Abstract
Background and Purpose
The objectives of the present study were to: a) noninvasively identify white matter reorganization and monitor its progress within 6 weeks after the onset of stroke, and b) quantitatively investigate the effect of recombinant human erythropoietin (rhEPO) treatment on this structural change using in vivo measurement of diffusion anisotropy.
Methods
Male Wistar rats were subjected to middle cerebral artery occlusion (MCAo) and treated with rhEPO intraperitoneally at a dose of 5000 units/kg of bodyweight (n=11) or the same volume of saline (n=7) daily for 7 days starting 24 hours after MCAo. MRI measurements of T2- and diffusion-weighted images and cerebral blood flow (CBF) were performed and neurological severity score was assessed at 1 day and weekly for 6 weeks after MCAo. Luxol fast blue and Bielschowsky staining were used to demonstrate myelin and axons, respectively.
Results
White matter reorganization occurred along the ischemic lesion boundary after stroke. The region of white matter reorganization seen on the tissue slice coincided with the elevated area on the fractional anisotropy (FA) map, which can be accurately identified. The increase in elevated FA pixels corresponded with progress of white matter reorganization and was associated with improvement of neurological function. Treatment with rhEPO after stroke significantly enhanced white matter reorganization, restored local CBF and expedited functional recovery.
Conclusions
White matter reorganization can be detected by FA. Elevated FA pixels may be a good MRI index to stage white matter remodeling and predict functional outcome.
Keywords: Erythropoietin, focal ischemia, fractional anisotropy, rat, white matter reorganization
1. Introduction
Diffusion anisotropy, as measured by fractional anisotropy (FA), is sensitive to alteration of white matter fiber integrity1–2 and has been successfully used to detect subtle abnormalities in a variety of diseases that involve disruption of white matter fibers, including Wallerian degeneration (WD),3 multiple sclerosis (MS),4 traumatic brain injury (TBI)5 and stroke6–10. For both human ischemia and experimental animal models of stroke, the change of diffusion anisotropy within the ischemic area discloses the degree of structural damage in the tissue and indicates functional potential.3, 9 Loss of structural integrity after stroke and its impact upon the outcome of neurological function have been studied in great detail.6–9 However, less attention has been paid to structural reorganization in fiber tracts beyond the area of the ischemic lesion,11–12 which may contribute to recovery of neurological function.13
Treatment of stroke with erythropoietin (EPO) promotes brain remodeling and improves neurological function. 14–17 Reorganization on a structural level is likely enhanced by such treatment and may account for improved functional recovery. However, the progress of structural reorganization in the ischemic brain after EPO intervention, which is an important part of the restorative process, has not been dynamically investigated. The objectives of the present study were to: a) noninvasively identify white matter reorganization and monitor its progress within 6 weeks after the onset of stroke, and b) quantitatively investigate the effect of EPO treatment on this structural change after stroke using in vivo measurement of diffusion anisotropy.
2. Materials and Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
2.1 Animal model and treatment groups
Male Wistar rats (300–350 g) were used in the present study. Middle cerebral artery occlusion (MCAo) was induced by placement of a single, intact, fibrin-rich, 24-hour-old, homologous white clot (~ 1ul) at the origin of MCA.18 Rats with embolic stroke were treated with recombinant human erythropoietin (rhEPO) (epoetin α, AM-GEN) intraperitoneally (i.p.) at a dose of 5000 units/kg of bodyweight (treated group; n = 11) or the same volume of saline (control group; n = 7) daily for 7 days starting 24 hours after MCAo. The dosage was chosen based on our previous study,14 which demonstrated the beneficial effect of rhEPO on functional recovery for this stroke model. All rats were euthanized 6 weeks post-MCAo.
2.2 Tissue preparation and histopathology
Immediately after the final MRI measurement at 6 weeks after stroke, rats were deeply anesthetized and transcardially perfused.19 The brain was removed shortly after death, immersed and fixed in 4% paraformaldehyde in PBS for 2 days, and then cut into 7 contiguous coronal blocks 2 mm thick. Coronal sections 6 µm thick were sliced from each block embedded in paraffin and stained for histological evaluation.
To identify structural changes after stroke, double staining for Luxol fast blue and Bielschowsky (LFB + B)15 was employed to demonstrate myelin and axons, respectively. Under an optical microscope, nuclei on the stained slice are colorless; myelin is blue and axons appear black on a pale blue background.
2.3 In vivo MRI acquisition and data processing
MRI was performed using a 7T, 20 cm-bore superconducting magnet (Magnex Scientific, Abingdon, U.K.) interfaced to a Bruker console (Bruker, Boston, U.S.)19. Animals were placed on a nonmagnetic holder equipped with a nose cone for administration of anesthetic gases and stereotaxic ear bars to minimize movement of the head. During the imaging procedure, anesthesia was maintained with 1.0% halothane in 69% N2O and 30% O2, and rectal temperature was kept at 37°C ± 1.0°C using a feedback-controlled water bath. T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI) and cerebral blood flow (CBF) were measured for all animals in both treated and control groups at 1 day and weekly for 6 weeks after the onset of stroke.
T2-weighted images were acquired using standard two-dimensional Fourier transform multi-slice (13 slices, 1 mm thick), multi-echo (6 echoes) MRI. Six sets of images (13 slices per set) were obtained using echo times (TE) of 15, 30, 45, 60, 75 and 90 ms and a repetition time (TR) of 8 s. Images were produced using a 32 × 32 mm2 field of view (FOV) and a 128 × 64 image matrix. The total sequence time was about 9 min.
Diffusion-weighted images were measured using the method described by Le Bihan et al,20 with diffusion gradients in the x, y and z directions. The spin-echo sequence (13 slices, 1 mm thick, 32 × 32 mm2 FOV, 128 × 64 image matrix, TR = 1500 ms, TE = 40 ms) was modified to include two 10 ms diffusion-weighting gradient pulses, one on either side of the refocusing 180° radio-frequency (RF) pulse. The diffusion-weighting gradient was increased in a nonlinear manner from 0 to ≈ 83 mT/m to obtain 3 images with gradient b-values of 20, 600 and 1200 s/mm2. Each image required a 5 min scan time, and the entire three-directional trace map sequence took about 15 min.
An arterial spin labeling technique was used to quantify CBF in cerebral tissue. Adiabatic inversion of arterial water protons was accomplished via an axial gradient of 0.3 kHz/mm and a 1-second continuous wave RF power of ≈ 0.3 kHz at a frequency offset of 6 kHz. This was followed by a spin echo imaging sequence with TR/TE = 1000 ms/20 ms. The labeled slice was 2 cm distal from the imaging slice and 1 mm thick. In order to eliminate gradient asymmetry in the axial direction, an image average was applied by switching around the gradient polarities. FOV was 32 × 32 mm2 and the image matrix was 64 × 64.
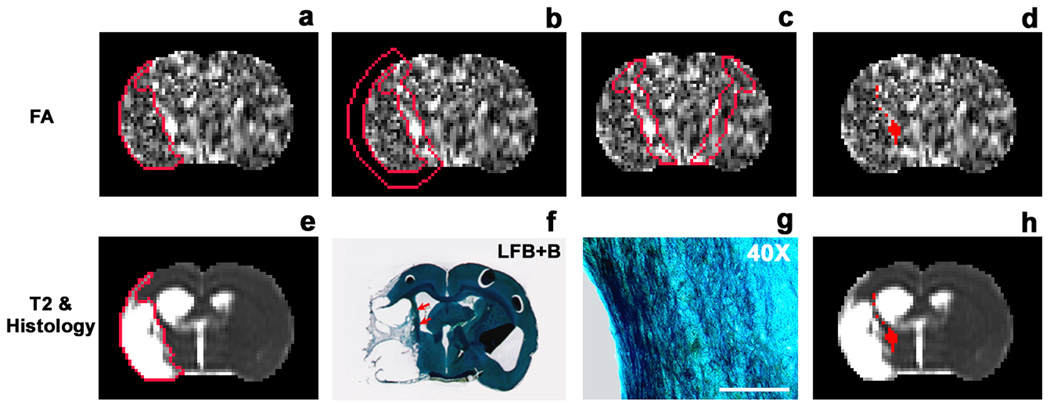
Based on the acquired images, we generated 13 equally spaced coronal slices of T2 and FA1 maps that covered the entire brain in identical slice locations for each animal. On histological evaluation, white matter reorganization after stroke was characterized by oriented bundles of myelin and axons extruding from the corpus callosum into the ipsilateral striatum along the boundary of the ischemic lesion (Fig. 1f & 1g). Careful comparison between LFB+B-stained tissue sections and corresponding MRI images demonstrated that the location of this structural reorganization occurred along the lesion boundary (Fig. 1f, red arrows) and coincided with the elevated area on the FA map (comparing Fig. 1b with 1f). For the animals studied, a 6-pixel-wide ribbon-like region in the non-ischemic area immediately adjacent to the edge of the lesion encompassed the area where FA values were elevated (Fig. 1b).
Fig. 1.
Identification of tissue areas undergoing white matter reorganization on a slice of FA map measured from a rhEPO-treated animal 6 weeks after MCAo. The upper row (a–d) shows a slice of FA map and the lower row presents the same slice of T2 map (e & h), corresponding LFB+B section (f) and a closer view of the section in the boundary region (g). The values of FA are relatively low in the ischemic area (a) determined by the T2 map (e), indicating demyelination induced by ischemia. White matter reorganization, characterized by oriented bundles of myelin and axons extruding from the corpus callosum into the ipsilateral striatum (f and g), takes place along the boundary of the lesion (f: LFB+B section, showing a narrow dark area (red arrows) along the ischemic boundary; g: higher magnification, revealing bundles of myelin and axons aligned along the ischemic boundary; bar = 100 µm). This region of structural change appears bright on the FA map (a) and dark on the T2 map (e). A 6-pixel wide ROI adjacent to the edge of the lesion encompasses the region (b), and FA mean+2SD, provided by homologous tissue region on the contralateral side (c), identifies the areas with elevated FA values (d). These elevated FA areas are located in the non-ischemic tissue along the lesion boundary (h).
To identify the areas of white matter reorganization represented on the FA map, a T2 map was used to detect the ischemic lesion (Fig. 1e). The lesion area was specified by those pixels with a T2 value higher than the mean plus twice the standard deviation (mean+2SD) provided by the normal tissue on the contralateral (non-ischemic) side.19 The outline of the lesion was copied onto the same slice of the FA map (Fig. 1a), and a region of interest (ROI) encompassing the area undergoing structural reorganization was created by expanding the rim of the lesion 6 pixels outward (Fig. 1b). The mean value of FA plus 2SD, measured from homologous tissue area on the contralateral side (Fig. 1c), was used as a threshold to identify the elevated FA pixels in the ipsilateral ROI (Fig. 1d). For each animal, ROIs were delineated on 13 slices of the FA map, based on the corresponding slices of the T2 map. The area of elevated FA in a specific perilesional region on each slice was identified, and the total number of elevated pixels throughout the brain was calculated. The mean value of FA in the identified elevated area for each slice was also measured. To detect changes in CBF in the reorganized area, the identified FA region at the 6-week time point was employed as a ROI to track the evolution of CBF within the experimental period. Data were normalized to the contralateral side for each slice to obtain relative FA and CBF, and averaged at the same time points for each group.
2.4 Behavioral testing
Neurological severity score (NSS),21 which grades the composite neurological function of an animal on motor, sensory, reflex and balance tests (normal score: 0; maximum deficit score: 18), was assessed at 1 day and weekly for 6 weeks after MCAo by an examiner blinded to the treatment groups and the corresponding MRI results.
2.5 Statistical analysis
A two-sample Wilcoxon exact test was used since our data were not normal. Statistical comparisons of MRI measurements between two treatment groups, including relative CBF and FA, lesion area, number of slices, number of pixels and NSS, were performed at each time point. p ≤ 0.05 was considered significant. For data illustration, the results are summarized as mean ± SE and presented at each time point.
3. Results
3.1 Elevated area on FA map and white matter reorganization
Histological evaluation based on LFB+B-stained tissue slices indicated that white matter reorganization occurred within a certain width along the ischemic lesion boundary after stroke (Fig. 1f, red arrows). Comparison between the tissue slices and the corresponding FA maps demonstrated that the region of white matter reorganization seen on the tissue slice coincided with the elevated area on the FA map (comparing Fig. 1b with 1f). These elevated areas along the ischemic lesion boundary on the FA map, which can be identified using the methods described in the previous section and illustrated in Fig. 1, then represent white matter reorganization.
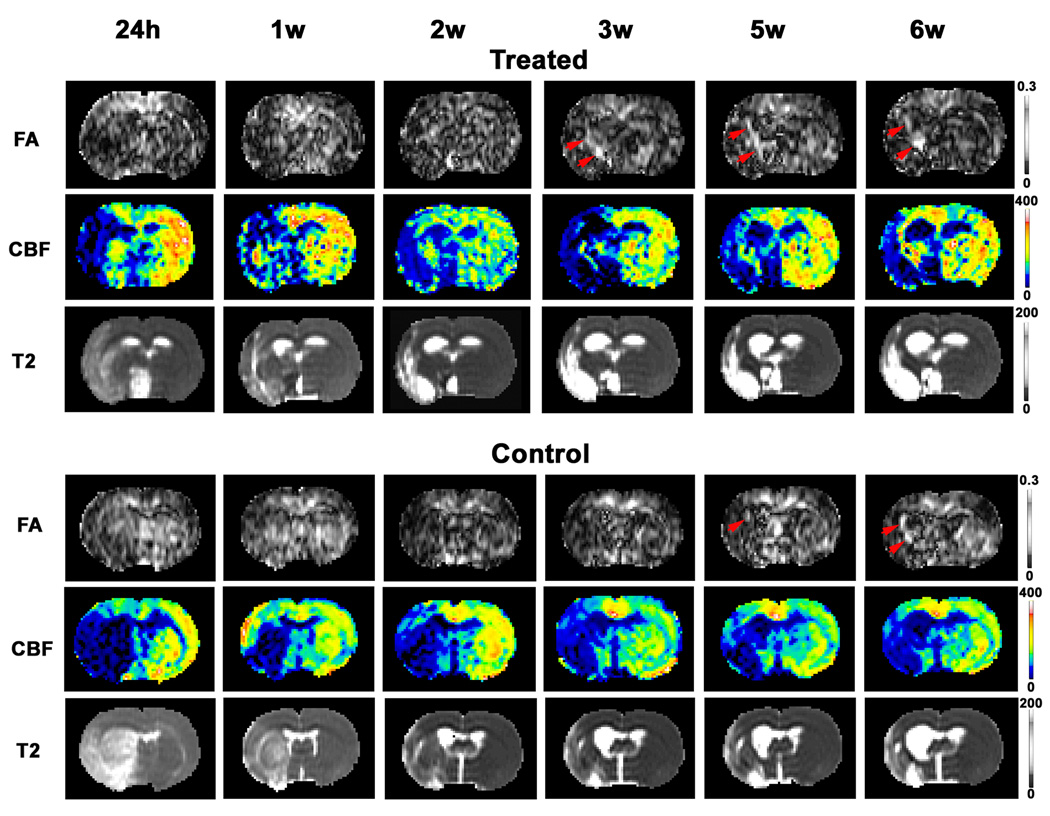
Fig. 2 gave a case comparison between the treated and control animals, showing that white matter reorganization characterized by FA map (red arrows on FA map) occurred earlier in the rhEPO-treated group than in the saline-treated controls. Our data also showed that no observable elevated FA areas were detected in the boundary region at 1 day post-MCAo for both rhEPO-treated and saline-treated animals. Visually apparent elevated areas on the FA map appeared as early as 1 week after stroke in the treated animals (3 of 11; 27%), but mostly after 3 weeks in the controls (6 of 7; 86%). White matter reorganization as indicated by the FA map took place significantly earlier in the rhEPO-treated group than in the non-treated group (p < 0.02). These dynamic MRI observations suggest that treatment with rhEPO accelerates white matter reorganization after stroke.
Fig. 2.
MRI maps showing changes in FA, CBF and T2 after MCAo for representative treated and control animals. Elevated areas on the FA map appeared earlier in the rhEPO-treated animals than in the controls (red arrows on FA map). In the treated animal, the area of white matter reorganization characterized by elevated pixels on the FA map coincided with the site of CBF restoration, whereas in the non-treated animal such a correlation was not apparent (comparing FA with CBF in elevated FA areas at each time point).
3.2 Measurements of elevated areas on FA map
FA value of the contralateral hemisphere, which is employed to determine the tissue area with structural change (Fig. 1), was measured and compared between the treated and control groups. No difference between the two groups was detected (p > 0.91).
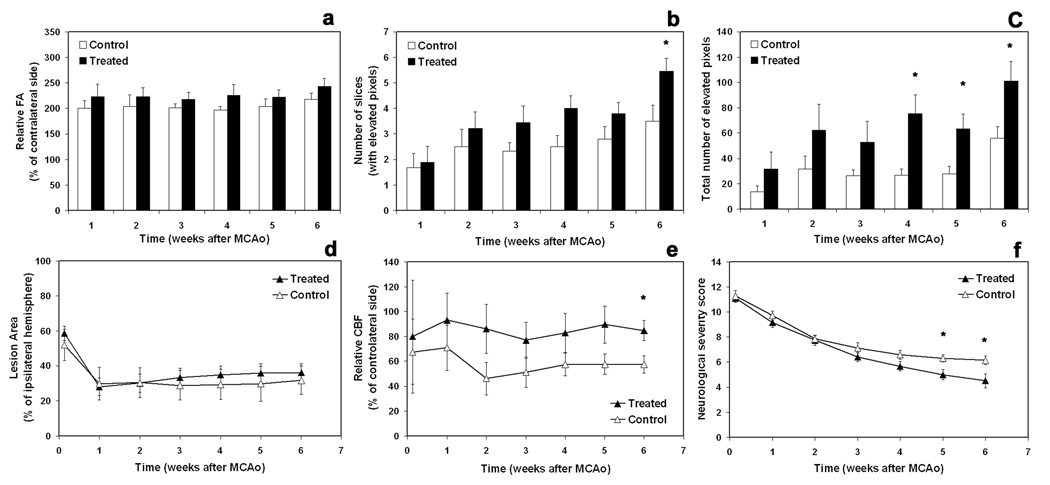
Changes in relative FA, number of slices with elevated FA pixels, and total number of elevated FA pixels throughout the brain are shown, respectively, in Fig. 3a–3c.
Fig. 3.
Quantitative data showing dynamic changes in relative FA (a), number of slices with elevated pixels (b), total number of elevated pixels (c), lesion area (d), relative CBF (e), and neurological severity scores (f) for both rhEPO-treated (n = 11) and control (n = 7) groups. * = p < 0.05 (based on ranked data), comparing two groups at the same time points.
As shown in Fig. 3a, relative FA was higher at each time point in the treated group than in the control group, although the difference did not achieve statistical significance. Unlike relative FA, number of slices with elevated FA pixels and total number of elevated FA pixels increased post-MCAo (Fig. 3b & 3c). By 6 weeks, a significantly higher number of slices containing elevated FA pixels were detected in the treated group than in the control group (Fig. 3b, 6-week, p < 0.05). Compared to the MCAo controls, treatment with rhEPO significantly increased the total number of elevated pixels on the FA map from 3 to 6 weeks after stroke (Fig. 3c), suggesting that rhEPO enhances structural reorganization.
3.3 Change in ischemic lesion size
Ischemic lesion size was identified by the T2 map and presented as a percentage of the ipsilateral hemisphere. Temporal profiles of the lesion area for the treated and control groups along with time post-MCAo are shown in Fig. 3d. We observed no significant difference between the two groups.
3.4 Evolution of CBF in the area of elevated FA
As shown in Fig. 2, the area of white matter reorganization characterized by the FA map (red arrows on FA map) coincided with the site of CBF restoration in treated animal, whereas no such evidence could be observed in non-treated animal (Fig. 2, comparing FA with CBF in elevated FA areas at each time point). More than half of the rhEPO-treated animals (6 of 11; 55%) and none of the controls exhibited this relationship. Quantitative data measured from two groups indicated that relative CBF values in the area of elevated FA were higher in the treated group than in the controls, and that a significant difference appeared by 6 weeks after stroke (Fig. 3e, p < 0.05).
3.5 Outcome of neurological function
Neurological severity scores (NSS) are given in Fig. 3f. Treatment with rhEPO significantly improved NSS during later stages of stroke (5 and 6 weeks) compared with the controls (p < 0.05).
4. Discussion
We investigated the progress of white matter reorganization in the rat brain with and without rhEPO intervention after embolic stroke both noninvasively and dynamically using in vivo MRI measurement of diffusion anisotropy. Our data demonstrate that the area of elevated fractional anisotropy (FA) in a specific region along the ischemic boundary after stroke reflects cerebral tissue undergoing white matter reorganization. This structural change can be identified and monitored on a FA map. The increase in elevated FA pixels corresponds with progress of white matter reorganization and is associated with improvement of neurological function, and therefore may be a good MRI index to stage the structural restorative process and predict potential functional outcome.
In the brain, diffusion of water varies significantly with direction, known as anisotropy. This anisotropic property of water diffusion can be quantified by the FA value as proposed by Basser and Pierpaoli.22 FA is a unitless measure, and a higher FA value indicates greater directionality. Anisotropic diffusion is most prominent in the white matter because it is organized into bundles of myelinated axonal fibers running in parallel.2, 7 Water diffuses more rapidly along the axon than across it, probably due to the presence of directional subcellular structures including the axonal membrane and the neurofilamentary cytoskeleton that serve as barriers to diffusion.23 A measure of anisotropy, such as FA, therefore provides an index of tissue microstructure and could possibly characterize the degree of fiber damage in diseases affecting the white matter.1–2 However, after stroke structural changes occur not only in the ischemic region but also in the cerebral tissue area undergoing reorganization.13 While a growing body of evidence has supported the ability of diffusion anisotropy to detect alteration of fiber tract integrity within the ischemic lesion after stroke,6–10 few data are available regarding structural reorganization at the ischemic boundary as measured by anisotropy.11–12 In addition, the dynamic relationship between structural reorganization and functional outcome is still relatively unknown. In the present study, we demonstrate that FA is a good measure of white matter reorganization, and that a larger area of white matter reorganization represented by elevated FA pixels in perilesional area indicates a better outcome of neurological function after stroke.
Our dynamic measurements of diffusion anisotropy revealed a unilateral asymmetric increase in FA adjacent to the lesion on the ipsilateral side, and these elevated areas on the FA map identified cerebral tissue undergoing white matter reorganization, which was confirmed histologically (Fig. 1). The temporal profile of relative FA in this area for both treated and control groups suggested that white matter reorganization, probably involving restoration and reformation of intact axonal membranes and reorganized fiber tracts, persisted to 6 weeks after stroke (Fig. 3a). This finding is well supported by other studies, which described long-term (3 years) improvement in white matter integrity in non-lesional brain region after human ischemic stroke.24 FA measurements showed not only that structural reorganization occurred earlier in the treated group than in the controls (Fig. 2), but also that the area undergoing white matter reorganization was larger in the treated animals than in the control animals (Fig. 3c), suggesting that rhEPO promotes structural reorganization after stroke. More importantly, the increase in reorganization area identified by FA map in the rhEPO-treated animals accompanied the improvement of neurological function evaluated by NSS (comparing Fig. 3c with Fig. 3f). A larger number of elevated FA pixels were associated with a lower NSS, or a better functional outcome. These data seem to imply that the increase in elevated pixels on the FA map characterizes the progress of white matter reorganization, and that this structural reorganization contributes to recovery of neurological function. Elevated FA values result from the increase in density and directionality of myelinated fiber tracts consistent with white matter reorganization after stroke. Our data indicated that rhEPO treatment enhanced such structural remodeling (Fig. 3c), and in turn, improved the neurological outcome (Fig. 3f).
Previous studies on FA recovery in lesion boundary tissue after transient MCAo were based on the ROIs where T2 recovered.11–12 FA increase resulted from white matter reorganization may have been attenuated, since the ROIs involved white and gray matter. In the present study, we directly identified the elevated FA area that was located in a specific perilesional region and represented white matter reorganization (Fig. 1). Discriminating this reorganization area from surrounding tissue allowed us to detect the effect of white matter reorganization on FA (Fig. 3a) and, particularly, to monitor the progress of these reorganization areas (Fig. 3c) along with time after stroke. FA data we presented here provide quantitative information about cerebral tissue undergoing structural change after stroke, which can not be revealed by ROI measurements.
As a member of the type I superfamily of cytokines, EPO is characterized by pleiotropic functionality.25 The biological activity of EPO extends well beyond erythropoiesis and includes several other important physiological processes, which expands the clinical use of EPO from the treatment of anemia to other pathological conditions such as stroke.26 Administration of EPO after focal ischemia protects cells,17, 27 tissues16, 28 and neural vessels29 and promotes angiogenesis14, 17 and neurogenesis14, 16, all of which may expedite structural reorganization after stroke by providing a restorative microenvironment.
The effect of EPO administration on ischemic lesion volume after stroke depends on treatment protocol.14, 30–31 Early treatment (e.g. ≤ 6 hours post stroke) with EPO reduces infarct volume,30–31 while delayed treatment (e.g. 24 hours post stroke) does not.14 Our long-term dynamic measurements confirm previous results that EPO administration (i.p.) initiated at 24 hours post stroke does not significantly alter lesion size (Fig. 3d).
Our findings also demonstrate that even without reduction of ischemic lesion size (Fig. 3d), rhEPO treatment improves neurological function (Fig. 3f) and restores local CBF (Fig. 3e), most likely the result of an EPO-induced integrative effect. Animals treated with rhEPO exhibited long-term restoration of CBF in the area of structural reorganization identified by the FA map (Fig. 2 & Fig. 3e), which may have been due to angiogenesis stimulated and enhanced by rhEPO.14, 17 Restoration of local blood supply in an ischemic brain plays a critical role in tissue repair and functional recovery.17 Our observation that CBF restoration and white matter reorganization co-existed strongly in rhEPO-treated animals (Fig. 2, comparing FA with CBF at elevated FA areas in the treated animal) suggests that local restoration of CBF may facilitate structural reorganization after stroke. However, such a combined effect was less evident in the controls, probably because angiogenesis was insufficient to restore CBF.
The method we used to identify the area of structural reorganization is an automatic operation without operator bias. But our data are still subject to errors. The FA value on the contralateral side provided a baseline in the current study to identify the cerebral tissue undergoing structural reorganization. Even though no difference in FA value on the contralateral hemisphere was detected, the threshold measured from a specific narrow tissue region could be affected by the noise of FA map. However, the noise affected all the animals in both groups. Given the restrictions of scan time, three-direction (x, y, z) instead of six-direction DWI were chosen to calculate FA. This measurement requires precise and consistent positioning of the animal in the scanner. Although a Tri-pilot sequence was used to precisely position the animal in the magnet before each scan, errors caused by minor change in position may influence FA. However, our data show that the FA map obtained from the current experimental setting reflects the microstructural changes (Fig. 2), as confirmed histologically (Fig. 1)
In summary, white matter reorganization in perilesional area during brain remodeling after stroke can be dynamically detected in vivo by measurement of diffusion anisotropy, such as FA map. Thus the FA map provides a noninvasive means for real-time visualization of structural reorganization following stroke. The degree of reorganization characterized by the area of elevated FA along the ischemic boundary can be helpful in estimating the potential for functional recovery. Treatment with rhEPO after stroke significantly enhances white matter reorganization, restores local CBF and expedites recovery of neurological function.
Acknowledgments
This work was supported by NINDS grants PO1 NS23393, PO1 NS42345, RO1 NS48349, RO1 NS38292, RO1 NS43324 and HL64766.
Footnotes
Disclosures
None.
References
- 1.van Gelderen P, de Vleeschouwer MH, DesPres D, Pekar J, van Zijl PC, Moonen CT. Water diffusion and acute stroke. Magn Reson Med. 1994;31:154–163. doi: 10.1002/mrm.1910310209. [DOI] [PubMed] [Google Scholar]
- 2.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concept and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 3.Liang Z, Zeng J, Liu S, Ling X, Xu A, Yu J, Ling L. A prospective study of secondary degeneration following subcortical infarction using diffusion tensor imaging. J Neurosurg Psychiatry. 2007;78:581–586. doi: 10.1136/jnnp.2006.099077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am J Neuroradiol. 1999;20:1491–1499. [PMC free article] [PubMed] [Google Scholar]
- 5.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meverand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 6.Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, Reese TG, Rosen BR, Wedeen VJ, Weisskoff RM. (1999) Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999;212:785–792. doi: 10.1148/radiology.212.3.r99se24785. [DOI] [PubMed] [Google Scholar]
- 7.Green HA, Pena A, Price CJ, Warburton FA, Pickard JD, Carpenter TA, Gillard JH. Increased anisotropy in acute stroke: a possible explanation. Stroke. 2002;33:1517–1521. doi: 10.1161/01.str.0000016973.80180.7b. [DOI] [PubMed] [Google Scholar]
- 8.Munoz Maniega S, Bastin ME, Armitage PA, Farrall AJ, Carpenter TK, Hand PJ, Cvoro V, Rivers CS, Wardlaw JM. Temporal evolution of water diffusion parameters is different in grey and white matter in human ischaemic stroke. Neurol Neurosurg Psychiatry. 2004;75:1714–1718. doi: 10.1136/jnnp.2003.033852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Bvblow WD. (2007) Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, D’Arceuil HE, Westmoreland S, He J, Duggan M, Gonzalez RG, Pryor J, de Crespigny AJ. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke. 2007;38:138–145. doi: 10.1161/01.STR.0000252127.07428.9c. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Q, Zhang ZG, Ding GL, Silver B, Zhang L, Meng H, Lu M, Pourabdillah-Nejed-D S, Wang L, Savant-Bhonsale S, Li L, Bagher-Ebadian H, Hu J, Arbab AS, Vanguri P, Ewing JR, Ledbetter KA, Chopp M. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32:1080–1089. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 12.van der Zijden JP, van der Toorn A, van der Marel K, DijKhuizen RM. Longitudinal in vivo MRI of alterations in perilesional tissue after transient ischemic stroke in rats. Exp Neurol. 2008;212:207–212. doi: 10.1016/j.expneurol.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Briwn CE, Li P, Boy JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Zhang Z, Wang Y, Zhang R, Choop M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Li Y, Cui Y, Chen J, Lu M, Elias SB, Chopp M. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034:34–39. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, Ferriero DM. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- 18.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Jiang Q, Ding GL, Zhang L, Zhang ZG, Ewing JR, Knight RA, Kapke A, Soltanian-Zadeh H, Chopp M. Map-ISODATA demarcates regional response to combination rt-PA and 7E3 F(ab’)2 treatment of embolic stroke in the rat. J Magn Reson Imaging. 2005;21:726–734. doi: 10.1002/jmri.20318. [DOI] [PubMed] [Google Scholar]
- 20.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motion: diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 21.Okazaki T, Magaki T, Takeda M, Kajiwara Y, Hanaya R, Sugiyama K, Arita K, Nishimura M, Kato Y, Kurisu K. Intravenous administration of bone marrow stromal cells increases survivin and Bcl-2 protein expression and improves sensorimotor function following ischemia in rats. Neurosci Lett. 2008;430:109–114. doi: 10.1016/j.neulet.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 22.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 23.Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, Thompson AJ. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neuol Neuosurg Psychiatry. 2000;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Stebbins GT, Nyenhuis DL, deToledo-Morrell L, Freels S, Gencheva E, Pedelty L, Sripathirathan K, Moseley ME, Turner DA, Gabrieli JD, Gorelick PB. Longitudinal changes in white matter following ischemic stroke: a three-year follow-up study. Neurobiol Aging. 2006;27:1827–1833. doi: 10.1016/j.neurobiolaging.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Selective modulation of the erythropoietic and tissue-protective effects of erythropoietin: time to reach the full therapeutic potential of erythropoietin. Biochim Biophys Acta. 2007;1776:1–9. doi: 10.1016/j.bbcan.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Torup L. Neuroprotection with or without erythropoiesis; sometimes less is more. Br J Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Lu ZY, Ogle M, Wei L. Erythropoietin prevents blood brain barrier damage induced by focal cerebral ischemia in mice. Neurochem Res. 2007;32:2132–2141. doi: 10.1007/s11064-007-9387-9. [DOI] [PubMed] [Google Scholar]
- 28.Chang YS, Mu D, Wendland M, Sheldon RA, Vexler ZS, McQuilen PS, Ferriero DM. Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatr Res. 2005;58:106–111. doi: 10.1203/01.PDR.0000163616.89767.69. [DOI] [PubMed] [Google Scholar]
- 29.Genc S, Koroglu TF, Genc K. Erythropoietin as a novel neuroprotectant. Restor Neurol Neurosci. 2004;22:105–119. [PubMed] [Google Scholar]
- 30.Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]