Abstract
A great deal of progress has been made recently in understanding the molecules and mechanisms that regulate transendothelial migration of leukocytes, or diapedesis, a critical step in the inflammatory response. This mini-review will focus mainly on the active role of the endothelial cell in this process as it occurs at endothelial cell borders. It discusses some of the many molecules that have been reported to play a role in transendothelial migration and asks why so many molecules seem to be involved. The concept is emerging that diapedesis itself can be dissected into sequential molecularly dissectible steps controlled by specific molecule(s) at the endothelial cell border. Several mechanisms have been shown to play a critical role in transendothelial migration including signals derived from clustering of apically-disposed intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), disruption or loosening of adherens junctions, and targeted recycling of platelet/endothelial cell adhesion molecule (PECAM) and other molecules from the recently-described lateral border recycling compartment. A hypothesis that integrates the various known mechanisms is proposed.
Keywords: Transendothelial migration, inflammation, leukocyte, endothelial cell, transmigration
The inflammatory response is the body's stereotyped reaction to tissue damage of any kind. It involves rapidly and transiently delivering preformed soluble elements in the blood to the site of injury followed by a more prolonged delivery of leukocytes. Since leukocytes cannot swim, they are recruited locally at the site of inflammation in a series of adhesive steps that allow them to attach to the vessel wall, locomote along the wall to the endothelial borders, traverse the endothelium and the subendothelial basement membrane and migrate through the interstitial tissue 1, 2. Transendothelial migration (TEM) or diapedesis is arguably the point-of-no-return in the inflammatory response. The preceding steps of leukocyte rolling, activation, adhesion, and locomotion are all reversible, and most leukocytes that initiate contact with the postcapillary venule at the site of inflammation re-enter the circulation. However, once the leukocyte commits to diapedesis, it does not go back—at least not as the same cell type 3. Most TEM takes place at endothelial borders. Recently there has been a flurry of interest in TEM through the endothelial cell body (transcellular migration). While this clearly can occur in vitro 4 and in vivo 5, in the interests of space, this Mini-Review will focus on transendothelial migration and specifically TEM at cell borders. Unfortunately, due to space limitations, a great many excellent publications on this subject cannot be cited.
MOLECULES REGULATING TRANSMIGRATION
A number of molecules have been implicated in transmigration due to the fact that genetic deletion or antibody blockade of these molecules impairs diapedesis. All of these molecules belong to a recognized family of adhesion molecules, are expressed on endothelial cells, and are enriched at cell borders. In addition to adhesive functions, these molecules have signaling functions that contribute to their role in TEM. Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) will be discussed in this section, as well. Although they are not localized to cell borders or involved in the diapedesis step per se, they seem to be involved in events directly preceding diapedesis and are recruited to the endothelial cell border during transmigration.
ICAM-1 and VCAM-1 are involved in firm adhesion of leukocytes to the apical surface of endothelial cells through interactions with leukocyte CD11a/CD18 and/or CD11b/CD18 and CD49a/CD29, respectively. Once adherent, ICAM-1 becomes enriched under the leukocyte as it migrates to the endothelial cell border and continues to surround it during transmigration 6. Thesrc-dependent phosphorylation of the actin-binding molecule cortactin is required for ICAM-1 clustering 7, 8. VCAM-1 clustering has been observed in the steps leading up to diapedesis. Both ICAM-1 and VCAM-1 are concentrated in actin-rich “docking structures” that form prior to TEM 9, 10.
ICAM-2, another CD11a/CD18 ligand, is constitutively expressed on endothelial cells, where it is concentrated at cell borders. Antibodies against ICAM-2 do not seem to have a major effect on TEM in vitro. However, in some inflammatory models in vivo, blocking antibodies or genetic deletion of ICAM-2 inhibit transmigration of neutrophils 11, 12.
Junctional adhesion molecule-A (JAM-A) and –C (JAM-C) are concentrated at endothelial cell borders. While JAM-A normally engages in homophilic adhesion, during inflammation it can bind to CD11a/CD18 on the leukocyte 13. Blocking JAM-A on human endothelial cells using a polyclonal antibody in vitro has been shown to reduce TEM 13; however, other investigators using polyclonal or monoclonal antibodies have seen no effect 6, 14, 15. On the other hand, in vivo studies show decreased inflammation 16 and transendothelial migration 12 when JAM-A is blocked. JAM-C can engage in homophilic adhesion, or heterophilic adhesion with JAM-B or CD11b/CD18. The latter interaction is implicated in transendothelial migration in vitro 17 and in vivo 18. For an extensive review of the roles of JAM family members in the inflammatory response, the reader is referred to a recent review 19.
Endothelial cell-selective adhesion molecule (ESAM) is molecularly related to the JAMs, but has a long cytoplasmic domain. As its name implies, its distribution is mostly limited to endothelial junctions, but it is expressed on activated platelets 20. It binds homophilically, and a ligand on leukocytes has not been described. ESAM-deficient mice have no defect in lymphocyte extravasation, but have a transient decrease in neutrophil emigration (marked decrease at two hours that had recovered by four hours) 21.
Platelet/endothelial cell adhesion molecule-1 (PECAM-1, PECAM, CD31) is an Ig superfamily member concentrated at the borders of endothelial cells as well as expressed diffusely on platelets and leukocytes. Homophilic interaction of leukocyte PECAM with endothelial PECAM is required for transendothelial migration 22, 23. Blockade with mAb against the amino-terminal homophilic interaction domain, soluble PECAM-Fc chimeras, and genetic deletion of PECAM inhibit transendothelial migration in vitro and in vivo (reviewed in 3.) When PECAM is transfected into cells that normally lack it, expression of PECAM imparts on them the ability to support TEM 24. This gain of function has not been demonstrated with other adhesion molecules. When PECAM-PECAM interactions are blocked, leukocytes are arrested tightly adherent to the apical surface of the cell 25 and actively migrate along the junctions as if searching for a place to transmigrate 15. In vivo, leukocytes are able to get to the postcapillary venules at the site of inflammation, but are unable to exit efficiently. They are seen apparently adherent to the endothelial cell luminal surface 26, 27, reminiscent of the block to TEM seen in vitro 22, 25.
This phenotype is seen with human cells and in all mouse strains examined except for C57BL/6 27, 28. Interestingly, this mouse strain seems to be unique in that genetic deletion of PECAM or administration of blocking antibody or mouse PECAM-Fc to these mice has no effect in a variety of inflammatory models 27, 29, 30. Even the closely related C57BL/10 strain responds to anti-PECAM therapy 28. The ability to circumvent the need for PECAM in the thioglycollate peritonitis model of inflammation has been linked to a small locus at the proximal end of chromosome 2 28. Therefore, earlier studies carried out in C57BL/6 mice that found no role or only a minor role for PECAM in inflammation need to be re-evaluated. See reference 3 for a detailed discussion of the role of PECAM in various in vivo models.
There is a role for leukocyte PECAM in traversing the basal lamina 31. C57BL/6 mice in which PECAM has been knocked out 29 or blocked with antibody 12 are defective in their ability to migrate across this extravascular barrier.
CD99 is a relatively unique molecule unrelated to any other molecule in the human genome except the closely-related paralog CD99-like 2 (CD99L2), which may have arisen from a common ancestral gene 32. Similar to PECAM, homophilic interaction between CD99 at the endothelial cell border and CD99 on monocytes 33 and neutrophils 34 is required for transmigration. However, CD99 regulates a later step in transmigration than PECAM. Leukocytes in which PECAM has been blocked can still be prevented from transmigrating if anti-CD99 is added after the anti-PECAM antibody has been washed away. Conversely, when CD99 interaction is first blocked, leukocytes can no longer be inhibited from transmigrating by anti-PECAM antibody when the anti-CD99 block is removed 33. In support of this, confocal images of leukocytes blocked in the act of transmigration by anti-CD99 show their leading edge under the endothelial cytoplasm, their cell body lodged at the border between endothelial cells, and the trailing uropod on the apical surface 33, 34. As long as the block continues, they migratealong the junctions over the surface of the endothelium in this manner, unable to finish transmigration 34. There is indirect evidence that CD99 cannot function unless PECAM acts first 24. In mice migration of T lymphocytes into skin 35 and neutrophils and monocytes into the peritoneal cavity 36 are blocked by anti CD99 antibodies.
CD99L2 expression in mice seems similar to that of CD99. That is, it is expressed on vascular endothelium of all tissues examined 37, 38 and is expressed at the borders of endothelial cells. It is expressed to varying degrees on all circulating blood cells. Only polyclonal antibodies against murine CD99L2 have been tested in vivo. They block neutrophil and monocyte influx in the thioglycollate peritonitis model 37, 38. It is tempting to speculate that the incomplete blockade of inflammation seen when interfering with either CD99 or CD99L2 is due to partial redundancy of the function of these molecules.
Vascular endothelial cell cadherin (VE-cadherin, cadherin 5) is the major adhesion molecule of the adherens junction. It negatively regulates transmigration. Antibodies against VE-cadherin enhance early migration into a site of inflammation in vivo 39. In vitro studies show that VE-cadherin is transiently removed from the site of transmigration at the cell junction 40, 41. Mutation of the cytoplasmic tail of VE-cadherin so that it cannot interact with p120 or β-catenin or mutation of the latter prevents clearance of VE-cadherin from the cell border and blocks transmigration 42, 43.
Why so many molecules?
Other endothelial molecules that have been shown to play a role in TEM by virtue of the inhibition of TEM by blocking antibodies include poliovirus receptor (CD155) 44, MUC18 (CD146) 45, activated leukocyte cell adhesion molecule (ALCAM/CD166) 46, integrin associated protein (IAP/CD47) 47, and nepmucin/CLM-9 48. It seems that each month brings a new report of an endothelial cell or leukocyte molecule that is implicated in diapedesis. When added to the well-characterized molecules discussed in the previous section, this raises the question of why so many molecules are required for TEM. Is this just an artifact of clogging up the junction with antibody or turning the cell junctions into immune complexes? This is unlikely, as most of these studies used control antibody, Fab or F(ab')2 fragments, soluble recombinant adhesion molecules, siRNA knockdown, or genetic deletion to buttress their claims.
The process of diapedesis itself can be further dissected into a series of molecularly-defined steps controlled by specific molecules acting in sequence. Sequential blocking experiments demonstrated that PECAM regulates a step in diapedesis that is “upstream” of the step regulated by CD99 33. Sequential blockade analysis has not been performed with other pairs of molecules, but images of leukocytes blocked by antibodies in vivo in C57BL/6 mice show that ICAM-2 arrests neutrophils on the apical surface of the endothelium, anti-JAM-A arrests them at the cell junctions, and anti-PECAM arrests them between the endothelial cell and basal lamina 12. This begs the questions of whether each molecule controls its own defined step in the sequence, whether multiple molecules control each step, and how many steps are there? Until sequential blockade studies can be performed with each of these molecules, this question will remain unanswered. The answer is likely to be different for different leukocyte types, vascular beds, and inflammatory stimuli, as well as the time after the initiation of the stimulus. However, it seems unlikely that there is a separate unique step in diapedesis controlled by each molecule reported to be important for transmigration.
What if most of the endothelial molecules reported to control transmigration were part of a large multimolecular complex, or a series of multimolecular complexes (one for each successive step in diapedesis) that combined to make a platform to support transmigration analogous to the way that multiple transcription factors and coactivators combine to make DNA accessible to transcription? Loss of, or interference with, any one of the molecules in that case could make the complex less efficient at supporting diapedesis and could account for the published results.
MECHANISMS REGULATING TRANSMIGRATION
Clustering surface ICAM-1 and VCAM-1
The adhesion step immediately upstream of diapedesis is an obvious prerequisite for diapedesis, and there is reason to think that some of the events that occur during this step signal the events that regulate transmigration. Clustering of ICAM-1 and VCAM-1 on the endothelial cell has been observed as the leukocyte approaches the endothelial cell border 9, 10. The initial leukocyte-faciliated clustering of ICAM-1 requires src-dependent phosphorylation of the actin-binding protein cortactin, which is also associated with actin filament remodeling that takes place during transmigration 7. On the other hand, ICAM-1 engagement or clustering induces src-dependent phosphorylation of cortactin 49. Rather than being results that are at odds, these observations may belie a self-amplification cycle: The initial recruitment of ICAM-1 and VCAM-1 may be due to adhesion to their leukocyte ligands. This clustering induces phosphorylation of cortactin, which leads to the actin polymerization and the recruitment of more ICAM-1 to the site of leukocyte adhesion, which induces more cortactin phosphorylation. Clustering of ICAM-1 and VCAM-1 stimulates signaling in the endothelial cells that promote diapedesis in ways that are discussed later.
Clustering of ICAM-1 and VCAM-1 may occur in three dimensions. Fingerlike projections of endothelial apical surface membrane reported to surround the lower portion of adherent leukocytes. The membrane is enriched in ICAM-1 and VCAM-1 and overlies cytoplasm enriched in f-actin and actin binding proteins. Sanchez-Madrid and co-workers first described these structures that engaged polyclonally activated lymphocytes and lymphoblasts adherent to cytokine-activated HUVEC 9. Subsequently, Carman et al. 50 demonstrated similar projections associated with transmigrating neutrophils, monocytes, and lymphocytes, at least under their experimental conditions that involved apical application of a chemokine or leukocyte activator and interaction with activated endothelium. They referred to these structures as “transmigratory cups” 10.
Disruption of the cytoskeleton abolished these structures, but had no effect on leukocyte adhesion. The authors commented that this might belie a role in transmigration. Interstingly, however, Barreiro, et al. had found that the docking structures rapidly vanished as lymphocytes began to migrate through the monolayers 9.
However, not everyone who reports rings of ICAM-1 enrichment around transmigrating leukocytes has seen docking structures or transmigratory cups. For example, Ridley's group, using a similar system (but without application of apical SDF-1) showed distinct ICAM-1 enrichment around transmigrating lymphoblasts, but no docking structures 51. Luscinskas' group also demonstrated local enrichment of ICAM-1 around transmigrating neutrophils undergoing transmigration 6 and commented that they did not see such actin-rich microvilli.
What do these docking structures represent and why are they not universally seen? One possibility is that they represent a response of the endothelial cell to leukocytes that are either highly activated or tightly adherent. The structures were seen under conditions where the leukocytes were adherent but could not transmigrate, allowing time for recruitment of additional ICAM-1 and/or VCAM-1 molecules 9, 52 or under which the leukocytes were additionally activated by the exogenous application of PAF or chemokines on the apical surface of the endothelial cells 10, 50. One could easily imagine that under these conditions, enhanced leukocyte integrin activation could result in greater recruitment of counter-receptors from the endothelial surface. In contrast, under conditions where ICAM-1 enrichment was not accompanied by formation of transmigratory cups, the transmigrating neutrophils 6 or lymphoblasts 51 were activated only by interactions with the cytokine-activated endothelium.
Loosening the Junctions
Several lines of evidence show that loosening the endothelial cell junctions is important for efficient transmigration. Clustering of ICAM-1 and VCAM-1 on endothelial cells transmits a number of signals into the endothelial cell (reviewed in 53), some of which appear to be relevant to diapedesis. Cross-linking VCAM-1 54 and ICAM-1 53 on the endothelial cell stimulates an increase in cytosolic free calcium ions, which has long been known to be a requirement for diapedesis 55. The increase in cytosolic free calcium ion has been shown to activate myosin light chain kinase (MLCK), leading to actin-myosin fiber contraction. This is believed to help endothelial cells separate 56.
Stimulation of ICAM-1 leads to phosphorylation of VE-cadherin, which is a prerequisite for adherens junction disassembly 57. In HUVEC the kinases Src and Pyk2 phosphorylate VE-cadherin on the p120 and β-catenin binding sites, tyrosine residues 658 and 731, respectively 42. This inhibits the binding of p120 and β-catenin to VE-cadherin. Since the interaction of these proteins with VE-cadherin is critical for retaining VE-cadherin at the adherens junction, this destabilizes the junctions.
Cross-linking VCAM-1 also activates Rac1 58 and stimulates an increase in reactive oxygen species in endothelial cells 59 that leads to loosening of adherens junctions. In other systems Rac1 activation leads to phosphorylation of VE-cadherin on serine 665, which signals its clathrin-dependent internalization 60. The net result is “loosening” of junctional structures.
Under resting conditions the vascular endothelial protein tyrosine phosphatase (VE-PTP) associates with VE-cadherin via plakoglobin (γ-catenin), maintaining it in a hypophosphorylated state at the junction. Interaction of leukocytes with cytokine-activated endothelial cells triggers rapid dissociation of VE-PTP from VE-cadherin, allowing it to be phosphorylated on tyrosine, increasing junctional permeability and facilitating transendothelial migration 61. A role for another VE-cadherin accessory molecule, p120 catenin, has been demonstrated recently 43. Overexpression of p120 prevented VE-cadherin phosphorylation and the formation of “gaps” in VE-cadherin staining along the endothelial junction during engagement of leukocytes. (These gaps were not spaces between cells, but disruption of the staining pattern of VE-cadherin.) This was associated with a significant decrease in transmigration. Interestingly, the authors did not find evidence for VE-cadherin internalization during gap formation 43.
In a similar manner, clustering of ICAM-1 activates RhoA, which activates Rho kinase (ROCK) (reviewed in 62). This in turn phosphorylates PP1c, the major phosphatase inactivating MLCK. The end result is potentiation of actin-myosin contraction. It is important to point out that, although intercellular gaps that are visible in the light microscope can be produced on endothelial cells cultured on glass coverslips, in vivo the gaps produced between endothelial cells by even the strongest inducers of vascular permeability (e.g. histamine and serotonin) are in the order of hundreds of angstroms 63, and these are re-sealed by the time most leukocytes are recruited. This does not mean that these gaps are not important, but it means that leukocytes must still crawl through closely adherent endothelial cells; they do not fall into holes between endothelial cells.
The Lateral Border Recycling Compartment (LBRC)
Even under steady state conditions there is a considerable amount of membrane movement taking place at the endothelial cell borders. Membrane is internalized into and recycled from an interconnected reticulum of tubulo-vesicular structures (the components of which are approximately 50 nm in diameter) that resides just beneath the plasma membrane of the endothelial cell borders 23. This compartment, the lateral border recycling compartment (LBRC) 64, is distinct from caveolae, typical recycling endosomes, and vesiculo-vacuolar organelles 23. About 30% of the cell's PECAM resides in this compartment and recycles with a half time of about 10 minutes 23. This compartment also contains CD99 and JAM-A, but not VE-cadherin (unpublished data). In high endothelial venule endothelium, the Ig superfamily molecule nepmucin (CLM-9), which promotes lymphocyte TEM, is in the LBRC 48.
The purpose of the constitutive recycling is not known. However, when a leukocyte transmigrates, membrane from the LBRC is redirected. It is targeted to the position along the cell junction where the leukocyte is transmigrating 23, 64. Membrane from the LBRC is exteriorized along the endothelial cell border at this site, providing an increase in surface area to accommodate the leukocyte as well as a source of PECAM, CD99, and JAM-A to interact with. Blocking PECAM-PECAM interactions between leukocyte and endothelial cell blocks targeted recycling from the LBRC and blocks transmigration. Moreover, there is accumulating evidence that targeted recycling from the LBRC is an essential step in TEM: LBRC membrane is trafficked to the site of transmigration by kinesin molecular motors along microtubules 64. Disrupting or bundling microtubules, or inhibiting the motor domain of kinesin blocks targeted recycling and blocks TEM. Activated lymphoblasts can transmigrate in a manner that cannot be blocked by anti-PECAM antibodies. Nevertheless, transmigration of lymphoblasts can be efficiently blocked by disrupting targeted recycling of the LBRC 64. A tyrosine→phenylalanine mutation on the cytoplasmic tail of PECAM blocks the ability of PECAM to support TEM. It turns out that this mutation interferes with the ability of PECAM to enter and leave the LBRC and markedly diminishes its ability to participate in targeted recycling 24. In confluent endothelial cell monolayers a minority of PECAM is phosphorylated; however, essentially all of the phosphorylated PECAM resides in the LBRC 65.
Targeted recycling of LBRC membrane during TEM potentially solves many of the “problems” inherent in the process. Rather than having to “unzip” high density homophilic adhesions of VE-cadherin, PECAM, JAM-A, CD99, etc., these molecules (and other structural components of the junction) may be pushed aside by membrane from the LBRC. This then presents unligated molecules that the leukocyte must interact with (e.g. PECAM, JAM-A, CD99, nepmucin) on its path across the endothelial cell while removing or diluting out those it needs to avoid (e.g. VE-cadherin). Hypothetically, once the leukocyte has moved across the junction, the LBRC may be pulled back into the cell, allowing the other components to diffuse back into place, re-establishing the endothelial junction without having to reform all of the complex three-dimensional interactions.
A unifying concept of transmigration?
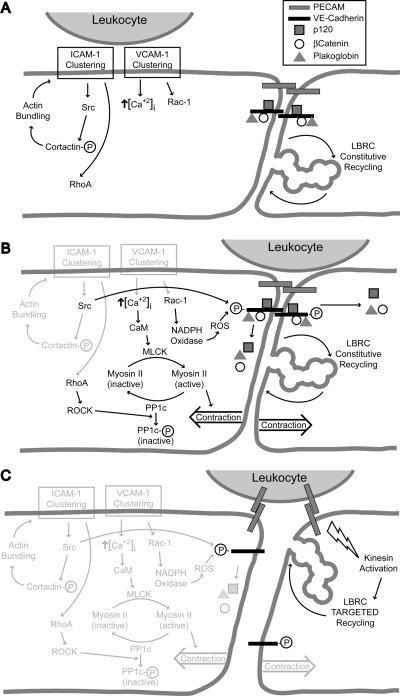
ICAM-1 and VCAM-1 signaling, cytosolic free calcium flux, RhoA and Rac1 activation, VE-cadherin removal from the junction, MLCK activation, and targeted recycling of the LBRC have all been shown to be necessary for efficient transmigration. How are these diverse phenomena related? Are they sequential links in a chain, or events occurring in parallel with all required for TEM to occur? Considering that many second messenger signaling systems interact with each other and feedback loops exist, this may be a question of semantics. However, the following undoubtedly oversimplified scheme seems to be consistent with all of the published data and at least provides a testable hypothesis (See Fig. 1).
A. Clustering of ICAM-1 and VCAM-1 initiate activation of src, RhoA and Rac-1, and increased cytosolic free calcium ion. B. These signals lead to activation of MLCK, inactivation of PP1c, and phosphorylation of VE-cadherin, inducing release of the associated catenins. C. Signals from the leukocyte activate kinesin molecular motors and stimulate targeted trafficking of LBRC membrane to surround the leukocyte. See text for detailed description. CaM = calmodulin; ROS = reactive oxygen species; circled P = phosphorylated state.
LFA-1 preferentially binds to ICAM-1 dimers 66, 67, which initiates clustering of ICAM-1. This stimulates phosphorylation of cortactin, enhancing the further actin-induced clustering of ICAM-1. This self-enhancing cycle leads to the enrichment of ICAM-1 around tightly adherent leukocytes. ICAM-1 multimerization leads to increases in cytosolic free calcium and activation of RhoA (Fig. 1A).
In the meantime, if the leukocytes express VLA-4 and the endothelial cells are expressing VCAM-1, clustering of VCAM-1 also stimulates an increase in cytosolic free calcium, activation of Rac-1, and production of reactive oxygen species in endothelial cells 68, 69. The latter activates PKCα 70. The net result is loosening of endothelial cell junctions (Fig. 1A, B).
ICAM-1 and VCAM-1 signaling additionally result in weakening of the endothelial junctions due to effects on phosphorylation of VE-cadherin. This dissociates VE-cadherin from its links to the actin cytoskeleton and it potentially (but not necessarily) becomes subject to endocytosis in a clathrin-dependent manner (Fig. 1B).
The increase in cysolic free calcium activates MLCK to induce tension in the endothelial cells. The activation of MLCK is augmented by the inactivation of PP1 phosphatase mediated by the RhoA activation stimulated by signals originated through ICAM-1 clustering. The net result of contraction of the endothelial cell body against weakened junctions would be to allow easier passage of leukocytes (Fig. 1B).
With leukocytes poised over weakened adherens junctions, the other homophilic junctional adhesion molecules still hold the endothelial borders apposed. PECAM-PECAM interactions between leukocyte and endothelial cell 23 or other signals 64 stimulate targeted trafficking of LBRC membrane to the site of leukocyte transmigration (Fig. 1C). Targeted recycling of the LBRC may displace structural molecules of adherens junctions and other junctions laterally, allowing transmigration to proceed even if the disruption of these junctions is incomplete. The additional membrane brought by the LBRC provides increased surface area and unligated molecules that the leukocytes want to interact with. The signals that trigger targeted recycling are not known, nor is it clear how the membrane is directed to the site of transmigration. However, weakening of the endothelial cell adherens junctions by brief extracellular calcium chelation leads to diffuse exteriorization of the LBRC along the endothelial cell border (unpublished data). It is possible that local weakening of the adherens junctions at the site of leukocyte engagement may allow for localized exteriorization of the LBRC.
It is possible, and even likely, that some of the many junctional molecules discussed earlier are also part of the LBRC or function to recruit it. That is, the LBRC may be one of the hypothetical multimolecular complexes controlling transmigration and other multimolecular complexes may function to recruit it to the site of TEM and reinternalize it after TEM.
Unanswered questions
While much has been learned about the mechanisms that regulate TEM, many important unanswered questions remain: Why are so many endothelial cell molecules implicated in this process? Where is actin-myosin contraction tension exerted in vivo, since endothelial cells in postcapillary venules don't have stress fibers? What directs LBRC targeted recycling? Finally, how are all of the molecules and mechanisms that have been identified to participate in transendothelial migration coordinated to ensure efficient leukocyte emigration?
ACKNOWLEGEMENTS
I wish to thank the members of my lab, past and present, for their contributions to the field and for helpful comments on the manuscript. I thank David Sullivan and Begum Kutay for preparing the Figure.
SOURCES OF FUNDING Supported by grants from the National Institutes of Health, R01HL046849 and R37HL064774.
Non-Standard Abbreviations and Acronyms
- ALCAM
activated leukocyte adhesion molecule
- CaM
calmodulin
- CD99L2
CD99-like molecule 2
- ESAM
endothelial cell-selective adhesion molecule
- HUVEC
human umbilical vein endothelial cell(s)
- IAP
integrin associated protein
- ICAM-1,-2
intercellular adhesion molecule-1 or -2
- JAM-A,-B,-C
junctional adhesion molecule-A, -B, or -C
- LFA-1
lymphocyte function associated antigen 1 (CD11a/CD18)
- LBRC
lateral border recycling compartment
- MLCK
myosin light chain kinase
- PECAM
platelet/endothelial cell adhesion molecule-1
- PKCα
protein kinase Cα
- ROCK
Rho A kinase
- ROS
reactive oxygen species
- SDF-1
stromal cell derived factor 1 (CXCL12)
- TEM
transendothelial migration
- VCAM-1
vascular cell adhesion molecule-1
- VE-cadherin
vascular endothelial cell specific cadherin (cadherin 5)
- VE-PTP
vascular endothelial protein tyrosine phosphatase
- VLA-4
very late antigen 4 (CD49d/CD29)
Footnotes
DISCLOSURES None.
REFERENCES
- 1.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends in Immunology. 2003;24:326–333. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Muller WA. PECAM: Regulating the start of diapedesis. In: Ley K, editor. Adhesion Molecules: Function and Inhibition. Birkhauser Verlag AG; Basel: 2007. pp. 201–220. [Google Scholar]
- 4.Carman CV, Springer TA. Trans-cellular migration: cell-cell contacts get intimate. Curr Opin Cell Biol. 2008;20:533–540. doi: 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to fMLP. Journal of Experimental Medicine. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200:1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res. 2006;98:394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang MT, Larbi KY, Scheiermann C, Woodfin A, Gerwin N, Haskard DO, Nourshargh S. ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood. 2006;107:4721–4727. doi: 10.1182/blood-2005-11-4683. [DOI] [PubMed] [Google Scholar]
- 12.Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostermann G, Weber KSC, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113:2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 15.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson-Leger C, Aurrand-Lions M, Beltraminelli N, Fasel N, Imhof BA. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood. 2002;100:2479–2486. doi: 10.1182/blood-2001-11-0098. [DOI] [PubMed] [Google Scholar]
- 18.Chavakis T, Keiper T, Matz-Westphal R, Hersemeyer K, Sachs UJ, Nawroth PP, Preissner KT, Santoso S. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem. 2004;279:55602–55608. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- 19.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 20.Nasdala I, Wolburg-Buchholz K, Wolburg H, Kuhn A, Ebnet K, Brachtendorf G, Samulowitz U, Kuster B, Engelhardt B, Vestweber D, Butz S. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J Biol Chem. 2002;277:16294–16303. doi: 10.1074/jbc.M111999200. [DOI] [PubMed] [Google Scholar]
- 21.Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, Li H, Nasdala I, Brandau O, Fassler R, Butz S, Krombach F, Vestweber D. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med. 2006;203:1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial cell surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 24.Dasgupta B, Dufour E, Mamdouh Z, Muller W. A novel and critical role for tyrosine 663 in PECAM trafficking and transendothelial migration. J. Immunol. 2009;182:5041–5051. doi: 10.4049/jimmunol.0803192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 [CD31] blocks acute inflammation in vivo. J Exp Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- 28.Seidman MA, Chew TW, Schenkel AR, Muller WA. PECAM-independent thioglycollate peritonitis is associated with a locus on murine chromosome 2. PLoS ONE. 2009;4:e4316. doi: 10.1371/journal.pone.0004316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- 30.Schenkel AR, Chew TW, Chlipala E, Harbord MW, Muller WA. Different susceptibilities of PECAM-deficient mouse strains to spontaneous idiopathic pneumonitis. Exp Mol Pathol. 2006;81:23–30. doi: 10.1016/j.yexmp.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakelin MW, Sanz M-J, Dewar A, Albelda SM, Larkin SW, Boughton-Smith N, Williams TJ, Nourshargh S. An anti-platelet/endothelial cell adhesion molecule-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through basement membrane. Journal of Experimental Medicine. 1996;184:229–239. doi: 10.1084/jem.184.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suh YH, Shin YK, Kook MC, Oh KI, Park WS, Kim SH, Lee IS, Park HJ, Huh TL, Park SH. Cloning, genomic organization, alternative transcripts and expression analysis of CD99L2, a novel paralog of human CD99, and identification of evolutionary conserved motifs. Gene. 2003;307:63–76. doi: 10.1016/s0378-1119(03)00401-3. [DOI] [PubMed] [Google Scholar]
- 33.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 34.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J. Immunol. 2007;178:1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- 35.Bixel G, Kloep S, Butz S, Petri B, Engelhardt B, Vestweber D. Mouse CD99 participates in T cell recruitment into inflamed skin. Blood. 2004;104:3205–3213. doi: 10.1182/blood-2004-03-1184. [DOI] [PubMed] [Google Scholar]
- 36.Dufour EM, Deroche A, Bae Y, Muller WA. CD99 Is Essential for Leukocyte Diapedesis In Vivo. Cell Commun Adhes. 2008:1–13. doi: 10.1080/15419060802442191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenkel AR, Dufour EM, Chew TW, Sorg E, Muller WA. The Murine CD99-Related Molecule CD99-Like 2 (CD99L2) Is an Adhesion Molecule Involved in the Inflammatory Response. Cell Commun Adhes. 2007;14:227–237. doi: 10.1080/15419060701755966. [DOI] [PubMed] [Google Scholar]
- 38.Bixel MG, Petri B, Khandoga AG, Khandoga A, Wolburg-Buchholz K, Wolburg H, Marz S, Krombach F, Vestweber D. A CD99-related antigen on endothelial cells mediates neutrophil, but not lymphocyte extravasation in vivo. Blood. 2007;109:5327–5336. doi: 10.1182/blood-2006-08-043109. [DOI] [PubMed] [Google Scholar]
- 39.Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J. Cell Sci. 1997;110:583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 40.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 42.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 43.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, Xerri L, Farnarier C, Cantoni C, Bottino C, Moretta A, Dubreuil P, Lopez M. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J Exp Med. 2004;199:1331–1341. doi: 10.1084/jem.20032206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bardin N, Blot-Chabaud M, Despoix N, Kebir A, Harhouri K, Arsanto JP, Espinosa L, Perrin P, Robert S, Vely F, Sabatier F, Le Bivic A, Kaplanski G, Sampol J, Dignat-George F. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2009;29:746–753. doi: 10.1161/ATVBAHA.108.183251. [DOI] [PubMed] [Google Scholar]
- 46.Masedunskas A, King JA, Tan F, Cochran R, Stevens T, Sviridov D, Ofori-Acquah SF. Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration. FEBS Lett. 2006;580:2637–2645. doi: 10.1016/j.febslet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW. Endothelial CD47 interaction with SIRPgamma is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood. 2008;112:1280–1289. doi: 10.1182/blood-2008-01-134429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin S, Umemoto E, Tanaka T, Shimomura Y, Tohya K, Kunizawa K, Yang BG, Jang MH, Hirata T, Miyasaka M. Nepmucin/CLM-9, an Ig domain-containing sialomucin in vascular endothelial cells, promotes lymphocyte transendothelial migration in vitro. FEBS Lett. 2008;582:3018–3024. doi: 10.1016/j.febslet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 49.Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud PO. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J Biol Chem. 1994;269:12536–12540. [PubMed] [Google Scholar]
- 50.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003;171:6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 51.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 52.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178:1279–1293. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Buul JD, Kanters E, Hordijk PL. Endothelial signaling by Ig-like cell adhesion molecules. Arterioscler Thromb Vasc Biol. 2007;27:1870–1876. doi: 10.1161/ATVBAHA.107.145821. [DOI] [PubMed] [Google Scholar]
- 54.Lorenzon P, Vecile E, Nardon E, Ferrero E, Harlan JM, Tedesco F, Dobrina A. Endothelial cell E-and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J. Cell Biol. 1998;142:1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang AJ, Manning JE, Bandak TM, Ratau MC, Hanser KR, Silverstein SC. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J. Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hixenbaugh EA, Goeckeler ZM, Papaiya NN, Wysolmerski RB, Silverstein SC, Huang AJ. Chemoattractant-stimulated neutrophils induce regulatory myosin light chain phosphorylation and isometric tension development in endothelial cells. American Journal of Physiology. 1997;273:H981–H988. doi: 10.1152/ajpheart.1997.273.2.H981. [DOI] [PubMed] [Google Scholar]
- 57.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 59.Cook-Mills JM, Johnson JD, Deem TL, Ochi A, Wang L, Zheng Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 61.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, Bixel MG, Butz S, Vestweber D. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205:2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cernuda-Morollon E, Ridley AJ. Rho GTPases and leukocyte adhesion receptor expression and function in endothelial cells. Circ Res. 2006;98:757–767. doi: 10.1161/01.RES.0000210579.35304.d3. [DOI] [PubMed] [Google Scholar]
- 63.Majno G, Palade GE. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J. Exp. Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dasgupta B, Muller WA. Endothelial Src kinase regulates membrane recycling from the lateral border recycling compartment during leukocyte transendothelial migration. Eur J Immunol. 2008;38:3499–3507. doi: 10.1002/eji.200838605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reilly PL, Woska JR, Jr., Jeanfavre DD, McNally E, Rothlein R, Bormann BJ. The native structure of intercellular adhesion molecule-1 (ICAM-1) is a dimer. J Immunol. 1995;155:529–532. Correlation with binding to LFA-1 [published erratum appears in J Immunol 1996 Apr 15;156(8):following 3088] [PubMed] [Google Scholar]
- 67.Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J Exp Med. 1995;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cook-Mills JM. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol Immunol. 2002;39:499–508. doi: 10.1016/s0161-5890(02)00206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285:C343–352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 70.Abdala-Valencia H, Cook-Mills JM. VCAM-1 signals activate endothelial cell protein kinase Calpha via oxidation. J Immunol. 2006;177:6379–6387. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]