Abstract
A series of vectors has been designed to enhance the versatility of targeted homologous recombination. Recombinase-mediated cassette exchange permits sequential targeting at any locus and improves flexibility in making user-defined mutations. Application of RMCE to delete an intronic microRNA gene is described.
HOMOLOGOUS recombination-based gene targeting has greatly enhanced the repertoire of tools available for genome manipulation in Drosophila melanogaster (Rong and Golic 2000, 2001, 2003). In addition to allowing targeted deletions to make specific loss of function mutations, “ends-out” homologous recombination allows sequence replacement with modified variants (Gong and Golic 2000, 2003). Using this method, gene-specific targeting has been reported at frequencies ranging from 1/200 to 1/350,000 (Manoli et al. 2005; Jones et al. 2007). Although versatile, current vectors do not allow repeated targeting to create variant alleles at a single locus. Each variant requires starting anew. Recombinase-mediated cassette exchange (RMCE) (Bateman et al. 2006) provides a means to overcome this limitation, allowing efficient generation of new alleles on the basis of a founder allele. Here, we present a series of ends-out gene targeting vectors, including one designed for RMCE and illustrate its use by generating knockout and knockin alleles for the intronic microRNA miR-31b.
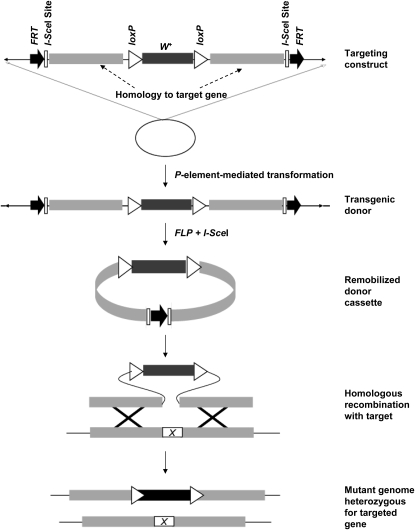
Figure 1 outlines the steps in replacement of an endogenous gene with vector encoded sequences. The original pW25 vector (Gong and Golic 2003) uses mini-white flanked by loxP sites to provide an eye-color marker, which can later be removed using Cre recombinase. To incorporate the Gal4/UAS system (Brand and Perrimon 1993) into the ends-out targeting strategy, we introduced Gal4-VP16 upstream of the 5′ loxP site in pW25 (pW25-Gal4, Figure 2B). Targeting with pW25-Gal4 produces alleles that direct GAL4 expression from the endogenous regulatory elements at the targeted locus.
Figure 1.—
Schematic representation of ends-out gene targeting by homologous recombination. Three- to 4-kb homology sequences flanking the target gene are cloned into the pW25 ends-out vector. P-element-mediated transformation gives rise to transgenic donor containing the targeting cassette. Induction of FLP recombinase is used to excise a circular DNA molecule containing the targeting vector, which is then linearized by cleavage with the I-SceI meganuclease. The linearized targeting vector can then recombine with the chromosomal target locus, replacing the endogenous gene with mini-white and generating a mutant heterozygous for the targeted gene.
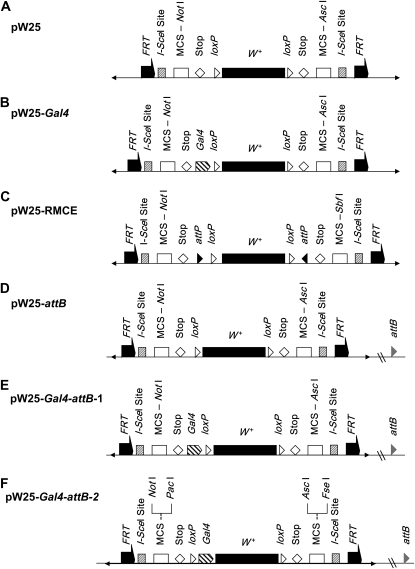
Figure 2.—
Modified ends-out gene targeting vectors. (A) pW25 vector. Arrowheads are P-element ends, half-arrows are FRT sequences, hatched boxes are I-SceI site, open boxes are cloning sites for homologous DNA sequences, diamonds are six-frame translation stop codons, and black boxes are the mini-white marker gene. (B) pW25-Gal4 vector. The Gal4 coding sequence, denoted by hatched pentagon, was cloned into the pW25 vector, between the 5′ six-frame stop codons and the loxP site. (C) pW25-RMCE vector. A 221-bp fragment for the phage attachment site (attP, denoted by black triangles,) was PCR amplified from the pTA-attP plasmid and inverted attP fragments were cloned into KpnI and AscI sites upstream and downstream of the mini-white sequence, respectively. (D and E) pW25-attB and pW25-Gal4-attB1 vectors. A 285-bp fragment containing the bacterial phage attachment site (attB, denoted by gray triangles) was cloned into both pW25 and pW25-Gal4 vector backbones at the NdeI site. (F) pW25-Gal4-attB2 vector. The Gal4 coding sequence was cloned between the 5′ loxP site and mini-white by composite cloning using pW25-attB as the vector backbone. This allows removal of the Gal4 driver together with the mini-white marker using the Cre-LoxP recombinase system. Restriction enzyme sites for PacI and FseI were introduced to the multiple cloning sites at the 5′ and 3′ end, respectively, to facilitate directional cloning of “homology arms.”
Recombinase-mediated cassette exchange:
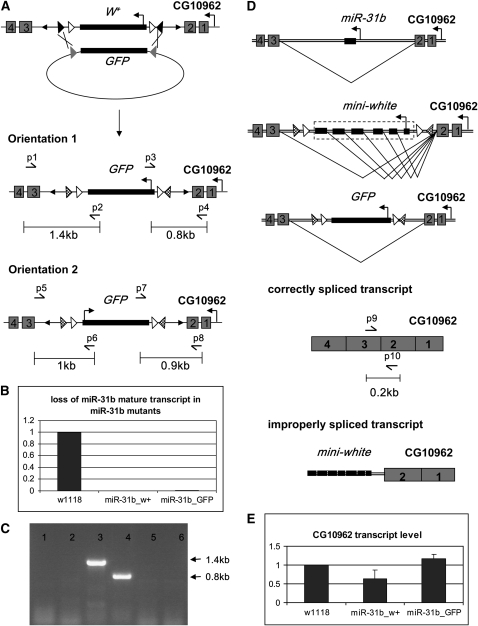
The bacteriophage ϕC31 integrase-mediated RMCE promotes directional site-specific recombination between a plasmid “donor cassette” and a chromosomal “acceptor cassette,” leading to replacement of the target cassette at frequencies of 5–20% (Bateman et al. 2006). We introduced an acceptor cassette into pW25 by flanking mini-white with inverted attP sites (pW25-RMCE; Figure 2C). Gene targeting with this vector introduces mini-white flanked by attP and loxP sites. Subsequent exchange of the acceptor cassette allows replacement of mini-white with any desired sequence. To test this system, we knocked out miR-31b. miR-31b is located in the second intron of the protein coding gene CG01962. Targeted knockout of miR-31b introduces the mini-white reporter into the intron of CG01962 (Figure 3A). Replacement was verified by mini-white expression and by loss of the mature microRNA (Figure 3B).
Figure 3.—
Site-specific integration via ϕC31 integrase-mediated RMCE. (A) Schematic representation of the targeting event replacing miR-31b with att-P and loxP flanked mini-white in the intron of CG10962. Exchange of the mini-white marker with GFP by crossover between the two inverted attP and attB sites leads to clean exchange of mini-white by GFP, resulting in a new attR site, denoted by checkered triangles. Cassette exchange can occur in both orientations. Primer pairs (arrows) were designed to distinguish the two outcomes. (B) Quantitative microRNA PCR to measure the level of miR-31b in total RNA from control male flies (w1118), males carrying the mini-white targeted allele (miR-31-w+) and males carrying the GFP replacement alllele (illustrated in C).(C) PCR genotyping of an RMCE candidate with GFP in orientation 1. Lanes 1 and 2: failure to amplify with primer pairs p5/p6 and p7/p8 indicates the absence of GFP in orientation 2. Lanes 3 and 4: the product amplified primer pairs p1/p2 and p3/p4 indicates the presence of GFP in orientation 1. Lanes 5 and 6 show the specificity of primer pairs p1/p2 and p3/p4 using initial mini-white-containing mutant genomic DNA as the template. (D) Illustration of possible splicing patterns of CG10962 in various miR-31b mutants. Upper panel: CG10962-RA has 4 exons, denoted by gray boxes 1–4. miR-31b is present in the second intron. The mini-white marker in the targeting vector contains introns and exons of the white locus and could interfere with splicing of CG10962. Replacement of mini-white with an intronless GFP cassette is illustrated. Lower panel: Primers p9/p10 amplify a product spanning the exon 2 and exon 3 junction and can be used in RT–PCR to measure the efficiency of removal of intron 2. (E) Quantitative RT–PCR using primers p9/10 (in D) showing exon 2/3 splicing of CG10962. Assays performed on total RNA from flies with genotypes as in B.
Cassette exchange was performed by injecting embryos from a cross between flies carrying the targeted miR-31b locus and flies expressing ϕC31 integrase (Bischof et al. 2007), with the piB-GFP donor plasmid, in which GFP is flanked by inverted attB sites (Bateman et al. 2006). Emerging adults were mated individually with w1118 partners. Putative RMCE events were identified by loss of the eye color marker. Molecular genotyping was carried out by PCR to determine the orientation of GFP insertion (Figure 3C). RMCE occurred in ∼25% of injected embryos in two separate trials. Fifty percent of the RMCE events resulted in the GFP reporter integrated in the same orientation as miR-31b. Expression profiling indicates low-level expression of CG01962 except at late larval and pupal stages (FlyBase). The knocked-in GFP reporter produced diffuse GFP expression at these stages. No specific spatial pattern was observed.
Use of RMCE to “cure” a gene trap:
The mini-white reporter contains introns (Klemenz et al. 1987) and so can serve as a “gene trap” when located in a host gene intron (Figure 3D). Flies homozygous for the miR-31b allele containing mini-white showed reduced levels of correctly spliced CG01962 transcript (Figure 3E). Curing the gene trap by exchange with the intronless GFP cassette restored CG01962 mRNA levels, illustrating the utility of RMCE to produce an intronic mutant with minimal disruption of the flanking locus.
The pW25-RMCE vector increases the versatility of current gene targeting strategies by allowing manipulation of a mutant genome after an initial targeting event. One useful application is genetic rescue, to validate a mutant phenotype by replacing the mutated gene with wild-type sequences. Replacement of protein-coding exons also permits direct comparison of protein sequence variants at the endogenous locus. While this work was in preparation Choi et al. (2009) reported use of RMCE for gene replacement at the atonal locus. In another variant, Huang et al. (2009) made use of single attP and loxP sites to create knockout flies that can serve as a docking site for recombinase-mediated integration for later manipulation. Both groups demonstrated the functionality of shorter attB and attP sites, while our RMCE system is faster and more efficient than the method described by Huang et al. (2009).
Other useful vectors:
The vectors discussed above rely on conventional P-element mediated transformation. Use of ϕC31-mediated site-specific transformation can improve overall efficiency. Ensuring that the initial transgenic “donor” strain is located on the desired chromosome facilitates the crossing schemes needed for targeting. To implement this modification, the attB site was cloned into the backbone of pW25 and pW25-Gal4 (Figure 2, D–F). Targeting constructs in these vectors were injected into embryos expressing ϕC31 integrase and containing an attP site at a defined location (Bischof et al. 2007). Alternatively, Gal4 was introduced in between the 5′loxP site and mini-white gene, which allows removal of Gal4 with mini-white by the Cre-loxP recombinase system, resulting in a “clean” knockout.
The series of modified gene targeting vectors presented here provides flexibility for gene targeting strategies. pW25-Gal4 and the pW25-attB series of vectors adds new options to the standard ends-out targeting vector pW25. The attB series of vectors were designed to make conventional gene targeting more efficient, which may be useful for large-scale mutagenesis studies. pW25-RMCE adds versatility to current gene targeting strategies, allowing further manipulation of a mutant genome after an initial gene targeting event.
Acknowledgments
We thank Sing-Fee Lim and Kah-Junn Tan for technical support and Thomas Sandmann for help with pW25-Gal4-attP2. This work has been supported by EU-FP6 grant “Sirocco” LSHG-CT-2006-037900 and Temasek Life Sciences Laboratory. Weng Ruifen is a recipient of a Singapore Millenium Foundation Scholarship.
References
- Bateman, J. R., A. M. Lee and C. T. Wu, 2006. Site-specific transformation of Drosophila via phiC31 integrase-mediated cassette exchange. Genetics 173: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof, J., R. K. Maeda, M. Hediger, F. Karch and K. Basler, 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104: 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Choi, C. M., S. Vilain, M. Langen, S. Van Kelst, N. De Geest et al., 2009. Conditional mutagenesis in Drosophila. Science 324: 54. [DOI] [PubMed] [Google Scholar]
- Gong, W. J., and K. G. Golic, 2003. Ends-out, or replacement, gene targeting in Drosophila. Proc. Natl. Acad. Sci. USA 100: 2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J, W. Zhou, W. Dong, A. M. Watson and Y. Hong, 2009 Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc. Natl. Acad. Sci. USA. 106: 8284–8289. [DOI] [PMC free article] [PubMed]
- Jones, W. D., P. Cayirlioglu, I. G. Kadow and L. B. Vosshall, 2007. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445: 86–90. [DOI] [PubMed] [Google Scholar]
- Klemenz, R., U. Weber and W. J. Gehring, 1987. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 15: 3947–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli, D. S., M. Foss, A. Villella, B. J. Taylor, J. C. Hall et al., 2005. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436: 395–400. [DOI] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2000. Gene targeting by homologous recombination in Drosophila. Science 288: 2013–2018. [DOI] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2001. A targeted gene knockout in Drosophila. Genetics 157: 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2003. The homologous chromosome is an effective template for the repair of mitotic DNA double-strand breaks in Drosophila. Genetics 165: 1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]