Abstract
Organic anion transporters (Oats) are located in the barrier epithelia of diverse organs, where they mediate the absorption and excretion of a wide range of metabolites, signaling molecules, and xenobiotics. Although their interactions with a broad group of substrates have been extensively studied and described, the primary physiological role of Oats remains elusive. The presence of overlapping substrate specificities among the different Oat isoforms, together with recent metabolomic data from the Oat1, Oat3, and renal-specific transporter (RST/URAT1) knockout mice, suggests a possible role in remote signaling wherein substrates excreted through one Oat isoform in one organ are taken up by another Oat isoform located in a different organ, thereby mediating communication between different organ systems, or even between different organisms. Here we further develop this “remote sensing and signaling hypothesis” and suggest how the regulation of SLC22 subfamily members (including those of the organic cation, organic carnitine, and unknown substrate transporter subfamilies) can be better understood by considering the organism's broader need to communicate between epithelial and other tissues by simultaneous regulation of transport of metabolites, signaling molecules, drugs, and toxins. This systems biology perspective of remote signaling (sensing) could help reconcile an enormous array of tissue-specific data for various SLC22 family genes and, possibly, other multispecific transporters, such as those of the organic anion transporting polypeptide (OATP, SLC21) and multidrug resistance-associated protein (MRP) families.
The transport of xenobiotics and endogenous metabolites across the epithelia of organs is mediated by a complex array of membrane transport systems, one of which is the organic anion transport (Oat) system. Members of the Oat family, which is characterized by a high structural and sequence homology, handle a diverse array of drugs that include loop and thiazide diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), angiotensin-converting enzyme inhibitors, β-lactam and sulfonamide antibiotics, and antiviral agents (Burckhardt and Burckhardt, 2003). When Oat1, the prototypical member of the Oat family was originally cloned and identified as novel kidney transporter (Lopez-Nieto et al., 1997), it was proposed that it, along with Oct1, was part of a larger subfamily of solute carrier (SLC) transporters, later designated SLC22 transporters. The SLC22 transporters have come to include the Oats, Octs, organic carnitine transporters, unknown substrate transporters, and urate transporters (Sweet et al., 2001; Eraly and Nigam, 2002; Eraly et al., 2004b; Wu et al., 2009).
Members of the Oat family continue to be identified and characterized, and include Oat2 [originally cloned as novel liver transporter in rat (Simonson et al., 1994)], Oat3 [originally cloned as ROCT (Brady et al., 1999; Kusuhara et al., 1999)], Oat4 (Cha et al., 2000), renal-specific transporter (RST)/URAT1 (Mori et al., 1997; Enomoto et al., 2002), Oat5 (Youngblood and Sweet, 2004), Oat6 (Monte et al., 2004), Oat7 (Shin et al., 2007), and Oat10 [originally identified as hORCTL3 (Bahn et al., 2008)]. Oats function mostly as exchangers, coupling entry of an organic anion to exit of another organic anion. Oat1 and Oat3, localized to the basolateral membrane of the renal proximal tubule (Kojima et al., 2002; Motohashi et al., 2002), couple organic anion entry into the cell with dicarboxylate exit (Sweet et al., 1997). This transport system is driven by concentration gradients produced by the Na+/K+-ATPase and the sodium-dicarboxylate cotransporter (NaDC3), which maintains high intracellular concentrations of dicarboxylates, especially α-ketoglutarate. Despite extensive characterization of the interaction between the Oats and a wide range of substrates, the primary physiological role of the Oats remains unclear. Oat1 knock-out mice were observed to manifest no apparent morphological or physiological abnormalities although reduced renal excretion of the prototypical substrate para-aminohippurate (PAH) and altered levels of endogenous organic anions such as benzoate, 3-hydroxybutyrate, 4-hydroxyphenyllactate, and N-acetylaspartate were observed in the plasma and/or urine (Eraly et al., 2006). Oat3 and RST knockout mice have also demonstrated alterations in levels of endogenous metabolites (Sweet et al., 2002; Eraly et al., 2008).
One possible important physiological role for the Oats is suggested by the overlapping substrate specificities between the different Oat isoforms and their localization in diverse organ systems. Oats and other multispecific transporters of the SLC22 subfamily (and possibly other SLC subfamilies as well as those of the multidrug resistance-associated protein, MRP, transporter family) may be involved in the transport of molecules between different tissue or fluid compartments, thereby participating in a “remote signaling” network between organ systems. This broader framework that considers diverse SLC22-expressing tissues and corresponding fluid compartments together with the demands placed on the whole organism can render the more fruitful interpretation of emerging data on tissue-specific expression and complex regulation of transporter expression and function during pre- and postnatal development and under conditions of stress and injury. Here, we explore the structure and tissue distribution of Oats, their potential role in chemosensation between organs and organisms, their function in maintaining whole body homeostasis, and their regulation by various factors.
Structure and Tissue Distribution of Oats
The Oats are generally 535 to 568 amino acids long and are considered to possess 12 transmembrane domains (TMD); the amino and carboxyl termini are located intracellularly (Sweet and Pritchard, 1999; Burckhardt and Wolff, 2000; Eraly et al., 2004a). A long hydrophilic extracellular loop is present between the first and second TMD and contains several N-linked glycosylation sites. Studies with hOat1 and hOat4 have shown that glycosylation is required for transporter trafficking to the plasma membrane (Tanaka et al., 2004; Zhou et al., 2005). A second long intracellular loop, present between the sixth and seventh TMDs, contains potential protein kinase C phosphorylation sites (Lopez-Nieto et al., 1997). Protein kinase C (PKC) activation has been reported to inhibit Oat activity (You, 2002; Terlouw et al., 2003) through a mechanism other than direct phosphorylation; PKC activation resulted in decreased mOat1 transport with no evidence of direct phosphorylation of the transporter (You et al., 2000), whereas mutation of canonical PKC consensus sites did not affect down-regulation of hOat1 transport by PKC activation (Wolff et al., 2003). In the case of hOat4, PKC activation resulted in the redistribution of the transporter from the cell surface to intracellular compartments (Zhou et al., 2007). Recent evidence also suggests that PKC inhibits Oat1 activity by regulating Oat1 internalization into recycling endosomes and that this internalization occurs partly through a dynamin- and clathrin-dependent pathway (Zhang et al., 2008).
Several conserved amino acids also seem to be crucial for transport function. Site-directed mutagenesis of histidine in TMD 1, lysine in TMD 8, and arginine in TMD 11 resulted in decreased transport of PAH in rat Oat3 and flounder renal Oat (Feng et al., 2001; Wolff et al., 2001). Leucine in TMD1 for hOat1 (Hong et al., 2004) and glycine in TMDs 5 and 8 of hOat4 (Zhou et al., 2004) have been implicated in directing of the transporters to the plasma membrane. Moreover, several amino acid residues in TMD7 have been shown to be essential for hOat1 transport activity and transporter stability (Hong et al., 2007).
The Oats are widely distributed in the epithelia of various organs (Table 1) (Anzai et al., 2006; Rizwan and Burckhardt, 2007; Zhou and You, 2007). Oat1 has been immunolocalized to the basolateral membrane of the proximal tubule in the human (Hosoyamada et al., 1999; Motohashi et al., 2002), rat (Tojo et al., 1999; Kojima et al., 2002) and mouse (Bahn et al., 2005) kidneys. Moreover, human Oat1 has also been identified in the brain and placenta (for review, see Ahn and Bhatnagar, 2008). Oats 2, 5, and 7 are expressed mainly in the liver (Simonson et al., 1994; Sun et al., 2001; Shin et al., 2007). Oat2 has also been localized to the basolateral membrane of human renal proximal tubule cells and to the apical membrane of the cortical collecting duct and the thick ascending limb in rats (Sweet, 2005). Oat3 is found in the basolateral membrane of the human, rat, and mouse proximal tubule and is distributed in the thick ascending limb of Henle's loop, distal convoluted tubule, and collecting duct in rats (Rizwan and Burckhardt, 2007). It is also expressed in the brain, specifically the choroid plexus, where it probably mediates the transport of drugs and metabolites from the cerebrospinal fluid to the blood (Sweet et al., 2002). Oat4 is found in both the placenta and the apical membrane of the proximal tubule (Cha et al., 2000). Oat5 has been localized to the apical membrane of rat proximal tubule cells (Sweet, 2005). The distribution of the Oats in diverse organs and the presence of the same Oat isoform in different tissues suggest that the Oats, by transporting signaling molecules and metabolites, may participate in a broader communication network between organs.
TABLE 1.
Gene loci and organ distribution of organic anion transporters (OATs)
| Oat Isoform | Gene | Gene Locus | Amino Acid Length | Endogenous Substrates | Tissue and [Membrane] Localization | Other Identified Homologs |
|---|---|---|---|---|---|---|
| hOAT1 | SLC22A6 | 11q13.1-13.2 | 506-563 | α-Ketoglutarate, urate, PGE2, PGF2α, folate, nicotinate, xanthine, hypoxanthine, neurotransmitter metabolites (5-methoxyindole-3-acetate, homovanillate, vanilmandelate, 3,4-dihydroxyphenyl acetate, 5-hydroxyindole-3-acetate, N-acetyl-5-hydroxytryptamine, melatonin) | Kidney [BL] | Rat, mouse, flounder, pig, rabbit, monkey, Caenorhabditis elegans |
| Brain [U] | ||||||
| Placenta [U] | ||||||
| hOAT2 | SLC22A7 | 6p21.2-21.1 | 546 and 548 | cAMP, propionate, DHEAS, ES, PGE2, PGF2α, l-ascorbate, α-ketoglutarate | Kidney [BL] | Mouse, rat |
| Liver [SM] | ||||||
| hOAT3 | SLC22A8 | 11q11.7 | 542 | α-Ketoglutarate, cAMP, cortisol, PGE2, PGF2α, DHEAS, ES, taurocholate, urate, estradiol-17 β-glucuronide | Kidney [BL] | Rat, mouse, pig, rabbit, monkey |
| Brain [LM] | ||||||
| Adrenals [U] | ||||||
| Skeletal muscle [U] | ||||||
| Developing bone [U] | ||||||
| hOAT4 | SLC22A11 | 11q13.1 | 550 | ES, DHEAS, estrone, 17β-estradiol-3-sulfate, PGE2, PGF2α, octanoate, succinate, cholate, urate, taurocholate | Kidney [LM] | |
| Adrenals [U] | ||||||
| Placenta [BL] | ||||||
| mOat5 | slc22a19 | 19 | 551 | ES, DHEAS | Kidney [LM] | Rat |
| mOat6 | slc22a20 | 19 | 556 | ES, propionate, methylbutyrate, benzoate, heptanoate, 2-ethylhexanoate, pyruvate, PGE2 | Olfactory mucosa [U] | Human, rat |
| Testis [U] | ||||||
| hOAT7 | SLC22A9 | 11q13.1 | 553 | ES, β-estradiol sulfate, DHEAS, butyrate, propionate, valerate, caproate, lactate, nicotinate, acetate | Liver [SM] | |
| hOAT10 | SLC22A13 | 3p21.3 | 551 | Nicotinate, urate, succinate, l-lactate, glutarate | Kidney [LM] | |
| Brain [U] | ||||||
| Heart [U] | ||||||
| Colon [U] | ||||||
| hURAT1 | SLC22A12 | 11q13.1 | 332 and 553 | Urate, acetoacetate, succinate, β-hydroxybutyrate, lactate, nicotinate, α-ketoglutarate | Kidney [LM] | Mouse |
hOAT, human organic anion transporter; mOat, mouse organic anion transporter; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α, DHEAS, dehydroepiandrosterone sulfate; ES, estrone-3-sulfate; BL, basolateral membrane; U, undetermined; SM, sinusoidal membrane; LM, luminal membrane.
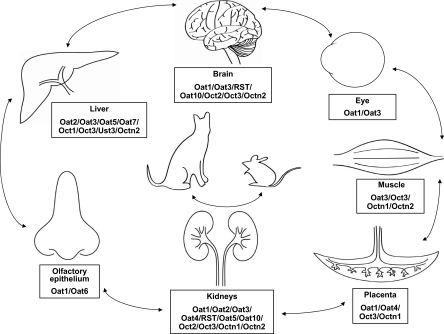
Oat1 and Oat6—A Potential Role in Olfactory Sensing?
The Oats are predominantly expressed in the kidney, choroid plexus, and liver (Eraly et al., 2004b; Robertson and Rankin, 2006). However, Oat6 (slc22a20) was found to be primarily expressed in the olfactory epithelium in mice, and to a lesser degree in testis and early embryonic tissues (Monte et al., 2004). It shares 40 and 60% sequence homology with Oat1 and Oat3, respectively, and contains the signature sequence motifs that are found in the slc22 family (Monte et al., 2004). Through in situ hybridization, it was observed that Oat6 was expressed in the olfactory epithelium but not in the neurons of the main olfactory epithelium or the vomeronasal organ (Kaler et al., 2006). Oat6 was found to possess a significant affinity for small volatile compounds that have been previously identified as odortype molecules in mouse urine (Singer et al., 1997; Willse et al., 2005). These odortype molecules have also been found at increased levels in the plasma of Oat1 knockout mice, suggesting that odortype substances excreted through an Oat1-mediated mechanism in the kidney may be taken up by the olfactory mucosa though an Oat6-mediated mechanism (Fig. 1). The possibility that a path like this may constitute part of a signaling mechanism between organisms is supported by the observation that mammals use odor cues in urine for identity recognition (Sherborne et al., 2007; Bates et al., 2008). Furthermore, Oat6 could conceivably modulate signaling from the olfactory epithelium to distant organs such as the brain, where other members of the SLC22 family, such as Oat1, Oat3, OCTN2. and RST are expressed (Kido et al., 2001; Imaoka et al., 2004; Kusuhara and Sugiyama, 2005). Although it remains unclear whether humans possess the Oat6 homolog, this alternate transport pathway that circumvents the blood-brain barrier raises the possibility of designing intranasally administered drugs targeted toward specific Oats and other SLC22 or multispecific transporters expressed in the olfactory mucosa and the brain.
Fig. 1.
Chemosensation between organs/organisms mediated by SLC22 family members. Organic anion transporters (Oats), organic cation transporters (Octs), organic carnitine transporters (Octns), and unknown substrate transporters (Usts) located in the barrier epithelia of certain organs may take up substrates excreted by transporters in other organs (arrows), thus mediating potential remote signaling and therefore communication between organs. This method of communication via the SLC22 transporters can also occur between organisms; for example, odor-type molecules excreted through Oat1 in the urine of cat may be taken up by the Oat6 in the olfactory mucosa of mouse.
Oat3 as a Potential Blood Pressure Regulator
Oat1 knockout mice, despite showing accumulation of several endogenous organic anions in the plasma as well as diminished responsiveness to diuretics under basal conditions (Vallon et al., 2008b), do not demonstrate apparent physiological abnormalities (Eraly et al., 2006). On the other hand, Oat3 knockout mice manifest a 10 to 15% lower blood pressure than wild-type mice, suggesting possible involvement of Oat3 in blood pressure regulation (Vallon et al., 2008a). Metabolomic analyses in Oat3 knockout mice revealed increased plasma levels of potential Oat3 substrates that may serve as endogenous blood pressure regulators. These compounds included thymidine, which when administered to mice in vivo resulted in a 10 to 15% reduction in blood pressure and was also found to be transported by Oat3 in vitro. Other known inhibitors of Oat3 (i.e., eosin-Y and probenecid) also resulted in reduction of blood pressure when administered to mice. Additional proposed modulators of blood pressure that are Oat3 substrates include cGMP and cAMP (Eraly, 2008), cyclic nucleotides that cause vasodilation.
An intriguing finding is the expression of Oat3 and Urat1 in the vascular smooth muscle cell (Hediger et al., 2005; Yamamoto et al., 2006). Oat3 and Urat1 transport uric acid, which has long been linked to hypertension (Hediger et al., 2005; Feig et al., 2008). Although the exact role of Oat3, and possibly Urat1, in blood pressure regulation remains unknown, a potential mechanism may involve regulation of their transport of endogenous blood pressure modulators. A remote sensing circuit may be present wherein changes in blood pressure sensed by the kidney are transmitted into changes in Oat3 expression or function (through regulatory mechanisms such as phosphorylation or glycosylation.) Changes in Oat3 expression or function, in turn, would result in altered excretion of blood pressure modulators. For example, elevated blood pressure sensed by the kidney could lead to decreased Oat3 expression or function and therefore reduced renal excretion of vasodilators. The vasodilator would accumulate in the body and subsequently be taken up by Oat3 expressed in the vascular smooth muscle, resulting in lowered blood pressure. This potential involvement of Oat3 in blood pressure regulation supports a central role for the Oats in responding to changes in the internal and external environment and thereby maintaining whole body homeostasis.
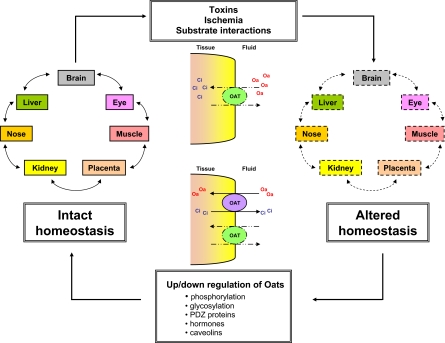
Role of Oats in Homeostasis—The Effect of Toxins, Ischemia and Substrate Interactions
Organisms are constantly exposed to environmental toxins and stressors that perturb whole-body homeostasis and can ultimately lead to tissue injury or death; therefore, the ability to eliminate these toxins and counteract the stressors is vital for the organism's survival. The Oats play an important role in this process by mediating the clearance of a vast array of toxins that include drugs (e.g., methotrexate, NSAIDs, antiviral agents, cephalosporins), environmental toxins (e.g., mycotoxins, mercuric conjugates) and some products of endogenous metabolism (e.g., uremic toxins such as indoxyl sulfate) (Sweet, 2005). Impaired elimination of these toxins, subsequent accumulation in the body and thus perturbation of homeostasis can conceivably be a result of either reduced Oat function or competitive inhibition of binding to the Oats by other substrates (drugs, environmental toxins, or metabolites) (Fig. 2). Furthermore, a broader consideration of the action of exogenous toxic substrates in the context of a remote sensing system involving communication between cells and tissues via small endogenous molecules may lead to the development of a new view on the effects of drugs and toxins that are Oat substrates on whole-organism physiology as well as cell and tissue-specific toxicities.
Fig. 2.
Potential role of organic anion transporters in homeostasis. By eliminating both endogenous and exogenous toxins, the Oats play a potentially critical role in maintaining whole-body homeostasis. Factors that impair the clearance of substrates by the Oats, such as toxins, ischemia, or competitive inhibition by other substrates, can disrupt Oat function and therefore the efficient exchange of organic anions (Oa) and their counter-ions (Ci) across the membrane. This can eventually lead to perturbed homeostasis. To compensate for the loss of function of other Oats and thereby restore homeostasis, enhanced expression and/or function of the Oats can occur on the transcriptional, translational or post-translational level in either the injured tissues or in other tissues participating in a remote-sensing network.
Endogenous and Exogenous Toxins
Decreased Oat function can result from the cytotoxicity conferred by the same toxins that they transport; significant tissue injury can result from these toxins because they are taken up by the Oats and accumulate within the cell. NSAIDs such as acetylsalicylate, salicylate, and ibuprofen, substrates of Oat1 and Oat3, have been associated with renal papillary necrosis (Muhalwas et al., 1981; Shah et al., 1981). Methotrexate, transported by hOAT2 (expressed in liver) and hOAT3 (expressed in kidney), has been linked to both hepatic and renal toxicity (Dubin and Harrell, 1969; Frei et al., 1975). Antiviral agents, such as adefovir and cidofovir, which are transported by hOAT1, induce renal proximal tubular injury that can be prevented by coadministration of NSAIDs (Cihlar et al., 1999; Mulato et al., 2000). Products of endogenous metabolism can also cause cytotoxicity and reduced Oat function. Indoxyl sulfate (IS), a product of dietary protein metabolism that interacts with Oat1, Oat3, and Oat4, accumulates within the proximal tubule cell during renal failure and triggers free radical production and eventually nephrotoxicity (Enomoto and Niwa, 2007). Furthermore, it has been shown that IS may also exert toxic effects on bone metabolism through its uptake by Oat3 expressed in bone osteoblasts (Nii-Kono et al., 2007). Accumulation of IS in bone osteoblasts has been associated with free radical generation and reduced levels of parathyroid hormone receptor expression (Nii-Kono et al., 2007).
Ischemia/Reperfusion Injury
Ischemia is another important cause of tissue injury and hence altered Oat function. PAH clearance has been reported to be decreased in recipients of cadaveric renal allografts 3 to 7 days after transplant; immunohistochemical analyses of these renal allografts have shown altered distribution of hOat1 from the basolateral membrane of proximal tubule cells to the cytoplasm (Kwon et al., 2007). In rat kidneys subjected to ischemia/reperfusion injury, reduced clearance of PAH was also observed, together with a decreased expression of both Oat1 and Oat3 protein and mRNA levels (Matsuzaki et al., 2007; Schneider et al., 2007; Di Giusto et al., 2008). It is interesting to consider the possibility that Oats or other multispecific transporters (e.g., OATPs, multidrug resistance-associated proteins) in other epithelial tissues may, through a remote signaling network, compensate in an effort to preserve organismal homeostasis until the injured tissue recovers.
Substrate Interactions
Competition for binding and transport to the Oats has been demonstrated by numerous drug-drug interactions, among the more frequently reported of which is the inhibition by probenecid, a well known substrate of Oat1 and Oat3, of the renal excretion of several drugs including methotrexate, cidofovir, and NSAIDs (Aherne et al., 1978; Schild and Roch-Ramel, 1988; Cundy et al., 1996). This competitive inhibition has been used to advantage in some clinical settings, such as coadministration with probenecid to reduce the nephrotoxic effects and prolong the serum half-life of β-lactam antibiotics or antiviral agents (e.g., adefovir and cidofovir).
Competitive binding to the Oats extends beyond xenobiotics to include endogenous substrates. In patients with renal failure, the accumulation of uremic toxins may inhibit the clearance of neurotransmitter metabolites from the brain by Oat1 and Oat3, leading to neurologic impairment (Sweet, 2005). In fact, homovanillic acid, a substrate of Oat1 and Oat3, has been detected at elevated levels in the cerebrospinal fluid of patients with uremic encephalopathy (Moe and Sprague, 1994; Alebouyeh et al., 2003). This interaction between substrates and Oats expressed in different organs is made more complex by the presence of overlapping substrate specificity among the Oats. Although substrate discrimination is present among the Oat isotypes as manifested by the presence of important differences in the structural binding determinants among Oat1, Oat3, and Oat6 (Kaler et al., 2007; Truong et al., 2008), a significant substrate overlap can still be appreciated among the different Oats. Furthermore, overlapping substrate specificity with other transporters such as the Octs (Ullrich et al., 1993) is also present. Thus, not only organic anions but also organic cations (especially in disease states leading to altered pH of body fluids) may compete for binding to the Oats, adding to the complexity of the interaction between Oat systems expressed in multiple organs and a vast array of both endogenous and exogenous substrates.
Reacting to perturbations in homeostasis brought about by the factors mentioned above and bringing the whole body system back to balance may be another important role played by the Oats. This restoration of homeostasis can potentially occur through up-regulation of the Oats on the transcriptional or translational level to compensate for decreased Oat function in the same cell, related cells, or distant cells participating in a remote signaling network. For instance, up-regulation of Oats in the intact proximal tubule cells may be one mechanism whereby the organism compensates for the loss of Oats in damaged proximal tubule cells. Indeed, it has been observed that proximal tubule cells exposed to ischemic insult initially show decreased expression of Oat1 and Oat3, but later on demonstrate up-regulated Oat1 and Oat3 expression accompanied by recovery of PAH clearance (Schneider et al., 2007). Furthermore, rats that had sustained liver injury by bile duct ligation demonstrated increased expression of Oat1 in the kidney cortex together with a higher urinary excretion of PAH, suggesting the presence of a compensation mechanism for the impaired hepatic route of substrate elimination (Brandoni et al., 2003).
Other Physiological Roles
Oat2 was initially cloned as novel liver-specific transporter from rat (Simonson et al., 1994) and since then has been cloned from human and mouse and functionally characterized (Sun et al., 2001; Kobayashi et al., 2002b). Oat2 substrates include prostaglandin E2, PAH, tetracycline, and salicylate (Anzai et al., 2006). Propionate, a 3-carbon short-chain fatty acid, was also found recently to be transported by Oat2 (Islam et al., 2008). Propionate is eventually converted to succinyl-CoA, an intermediate of the tricarboxylic acid cycle, and also serves as a ligand for GPR41 (Brown et al., 2003), a G-protein-coupled receptor that stimulates leptin release (Xiong et al., 2004). Therefore, through modulation of propionate transport, Oat2 may regulate cellular metabolism in different organs. A role in remote signaling for Oat2 is also supported by the observation that it avidly transports cGMP, the second messenger that modulates intracellular responses to external stimuli in diverse cell types (Cropp et al., 2008).
The association of serum uric acid levels with multiple disorders, including hypertension, the metabolic syndrome, coronary artery disease, cerebrovascular disease, and kidney disease (for review, Feig et al., 2008) has placed urate metabolism and transport at the center of much interest. Urate excretion through the kidneys seems to be mediated in part by the Oats. Oat1 and Oat3 knockout mice showed slightly decreased urine urate levels, whereas RST (murine ortholog of the human uric acid transporter URAT1) knockout mice demonstrated elevated urine urate levels, suggesting that Oat1 and Oat3 mediate the renal secretion of urate, whereas RST mediates the renal reabsorption of urate (Eraly et al., 2008). It is noteworthy that recent genome-wide association studies failed to show an association between URAT1 and serum urate levels; instead, a link was demonstrated with the locus encoding GLUT9 (Li et al., 2007; Döring et al., 2008; Vitart et al., 2008; Wallace et al., 2008), indicating that other transporters may be involved in urate excretion.
Regulation of Oats
The vital role that the Oats play in whole-body homeostasis through toxin clearance and their potential role in mediating an intricate communication network between organ systems by transporting “signaling” molecules between tissues may explain the presence of complex regulatory mechanisms that control Oat activity. These regulatory mechanisms are apparent from embryogenesis, where Oat1 and Oat3 expression begins at midgestation in concert with proximal tubule differentiation in murine kidneys and slowly increases through nephron development (Lopez-Nieto et al., 1997; Pavlova et al., 2000; Sweet et al., 2006). Oat expression was also detected in extrarenal tissues during embryogenesis with Oat1 transcripts detected in the murine brain and Oat2 in liver, lung, intestine, and developing bone and cartilage (Pavlova et al., 2000).
The need to finely regulate Oat activity is also evidenced by the regulation of Oat activity, not only at the translational level but also at the transcriptional level. Computational analysis of the murine and human genomic loci of OAT1-3 have revealed several conserved binding sites for transcriptional factors important in kidney development, such as WT-1, Pbx, Tcf, and hepatocyte nuclear factor (HNF-1) (Barasch, 2001; Schnabel et al., 2001; Eraly et al., 2003b). HNF-1 regulates the transcription of other renal transporters such as type II sodium-glucose cotransporter (Pontoglio et al., 2000) and sodium/phosphate cotransporters (Soumounou et al., 2001; Cheret et al., 2002), therefore raising the possibility of its being a transcriptional regulator of the Oats (Eraly et al., 2003a). Subsequently, several studies have shown that HNF isoforms activate Oat1, Oat3, and Urat1 promoter activities. Oat1, Oat2, and Oat3 expression was found to be markedly down-regulated in kidneys of HNF-1α-null mice compared with wild type (Maher et al., 2006), and HNF-1α/β was subsequently found to transactivate human and mouse Oat1 promoters (Saji et al., 2008). Furthermore, hOat1 promoter activity is also regulated by HNF-4α, which acts through a response element consisting of an inverted repeat of hexamers separated by eight nucleotides (Ogasawara et al., 2007). The HNF-1α homodimer and the HNF-1α/β heterodimer have been shown to increase hOAT3 promoter activity in human embryonic kidney (HEK) 293 cells, whereas DNA methylation was shown to repress the promoter activity (Kikuchi et al., 2006). A similar regulatory mechanism was observed for Urat1, where mouse/human URAT1 promoter activity was activated by HNF-1α/β heterodimer and repressed by DNA methylation (Kikuchi et al., 2007).
Recent interest has focused on the role of proteins such as caveolins and PDZ proteins in regulation of OAT activity and expression (Zhou and You, 2007). PDZ proteins, which bind to PDZ consensus binding sites at the carboxyl terminus of transporter proteins, have been implicated in the selective targeting of the transporters to the plasma membrane and their retention and regulation at the membrane (Brône and Eggermont, 2005). PDZK1 was found to interact with Urat1 in yeast two-hybrid assays and transfection of Urat1 expressing HEK293 cells with PDZK1 resulted in increased surface expression and transport activity of Urat1 (Anzai et al., 2004). Further studies have shown that the PDZ domain-containing proteins PDZK1 and NHERF1 interacted with hOAT4 and that they increased estrone-3-sulfate transport in hOAT4-expressing HEK293 cells (Miyazaki et al., 2005). Moreover, hOAT4 cell surface expression in kidney LLC-PK1 cells was found to be stimulated by PDZK1 and NHERF1, whereas no effect was observed in human placenta BeWo cells, suggesting tissue-specific regulation (Zhou et al., 2008).
Oat expression also seems to be gender-dependent (Kobayashi et al., 2002a; Kudo et al., 2002; Buist and Klaassen, 2004) and may explain certain differences in pharmacokinetics between genders. Oat1 mRNA expression in the kidney is higher in male rodents compared with female rodents (Buist and Klaassen, 2004), which is consistent with previous observations that PAH transport is higher in intact male rat renal cortical slices than in those of orchiectomized male rats (Reyes et al., 1998). Oat3 mRNA expression in the liver was also observed to be higher in male rats than in female rats (Buist et al., 2002). On the other hand, Oat2 mRNA and protein levels are higher in female than in male rodent kidneys, and its expression is weakly stimulated by estradiol and progesterone and inhibited by androgens (Ljubojević et al., 2007). Human OAT4 also seems to be under hormonal regulation with progesterone exposure resulting in down-regulation (Zhou et al., 2007).
Thus, the regulation of Oat expression and function is quite complex; if the remote sensing hypothesis has merit, the data discussed above already suggest multiple points at which such a remote sensing (signaling) network can undergo dynamic regulation.
Conclusions
The development of Oat knockout mice and the use of methods such as metabolomic and computational analysis have brought great strides in our understanding of the structure and binding properties of Oats and the endogenous substrates transported by the Oats. The continuing expansion of the list of endogenous substrates transported by the Oats provides important clues to their physiological role. The overlap between substrates transported by the different Oats and their potential role as signaling molecules, suggest that the Oats may play essential roles in a broad “remote signaling” network among different organs and organisms. By considering the differing tissue expression patterns of Oats and other SLC22 as well as non-SLC22 multispecific transporters (e.g., organic anion-transporting polypeptides, Oatps, and multidrug resistance proteins) and their complex regulation in the framework of this remote signaling hypothesis and the demands placed on the whole organism in normal physiology (including pre- and postnatal development) and states of extreme stress (e.g., acute ischemic injury or toxin exposure), it may be possible to understand what currently seems like a bewildering array of data on many genes with varied tissue expression, regulation, and functional transport.
This work was supported by the National Institutes of Health National Institute of Allergy and Infectious Diseases [Grant R01-AI057695], the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK079784], and the National Institutes of Health National Institute of Neurological Disorders and Stroke[Grant RO1-NS062156] (all to S.K.N.).
ABBREVIATIONS: Oat, organic anion transporter; NSAID, nonsteroidal anti-inflammatory drug; SLC, solute carrier; Oct, organic cation transporter; PAH, para-aminohippurate; TMD, transmembrane domain; PKC, protein kinase C; IS, indoxyl sulfate; HNF, hepatocyte nuclear factor; HEK, human embryonic kidney; PDZ, postsynaptic density 95/disc-large/zona occludens; Urat, uric acid transporter.
References
- Aherne GW, Piall E, Marks V, Mould G, and White WF (1978) Prolongation and enhancement of serum methotrexate concentrations by probenecid. Br Med J 1 1097-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SY and Bhatnagar V (2008) Update on the molecular physiology of organic anion transporters. Curr Opin Nephrol Hypertens 17 499-505. [DOI] [PubMed] [Google Scholar]
- Alebouyeh M, Takeda M, Onozato ML, Tojo A, Noshiro R, Hasannejad H, Inatomi J, Narikawa S, Huang XL, Khamdang S, et al. (2003) Expression of human organic anion transporters in the choroid plexus and their interactions with neurotransmitter metabolites. J Pharmacol Sci 93 430-436. [DOI] [PubMed] [Google Scholar]
- Anzai N, Kanai Y, and Endou H (2006) Organic anion transporter family: current knowledge. J Pharmacol Sci 100 411-426. [DOI] [PubMed] [Google Scholar]
- Anzai N, Miyazaki H, Noshiro R, Khamdang S, Chairoungdua A, Shin HJ, Enomoto A, Sakamoto S, Hirata T, Tomita K, et al. (2004) The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem 279 45942-45950. [DOI] [PubMed] [Google Scholar]
- Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, Burckhardt BC, Sabolic I, and Burckhardt G (2008) Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem 283 16332-16341. [DOI] [PubMed] [Google Scholar]
- Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolic I, Burckhardt G, and Hagos Y (2005) Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol 289 C1075-1084. [DOI] [PubMed] [Google Scholar]
- Barasch J (2001) Genes and proteins involved in mesenchymal to epithelial transition. Curr Opin Nephrol Hypertens 10 429-436. [DOI] [PubMed] [Google Scholar]
- Bates LA, Sayialel KN, Njiraini NW, Poole JH, Moss CJ, and Byrne RW (2008) African elephants have expectations about the locations of out-of-sight family members. Biol Lett 4 34-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KP, Dushkin H, Förnzler D, Koike T, Magner F, Her H, Gullans S, Segre GV, Green RM, and Beier DR (1999) A novel putative transporter maps to the osteosclerosis (oc) mutation and is not expressed in the oc mutant mouse. Genomics 56 254-261. [DOI] [PubMed] [Google Scholar]
- Brandoni A, Quaglia NB, and Torres AM (2003) Compensation increase in organic anion excretion in rats with acute biliary obstruction: role of the renal organic anion transporter 1. Pharmacology 68 57-63. [DOI] [PubMed] [Google Scholar]
- Brône B and Eggermont J (2005) PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes. Am J Physiol Cell Physiol 288 C20-29. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. (2003) The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278 11312-11319. [DOI] [PubMed] [Google Scholar]
- Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, and Klaassen CD (2002) Gender-specific and developmental influences on the expression of rat organic anion transporters. J Pharmacol Exp Ther 301 145-151. [DOI] [PubMed] [Google Scholar]
- Buist SC and Klaassen CD (2004) Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1-3; Slc22a6-8) mRNA levels. Drug Metab Dispos 32 620-625. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC and Burckhardt G (2003) Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol 146 95-158. [DOI] [PubMed] [Google Scholar]
- Burckhardt G and Wolff NA (2000) Structure of renal organic anion and cation transporters. Am J Physiol Renal Physiol 278 F853-866. [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, and Endou H (2000) Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem 275 4507-4512. [DOI] [PubMed] [Google Scholar]
- Cheret C, Doyen A, Yaniv M, and Pontoglio M (2002) Hepatocyte nuclear factor 1 alpha controls renal expression of the Npt1-Npt4 anionic transporter locus. J Mol Biol 322 929-941. [DOI] [PubMed] [Google Scholar]
- Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, and Sweet DH (1999) The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol 56 570-580. [DOI] [PubMed] [Google Scholar]
- Cropp CD, Komori T, Shima JE, Urban TJ, Yee SW, More SS, and Giacomini KM (2008) Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol 73 1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundy KC, Li ZH, and Lee WA (1996) Effect of probenecid on the distribution, metabolism, and excretion of cidofovir in rabbits. Drug Metab Dispos 24 315-321. [PubMed] [Google Scholar]
- Di Giusto G, Anzai N, Endou H, and Torres AM (2008) Elimination of organic anions in response to an early stage of renal ischemia-reperfusion in the rat: role of basolateral plasma membrane transporters and cortical renal blood flow. Pharmacology 81 127-136. [DOI] [PubMed] [Google Scholar]
- Döring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, Fischer G, Henke K, Klopp N, Kronenberg F, et al. (2008) SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet 40 430-436. [DOI] [PubMed] [Google Scholar]
- Dubin HV and Harrell ER (1969) Absorption of methotrexate and hepatotoxicity. JAMA 210 1104. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, et al. (2002) Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417 447-452. [DOI] [PubMed] [Google Scholar]
- Enomoto A and Niwa T (2007) Roles of organic anion transporters in the progression of chronic renal failure. Ther Apher Dial 11 (Suppl 1): S27-S31. [DOI] [PubMed] [Google Scholar]
- Eraly SA (2008) Organic anion transporter 3 inhibitors as potential novel antihypertensives. Pharmacol Res 58 257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Blantz RC, Bhatnagar V, and Nigam SK (2003a) Novel aspects of renal organic anion transporters. Curr Opin Nephrol Hypertens 12 551-558. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Bush KT, Sampogna RV, Bhatnagar V, and Nigam SK (2004a) The molecular pharmacology of organic anion transporters: from DNA to FDA? Mol Pharmacol 65 479-487. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Hamilton BA, and Nigam SK (2003b) Organic anion and cation transporters occur in pairs of similar and similarly expressed genes. Biochem Biophys Res Commun 300 333-342. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Monte JC, and Nigam SK (2004b) Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol Genomics 18 12-24. [DOI] [PubMed] [Google Scholar]
- Eraly SA and Nigam SK (2002) Novel human cDNAs homologous to Drosophila Orct and mammalian carnitine transporters. Biochem Biophys Res Commun 297 1159-1166. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Rieg T, Gangoiti JA, Wikoff WR, Siuzdak G, Barshop BA, and Nigam SK (2008) Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics 33 180-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, et al. (2006) Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem 281 5072-5083. [DOI] [PubMed] [Google Scholar]
- Feig DI, Kang DH, and Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359 1811-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Dresser MJ, Shu Y, Johns SJ, and Giacomini KM (2001) Arginine 454 and lysine 370 are essential for the anion specificity of the organic anion transporter, rOAT3. Biochemistry 40 5511-5520. [DOI] [PubMed] [Google Scholar]
- Frei E 3rd, Jaffe N, Tattersall MH, Pitman S, and Parker L (1975) New approaches to cancer chemotherapy with methotrexate. N Engl J Med 292 846-851. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Johnson RJ, Miyazaki H, and Endou H (2005) Molecular physiology of urate transport. Physiology 20 125-133. [DOI] [PubMed] [Google Scholar]
- Hong M, Zhou F, Lee K, and You G (2007) The putative transmembrane segment 7 of human organic anion transporter hOAT1 dictates transporter substrate binding and stability. J Pharmacol Exp Ther 320 1209-1215. [DOI] [PubMed] [Google Scholar]
- Hong M, Zhou F, and You G (2004) Critical amino acid residues in transmembrane domain 1 of the human organic anion transporter hOAT1. J Biol Chem 279 31478-31482. [DOI] [PubMed] [Google Scholar]
- Hosoyamada M, Sekine T, Kanai Y, and Endou H (1999) Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol 276 F122-F128. [DOI] [PubMed] [Google Scholar]
- Imaoka T, Kusuhara H, Adachi-Akahane S, Hasegawa M, Morita N, Endou H, and Sugiyama Y (2004) The renal-specific transporter mediates facilitative transport of organic anions at the brush border membrane of mouse renal tubules. JAmSoc Nephrol 15 2012-2022. [DOI] [PubMed] [Google Scholar]
- Islam R, Anzai N, Ahmed N, Ellapan B, Jin CJ, Srivastava S, Miura D, Fukutomi T, Kanai Y, and Endou H (2008) Mouse organic anion transporter 2 (mOat2) mediates the transport of short chain fatty acid propionate. J Pharmacol Sci 106 525-528. [DOI] [PubMed] [Google Scholar]
- Kaler G, Truong DM, Khandelwal A, Nagle M, Eraly SA, Swaan PW, and Nigam SK (2007) Structural variation governs substrate specificity for organic anion transporter (OAT) homologs. Potential remote sensing by OAT family members. J Biol Chem 282 23841-23853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler G, Truong DM, Sweeney DE, Logan DW, Nagle M, Wu W, Eraly SA, and Nigam SK (2006) Olfactory mucosa-expressed organic anion transporter, Oat6, manifests high affinity interactions with odorant organic anions. Biochem Biophys Res Commun 351 872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido Y, Tamai I, Ohnari A, Sai Y, Kagami T, Nezu J, Nikaido H, Hashimoto N, Asano M, and Tsuji A (2001) Functional relevance of carnitine transporter OCTN2 to brain distribution of L-carnitine and acetyl-L-carnitine across the blood-brain barrier. J Neurochem 79 959-969. [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Hattori N, Kim I, Shiota K, Gonzalez FJ, and Sugiyama Y (2007) Regulation of tissue-specific expression of the human and mouse urate transporter 1 gene by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. Mol Pharmacol 72 1619-1625. [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Hattori N, Shiota K, Kim I, Gonzalez FJ, and Sugiyama Y (2006) Regulation of the expression of human organic anion transporter 3 by hepatocyte nuclear factor 1alpha/beta and DNA methylation. Mol Pharmacol 70 887-896. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Hirokawa N, Ohshiro N, Sekine T, Sasaki T, Tokuyama S, Endou H, and Yamamoto T (2002a) Differential gene expression of organic anion transporters in male and female rats. Biochem Biophys Res Commun 290 482-487. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Ohshiro N, Shibusawa A, Sasaki T, Tokuyama S, Sekine T, Endou H, and Yamamoto T (2002b) Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in mice. Mol Pharmacol 62 7-14. [DOI] [PubMed] [Google Scholar]
- Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, and Endou H (2002) Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J Am Soc Nephrol 13 848-857. [DOI] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, and Kawashima Y (2002) Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem Biol Interact 139 301-316. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, and Endou H (1999) Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem 274 13675-13680. [DOI] [PubMed] [Google Scholar]
- Kusuhara H and Sugiyama Y (2005) Active efflux across the blood-brain barrier: role of the solute carrier family. NeuroRx 2 73-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O, Hong SM, and Blouch K (2007) Alteration in renal organic anion transporter 1 after ischemia/reperfusion in cadaveric renal allografts. J Histochem Cytochem 55 575-584. [DOI] [PubMed] [Google Scholar]
- Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, Lai S, Dei M, Orrù M, Albai G, et al. (2007) The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet 3 e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubojević; M, Balen D, Breljak D, Kusan M, Anzai N, Bahn A, Burckhardt G, and Sabolić; I (2007) Renal expression of organic anion transporter OAT2 in rats and mice is regulated by sex hormones. Am J Physiol Renal Physiol 292 F361-372. [DOI] [PubMed] [Google Scholar]
- Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, and Nigam SK (1997) Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J Biol Chem 272 6471-6478. [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Callaghan TN, Cheng X, Cheung C, Gonzalez FJ, and Klaassen CD (2006) Alterations in transporter expression in liver, kidney, and duodenum after targeted disruption of the transcription factor HNF1alpha. Biochem Pharmacol 72 512-522. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Watanabe H, Yoshitome K, Morisaki T, Hamada A, Nonoguchi H, Kohda Y, Tomita K, Inui K, and Saito H (2007) Downregulation of organic anion transporters in rat kidney under ischemia/reperfusion-induced acute [corrected] renal failure. Kidney Int 71 539-547. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Anzai N, Ekaratanawong S, Sakata T, Shin HJ, Jutabha P, Hirata T, He X, Nonoguchi H, Tomita K, et al. (2005) Modulation of renal apical organic anion transporter 4 function by two PDZ domain-containing proteins. J Am Soc Nephrol 16 3498-3506. [DOI] [PubMed] [Google Scholar]
- Moe SM and Sprague SM (1994) Uremic encephalopathy. Clin Nephrol 42 251-256. [PubMed] [Google Scholar]
- Monte JC, Nagle MA, Eraly SA, and Nigam SK (2004) Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem Biophys Res Commun 323 429-436. [DOI] [PubMed] [Google Scholar]
- Mori K, Ogawa Y, Ebihara K, Aoki T, Tamura N, Sugawara A, Kuwahara T, Ozaki S, Mukoyama M, Tashiro K, et al. (1997) Kidney-specific expression of a novel mouse organic cation transporter-like protein. FEBS Lett 417 371-374. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, and Inui K (2002) Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol 13 866-874. [DOI] [PubMed] [Google Scholar]
- Muhalwas KK, Shah GM, and Winer RL (1981) Renal papillary necrosis caused by long-term ingestion of pentazocine and aspirin. Jama 246 867-868. [PubMed] [Google Scholar]
- Mulato AS, Ho ES, and Cihlar T (2000) Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adefovir mediated by the human renal organic anion transporter 1. J Pharmacol Exp Ther 295 10-15. [PubMed] [Google Scholar]
- Nii-Kono T, Iwasaki Y, Uchida M, Fujieda A, Hosokawa A, Motojima M, Yamato H, Kurokawa K, and Fukagawa M (2007) Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int 71 738-743. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Terada T, Asaka J, Katsura T, and Inui K (2007) Hepatocyte nuclear factor-4{alpha} regulates the human organic anion transporter 1 gene in the kidney. Am J Physiol Renal Physiol 292 F1819-F1826. [DOI] [PubMed] [Google Scholar]
- Pavlova A, Sakurai H, Leclercq B, Beier DR, Yu AS, and Nigam SK (2000) Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and Roct. Am J Physiol Renal Physiol 278 F635-F643. [DOI] [PubMed] [Google Scholar]
- Pontoglio M, Prié D, Cheret C, Doyen A, Leroy C, Froguel P, Velho G, Yaniv M, and Friedlander G (2000) HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep 1 359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JL, Meléndez E, Alegría A, and Jaramillo-Juárez F (1998) Influence of sex differences on the renal secretion of organic anions. Endocrinology 139 1581-1587. [DOI] [PubMed] [Google Scholar]
- Rizwan AN and Burckhardt G (2007) Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res 24 450-470. [DOI] [PubMed] [Google Scholar]
- Robertson EE and Rankin GO (2006) Human renal organic anion transporters: characteristics and contributions to drug and drug metabolite excretion. Pharmacol Ther 109 399-412. [DOI] [PubMed] [Google Scholar]
- Saji T, Kikuchi R, Kusuhara H, Kim I, Gonzalez FJ, and Sugiyama Y (2008) Transcriptional regulation of human and mouse organic anion transporter 1 by hepatocyte nuclear factor 1 alpha/beta. J Pharmacol Exp Ther 324 784-790. [DOI] [PubMed] [Google Scholar]
- Schild L and Roch-Ramel F (1988) Transport of salicylate in proximal tubule (S2 segment) isolated from rabbit kidney. Am J Physiol 254 F554-F561. [DOI] [PubMed] [Google Scholar]
- Schnabel CA, Selleri L, Jacobs Y, Warnke R, and Cleary ML (2001) Expression of Pbx1b during mammalian organogenesis. Mech Dev 100 131-135. [DOI] [PubMed] [Google Scholar]
- Schneider R, Sauvant C, Betz B, Otremba M, Fischer D, Holzinger H, Wanner C, Galle J, and Gekle M (2007) Downregulation of organic anion transporters OAT1 and OAT3 correlates with impaired secretion of para-aminohippurate after ischemic acute renal failure in rats. Am J Physiol Renal Physiol 292 F1599-F1605. [DOI] [PubMed] [Google Scholar]
- Shah GM, Muhalwas KK, and Winer RL (1981) Renal papillary necrosis due to ibuprofen. Arthritis Rheum 24 1208-1210. [DOI] [PubMed] [Google Scholar]
- Sherborne AL, Thom MD, Paterson S, Jury F, Ollier WE, Stockley P, Beynon RJ, and Hurst JL (2007) The genetic basis of inbreeding avoidance in house mice. Curr Biol 17 2061-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HJ, Anzai N, Enomoto A, He X, Kim do K, Endou H, and Kanai Y (2007) Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology 45 1046-1055. [DOI] [PubMed] [Google Scholar]
- Simonson GD, Vincent AC, Roberg KJ, Huang Y, and Iwanij V (1994) Molecular cloning and characterization of a novel liver-specific transport protein. J Cell Sci 107 1065-1072. [DOI] [PubMed] [Google Scholar]
- Singer AG, Beauchamp GK, and Yamazaki K (1997) Volatile signals of the major histocompatibility complex in male mouse urine. Proc Natl Acad Sci U S A 94 2210-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumounou Y, Gauthier C, and Tenenhouse HS (2001) Murine and human type I Na-phosphate cotransporter genes: structure and promoter activity. Am J Physiol Renal Physiol 281 F1082-F1091. [DOI] [PubMed] [Google Scholar]
- Sun W, Wu RR, van Poelje PD, and Erion MD (2001) Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun 283 417-422. [DOI] [PubMed] [Google Scholar]
- Sweet DH (2005) Organic anion transporter (Slc22a) family members as mediators of toxicity. Toxicol Appl Pharmacol 204 198-215. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Bush KT, and Nigam SK (2001) The organic anion transporter family: from physiology to ontogeny and the clinic. Am J Physiol Renal Physiol 281 F197-F205. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Eraly SA, Vaughn DA, Bush KT, and Nigam SK (2006) Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int 69 837-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, and Nigam SK (2002) Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem 277 26934-26943. [DOI] [PubMed] [Google Scholar]
- Sweet DH and Pritchard JB (1999) The molecular biology of renal organic anion and organic cation transporters. Cell Biochem Biophys 31 89-118. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Wolff NA, and Pritchard JB (1997) Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J Biol Chem 272 30088-30095. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Xu W, Zhou F, and You G (2004) Role of glycosylation in the organic anion transporter OAT1. J Biol Chem 279 14961-14966. [DOI] [PubMed] [Google Scholar]
- Terlouw SA, Masereeuw R, and Russel FG (2003) Modulatory effects of hormones, drugs, and toxic events on renal organic anion transport. Biochem Pharmacol 65 1393-1405. [DOI] [PubMed] [Google Scholar]
- Tojo A, Sekine T, Nakajima N, Hosoyamada M, Kanai Y, Kimura K, and Endou H (1999) Immunohistochemical localization of multispecific renal organic anion transporter 1 in rat kidney. J Am Soc Nephrol 10 464-471. [DOI] [PubMed] [Google Scholar]
- Truong DM, Kaler G, Khandelwal A, Swaan PW, and Nigam SK (2008) Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J Biol Chem 283 8654-8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich KJ, Rumrich G, David C, and Fritzsch G (1993) Bisubstrates: substances that interact with renal contraluminal organic anion and organic cation transport systems. I. Amines, piperidines, piperazines, azepines, pyridines, quinolines, imidazoles, thiazoles, guanidines and hydrazines. Pflugers Arch 425 280-299. [DOI] [PubMed] [Google Scholar]
- Vallon V, Eraly SA, Wikoff WR, Rieg T, Kaler G, Truong DM, Ahn SY, Mahapatra NR, Mahata SK, Gangoiti JA, et al. (2008a) Organic anion transporter 3 contributes to the regulation of blood pressure. J Am Soc Nephrol 19 1732-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, and Nigam SK (2008b) Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol 294 F867-F873. [DOI] [PubMed] [Google Scholar]
- Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, et al. (2008) SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40 437-442. [DOI] [PubMed] [Google Scholar]
- Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, Ahmadi K, Dobson RJ, Marçano AC, Hajat C, et al. (2008) Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet 82 139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willse A, Belcher AM, Preti G, Wahl JH, Thresher M, Yang P, Yamazaki K, and Beauchamp GK (2005) Identification of major histocompatibility complex-regulated body odorants by statistical analysis of a comparative gas chromatography/mass spectrometry experiment. Anal Chem 77 2348-2361. [DOI] [PubMed] [Google Scholar]
- Wolff NA, Grünwald B, Friedrich B, Lang F, Godehardt S, and Burckhardt G (2001) Cationic amino acids involved in dicarboxylate binding of the flounder renal organic anion transporter. J Am Soc Nephrol 12 2012-2018. [DOI] [PubMed] [Google Scholar]
- Wolff NA, Thies K, Kuhnke N, Reid G, Friedrich B, Lang F, and Burckhardt G (2003) Protein kinase C activation downregulates human organic anion transporter 1-mediated transport through carrier internalization. J Am Soc Nephrol 14 1959-1968. [DOI] [PubMed] [Google Scholar]
- Wu W, Baker ME, Eraly SA, Bush KT and Nigam SK (2009) Analysis of a large cluster of Slc22 transporter genes, including novel USTS, reveals species-specific amplification of subsets of family members. Physiol Genomics doi: 10.1152/physiolgenomics.90309.2008 [DOI] [PMC free article] [PubMed]
- Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, and Yanagisawa M (2004) Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A 101 1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Tsuruoka S, Ioka T, Ando H, Ito C, Akimoto T, Fujimura A, Asano Y, and Kusano E (2006) Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int 69 1780-1785. [DOI] [PubMed] [Google Scholar]
- You G (2002) Structure, function, and regulation of renal organic anion transporters. Med Res Rev 22 602-616. [DOI] [PubMed] [Google Scholar]
- You G, Kuze K, Kohanski RA, Amsler K, and Henderson S (2000) Regulation of mOAT-mediated organic anion transport by okadaic acid and protein kinase C in LLC-PK(1) cells. J Biol Chem 275 10278-10284. [DOI] [PubMed] [Google Scholar]
- Youngblood GL and Sweet DH (2004) Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol Renal Physiol 287 F236-F244. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Hong M, Duan P, Pan Z, Ma J, and You G (2008) Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem 283 32570-32579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Hong M, and You G (2007) Regulation of human organic anion transporter 4 by progesterone and protein kinase C in human placental BeWo cells. Am J Physiol Endocrinol Metab 293 E57-E61. [DOI] [PubMed] [Google Scholar]
- Zhou F, Tanaka K, Pan Z, Ma J, and You G (2004) The role of glycine residues in the function of human organic anion transporter 4. Mol Pharmacol 65 1141-1147. [DOI] [PubMed] [Google Scholar]
- Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, and You G (2005) The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol 67 868-876. [DOI] [PubMed] [Google Scholar]
- Zhou F, Xu W, Tanaka K, and You G (2008) Comparison of the interaction of human organic anion transporter hOAT4 with PDZ proteins between kidney cells and placental cells. Pharm Res 25 475-480. [DOI] [PubMed] [Google Scholar]
- Zhou F and You G (2007) Molecular insights into the structure-function relationship of organic anion transporters OATs. Pharm Res 24 28-36. [DOI] [PubMed] [Google Scholar]