Abstract
Naturally occurring variation in gene copy number is increasingly recognized as a heritable source of susceptibility to genetically complex diseases. Here we report strong association between FCGR3B copy number and risk of systemic lupus erythematosus (P = 2.7 × 10-8), microscopic polyangiitis (P = 2.9 × 10-4) and Wegener’s granulomatosis in two independent cohorts from the UK (P = 3 × 10-3) and France (P = 1.1 × 10-4). We did not observe this association in the organ-specific Graves’ disease or Addison’s disease. Our findings suggest that low FCGR3B copy number, and in particular complete FCGR3B deficiency, has a key role in the development of systemic autoimmunity.
Structural variation is now recognized as a rich source of genetic diversity in the human genome, and during the past 3 years thousands of common copy number variants (CNVs) have been described1-4. Although rare CNVs have been established as a cause of mental retardation and single-gene disorders5,6, the role of more common, transmissible CNVs in evolutionary selection and disease susceptibility is largely unknown.
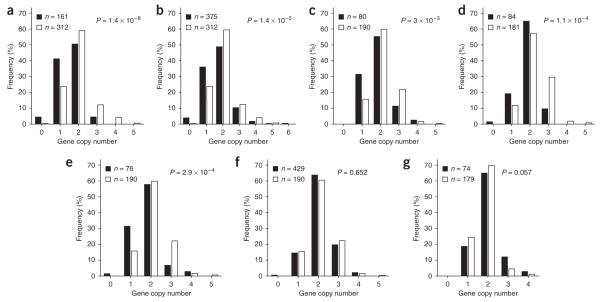
We recently reported an association between low FCGR3B copy number and autoimmune glomerulonephritis in a sample of individuals with systemic lupus erythematosus (SLE) from UK nuclear families7. In the present study, the previously reported association (P = 1 × 10-3) was confirmed and strengthened (P = 1.4 × 10-8, 95% c.i. = 0 to 2.9 × 10-5; Fig. 1a) in a larger sample comprising 161 SLE patients with glomerulonephritis from the UK and in 312 independent controls from the UK 1958 birth cohort (Supplementary Methods and Supplementary Table 1 online). Compared with individuals possessing two copies of FCGR3B (the median copy number in the control population), individuals with fewer than two copies had a significantly higher risk of glomerulonephritis (odds ratio (OR) = 2.43, P = 1 × 10-3, Table 1).
Figure 1.
Distribution of FCGR3B copy number in different groups of affected individuals (filled bars) versus unaffected controls (open bars). (a) SLE with nephritis. (b) SLE without nephritis. (c) Wegener’s granulomatosis (UK). (d) Wegener’s granulomatosis (France). (e) Microscopic polyangiitis. (f) Graves’ disease. (g) Addison’s disease. A nonparametric Mann-Whitney U test was applied to assess association with gene copy number at FCGR3B. Two-tailed P values for significance were estimated by 100,000 Monte Carlo simulations. n, number of subjects in each group.
Table 1. Estimated risk of several autoimmune disorders according to FCGR3B gene copy number.
| Autoimmune disease | Cases/controlsa | Copy number | OR | 95% c.i. | P valueb |
|---|---|---|---|---|---|
| SLE with nephritis | 152/312 | <2 | 2.43 | 1.47-4.03 | 0.001 |
| >2 | 0.40 | 0.15-1.07 | 0.07 | ||
| SLE without nephritis | 359/312 | <2 | 2.21 | 1.13-3.40 | 0.0003 |
| >2 | 1.13 | 0.63-2.00 | 0.684 | ||
| Wegener’s granulomatosis (UK) | 80/188 | <2 | 2.46 | 1.19-5.10 | 0.015 |
| >2 | 0.76 | 0.32-1.79 | 0.525 | ||
| Wegener’s granulomatosis (France) | 77/181 | <2 | 1.58 | 0.76-3.27 | 0.220 |
| >2 | 0.28 | 0.12-0.62 | 0.002 | ||
| Microscopic polyangiitis | 76/188 | <2 | 2.56 | 1.22-5.39 | 0.013 |
| >2 | 0.52 | 0.19-1.42 | 0.204 | ||
| Graves’ disease | 278/188 | <2 | 0.87 | 0.51-1.49 | 0.621 |
| >2 | 0.88 | 0.56-1.40 | 0.599 | ||
| Addison’s disease | 71/179 | <2 | 0.69 | 0.33-1.43 | 0.327 |
| >2 | 2.89 | 1.15-7.28 | 0.023 |
SLE, systemic lupus erythematosus; OR, odds ratio (risk of acquiring disease); c.i., confidence interval.
Subjects with missing age or gender data were discarded from the logistic regression analysis
P value: level of significance of the OR.
We estimated gene copy number as described in Supplementary Methods and Supplementary Table 2 online and as previously reported7. Additionally, we validated the quantitative PCR assay by semiquantitative PCR (Supplementary Fig. 1 online) and by array comparative genomic hybridization analysis (Supplementary Fig. 2 online). Statistical analyses are described in Supplementary Methods.
We then investigated the involvement of CNV at FCGR3B in the development of SLE with no known renal involvement in a set of 375 unrelated cases recruited from the UK (Supplementary Methods and Supplementary Table 1). We found a new, strong association (P = 1.4 × 10-5; 95% c.i. = 0 to 2.9 × 10-5; Fig. 1b and Table 1) and an increased risk for development of SLE in individuals with fewer than two copies of FCGR3B (OR = 2.21, P = 3 × 10-4, Table 1). When we considered all SLE cases together (n = 536), regardless of renal involvement, the P value of association was 2.7 × 10-8 (95% c.i. = 0 to 2.9 × 10-5). However, we did not observe any significantly higher risk of acquiring glomerulonephritis in individuals with SLE who possessed fewer than two copies of FCGR3B (OR = 1.09; 95% c.i. = 0.73-1.62, P = 0.660).
Given these findings and the key role of Fc receptors in regulation of inflammatory and immune responses8-10, we hypothesized that CNV in FCGR3B may confer susceptibility to a range of autoimmune disorders, with or without glomerular involvement. Therefore, we first studied anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, a systemic autoimmune disorder in which renal disease occurs commonly. Individuals with ANCA-associated vasculitis typically present with one of two clinical syndromes: Wegener’s granulomatosis or microscopic polyangiitis (MPA); we considered these two subtypes separately (Supplementary Methods and Supplementary Table 1).
In two independent cohorts of individuals with Wegener’s granulomatosis from the University of Birmingham and from the Cochin Hospital in Paris, we found association between FCGR3B copy number and susceptibility to Wegener’s granulomatosis (Birmingham cohort, P = 3 × 10-3 and 95% c.i. = 2.5 × 10-3 to 3.1 × 10-3; Paris cohort, P = 1.1 × 10-4 and 95% c.i. = 4.5 × 10-5 to 1.7 × 10-4; Fig. 1c,d). Similarly, we observed a significant association between FCGR3B copy number and MPA in a separate Birmingham cohort (P = 2.9 × 10-4; 95% c.i. = 1.8 × 10-4 to 3.9 × 10-4; Fig. 1e).
We also studied individuals with Graves’ disease and isolated autoimmune Addison’s disease, as examples of organ-specific autoimmune diseases with no renal involvement (Supplementary Methods and Supplementary Table 1). We did not see any significant association between FCGR3B copy number and either Graves’ disease (in a large cohort that included 429 cases and 190 controls; P = 0.65; 95% c.i. = 0.64-0.65; Fig. 1f) or Addison’s disease (74 cases, 179 controls; P = 0.057; 95% c.i. = 0.056-0.059; Fig. 1g).
Logistic regression models indicated an increased risk of Wegener’s granulomatosis and MPA in UK individuals with a low FCGR3B copy number, with OR = 2.46 (P = 0.015) and OR = 2.56 (P = 0.013), respectively. Individuals in the French Wegener’s granulomatosis cohort did not show significantly increased risk with low copy number, although we saw a reduced risk in those with high copy number (Table 1). Consistent with the association results, logistic regression models did not show any increased risk in individuals with low FCGR3B copy numbers for either Graves’ or Addison’s disease (Table 1).
The frequency of individuals with zero copies of FCGR3B (indicating complete genomic absence of FCGR3B and total deficiency of FCGR3B protein11,12) differed markedly between cases and controls. We observed zero copies of FCGR3B in 25 out of 1,279 (2%) individuals with autoimmunity (7 SLE patients with glomerulonephritis, 14 with SLE, one with MPA, 1 with Wegener’s granulomatosis and 2 with Graves’ disease) but in only 1 of 862 controls (0.1%). This suggests that complete FCGR3B deficiency confers a very high risk of autoimmunity, particularly for SLE.
Our data show an association between low FCGR3B copy number and risk of development of SLE and ANCA-associated vasculitis, both of which have systemic inflammation as a key feature and commonly involve the glomerulus. In contrast, we did not find any significant association with Graves’ or Addison’s diseases, both of which are organ-specific autoimmune disorders, suggesting a different role for FCGR3B CNV in development of systemic and organ-specific autoimmunity.
FCGR3B is expressed by neutrophils and eosinophils and is important in tethering of neutrophils to immune complexes and in clearance of immune complexes13. We hypothesize that in individuals with SLE, reduced clearance of immune complexes predisposes to disease in glomeruli and at other sites. In ANCA-associated vasculitis, in which immune complexes seem not to have a central pathogenic role14, our data suggest that reduced or absent expression of FCGR3B may be associated with enhanced neutrophil activation by ANCA.
These findings suggest that low FCGR3B copy number may be a common genomic mechanism in the development of SLE, Wegener’s granulomatosis and MPA. The data also support the hypothesis that frequent, heritable CNV in the human genome confers risk to a range of common human diseases. As CNVs have been reported frequently in genes that act in inflammation and immune response pathways3,4,15, structural variation in such genes may contribute to the evolution of predisposition to a range of infectious and inflammatory disorders.
All study participants gave written informed consent. Ethics committee approval was obtained for all studies as detailed in the Supplementary Methods.
ACKNOWLEDGMENTS
We thank C. Neuwirth and Y. Tan (Clinical Sciences Centre); A. Wong (Imperial College); J. Cedric and M. Delpech (INSERM U567); M. Marre (INSERM U695) and B. Balkau (INSERM U780-IFR69) for their role in collecting patients and controls. We thank A. Tsalenko, A. Scheffer, N. Sampas, P. Tsang and L. Bruhn from Agilent Laboratories for contributions to the array comparative genomic hybridization results. We acknowledge intramural funding from the Clinical Sciences Centre (to T.J.A.) and support from the Wellcome Trust Cardiovascular Functional Genomics award (T.J.A.), from the FP6 EURATools (European Union contract number LSHG-CT-2005-019015) award (T.J.A.), from the UK MRC (T.J.A., H.T.C.) and from a Wellcome Trust Senior Fellowship (T.J.V.). We acknowledge use of genotype data from the British 1958 Birth Cohort DNA collection, funded by the UK Medical Research Council grant G0000934 and the Wellcome Trust grant 068545/Z/02.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Note: Supplementary information is available on the Nature Genetics website
References
- 1.Sebat J, et al. Science. 2004;305:525–528. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- 2.Iafrate AJ, et al. Nat. Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- 3.Tuzun E, et al. Nat. Genet. 2005;37:727–732. doi: 10.1038/ng1562. [DOI] [PubMed] [Google Scholar]
- 4.Redon R, et al. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stankiewicz P, et al. Trends Genet. 2002;18:74–82. doi: 10.1016/s0168-9525(02)02592-1. [DOI] [PubMed] [Google Scholar]
- 6.Lupski JR. Nat. Genet. 2006;38:974–976. doi: 10.1038/ng0906-974. [DOI] [PubMed] [Google Scholar]
- 7.Aitman TJ, et al. Nature. 2006;439:851–855. doi: 10.1038/nature04489. [DOI] [PubMed] [Google Scholar]
- 8.Salmon JE, et al. Arthritis Rheum. 2001;44:739–750. doi: 10.1002/1529-0131(200104)44:4<739::AID-ANR129>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Takai T. J. Clin. Immunol. 2005;25:1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, et al. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 11.de Haas M, et al. Blood. 1995;86:2403–2413. [PubMed] [Google Scholar]
- 12.Clark MR, et al. J. Clin. Invest. 1990;86:341–346. doi: 10.1172/JCI114706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coxon A, et al. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 14.Harper L, et al. J. Pathol. 2000;190:349–359. doi: 10.1002/(SICI)1096-9896(200002)190:3<349::AID-PATH524>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez E, et al. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]