-WP9QY hydrogels can be used to prevent hTNFα-induced mouse β-cell apoptosis. Indeed, while mouse islets encapsulated in un-modified PEG hydrogels show an increased caspase 3/7 activity (Figure 5a), impaired cell survival (Figure 5b), and reduced insulin secretion (Figure 5c) under hTNFα challenge, no significant difference was found, with or without hTNFα challenge, when islets were encapsulated within PEG-WP9QY(100μM) functionalized hydrogels.
Figure 5.
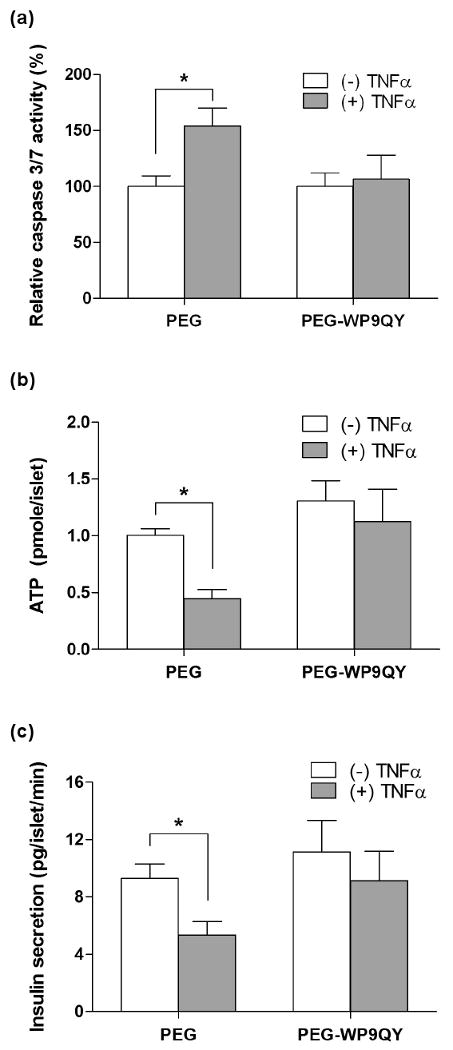
Cyto-protective effects of PEG-WP9QY hydrogels to encapsulated mouse islets under TNFα (50ng/mL) challenge. (a) Relative caspase 3/7 activity (normalized to the number of islets in the gels and then to the caspase 3/7 activity of non-treated islets) 2 days after TNFα challenge. (b) Metabolic activity (ATP concentration) of encapsulated islets under 5 days of TNFα challenge. (c) Insulin secretion in high glucose buffer (5 days of TNFα challenge). Asterisks represent statistical significance (p<0.05) between the indicated groups (N=3, Mean ± SEM).
The use of hydrogels to encapsulate human mesenchymal stem cells (hMSCs) has proven valuable in providing a 3D environment for supporting their osteogenic [1, 10-13], chondrogenic [9], and adipogenic [32] differentiation to restore damaged tissues. However, one question remaining to be answered is that, in the highly inflammatory environments of the wound sites, can the encapsulated hMSCs still undergo their desired differentiation pathways in the presence of pro-inflammatory cytokines? In 2D culture, several publications have revealed the molecular mechanisms of the inhibitory effects of TNFα on osteogenic differentiation of hMSCs [33-35]. In an attempt to promote 3D hMSCs osteogenic differentiation under TNFα challenge, we photo-encapsulated hMSC in PEG-RGD (3mM CGRGDS) or PEG-RGD-WP9QY (100μM) hydrogels and studied the effects of the infiltrated TNFα on the survival and osteogenic differentiation potential of hMSCs. Our results show that, similar to PC12 (Figure 4b) and islet (Figure 5a) encapsulation, encapsulated hMSCs exhibit higher caspase 3/7 activity under TNFα challenge in PEG-RGD hydrogels, but not in PEG-RGD-WP9QY hydrogels (Figure 6a). Differing from the decreased cell survival in PC12 cells and islets under TNFα challenge, however, is that hMSCs encapsulated in PEG-RGD hydrogels are more proliferative than in PEG-RGD-WP9QY hydrogels in the presence of TNFα. This result is in accordance with a recent report that TNFα induces hMSC proliferation [36]. We verified the proliferative effects of TNFα on hMSC in 2D culture (Figure S3a), as well as in 3D hydrogels (Figure 6b). Since proliferative hMSCs have less differentiation potential and the treatment of TNFα increases proliferation of hMSCs [36], a less osteogenic differentiation might be expected under TNFα challenge. As shown in Figure S3b, when challenged with TNFα in 2D culture, hMSCs show less (on a per cell basis) alkaline phosphatase (ALP) activity, which demonstrates the potential effects of TNFα in inhibiting hMSC osteogenic differentiation. Finally, we show that the use of PEG-RGD-WP9QY functionalized hydrogels successfully prevents the inhibitory effects of TNFα on the osteogenic differentiation of encapsulated hMSCs in a 3D hydrogel culture. As illustrated in Figure 6c, the relative ALP activity of hMSCs encapsulated in PEG-RGD-WP9QY gels is maintained, under the challenge of TNFα, but not in PEG-RGD hydrogels.
Figure 6.
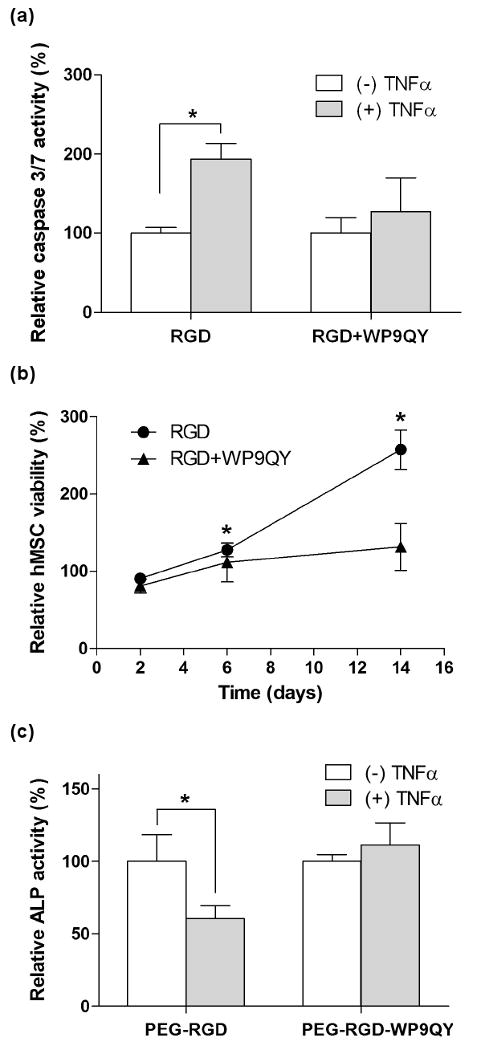
Cyto-protective effects of PEG-WP9QY hydrogels to encapsulated hMSCs under TNFα (50ng/mL) challenge. (a) Normalized caspase 3/7 activity. (b) Relative encapsulated hMSCs proliferation (with or without TNFα challenge) in PEG-RGD (3mM CGRGDS) or PEG-RGD-WP9QY (100μM acryl-WP9QY) hydrogels. Asterisks represent statistical significance (p<0.05) to the non treated groups with the same gel composition. (c) Relative ALP activity of encapsulated hMSCs. Asterisks represent statistical significance (p<0.05) between the indicated groups (N=3, Mean ± SEM).
We show that PEG-WP9QY hydrogels antagonize TNFα for two weeks (Figure 6b). However, one concern remains regarding the long-term cytokine antagonistic effect of the hydrogels. It should be noted that at sufficiently high peptide concentrations, the release of bound cytokine from immobilized peptide is minimal [24]. It is also likely that the bound cytokine will denature after long-term binding to the immobilized peptide. This study opens an avenue for fabricating functional biomaterials capable of interacting with small cytokines to improve the survival and function of encapsulated cells for in vivo tissue engineering and regenerative medicine applications. The therapeutic efficacy of these cytokine-antagonizing PEG hydrogels can be further expanded through immobilizing other, synergistic peptidomimetic antagonists for additional cytotoxic cytokines, such as interleukin-1β (IL-1β) and interferon γ (IFN- γ). Such peptide-functionalized hydrogel platforms might also serve as an interactive affinity depot, loaded with therapeutic agents, to recruit cells for promoting tissue regeneration.
4. Conclusions
In summary, we synthesized WP9QY-functionalized, TNFα-antagonizing PEG hydrogels capable of sequestering cytotoxic TNFα and prolonging the survival and function of encapsulated PC12 cells and mouse islets. Furthermore, WP9QY-functionalized PEG hydrogels not only prevented TNFα-induced proliferation of hMSCs, but also maintained their osteogenic differentiation potential in 3D hydrogel culture. This study offers a new approach in the fabrication of immuno-isolating hydrogels for cell-based therapies and should find useful applications in in vivo tissue engineering applications.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (5R01DK076084), the Howard Hughes Medical Institute, and the Juvenile Diabetes Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, Anseth KS. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Progress in Polymer Science. 2008;33:167–179. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 3.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. Journal of Biomedical Materials Research. 1998;39:266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 5.Li RH. Materials for immunoisolated cell transplantation. Advanced Drug Delivery Reviews. 1998;33:87–109. doi: 10.1016/s0169-409x(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 6.Weber LM, He J, Bradley B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled beta-cell microenvironments. Acta Biomaterialia. 2006;2:1–8. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Weber LM, Hayda KN, Haskins K, Anseth KS. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004–3011. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Mahoney MJ, Anseth KS. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27:2265–2274. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Salinas CN, Cole BB, Kasko AM, Anseth KS. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Engineering. 2007;13:1025–1034. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 10.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biology. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Nuttelman CR, Tripodi MC, Anseth KS. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. Journal of Biomedical Materials Research Part A. 2006;76A:183–195. doi: 10.1002/jbm.a.30537. [DOI] [PubMed] [Google Scholar]
- 12.Nuttelman CR, Benoit DSW, Tripodi MC, Anseth KS. The effect of ethylene glycol methacrylate phosphate in PEG hydrogels on mineralization and viability of encapsulated hMSCs. Biomaterials. 2006;27:1377–1386. doi: 10.1016/j.biomaterials.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Benoit DSW, Collins SD, Anseth KS. Multifunctional hydrogels that promote osteogenic human mesenchymal stem cell differentiation through stimulation and sequestering of bone morphogenic protein 2. Advanced Functional Materials. 2007;17:2085–2093. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung CY, McCartney SJ, Anseth KS. Synthesis of Polymerizable Superoxide Dismutase Mimetics to Reduce Reactive Oxygen Species Damage in Transplanted Biomedical Devices. Advanced Functional Materials. 2008;18:3119–3126. [Google Scholar]
- 15.Takasaki W, Kajino Y, Kajino K, Murali R, Greene MI. Structure-based design and characterization of exocyclic peptidomimetics that inhibit TNF alpha binding to its receptor. Nature Biotechnology. 1997;15:1266–1270. doi: 10.1038/nbt1197-1266. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Kojima T, Takahashi M, Horne WC, Baron R, Amagasa T, et al. A tumor necrosis factor receptor loop peptide mimic inhibits bone destruction to the same extent as antitumor necrosis factor monoclonal antibody in murine collagen-induced arthritis. Arthritis and Rheumatism. 2007;56:1164–1174. doi: 10.1002/art.22495. [DOI] [PubMed] [Google Scholar]
- 17.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annual Review Of Immunology. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 18.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. European Journal of Biochemistry. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 19.Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s) Microscopy Research and Technique. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S. A decision between life and death during TNF-alpha-induced signaling. Journal of Clinical Immunology. 2002;22:185–194. doi: 10.1023/a:1016089607548. [DOI] [PubMed] [Google Scholar]
- 21.Eizirik DL, Mandrup-Poulsen T. A choice of death - the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 22.Dempsey PW, Doyle SE, He JQ, Cheng GH. The signaling adaptors and pathways activated by TNF superfamily. Cytokine & Growth Factor Reviews. 2003;14:193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 23.Lin CC, Metters AT. Metal-chelating affinity hydrogels for sustained protein release. Journal of Biomedical Materials Research Part A. 2007;83A:954–964. doi: 10.1002/jbm.a.31282. [DOI] [PubMed] [Google Scholar]
- 24.Lin CC, Anseth KS. Controlling Affinity Binding with Peptide-Functionalized Poly(ethylene glycol) Hydrogels. Advanced Functional Materials. 2009 doi: 10.1002/adfm.200900107. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace HJ, Stacey MC. Levels of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in chronic venous leg ulcers - Correlations to healing status. Journal of Investigative Dermatology. 1998;110:292–296. doi: 10.1046/j.1523-1747.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 27.Salinas CN, Anseth KS. Mixed mode thiol-acrylate photopolymerizations for the synthesis of PEG-peptide hydrogels. Macromolecules. 2008;41:6019–6026. [Google Scholar]
- 28.Pittier R, Sauthier F, Hubbell JA, Hall H. Neurite extension and in vitro myelination within three-dimensional modified fibrin matrices. Journal of Neurobiology. 2005;63:1–14. doi: 10.1002/neu.20116. [DOI] [PubMed] [Google Scholar]
- 29.Park KH, Yun K. Immobilization of Arg-Gly-Asp (RGD) sequence in a thermosensitive hydrogel for cell delivery using pheochromocytoma cells (PC12) Journal of Bioscience and Bioengineering. 2004;97:374–377. doi: 10.1016/S1389-1723(04)70221-2. [DOI] [PubMed] [Google Scholar]
- 30.Mielke K, Herdegen T. Fatal shift of signal transduction is an integral part of neuronal differentiation: JNKs realize TNF alpha-mediated apoptosis in neuronlike, but not naive, PC12 cells. Molecular and Cellular Neuroscience. 2002;20:211–224. doi: 10.1006/mcne.2002.1132. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Xing D, Liu L, Gao XJ, Chen MJ. TNF alpha induces apoptosis through JNK/Bax-Dependent pathway in differentiated, but not naive PC12 cells. Cell Cycle. 2007;6:1479–1486. [PubMed] [Google Scholar]
- 32.Vashi AV, Keramidaris E, Abberton KM, Morrison WA, Wilson JL, O'Connor AJ, et al. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials. 2008;29:573–579. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert LC, Rubin J, Nanes MS. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. American Journal of Physiology-Endocrinology and Metabolism. 2005;288:E1011–E1018. doi: 10.1152/ajpendo.00534.2004. [DOI] [PubMed] [Google Scholar]
- 34.Byun CH, Koh JM, Kim DK, Park SI, Lee KU, Kim GS. alpha-Lipoic acid inhibits TNF-alpha-induced apoptosis in human bone marrow stromal cells. Journal of Bone and Mineral Research. 2005;20:1125–1135. doi: 10.1359/JBMR.050302. [DOI] [PubMed] [Google Scholar]
- 35.Abbas S, Zhang YH, Clohisy JC, Abu-Amer Y. Tumor necrosis factor-alpha inhibits preosteoblast differentiation through its type-1 receptor. Cytokine. 2003;22:33–41. doi: 10.1016/s1043-4666(03)00106-6. [DOI] [PubMed] [Google Scholar]
- 36.Bocker WG, Docheva D, Prall WC, Egea V, Pappou E, Rossmann O, et al. IKK-2 is required for TNF-alpha-induced invasion and proliferation of human mesenchymal stem cells. Journal of Molecular Medicine-Jmm. 2008;86:1183–1192. doi: 10.1007/s00109-008-0378-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


