Abstract
Encapsulating pancreatic islets in a semipermeable poly(ethylene glycol) (PEG) hydrogel membrane holds potential as an immuno-isolation barrier for the treatment of type 1 diabetes mellitus. The semipermeable PEG hydrogel not only permits free diffusion of nutrients, metabolic waste, and insulin produced from the encapsulated β-cells, but also provides a size-exclusion effect to prevent direct contact of entrapped islets to host immune cells and antibodies. However, the use of unmodified PEG hydrogels for islet encapsulation is not ideal, as there is no bioactive cue to promote the long-term survival and function of the encapsulated cells. Herein, we report the synthesis and characterization of a bioactive glucagon-like peptide 1 (GLP-1) analog, namely, GLP-1-cysteine or GLP-1C, and the fabrication of functional GLP-1 immobilized PEG hydrogels via a facile thiol−acrylate photopolymerization. The immobilization of bioactive GLP-1C within PEG hydrogels is efficient and does not alter the bulk hydrogel properties. Further, the GLP-1 immobilized PEG hydrogels enhance the survival and insulin secretion of encapsulated islets. Overall, this study demonstrates a strategy to modify PEG hydrogels with bioactive peptide moieties that can significantly enhance the efficacy of islet encapsulation.
Introduction
Type 1 diabetes mellitus (DM), also known as juvenile diabetes or insulin-dependent diabetes mellitus (IDDM), is an autoimmune disease in which the insulin-producing β-cells in the pancreatic islets of Langerhans are destroyed by the patient’s autoimmunity.(1) The damaged β-cells fail to produce insulin to maintain a normal glycemia condition. Currently, administration of insulin through daily multiple injections is a standard treatment for type 1 DM. To alleviate patients’ reliance on daily insulin injections, several alternatives have been proposed, including closed-loop insulin delivery,(2) oral or nasal insulin delivery,3−5 and islet transplantation.6,7 Among these alternatives, islet transplantation to replace lost β-cells is considered a better option to free the diabetic patients from insulin dependence. Unfortunately, in conjunction with islet transplantation is life-long administration of immunosuppressants. Therefore, this approach is only executed in diabetic patients accepting other organ transplantations (e.g., kidney), because of the side effects caused by the immunosuppressants.(8) Beyond problems associated with immune rejection, the lack of long-term survival of transplanted islets and the insufficient supply of donor islets largely hinder the clinical prevalence of islet transplantation.(9) In this regard, strategies to enhance insulin secretion from islet β-cells (and hence decrease the number of islets needed for transplantation) should prove valuable in the treatment of type 1 DM.
Recently, islets encapsulated in semipermeable materials such as alginate-based microcapsules10−12 and poly(ethylene glycol) (PEG) hydrogels13−19 have been suggested as an attractive means to prevent transplanted islets from host immune destruction and to potentially decrease the needs of administrating immunosuppressants. In particular, a semipermeable PEG hydrogel barrier permits facile exchange of nutrients and waste to maintain islet survival, while preventing the infiltration of large immune cells and antibodies from recognizing the allogenic or xenogenic islet grafts. More importantly, a thin layer of PEG hydrogel does not significantly hinder the diffusion of insulin secreted by encapsulated islets. Cross-linked PEG hydrogel networks also provide a platform for facile incorporation of bioactive moieties. In vivo animal studies have revealed the therapeutic efficacy of islet-encapsulated PEG hydrogels to treat insulin deficiency.(16) Although the use of PEG hydrogels provides certain immuno-isolation to the encapsulated islets, the encapsulated islet grafts are still challenged under the sophisticated host immune response and fail to function normally in the long term. The failure of size-exclusion barriers can be attributed to multiple factors, such as small cytotoxic cytokines, reactive oxygen species (ROS), and hypoxia within the gel environments. Recent studies are therefore focused on synthesizing bioactive hydrogels, rather than unmodified PEG hydrogels, that can actively protect encapsulated islets from the damage originating from T-cells and ROS.15,18 Although intelligent, the functional moieties in these strategies do not actively interact with the encapsulated β-cells. Therefore, identifying bioactive cues that can actively protect β-cells as well as developing strategies to immobilize these bioactive cues within PEG hydrogels may enhance the long-term survival and function of encapsulated cells.
Several therapeutic agents have been proposed to increase the insulinotropic effect of pancreatic β-cells and to actively protect them from apoptosis, including glucagon-like peptide 1 (GLP-1),20−23 exendin-4 (Ex-4),20,24 and the recently discovered α1-antitrypsin.25−27 The common rationale is to increase the amount of insulin secretion and the susceptibility of islet β-cells against apoptotic signals. Intense research efforts are currently in progress to examine the beneficial effects, as well as the molecular mechanisms, of these therapeutic agents.23,24 Among these therapeutic agents, GLP-1 has been extensively studied, both on its glucose-dependent insulinotropic and antiapoptotic effects to islet β-cells.20−24 GLP-1 (7−37) is a 31-mer peptide with a bioactive site located at the N-terminus of the peptide (7His-Ala) and its inactivation is caused by the removal of the peptide’s first two bioactive residues by dipeptidyl peptidase IV (DPP-IV).22,24 Consequently, GLP-1 has an extremely short half-live of less than 2 min in vivo.(28) To extend the circulation half-life of GLP-1, several chemical modification schemes have been proposed, including PEGylation,28−30 biotinylation,31,32 amino acid substitution,(33) and N-terminal acetylation.(34) Due to its insulinotropic effect, GLP-1 has also been conjugated to polymers and physically entrapped within alginate capsules for islet encapsulation and treatment of type 1 diabetic animals.35,36 While demonstrating an important advance, this approach requires laborious modification of native GLP-1 and the reaction schemes used in these studies to immobilized GLP-1 on polymer chains was a random conjugation between carboxylates of the polymer chains and primary amines of GLP-1, including its bioactive site at the N-terminus. As a result, only a limited amount of the conjugated GLP-1 maintained bioactivity with an unmodified N-terminus.(36) Therefore, a strategy to immobilize GLP-1 in a polymer network without modifying the GLP-1 sequence chemically should be beneficial to islet encapsulations.
Here, we aimed to fabricate bioactive GLP-1 functionalized PEG hydrogels via thiol−acrylate photopolymerizations. Photopolymerized PEG hydrogels provide a platform for facile copolymerization of bioactive moieties to promote the survival and functions of the encapsulated cells. Several studies have revealed the beneficial effects of incorporating extracellular matrix (ECM) proteins in PEG hydrogels to promote the survival of and insulin secretion from β-cells.17,19 However, the copolymerization of bioactive GLP-1 within photopolymerized PEG hydrogels for promoting β-cells survival and functions remains unexplored. This contribution presents an efficient conjugation scheme, namely, thiol−acrylate photopolymerizations,37,38 to immobilize a GLP-1 analog, GLP-1-Cysteine or GLP-1C, within PEG-diacrylate (PEGDA) hydrogels. GLP-1C was synthesized with an additional cysteine residue at the C-terminus of the original GLP-1 (7−37) sequence. No additional chemical conjugation is required following peptide synthesis and purification. Further, because the immobilization of GLP-1C in PEG hydrogels was through a thiol−acrylate reaction, the resulting GLP-1 immobilized PEG hydrogels should retain the full bioactivity of GLP-1 as no conjugation/reaction occurs at the bioactive site (N-terminus) of the peptide. Results herein show that the bioactive GLP-1 immobilized PEG hydrogels not only enhance the survival of pancreatic β-cells in gels but also increases insulin secretion from islet β-cells.
Materials and Methods
Materials
Amino acids and reagents for peptide synthesis were purchased from Anaspec (San Jose, CA). Pseudoprolinedipeptide (Fmoc-Val-Ser(ΨMe,Mepro)-OH) was obtained from EMD Biosciences (Gibbstown, NJ). Mouse TNFα was obtained from Abcam (Cambridge, MA). Fluoraldehyde reagent was purchased from PIERCE (Rockford, IL). RPMI1640, fetal bovine serum, penicillin-streptomycin, and fungizone were obtained from Gibco (Carlsbad, CA). cAMP-Glo assay kit, Caspase 3/7 Glo, and CellTiter-Glo cell viability kit were acquired from Promega (Madison, WI). The mouse and rat insulin ELISA kits were purchased from Mercodia (Winston Salem, NC). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless noted otherwise.
Peptide Synthesis, Purification, and Characterization
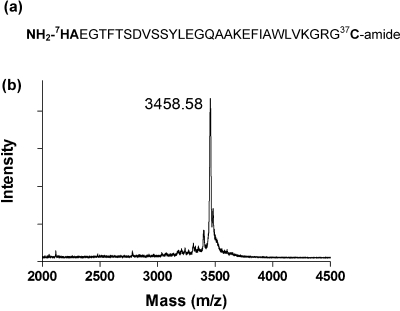
GLP-1C (7−37C, Figure 1a) with an additional cysteine residue added to the C-terminus of the GLP-1 (3−37) peptide sequence was synthesized via a solid-phase peptide synthesizer (SPPS, Applied Biosystems, Model 433A). A pseudoprolinedipeptide, Fmoc-Val-Ser(ΨMe,Mepro)-OH containing Val-Ser (16−17) residues, was used in the synthesis to facilitate the synthesis of the long peptide sequence. Synthesized GLP-1C was deprotected and cleaved from the resin for 2 h at room temperature using a cleavage cocktail, containing 5 wt % phenol in 95% TFA, 2.5% triisopropylsilane (TIPS), and 2.5% water. The cleaved peptide was precipitated in cold ether three times and dried at room temperature under vacuum. Reverse phase HPLC (RP-HPLC) was used to purify GLP-1C and the molecular weights of the collected purified fractions were characterized with MALDI-TOF mass spectroscopy. The collected fractions (with 95% purity) were lyophilized to obtain the pure GLP-1C.
Figure 1.
(a) Amino acid sequence of GLP-1C with its active center (His-Ala) located at the N-terminus of the peptide and additional cysteine residue (for thiol−acrylate photopolymerization) at the C-terminus. (b) Mass spectrum of purified GLP-1C (7−37C) determined by MALDI-TOF mass spectroscopy. Calculated/measured mass: 3458.80/3458.58 Da.
The laminin-derived cell adhesive peptide sequence IKVAV was synthesized similar to the process described above. To increase the solubility of the hydrophobic IKVAV peptide and to facilitate its copolymerization with PEGDA, pure IKVAV was conjugated with acryl-PEG-N-hydroxysuccinimide (acryl-PEG-NHS, Layson Bio. Inc.) according to the literature.(39) After reaction, acryl-PEG-IKVAV was dialyzed (MWCO: 500 Da) against dH2O for 48 h to remove unreacted peptide. The final product was lyophilized and stored at −20 °C until use.
GLP-1C Bioactivity Assays
1. cAMP Production
The bioactivity of the synthesized GLP-1C was assayed in a rat insulinoma cell line RIN-m5F acquired from American type Culture Collection (ATCC). The ability of GLP-1C to elicit intracellular elevation of cAMP level was determined by cAMP-Glo assay kit, using commercially obtained GLP-1 (Abcam Inc., Cambridge, MA) as positive controls. Briefly, RIN-m5F cells were seeded in a 96-well plate at a concentration of 50000 cells per well for 3 days. Before GLP-1C stimulation, cells were incubated in serum-free RPMI for overnight. Cells were washed with HBSS (Hank’s balanced salt solution, Invitrogen) and incubated in serum-free media containing 500 μM IBMX (3-isobutyl-1-methylxanthine) for 15 min to inhibit the enzymatic degradation of intracellular cAMP. Cells were then incubated in serum-free culture media containing different concentrations of GLP-1 or GLP-1C for 10 min. Equal volume (20 μL) of cell lysis buffer was added and incubated for another 15 min on an orbital shaker at room temperature. The concentrations of cAMP were quantified according to the manufacturer’s protocol.
2. Insulin Secretion
Insulin secretion from in vitro culture of RIN-m5F cells was used to examine the bioactivity of synthesized GLP-1C. Briefly, RIN-m5F cells (250k cells/well) were seeded in a 24-well plate and culture for 3 days before glucose and GLP-1C/GLP-1 stimulation. Cells were washed with HBSS and incubated in 1 mL of Kreb-Ringer buffer solution containing 0.2 g/L glucose for 45 min, followed by incubation in a Kreb-Ringer buffer solution containing 4.5 g/L glucose buffer for 1 h. Insulin concentrations in the high glucose buffer solutions were measured by a rat insulin ELISA kit according to manufacturer’s instruction.
GLP-1 Immobilized PEG Hydrogel Synthesis and Characterization
PEGDA was synthesized by reacting 10 kDa PEG with acryloyl chloride in toluene as described elsewhere.40,41 PEGDA (final concentration: 10 wt %), required amount of GLP-1C, and photoinitiator I-2959 (0.025 wt % Irgacure-2959, Ciba chemical) were dissolved in PBS and sterile-filtered through a 0.2 μm filter. The macromer solutions were exposed to UV light (365 nm, 8 mW/cm2) for 10 min to obtain GLP-1 functionalized PEG hydrogels. To quantify the GLP-1C incorporation efficiency, PEG hydrogels containing predetermined concentrations of GLP-1C (5 mm diameter × 1 mm thick) were fabricated as described and incubated in PBS at room temperature for 48 h. After which, the supernatants containing leached-out GLP-1C were collected and their concentrations determined by fluoraldehyde reagent according to manufacturer’s protocol. PEG-only hydrogels encapsulated with commercially available GLP-1 (obtained from Abcam) were used as a control. Equilibrium mass swelling ratios were measured gravimetrically and presented as a ratio of the hydrogel swollen weight to the dried weight. The diffusion property of GLP-1 functionalized PEG hydrogels was determined by measuring the release of bovine insulin from hydrogels. Briefly, PEG hydrogels and GLP-1 functionalized PEG hydrogels containing 100 μM or 200 μM GLP-1C were fabricated and incubated in 1 mL bovine insulin (2 mg/mL) solution for 72 h. Hydrogels containing bovine insulin were transferred to fresh PBS solutions to characterize insulin release. At predetermined time intervals, gels were transferred to an equal volume of fresh PBS and the collected samples were stored at −80 °C until protein quantification. Released insulin concentrations were determined by a fluorescamine assay.(42) The release rates of insulin were not affected by the incorporation of GLP-1 within permissive PEG hydrogels (data not shown).
Encapsulation of Rat Insulinoma Cells RIN-m5F and Mouse Islets
For encapsulation of RIN-m5F cells in PEG hydrogels, 40 μL of trypsinized RIN-m5F cells at a final density of 5 × 106 cells/mL were suspended in 10 wt % PEGDA macromer solutions containing 0.025 wt % photoinitiator I-2959 and photopolymerized for 10 min. Perpolymer solutions containing dispersed RIN-m5F β-cells were cast inside the tip of a 1 mL syringe with its tip cutoff. The following four groups of hydrogels were prepared for RIN-m5F encapsulation: (1) PEG-only hydrogels; (2) PEG hydrogels copolymerized with 2.5 mM of laminin-derived cell adhesive peptide IKVAV; (3) PEG hydrogels copolymerized with 10 μM GLP-1C; and (4) PEGDA hydrogels copolymerized with 2.5 mM acryl-PEG-IKVAV and 10 μM GLP-1C. IKVAV peptide was used to enhance the survival of encapsulated dispersed β-cells. Encapsulated cells were maintained in RPMI1640 medium supplemented with10% FBS, 1% penicillin-streptomycin, and 0.5 μg/mL fungizone and placed on an orbital shaker (40 rpm).
Mouse islets were isolated from adult Balb/c mice in the Diabetes and Endocrinology Research Center at the Barbara Davis Center for Childhood Diabetes (Denver, CO). The isolated islets were digested by type V collagenase and were purified on a Histopaque density gradient. Isolated islets were encapsulated in PEG hydrogels with or without GLP-1C immobilization. Briefly, islets were suspended in 10 wt % PEGDA (10 kDa) solutions containing required amount of GLP-1C (final concentration in the prepoplymer solution: 0 or 10 μM) and 0.02 wt % photoinitiator I-2959. The islet-macromer solutions, containing approximately 20 islets per aliquot (30 μL), were injected in a perfusion chamber mold (9 mm diameter × 0.5 mm thick, Grace Bio-Laboratories, Bend, OR) and photopolymerized for 10 min. Note that a IKVAV peptide is not required in the encapsulation of whole islets. Encapsulated islets were cultured in RMPI 1640 culture media on an orbital shaker. Culture media was changed every other day.
Encapsulated β-Cell Viability and Apoptosis Assay
Viability of the encapsulated β-cells was measured by either CellTiter-Glo reagent or AlamarBlue reagent. CellTiter-Glo reagent quantified intracellular ATP concentration after cell lysis, while AlamarBlue reagent measures the metabolic activity of viable cells. For the AlmarBlue assay, 500 μL of culture media containing 10% of the AlamarBlue reagent was added into culture media containing cell-laden gels for 4 h. The fluorescent signals (Ex: 560 nm, Em: 590 nm) of the AlamarBlue are proportional to the number of viable cells. For CellTiter-Glo assay, cell-laden gels were transferred to a 48-well plate containing 300 μL of fresh culture media containing 50% of CellTiter-Glo reagent and incubated at room temperature on an orbital shaker for 1 h. After incubation, 200 μL of the solutions were transferred to a white-wall 96-well plate for luminescence measurements in a microplate reader. To quantify the degree of cell apoptosis, the activity of caspase 3 was measured using Caspase 3/7 Glo reagent with a procedure similar to the CellTiter-Glo assay described above. The obtained caspase 3 activity was normalized to the fluorescence signal obtained from AlamarBlue viability test in the respective gel sample. For these experiments, PEG hydrogels without the incorporation of GLP-1C were used as controls.
Live/Dead Staining and Confocal Imaging
Cell-laden gels were incubated in Live/Dead viability staining reagent (Invitrogen, Eugene, OR.) containing green fluorescent dye calcein AM (stains live cells) and red fluorescent dye ethidium homodimer-1 (stains dead cells) at room temperature for 1 h on an orbital shaker. Stained cells were imaged using a Zeiss LSM 5 Pascal confocal microscope. A series of z-section images (total thickness: 500 μm) at 10 μm intervals were projected into single plane images.
Insulin Secretion from Encapsulated Islets
The encapsulated mouse islets were exposed to glucose solutions, and insulin secretion was subsequently monitored. Briefly, islets encapsulated in gel samples were first placed in a Kreb-Ringer buffer solution containing 0.2 g/L glucose for 45 min, followed by incubation in a Kreb-Ringer buffer solution containing 4.5 g/L glucose buffer for 1 h. Samples were collected and stored at −80 °C until assay. The insulin concentrations in the high glucose buffer solutions were measured by a mouse insulin ELISA kit according to manufacturer’s instruction.
Statistical Analysis
All results are presented as mean ± SEM. Statistical significance was performed using a two-tailed, unpaired Student’s t-test. Differences between groups were considered statistically significant when p value was less than 0.05.
Results and Discussion
GLP-1C Synthesis and Characterization
The applications of GLP-1 to enhance insulinotropic and antiapoptotic effects to pancreatic β-cells are well-documented.20,23,24 However, to effectively immobilize GLP-1 within hydrogels without compromising its bioactivity remains challenging with conventional conjugation schemes that involve reactions with primary amines.35,36 Because the bioactive site of GLP-1 is located at its N-terminus, random reactions on primary amines result in the loss of the peptide bioactivity due to the conjugation to the N-terminal primary amine. Alternatively, selective protection−deprotection strategies can be used to achieve site-specific conjugations, such that no reactions occur at the N-terminus of GLP-1.(43) These strategies, however, require multiple reactions and purification processes. Previously, thiol−acrylate photopolymerizations have been used to immobilize a variety of bioactive peptides through the copolymerization of an additional cysteine residue on the oligopeptides with acrylate (or ene) functionalized PEGs.37,38 This conjugation scheme is particular useful in immobilizing GLP-1 within PEG hydrogels, as GLP-1 contains no cysteine residue. Thus, the conjugation/immobilization can be designed to occur selectively between the additional cysteine residues at the peptides’ C-terminus with unsaturated acrylates on PEGDA macromers, leaving all the primary amines on the GLP-1 sequence intact. To achieve this, we synthesized a GLP-1 analog containing an extra cysteine residue at the C-terminus of GLP-1 (7−37), or GLP-1C (Figure 1a), using standard Fmoc chemistry in a peptide synthesizer. After peptide synthesis and cleavage, GLP-1C was purified by RP-HPLC and its molecular weight characterized by MALDI-TOF mass spectroscopy. As shown in Figure 1b, we successfully synthesized and purified GLP-1C with high purity (<95%, after HPLC purification) and accuracy (calculated/measured M.W.: 3458.80/3458.58).
Bioactivity of GLP-1C
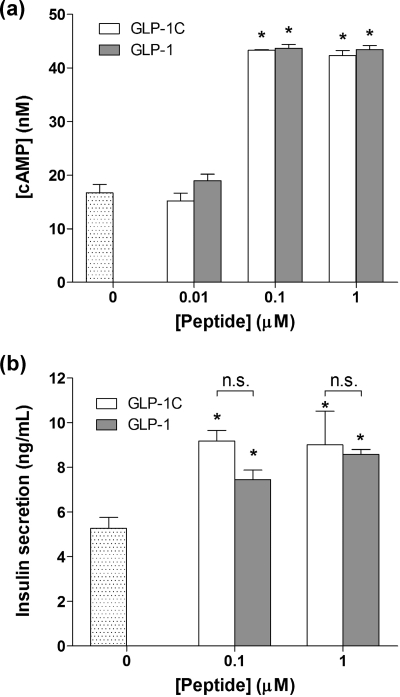
The bioactivity of the synthesized GLP-1C was determined using a rat insulinoma cell line, RIN-m5F. The mechanisms by which GLP-1-receptor binding augment glucose-dependent insulin secretion are well-documented.23,44 The insulinotropic effect of GLP-1 (or GLP-1C) is attributed to the intracellular signaling events triggered by the binding of GLP-1 to its cell surface receptors, GLP-1R. This binding causes the elevation of intracellular cyclic AMP (cAMP) concentration. To examine whether the synthesized GLP-1C elicits GLP-1R binding, we quantified cAMP concentrations in an in vitro RIN-m5F cell culture. Figure 2a shows that the intracellular cAMP concentration increases about 2.4-fold when RIN-m5F cells were incubated in serum-free media containing 0.1 μM of GLP-1C, indicating that GLP-1C binds to GLP-1R and elicits intracellular signaling. Control experiments using commercially available GLP-1 show a similar response.
Figure 2.
Bioactivity of synthesized GLP-1C. (a) Intracellular cAMP production in RIN-m5F cells upon treatment of GLP-1C or GLP-1 (mean ± SEM, n = 4). Asterisks indicate statistical significance compared to the nontreated group (*p < 0.05). (b) In vitro insulin secretion from RIN-m5F cells cultured in Kreb-Ringer buffer solution containing 4.5 g/L glucose with GLP-1C or GLP-1 (mean ± SEM, n = 4). Asterisks indicate statistical significance compared to the nontreated group (*p < 0.05).
GLP-1 has also been shown to increase insulin secretion at high glucose concentrations.(23) To examine whether the synthesized GLP-1C exerts similar insulinotropic effects on pancreatic β-cells, we incubated RIN-m5F cells in buffer solutions containing GLP-1C or GLP-1. As shown in Figure 2b, a 1.8-fold and 1.5-fold increase in insulin secretion were obtained when cells were treated with 0.1 μM GLP-1C and GLP-1, respectively. Taken together, the enhanced intracellular cAMP production and increased insulin secretion demonstrate that the synthesized GLP-1C preserves bioactivity for pancreatic β-cells, to a degree comparable to GLP-1.
GLP-1C Immobilized PEG Hydrogel Fabrication and Characterization
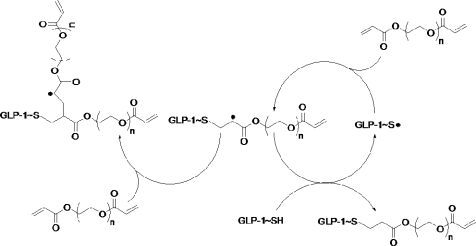
Thiol−acrylate photopolymerization is a facile way of incorporating thiol-containing molecules, such as cysteine-containing bioactive peptides, within PEGDA hydrogels for enhancing the bioactivity of the otherwise inert PEG-based hydrogels (Scheme 1). GLP-1 is an ideal candidate for this type of incorporation because of its insulinotropic effect and that it does not contain any native cysteine functionality in its sequence (Figure 1a). Unlike conventional conjugation schemes that usually involve the reactions on the primary amines of GLP-1 (including N-terminal bioactive site and two lysine residues), the conjugation of GLP-1C in PEG hydrogels through thiol−acrylate photopolymerization does not involve the reaction on the primary amines. Therefore, the bioactive N-terminus of GLP-1 should remain unmodified during polymerization and preserve full bioactivity. Furthermore, after the peptide is synthesized and purified, no additional conjugation is required to render the peptide cross-linkable. A peptide release/leaching experiment was conducted to reveal the efficiency of peptide incorporation in PEG hydrogels via a thiol−acrylate photopolymerization. While almost all of the GLP-1 loaded in the PEG gels readily diffused out of the gels within 2 h, about 80% of the incorporated GLP-1C remained in the cross-linked PEG hydrogels (Table 1).
Scheme 1. Mechanism of Thiol−Acrylate Photopolymerization with −SH Groups from GLP-1C and Acrylate Groups from PEGDA.
Reaction is shown only on one side of the PEGDA.
Table 1. Characterization of Hydrogel Swelling and GLP-1C Incorporation.
| peptide leaching (%) |
|||||
|---|---|---|---|---|---|
| [GLP-1C] (μM) | Qma | 1 h | 2 h | 4 h | 24 hb |
| 0 | 15.4 ± 0.59 | ||||
| 100 | 15.1 ± 0.56 | 12.8 ± 0.64 | 16.2 ± 1.43 | 16.9 ± 1.17 | 18.4 ± 0.29 |
| 200 | 14.8 ± 0.75 | 16.5 ± 1.53 | 23.2 ± 4.39 | 20.7 ± 1.32 | 22.4 ± 1.13 |
| 100 μM GLP-1 | 16.0 ± 1.45 | 79.3 ± 6.09 | 97.2 ± 4.49 | 101 ± 0.17 | n.d. |
Qm = swollen gel wt/dried polymer wt.
No further GLP-1C leaching was detected after 24 h.
When introducing bioactive peptides within hydrogels through covalent linkages, one critical consideration is that the incorporation of these peptides should not negatively affect the bulk properties of the hydrogels. Swelling, for example, is particularly important as it dictates many physical properties of PEG hydrogels, such as mechanical properties, degradability, and diffusivity of molecules in the cross-linked network. For islet encapsulation, the degree of hydrogel swelling will affect the rate of insulin diffusion, as well as nutrient−waste exchange that is critical for maintaining islet survival in a gel environment. It was found that the incorporation of GLP-1C within PEG hydrogels does not alter the equilibrium mass swelling ratio (Table 1) of PEG hydrogels and the release insulin from cell-laden gels (data not shown), suggesting that the diffusion of small molecules (e.g., glucose and insulin) in GLP-1C modified PEG hydrogels was not affected. Furthermore, the geometry of the photopolymerized PEG hydrogels can be easily manipulated through facile molding to fulfill specific needs for other applications. Because the copolymerization of thiol-containing peptide with PEGDA is completed in a single step, this strategy is also compatible with other islet encapsulation techniques, such as the formation of microspheres and conformal coatings.
Enhanced β-Cells Survival in GLP-1C Immobilized Hydrogels
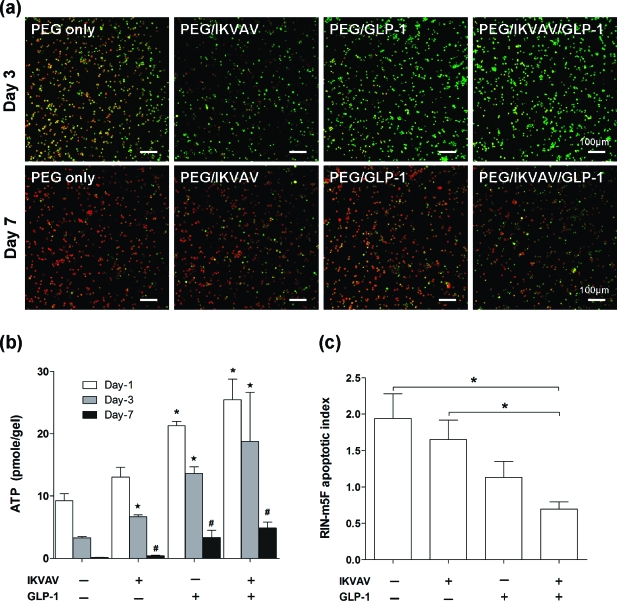
To assess the ability of GLP-1C immobilized PEG hydrogels to promote pancreatic β-cells survival, we first encapsulated dispersed RIN-m5F cells in one of the following four PEG gel formulations: (1) unmodified PEG hydrogels; (2) PEG hydrogels copolymerized with IKVAV; (3) PEG hydrogels copolymerized with GLP-1C; and (4) PEG hydrogels copolymerized with both IKVAV and GLP-1C. It is known that the survival of pancreatic β-cells requires either cell−cell or cell−matrix interactions.17,19 A laminin-derived IKVAV peptide was used in this study, because it has been shown previously by our group that the copolymerization of this peptide in PEG hydrogels enhances the survival and function of dispersed pancreatic β-cells (without cell−cell contact) in 3D hydrogel environments.(17) To visualize cell survival in PEG-based hydrogels qualitatively, we first stained the encapsulated dispersed RIN-m5F cells with a live/dead staining kit and imaged the encapsulated cells with confocal microscopy (Figure 3a). Quantitatively, we also assayed the viability of RIN-m5F cells encapsulated in PEG-based hydrogels using an AlamarBlue reagent as well as a CellTiter-Glo reagent. AlamarBlue reagent reduces the nonfluorescent indicator dye, resazurin, into red-fluorescent resorufin by metabolically active cells, while CellTiter-Glo reagent measures the ATP production in cells. As shown in Figure 3a,b, the viability of dispersed RIN-m5F cells encapsulated in unmodified PEG hydrogels decreased rapidly over time and almost no viable cells were detected after 7 days in gels (Figure 3a, PEG-only panel). This result verifies the importance of cell−cell and cell−matrix interactions in supporting pancreatic β-cells survival in synthetic biomaterial environment. Figure 3a also shows that while copolymerizing IKVAV slightly enhances the viability of RIN-m5F cells, the immobilization of a small quantity of GLP-1 (10 μM) within PEG hydrogels greatly increases the viability of encapsulated RIN-m5F cells, suggesting that the pro-survival effect of GLP-1 may be superior to the laminin-derived IKVAV peptide. Further, when RIN-m5F cells were encapsulated in IKVAV/GLP-1C copolymerized PEG gels, significantly more viable cells, compared to PEG-only, PEG/IKVAV, and PEG/GLP-1C gels, are seen after a 7 day 3D culture (Figure 3a, PEG/ILVAV/GLP-1C panel). Quantitative assessments of cell viability agree with the qualitative observations (Figure 3b). Although the incorporation of GLP-1C in PEG hydrogels only delays, but not prevents, the death of dispersed β-cells in PEG hydrogels, this is the first example of using incretin GLP-1 to promote the survival of dispersed β-cells. Mechanistically, the binding of IKVAV to laminin receptors on cell surface activates mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway and promotes the survival of anchorage-dependent cells. Recently, studies also suggest that the binding of GLP-1 to its trans-membrane G protein-coupled receptor (GLP-1R) activates the MAPK/ERK pathway.(45) From our RIN-m5F cell encapsulation results, it is possible that the binding of immobilized GLP-1 to GLP-1R activates the signaling of MAPK/ERK pathway and rescues dispersed β-cells from apoptosis induced by the deprivation of cell−cell/cell-matrix interactions. However, further investigations on the intracellular signaling pathway are needed to reveal the molecular mechanisms related to GLP-1R signaling and cell survival in 3D hydrogels.
Figure 3.
(a) Representative confocal z-section stacking images of encapsulated RIN-m5F cells at 3 days and 7 days. Cells were stained with Live/Dead cell viability kit (scale bar = 100 μm). (b) Quantification of encapsulated RIN-m5F cell survival in PEG hydrogels with indicated compositions determined by CellTiter-Glo assay (mean ± SEM, n = 4). Asterisks indicate statistical significance between experimental groups and the unmodified PEG gels [(−) IKVAV, (−) GLP-1C] at the respective time point (p < 0.05). (c) Antiapoptotic effect of immobilized GLP-1C on encapsulated RIN-m5F cells 3 days after encapsulation. Luminescence signals of the caspase 3/7 activity were normalized by the AlamarBlue fluorescence of the respective gel sample to account for the variance of viable cells (mean ± SEM, n = 3; *p < 0.05).
Mouse Islet Encapsulation in GLP-1C Functionalized PEG Hydrogels
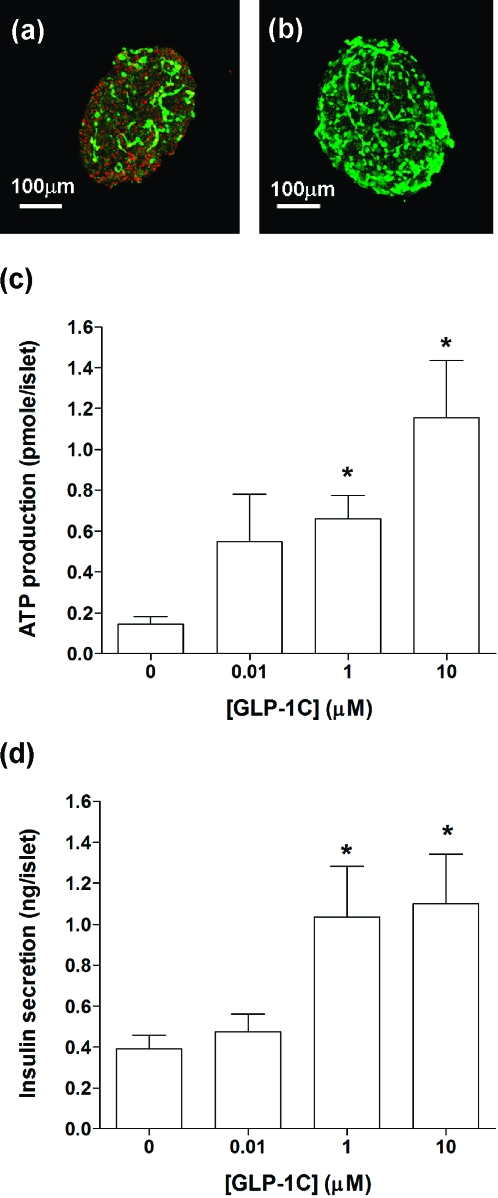
In previous publications, PEG hydrogels formed via photopolymerization techniques have been successfully used to encapsulate mouse islets for in vitro as well as in vivo studies.16,17,19 Although the fabrication of PEG hydrogels via photopolymerizations produces a stable network rapidly and has been applied to encapsulate a variety of cell types, the generation of free radicals during the chain polymerization reaction may cause a certain degree of cell damage. To reveal the photopolymerization-induced damage on encapsulated islets, we stained and imaged encapsulated mouse islets right after photopolymerization. As shown in Figure 4a, dead cells (evidenced by the binding of red fluorescent dye, ethidium homodimer-1, to the exposed DNA of dead cells) are clearly visible on the photoencapsulated islet cells, particularly at the surface of the islet. Because the staining was done immediately after photopolymerization, it is reasonable to suggest that the cell damage was caused by the polymerization process and could be a result of radical-mediated cell death or apoptosis. Interestingly, when isolated islets were photoencapsulated in PEG hydrogels in the presence of GLP-1C, the amount of dead cells decreased dramatically (Figure 4b). This result suggests a potential cyto-protective effect of GLP-1 to islet cells during radical-mediated photoencapsulation. One previous study revealed an antiapoptotic effect of GLP-1, via PI3-kinase (PI3K) pathway, to pancreatic β-cells under the challenge of H2O2.(46) We reason that GLP-1 (or GLP-1C) may exhibit a similar protective mechanism for islet cells during radical-mediated photopolymerization. Further studies, however, are required to validate this hypothesis.
Figure 4.
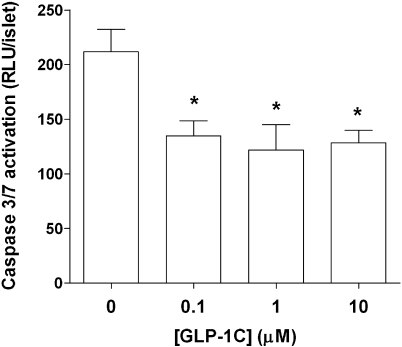
(a) Representative confocal z-section stacking image of a mouse islet encapsulated in PEGDA-only hydrogel. (b) Representative confocal z-section stacking image of a mouse islet encapsulated in GLP-1 immobilized PEGDA hydrogel. (Encapsulated islets were stained with live/dead staining kit and imaged right after photopolymerization.) (c) Encapsulated mouse islet survival after 7 days (CellTiter-Glo ATP production assay) in PEG hydrogels incorporated with various GLP-1C concentrations (mean ± SEM, n = 4). (d) Insulin secretion from mouse islets encapsulated in PEG hydrogels (7 days) incorporated with various GLP-1C concentrations (mean ± SEM, n = 4). Asterisks indicate statistical significance compared to unmodified PEG gels (*p < 0.05).
To assess the protective effects of immobilized GLP-1 on encapsulated islets, we incorporated GLP-1C at different concentrations in PEG hydrogels via thiol−acrylate photopolymerizations. Figure 4c shows that in GLP-1C functionalized PEG hydrogels, the level of intracellular ATP was significantly higher at high GLP-1C concentrations, compared to unmodified PEG hydrogels (7 days after photoencapsulation). This demonstrates that the immobilization of GLP-1C within PEG hydrogels enhances the survival of isolated islets. Further, Figure 4d shows the results of glucose-stimulated insulin secretion from encapsulated islets 7 days after photoencapsulation. It can be seen that the incorporation of GLP-1C in PEG hydrogels greatly enhances insulin secretion by about 2.5-fold, when compared to unmodified PEG hydrogels. We also discovered that the immobilization of GLP-1C in PEG hydrogels does not significantly change the level of insulin secretion at low glucose (0.2 g/L) concentration (data not shown). This phenomenon coincides with previous studies that the insulinotropic effect of GLP-1 is dependent on glucose concentrations.(22)
Antiapoptotic Effect of Immobilized GLP-1 to Encapsulated β-Cells
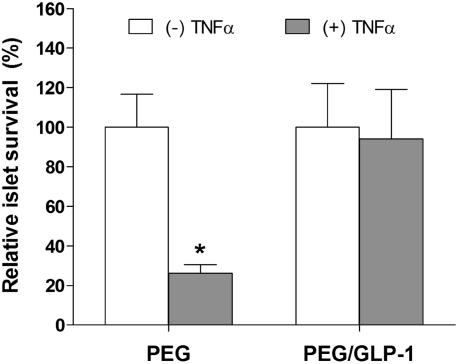
Previous studies have shown that the survival of pancreatic β-cells encapsulated in PEG hydrogels relies heavily on cell−cell and cell−matrix interactions.16,17,19 As a result, β-cells undergo apoptosis rapidly when encapsulated as single cells in PEG hydrogels. Although GLP-1 is not an extracellular matrix protein, it does provide antiapoptotic effects to stimuli-challenged β-cells via activation of PI3-kinase (PI3K) and MAPK/ERK pathways.45−47 The immobilization of GLP-1 within PEG hydrogels may therefore provide antiapoptotic to the encapsulated cells. As shown in Figure 3c, when encapsulated as dispersed cells, RIN-m5F cells undergo a higher level of apoptosis in PEG-only hydrogels than in IKVAV or GLP-1C functionalized PEG hydrogels. Interestingly, the antiapoptotic effect was more significant when both IKVAV and GLP-1 were copolymerized within PEG hydrogels, suggesting a potential synergistic effect of the ECM peptide IKVAV and incretin hormone GLP-1 on the intracellular antiapoptotic signaling. Figure 5 shows a similar antiapoptotic effect of immobilized GLP-1 on encapsulated mouse islets.
Figure 5.
Antiapoptotic effect of immobilized GLP-1 on isolated mouse islets encapsulated in PEG gels (3 days after encapsulation, mean ± SEM, n = 3). The relative luminescence units (RLU) of the caspase 3/7 activity were normalized by the number of islets encapsulated in the respective gel (*p < 0.05).
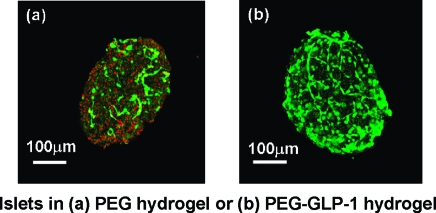
The signaling of GLP-1 to pancreatic β-cells has been a subject of intensive research. In addition to its glucose-dependent insulinotropic effect, GLP-1 or its receptor agonists (e.g., Exendin 4) also exhibits pro-survival or antiapoptotic effects on pancreatic β-cells under a variety of biological/chemical stimuli, including pro-inflammatory cytokines (e.g., TNFα, IL-β).(45) Recent studies have revealed that the beneficial effects of GLP-1 on β-cells in the presence of pro-apoptotic signals are the results of GLP-1R activated signaling pathways, including cAMP/protein kinase A (PKA), PI3K, and MAPK/ERK pathways.23,45−47 To elucidate the antiapoptotic effect of immobilized GLP-1 against cytokine challenge, we incubated encapsulated islets in media supplemented with TNFα (50 ng/mL).48−50 Because TNFα is a small molecule with a molecular weight of approximately 17.4 kDa, it infiltrates the highly permeable PEG hydrogels rapidly (data not shown). Upon binding to TNF-R, TNFα triggers an apoptotic pathway that leads to islet cell death. As shown in Figure 6, the survival of islets encapsulated in GLP-1 immobilized PEG hydrogels was higher than in unmodified PEG hydrogels under TNFα challenge.
Figure 6.
Relative islet cell survival in PEG hydrogels or PEG-GLP-1 hydrogels (10 μM GLP-1C) under 50 ng/mL TNFα challenge for 48 h (3 days after encapsulation) as determined by CellTiter-Glo assay for ATP production (mean ± SEM, n = 3; *p < 0.05 compared to islets in PEG hydrogels without TNFα challenge).
Conclusions
In conclusion, we have successfully synthesized a GLP-1 analog, GLP-1C, and fabricated bioactive GLP-1 functionalized hydrogels via a thiol−acrylate photopolymerization for pancreatic β-cell encapsulation. The physical properties, such as gel swelling and molecular transport, of PEG hydrogels were not affected by the copolymerization with GLP-1C. Compared to PEG hydrogels, pancreatic β-cells (RIN-m5F) and mouse islets encapsulated in GLP-1C immobilized PEG hydrogels had higher viability and secreted more insulin upon glucose stimulation. Further, β-cells encapsulated in bioactive GLP-1C immobilized PEG hydrogels were less apoptotic than those encapsulated in unmodified PEG hydrogels. The current work augments the therapeutic efficacy of PEG hydrogels in encapsulating pancreatic islets to treat type 1 DM and should prove useful in future in vivo studies.
Acknowledgments
This work was supported by NIH (5R01DK076084) and the Howard Hughes Medical Institute. The authors thank Philip Pratt (Barbara Davis Center for Childhood Diabetes) for the isolation of mouse islets and Alex Aimetti for his assistance on peptide synthesis and purification.
Funding Statement
National Institutes of Health, United States
References
- Mathis D.; Vence L.; Benoist C. Nature 2001, 414, 792–798. [DOI] [PubMed] [Google Scholar]
- Steil G. M.; Panteleon A. E.; Rebrin K. Adv. Drug Delivery Rev. 2004, 56, 125–144. [DOI] [PubMed] [Google Scholar]
- Owens D. R.; Zinman B.; Bolli G. Diabetic Med. 2003, 20, 886–898. [DOI] [PubMed] [Google Scholar]
- Peppas N. A.; Wood K. M.; Blanchette J. O. Expert Opin. Biol. Ther. 2004, 4, 881–887. [DOI] [PubMed] [Google Scholar]
- Morishita M.; Peppas N. A. Drug Discovery Today 2006, 11, 905–910. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A.; Eisenbarth G. S. Lancet 2001, 358, 221–229. [DOI] [PubMed] [Google Scholar]
- Vaithilingam V.; Sundaram G.; Tuch B. E. Curr. Opin. Organ Transplant. 2008, 13, 633–638. [DOI] [PubMed] [Google Scholar]
- Spencer C. M.; Goa K. L.; Gillis J. C. Drugs 1997, 54, 925–975. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S.; Weir G. C. Nat. Biotechnol. 2005, 23, 857–861. [DOI] [PubMed] [Google Scholar]
- Wilson J. T.; Chaikof E. L. Adv. Drug Delivery Rev. 2008, 60, 124–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Lacik I.; Brissova M.; Anilkumar A. V.; Prokop A.; Hunkeler D.; Green R.; Shahrokhi K.; Powers A. C. Nat. Biotechnol. 1997, 15, 358–362. [DOI] [PubMed] [Google Scholar]
- Soon-Shiong P. Adv. Drug Delivery Rev. 1999, 35, 259–270. [DOI] [PubMed] [Google Scholar]
- Cruise G. M.; Hegre O. D.; Scharp D. S.; Hubbell J. A. Biotechnol. Bioeng. 1998, 57, 655–665. [DOI] [PubMed] [Google Scholar]
- Cruise G. M.; Hegre O. D.; Lamberti F. V.; Hager S. R.; Hill R.; Scharp D. S.; Hubbell J. A. Cell Transplant. 1999, 8, 293–306. [DOI] [PubMed] [Google Scholar]
- Cheung C. Y.; Anseth K. S. Bioconjugate Chem. 2006, 17, 1036–1042. [DOI] [PubMed] [Google Scholar]
- Weber L. M.; He J.; Bradley B.; Haskins K.; Anseth K. S. Acta Biomater. 2006, 2, 1–8. [DOI] [PubMed] [Google Scholar]
- Weber L. M.; Hayda K. N.; Haskins K.; Anseth K. S. Biomaterials 2007, 28, 3004–3011. [DOI] [PubMed] [Google Scholar]
- Cheung C. Y.; McCartney S. J.; Anseth K. S. Adv. Funct. Mater. 2008, 18, 3119–3126. [Google Scholar]
- Weber L. M.; Anseth K. S. Matrix Biol. 2008, 27, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J. Curr. Pharm. Des. 2001, 7, 1399–1412. [DOI] [PubMed] [Google Scholar]
- Drucker D. J. Endocrinology 2001, 142, 521–527. [DOI] [PubMed] [Google Scholar]
- Drucker D. J. Gastroenterology 2002, 122, 531–544. [DOI] [PubMed] [Google Scholar]
- Brubaker P. L.; Drucker D. J. Endocrinology 2004, 145, 2653–2659. [DOI] [PubMed] [Google Scholar]
- Holz G. G.; Chepurny O. G. Curr. Med. Chem. 2003, 10, 2471–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. C.; Shapiro L.; Bowers O. J.; Dinarello C. A. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 12153–12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. H.; Molano R. D.; Pileggi A.; Lu Y. Q.; Wasserfall C.; Campbell-Thompson M.; Zahr E.; Sanjose S.; Ricordi C.; Atkinson M. A.; Inverardi L. Diabetes 2007, 56, A525−A525. [Google Scholar]
- Zhang B.; Lu Y. Q.; Campbell-Thompson M.; Spencer T.; Wasserfall C.; Atkinson M.; Song S. H. Diabetes 2007, 56, 1316–1323. [DOI] [PubMed] [Google Scholar]
- Lee S. H.; Lee S.; Youn Y. S.; Na D. H.; Chae S. Y.; Byun Y.; Lee K. C. Bioconjugate Chem. 2005, 16, 377–382. [DOI] [PubMed] [Google Scholar]
- Lee S.; Youn Y. S.; Lee S. H.; Byun Y.; Lee K. C. Diabetologia 2006, 49, 1608–1611. [DOI] [PubMed] [Google Scholar]
- Youn Y. S.; Jeon J. E.; Chae S. Y.; Lee S.; Lee K. C. Diabetes, Obes. Metab. 2008, 10, 343–346. [DOI] [PubMed] [Google Scholar]
- Chae S. Y.; Jin C. H.; Shin H. J.; Youn Y. S.; Lee S.; Lee K. C. Bioconjugate Chem. 2008, 19, 334–341. [DOI] [PubMed] [Google Scholar]
- Youn Y. S.; Chae S. Y.; Lee S.; Kwon M. J.; Shin H. J.; Lee K. C. Eur. J. Pharm. Biopharm. 2008, 68, 667–675. [DOI] [PubMed] [Google Scholar]
- Miranda L. P.; Winters K. A.; Gegg C. V.; Patel A.; Aral J.; Long J. S.; Zhang J. W.; Diamond S.; Guido M.; Stanislaus S.; Ma M.; Li H. Y.; Rose M. J.; Poppe L.; Veniant M. M. J. Med. Chem. 2008, 51, 2758–2765. [DOI] [PubMed] [Google Scholar]
- John H.; Maronde E.; Forssmann W. G.; Meyer M.; Adermann K. Eur. J. Med. Res. 2008, 13, 73–78. [PubMed] [Google Scholar]
- Kim S.; Bae Y. H. Tissue Eng. 2004, 10, 1607–1616. [DOI] [PubMed] [Google Scholar]
- Kim B.; Kim S. W.; Bae Y. H. Biomaterials 2005, 26, 3597–3606. [DOI] [PubMed] [Google Scholar]
- Salinas C. N.; Anseth K. S. Macromolecules 2008, 41, 6019–6026. [Google Scholar]
- Salinas C. N.; Anseth K. S. Biomaterials 2008, 29, 2370–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hern D. L.; Hubbell J. A. J. Biomed. Mater. Res. 1998, 39, 266–276. [DOI] [PubMed] [Google Scholar]
- Cruise G. M.; Scharp D. S.; Hubbell J. A. Biomaterials 1998, 19, 1287–1294. [DOI] [PubMed] [Google Scholar]
- Lin C. C.; Metters A. T. J. Biomed. Mater. Res. 2007, 83A, 954–964. [DOI] [PubMed] [Google Scholar]
- Lin C. C.; Metters A. T. Pharm. Res. 2006, 23, 614–622. [DOI] [PubMed] [Google Scholar]
- Youn Y. S.; Chae S. Y.; Lee S.; Jeon J. E.; Shin H. G.; Lee K. C. Biochem. Pharmacol. 2007, 73, 84–93. [DOI] [PubMed] [Google Scholar]
- Perry T.; Greig N. H. Trends Pharm. Sci. 2003, 24, 377–383. [DOI] [PubMed] [Google Scholar]
- Blandino-Rosano M.; Perez-Arana G.; Mellado-Gil J. M.; Segundo C.; Aguilar-Diosdado M. J. Mol. Endocrinol. 2008, 41, 35–44. [DOI] [PubMed] [Google Scholar]
- Hui H. X.; Nourparvar A.; Zhao X. N.; Perfetti R. Endocrinology 2003, 144, 1444–1455. [DOI] [PubMed] [Google Scholar]
- Buteau J.; Foisy S.; Rhodes C. J.; Carpenter L.; Biden T. J.; Prentki M. Diabetes 2001, 50, 2237–2243. [DOI] [PubMed] [Google Scholar]
- Blandino-Rosano M.; Perez-Arana G.; Mellado-Gil J. M.; Segundo C.; Aguilar-Diosdado M. J. Mol. Endocrinol. 2008, 41, 35–44. [DOI] [PubMed] [Google Scholar]
- Hoorens A.; Pipeleers D. Diabetologia 1999, 42, 55–59. [DOI] [PubMed] [Google Scholar]
- Wu J. J.; Chen X. J.; Cao X. C.; Baker M. S.; Kaufman D. B. J. Surg. Res. 2001, 101, 190–195. [DOI] [PubMed] [Google Scholar]